Abstract
Background
Clinical trials have demonstrated the superior efficacy of immune checkpoint inhibitor (ICI)-based combination therapy over sunitinib, a multi-target tyrosine kinase inhibitor (TKI), in patients with advanced renal cell carcinoma. However, such benefits have not been elucidated in populations outside of clinical trials.
Methods
We retrospectively evaluated data from 467 patients with advanced renal cell carcinoma who received ICI-based combination therapy or TKIs, as first-line therapy. Clinical outcome was compared between ICI-based combination therapy and TKIs in each population divided according to trial eligibility.
Results
Among 152 patients treated with ICI-based combination therapy and 315 patients treated with TKIs, 76 (50.0%) and 156 (49.5%) were trial ineligible, respectively. Overall survival (p = 0.0072) and objective response rate (p < 0.0001) were significantly higher in ICI-based combination therapy than in TKIs, but progression-free survival was comparable (p = 0.681). In the trial-eligible population, overall survival was longer (p = 0.0906) and the objective response rate was significantly higher (p = 0.0124) in ICI-based combination therapy than in TKIs. In the trial-ineligible population, overall survival (p = 0.0208) and objective response rate (p = 0.0006) were significantly higher with ICI-based combination therapy than with TKIs. A multivariate analysis also showed that ICI-based combination therapy was independently associated with prolonged overall survival (hazard ratio, 0.47; p = 0.0016). Regardless of trial eligibility, progression-free survival did not differ between ICI-based combination therapy and TKIs (trial eligible: p = 0.287; trial ineligible: p = 0.0708).
Conclusions
The present study, using real-world data, provides evidence indicating the therapeutic benefit of ICI-based combination therapy over TKIs for advanced renal cell carcinoma was more statistically significant in the trial-ineligible population than in the trial-eligible population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Half of the real-world patients with advanced renal cell carcinoma were outside clinical trials in a systemic therapy setting, including immune-checkpoint inhibitor-based combination therapy and tyrosine kinase inhibitors. |
Immune-checkpoint inhibitor-based combination therapy was more successful than tyrosine kinase inhibitors with regard to the improvement of clinical outcomes in the general population. |
The therapeutic advantage of immune-checkpoint inhibitor-based combination therapy was statistically more significant among the trial-ineligible population than in the trial-eligible population. |
1 Introduction
The therapeutic landscape for advanced renal cell carcinoma (RCC) has undergone rapid advancement. In the era of tyrosine kinase inhibitors (TKIs), the mainstay of systemic therapy has shifted to immune checkpoint inhibitors (ICIs). In the first-line setting, pivotal clinical trials have demonstrated the superior efficacy of ICI dual combination therapy (i.e., immunotherapy [IO]-IO) and ICI plus TKI combination therapy (i.e., IO-TKI) over sunitinib, a multi-target TKI [1,2,3,4,5]. Currently, ICI-based combination therapy plays a central role in systemic therapy for advanced RCC [6,7,8].
Confirming findings from clinical trials, real-world data have also supported the superior outcomes in the ICI-based combination therapy era compared with the previous TKI era [9, 10]. In the real world, patient heterogeneity is much greater than in clinical trials. Furthermore, there are more patients with poor prognosticators in the real world, as clinical trials generally set strict enrollment criteria and result in the exclusion of these potentially high-risk patients. In fact, multiple studies have indicated that the oncological outcome of patients not meeting the trial eligibility criteria was inferior to that of trial-eligible patients with various types of cancer treated with systemic therapy, including cytotoxic chemotherapy and molecular-targeted therapy [11,12,13,14,15,16].
In the context of ICI-based therapy, such evidence is still limited. We recently reported that patients with advanced RCC, determined to be trial ineligible, had a comparable outcome profile to that of a trial-eligible population in ICI-based combination therapy [17]. Another recent study also suggested that survival was not inferior in trial-eligible compared to trial-ineligible populations treated with pembrolizumab for previously treated advanced urothelial carcinoma [18]. Conversely, a large cohort study using a database of multiple cancer types showed inferior survival in a trial-ineligible population than in a trial-eligible population receiving ICI-based therapy [19]. Therefore, as data on the efficacy of ICI-based combination therapy for cancer, including RCC, in the population outside clinical trials remain limited and conflicting, further knowledge about this field is necessary.
Clinical trials have demonstrated the feasible efficacy of ICI-based combination therapy for advanced RCC [1,2,3,4,5], but caution is needed for the direct application of trial data to real-world patients because a subset of them lies outside the trials and we do not have solid data on outcome profiles in such populations. In other words, evidence that shows the superiority of ICI-based combination therapy over TKIs based on trial eligibility in real-world patients is not fully available.
In this context, we retrospectively analyzed the differences in outcomes between ICI-based combination therapy and TKIs for patients with advanced RCC based on trial eligibility using data from multiple institutions in a real-world setting.
2 Patients and Methods
2.1 Patient Selection and Study Design
The study protocol was approved by the Institutional Ethics Review Board of Tokyo Women’s Medical University (ID:2020-0009). This study was performed in accordance with the principles outlined in the Declaration of Helsinki of 1964 and its subsequent amendments. The requirement of informed consent was waived owing to the retrospective observational nature of this study. All clinical and laboratory data were obtained from electronic databases and medical records.
At our department and at four affiliated institutions, 507 patients with advanced RCC received at least one administration of TKIs or ICIs as first-line therapy between January 2008 and August 2022. From these, we excluded 12 patients who received drugs as adjuvant therapy after radical surgery and 28 patients whose clinical data, including the follow-up period, were insufficient. The remaining 467 patients were evaluated in this retrospective multi-institutional study.
Patients were assessed for trial ineligibility when they met at least one of the following criteria: Karnofsky Performance Status score < 70%, hemoglobin level < 9.0 g/dL, estimated glomerular filtration rate < 40 mL/min/1.73 m2, platelet count < 100,000/µL, neutrophil count < 1500/ µL, and non-clear cell histology or brain metastasis, according to a previous study [19]. Even if data were missing for one or several factors, patients were determined to be trial ineligible if they had at least one trial-ineligible factor. Based on a previous study, patients were determined to be trial eligible if they had no trial-ineligible factors, even if data were missing for the other factors [11].
The patients were further divided according to treatment class into ICI-based combination therapy and TKIs. We compared progression-free survival (PFS) and overall survival (OS) after initiation of treatment and the objective response rate (ORR) during treatment based on trial eligibility between ICI-based combination therapy and TKIs.
2.2 Treatment Protocol of ICI-Based Combination Therapy and TKIs
Monotherapy with a TKI was the standard of care for patients with advanced RCC between January 2008 and August 2018. Sorafenib was administered as first-line therapy but was subsequently replaced by sunitinib or pazopanib. The sunitinib schedule adopted in our institutions consisted of an alternative 2-week-on/1-week-off pattern, based on previous studies [20,21,22]. Sorafenib or pazopanib was preferred in patients with severe comorbidity such as end-stage renal disease requiring maintenance dialysis therapy [23,24,25]. Axitinib or cabozantinib has not been used as first-line therapy to date.
In September 2018, ICI-based combination therapy was implemented. Nivolumab plus ipilimumab was selected for intermediate-risk or high-risk patients with the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk classification. From December 2019, pembrolizumab plus axitinib was selected in all patients, regardless of the IMDC risk, as presurgical therapy for locally advanced cases, or in patients histopathologically diagnosed with papillary RCC, based on our previous findings [26]. At approximately the same time, avelumab plus axitinib was preferentially selected in IMDC favorable-risk patients, older aged patients, or those with severe comorbidities who were considered intolerable to steroid administration when immune-related adverse events (AEs) developed (e.g., uncontrolled diabetes mellitus) as this combination therapy was reported to relatively lower the risk of immune-related AE development requiring corticosteroid therapy than other regimens [3]. From September 2021, cabozantinib plus nivolumab was selected in all patients regardless of the risk of IMDC but was preferred in patients who required early tumor shrinkage because of disease-related symptoms or as presurgical therapy for locally advanced cases, as this combination therapy was reported to have a relatively higher effect on tumor response than other regimens [4]. From February 2022, lenvatinib plus pembrolizumab was selected based on the same criteria as those applied to cabozantinib plus nivolumab, as this combination therapy had a higher effect on tumor response than other regimens [5].
Until August 2018, selection of treatment classes was limited to TKI monotherapy because of drug availability. Since September 2018, treatment classes including ICI-based combination therapy and TKI monotherapy could be selected; however, ICI-based combination therapy was selected at our affiliated institutions by principle. Nevertheless, whether ICI-based combination therapy or TKI monotherapy was selected and which regimens or drugs among ICI-based combination therapy or TKI monotherapy were selected, the final decision depends on the choice of physicians.
Post-treatment follow-up computed tomography of the chest, abdomen, and pelvis was performed at regular 4-week to 12-week intervals depending on the patient’s condition. Magnetic resonance imaging or positron emission tomography/computed tomography was performed when necessary. A brain scan was also performed when necessary. Any drugs were administered until radiographic or clinical disease progression or intolerable AEs occurred. Tumor response was assessed using RECIST version 1.1 [27].
2.3 Statistical Analysis
Continuous variables were analyzed using the Mann–Whitney U test and categorical variables were analyzed using the Fisher’s exact test. Progression-free survival was calculated from the initiation of treatment until disease progression or death, whichever occurred first. Overall survival was calculated from the initiation of treatment to death from any cause. Patients lost to follow-up were censored at the time of last contact. Survival data up to the end of October 2022 were obtained. Survival was calculated using the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate analyses using Cox proportional hazard regression models were conducted to identify risk factors for survival. Risks are expressed as hazard ratios with 95% confidence intervals (CIs). Statistical significance was set at p < 0.05.
3 Results
3.1 Trial Eligibility Profile and Patient Background
Of a total of 467 patients, 152 (32.5%) and 315 (67.4%) were treated with ICI-based combination therapy or TKIs, respectively. In each treatment group, 76 (50.0%) and 156 (49.5%) patients, respectively, did not meet the trial eligibility criteria and this rate was comparable between the two treatment groups (p = 1.000). Detailed trial eligibility profiles are shown in Table 1. The number of patients with multiple factors who were determined to be trial ineligible was comparable between the two treatment groups (n = 25 [16.4%] vs n = 51 [16.2%], p = 0.995). The most common factor was kidney dysfunction (i.e., low estimated glomerular filtration rate) in both groups (ICI-based combination therapy: n = 35 [23.0%]; TKIs: n = 103 [32.7%]), and this rate was significantly higher in TKIs than in ICI-based combination therapy (p = 0.0395).
We aimed to evaluate outcome differences between ICI-based combination therapy and TKIs based on trial eligibility, we first compared patient backgrounds between ICI-based combination therapy and TKIs in trial-eligible and trial-ineligible populations (Table 2). The number of patients who underwent nephrectomy prior to the initiation of systemic therapy was significantly lower for ICI-based combination therapy compared with TKIs in the trial-eligible population (n = 51 [67.1%] vs n = 145 [91.2%], p < 0.0001) and in the trial-ineligible population (n = 51 [67.1%] vs n = 136 [87.2%], p = 0.0006). Conversely, other factors regarding patient background were not significantly different between the two treatment groups, in both the trial-eligible and trial-ineligible populations (all, p > 0.05).
3.2 Survival Between ICI-Based Combination Therapy and TKIs Based on Trial Eligibility
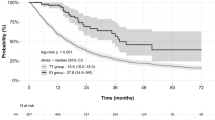
Overall, 335 and 240 patients experienced disease progression and died of any cause, respectively, during the median follow-up period of 18.0 months (interquartile range: 7.89–35.4). Progression-free survival was not significantly different between ICI-based combination therapy and TKIs (median: 10.2 [95% CI 6.71–16.1] vs 9.86 [95% CI 8.58–12.1] months, p = 0.681) (Fig. 1a), while OS was significantly longer in ICI-based combination therapy than in TKIs (median: not reached (N.R.) [31.0–N.R.] vs 26.1 [21.1–32.6] months, p = 0.0072) (Fig. 1b).
Furthermore, we compared survival between ICI-based combination therapy and TKIs according to trial eligibility. In the trial-eligible population, PFS was not significantly different between the two treatment groups (median: 13.1 [7.66–24.0] vs 14.7 [12.0–19.1] months, p = 0.287) [Fig. 2a and Table 1 of the Electronic Supplementary Material [ESM]). Conversely, OS was likely to be longer in ICI-based combination therapy than in TKIs, but the difference was not statistically significant (median: N.R. [31.0–N.R.] vs 43.0 [31.5–74.3] months, p = 0.0906) [Fig. 2b and Table 1 of the ESM]. In the trial-ineligible population, PFS was likely to be longer in ICI-based combination therapy than in TKIs, but the difference was not statistically significant (median: 7.36 [4.54–13.3] vs 6.15 [5.06–7.89] months, p = 0.0708) [Fig. 2a and Table 1 of the ESM]. In contrast, OS was significantly longer in ICI-based combination therapy than in TKIs (median: 33.7 [20.8–38.4] vs 17.8 [14.1–22.5] months, p = 0.0208) [Fig. 2b and Table 1 of the ESM]. Furthermore, we performed univariate and multivariate analyses to determine whether differences in systemic therapy (i.e., ICI-based combination therapy vs TKIs) were independently associated with OS in the trial-ineligible population. The analyses showed that ICI-based combination therapy was independently associated with prolonged OS (hazard ratio: 0.47 [95% CI 0.29–0.75], p = 0.0016) (Table 3).
3.3 Tumor Response Between ICI-Based Combination Therapy and TKIs Based on Trial Eligibility
Next, we compared tumor response rates between ICI-based combination therapy and TKIs based on trial eligibility. In general, the ORR was significantly higher in ICI-based combination therapy than in TKIs (48.7% vs 27.6%, p < 0.0001) [Fig. 3 and Table 2 of the ESM]. When analyzed according to trial eligibility, ORRs were significantly higher in ICI-based combination therapy than in TKIs both in trial-eligible populations (51.3% vs 34.0%, p = 0.0124) and in trial-ineligible populations (46.1% vs 21.2%, p = 0.0006).
Comparison of tumor response between immune checkpoint inhibitor (ICI)-based combination therapy and tyrosine kinase inhibitors (TKIs) based on trial eligibility. The number at the top of the bar indicates the objective response rate (the sum of the completed response [CR] and partial response [PR] rates)
3.4 Impact of Trial Ineligibility on Survival and Tumor Response
We previously reported that a substantial number of patients harboring multiple factors were considered to be ineligible for participation in clinical trials in the real-world setting of molecular-targeted therapy for advanced RCC, and notably, such patients had worse OS [16]. Indeed, the present cohort showed that 16.3% of the patients (76/467) exhibited multiple trial-ineligible factors (Table 1).
We focused on survival and tumor response rates in this population. In patients with a single trial-ineligible factor, OS was not significantly different between ICI-based combination therapy and TKIs (median: 33.7 [95% CI 20.8–N.R.] vs 22.5 [15.5–28.0] months, p = 0.205) [Fig. 4a and Table 3 of the ESM]. Conversely, in patients with multiple trial-ineligible factors, OS was significantly longer in ICI-based combination therapy than in TKIs (median: 38.4 [11.4–38.4] vs 11.6 [5.62–17.8] months, p = 0.0248). Regarding ORRs, both in patients with single and multiple trial-ineligible factors, ORRs were significantly higher in ICI-based combination therapy than in TKIs (patients with a single trial-ineligible factor: 47.1% vs 25.7%, p = 0.0295; patients with multiple factors: 44.0% vs 11.8%, p = 0.0056) [Fig. 4b and Table 2 of the ESM].
Comparison of overall survival and tumor response between immune checkpoint inhibitor (ICI)-based combination therapy and tyrosine kinase inhibitors (TKIs) based on the burden of trial ineligibility. a Overall survival and b objective tumor response. The number at the top of the bar indicates an objective response rate (the sum of rates of complete response [CR] and partial response [PR]). CI confidence interval, NR not reached
4 Discussion
This retrospective study evaluating systemic therapy in patients with advanced RCC in a real-world setting showed that half of the patients would have been ineligible for clinical trials. In the overall cohort, ICI-based combination therapy induced significantly higher efficacy than TKIs in terms of OS and ORR, which was in line with previous findings from pivotal clinical trials [1,2,3,4,5]. According to trial eligibility, in the trial-eligible population, OS was likely to be higher and the ORR was significantly higher in ICI-based combination therapy than in TKIs. In contrast, in the trial-ineligible population, OS and ORR were significantly higher in ICI-based combination therapy than in TKIs. The multivariate analysis also showed that ICI-based combination therapy was independently associated with prolonged OS. These data suggest that the superiority of oncological efficacy of ICI-based combination therapy over TKIs was more significant in populations outside clinical trials than in trial-eligible populations.
Clinical trials are designed to assess the mean effect of the experimental treatment in a highly selected study population. Patients with potentially unfavorable clinical factors, such as organ dysfunction or poor general condition, are generally excluded. Thus, the effects of a new drug examined in clinical trials may not always be the same in a clinical setting [28]. Importantly, the real-world population generally harbors strong heterogeneity, simultaneously involving the aforementioned risk factors. According to previous reports, approximately 30–60% of patients in the real world are outside of clinical trial eligibility [11, 13, 14, 16,17,18,19, 29]. Importantly, this population had poorer outcomes following systemic therapy, including molecular-targeted therapy or cytotoxic chemotherapy, compared with the trial-eligible population [11,12,13,14, 16].
We found that ICI-based combination therapy improved the clinical outcomes of patients in the real world compared with TKIs in the overall cohort, which agreed with previous findings of clinical trials [1,2,3,4,5]. In particular, such a therapeutic benefit was more prominent in the population outside of clinical trials than in the population eligible for trials. This finding can be explained by several hypotheses. First, previous trials have shown that ICI-based combination therapy successfully maintained quality of life better than sunitinib [4, 30, 31]. Furthermore, the profile of AEs, especially in severe cases (i.e., grade 3 or higher) during ICI-based combination therapy, was comparable between the trial-eligible and trial-ineligible populations, as previously reported [17]. Taken together, ICIs can exhibit an acceptable safety profile even in this challenging population [32, 33], resulting in avoidance of withdrawal from treatment. Otherwise, the development of AEs often requires a dose reduction or interruption of administration, and this event directly decreases dose intensity. In TKI treatment, such a reduction in dose intensity can decrease the efficacy of treatment [34,35,36]. In contrast, in ICI-based therapy, AEs (immune-related AEs) may not be associated with the deterioration of an outcome [37,38,39]. Taken together, the absence of the negative impact of AEs in ICI-based combination therapy may contribute to improved outcomes in trial-ineligible populations.
Another possible reason is the higher efficacy of ICI-based combination therapy in patients with non-clear-cell histology such as papillary RCC. Cabozantinib is an inhibitor of MET, which has been identified as constitutionally activated in inherited papillary RCC and in a subset of sporadic cases [40, 41]. Several trials have demonstrated a feasible effect of cabozantinib-containing therapy in patients with papillary RCC [42, 43]. In addition, it is expected that patients with advanced RCC have a higher prevalence of chronic kidney disease because a substantial number of patients have undergone nephrectomy prior to the initiation of systemic therapy. The outcome in patients with severe kidney dysfunction, including end-stage renal disease, was reported to be worse than that in the general population [24, 44]. In particular, recent studies have shown the feasible efficiency and safety profile of nivolumab in patients with end-stage renal disease [45, 46]. Thus, ICI-based combination therapy improved results even in trial-ineligible populations, including patients with kidney dysfunction.
Interestingly, based on the PFS or OS even in trial-eligible populations, our data did not show the distinct therapeutic advantage of ICI-based combination therapy over TKIs, which is inconsistent with the findings of previous clinical trials [1,2,3,4,5], although it may be still difficult to interpret our findings owing to a relatively short duration of follow-up. Indeed, the tendency of longer OS in ICI-based combination therapy over TKIs was observed (p = 0.0906, Table 1 of the ESM). Even so, this finding may be explained by the hypothesis that some trial-eligible populations in the real world inherently harbor poor prognostic factors that are not clearly manifested. Otherwise, even eligible patients in the real world frequently have comorbidities and may be older adults, which potentially achieve fewer therapeutic benefits from ICI-based combination therapy. Potentially existing differences in race or medical insurance systems between Japan and other areas of the world may also affect the findings. Some Japanese subgroup analyses of pivotal trials showed the absence of a statistically significant survival benefit of ICI-based combination therapy over sunitinib, although the data are difficult to interpret statistically because of the small sample size [47,48,49].
This study has several limitations. First, the retrospective nature of the study, which was conducted with a relatively small sample size, inevitably induced selection bias, which might have affected the findings. Second, the short duration of follow-up made it difficult to interpret survival data, especially in the OS of patients treated with ICI-based combination therapy. Third, we compared the efficacy of ICI-based combination therapy with TKIs as a historical control, but the strategy of subsequent therapy has also improved in the ICI-based combination therapy era compared with the TKI era, potentially affecting survival, especially OS.
5 Conclusions
The present study indicated that there are a substantial number of patients outside of clinical trial eligibility in the setting of systemic therapy for advanced RCC. Immune checkpoint inhibitor-based combination therapy contributed to improve clinical outcomes compared with TKIs in the general population. Furthermore, this therapeutic benefit was more significant in populations outside of clinical trial eligibility than in populations eligible for trials. These findings support the application of ICI-based combination therapy for patients with advanced RCC in the real world, even though the real-world population comprises patients with strong heterogeneity, as well as those potentially having poor prognosticators.
References
Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–90.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–27.
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–15.
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384:829–41.
Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–300.
Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European Association of Urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82(4):399–410.
Motzer RJ, Jonasch E, Agarwal N, Alva A, Baine M, Beckermann K, et al. Kidney cancer, Version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:71–90.
Rathmell WK, Rumble RB, Van Veldhuizen PJ, Al-Ahmadie H, Emamekhoo H, Hauke RJ, et al. Management of metastatic clear cell renal cell carcinoma: ASCO guideline. J Clin Oncol. 2022;40:2957–95.
Stühler V, Herrmann L, Rausch S, Stenzl A, Bedke J. Real world data on IO-based therapy for metastatic renal cell carcinoma. J Cancer Res Clin Oncol. 2022. https://doi.org/10.1007/s00432-022-04173-0.
Ishihara H, Nemoto Y, Nakamura K, Tachibana H, Fukuda H, Yoshida K, et al. Changes in real-world outcomes in patients with metastatic renal cell carcinoma from the molecular-targeted therapy era to the immune checkpoint inhibitor era. Target Oncol. 2022;17:307–19.
Heng DY, Choueiri TK, Rini BI, Lee J, Yuasa T, Pal SK, et al. Outcomes of patients with metastatic renal cell carcinoma that do not meet eligibility criteria for clinical trials. Ann Oncol. 2014;25:149–54.
Mol L, Koopman M, van Gils CW, Ottevanger PB, Punt CJ. Comparison of treatment outcome in metastatic colorectal cancer patients included in a clinical trial versus daily practice in The Netherlands. Acta Oncol. 2013;52:950–5.
Knauf W, Aldaoud A, Hutzschenreuter U, Klausmann M, Dille S, Wetzel N, et al. Survival of non-transplant patients with multiple myeloma in routine care differs from that in clinical trials: data from the prospective German Tumour Registry Lymphatic Neoplasms. Ann Hematol. 2018;97:2437–45.
Marschner N, Staehler M, Müller L, Nusch A, Harde J, Koska M, et al. Survival of patients with advanced or metastatic renal cell carcinoma in routine practice differs from that in clinical trials: analyses from the German Clinical RCC Registry. Clin Genitourin Cancer. 2017;15:e209–15.
Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10:757–63.
Ishihara H, Tachibana H, Fukuda H, Yoshida K, Kobayashi H, Takagi T, et al. Prognostic impact of trial-eligibility criteria in patients with metastatic renal cell carcinoma. Urol Int. 2022;106(4):368–75.
Nemoto Y, Ishihara H, Nakamura K, Tachibana H, Fukuda H, Yoshida K, et al. Efficacy and safety of immunotherapy-based combinations as first-line therapy for metastatic renal cell carcinoma in patients who do not meet trial eligibility criteria. Target Oncol. 2022;17:475–82.
Fukuokaya W, Yanagisawa T, Hashimoto M, Yamamoto S, Koike Y, Imai Y, et al. Effectiveness of pembrolizumab in trial-ineligible patients with metastatic urothelial carcinoma. Cancer Immunol Immunother. 2022. https://doi.org/10.1007/s00262-022-03291-5.
Gan CL, Stukalin I, Meyers DE, Dudani S, Grosjean HAI, Dolter S, et al. Outcomes of patients with solid tumour malignancies treated with first-line immuno-oncology agents who do not meet eligibility criteria for clinical trials. Eur J Cancer. 2021;151:115–25.
Lee JL, Kim MK, Park I, Ahn JH, Lee DH, Ryoo HM, et al. RandomizEd phase II trial of Sunitinib four weeks on and two weeks off versus two weeks on and one week off in metastatic clear-cell type REnal cell carcinoma: RESTORE trial. Ann Oncol. 2015;26:2300–5.
Bracarda S, Iacovelli R, Boni L, Rizzo M, Derosa L, Rossi M, et al. Sunitinib administered on 2/1 schedule in patients with metastatic renal cell carcinoma: the RAINBOW analysis. Ann Oncol. 2015;26:2107–13.
Kondo T, Takagi T, Kobayashi H, Iizuka J, Nozaki T, Hashimoto Y, et al. Superior tolerability of altered dosing schedule of sunitinib with 2-weeks-on and 1-week-off in patients with metastatic renal cell carcinoma—comparison to standard dosing schedule of 4-weeks-on and 2-weeks-off. Jpn J Clin Oncol. 2014;44:270–7.
Kennoki T, Kondo T, Kimata N, Murakami J, Ishimori I, Nakazawa H, et al. Clinical results and pharmacokinetics of sorafenib in chronic hemodialysis patients with metastatic renal cell carcinoma in a single center. Jpn J Clin Oncol. 2011;41:647–55.
Ishihara H, Fukuda H, Tachibana H, Yoshida K, Kobayashi H, Takagi T, et al. Outcome of advanced renal cell carcinoma arising in end-stage renal disease: comparison with sporadic renal cell carcinoma. Clin Exp Nephrol. 2021;25:674–82.
Frampton JE. Pazopanib: a review in advanced renal cell carcinoma. Target Oncol. 2017;12:543–54.
Tachibana H, Kondo T, Ishihara H, Fukuda H, Yoshida K, Takagi T, et al. Modest efficacy of nivolumab plus ipilimumab in patients with papillary renal cell carcinoma. Jpn J Clin Oncol. 2021;51:646–53.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Rizzo M, Cartenì G, Pappagallo G. We need both randomized trials and real-world data: the example of everolimus as second-line therapy for mRCC. Future Oncol. 2014;10:1893–6.
Parikh RB, Min EJ, Wileyto EP, Riaz F, Gross CP, Cohen RB, et al. Uptake and survival outcomes following immune checkpoint inhibitor therapy among trial-ineligible patients with advanced solid cancers. JAMA Oncol. 2021;7:1843–50.
Cella D, Grünwald V, Escudier B, Hammers HJ, George S, Nathan P, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol. 2019;20:297–310.
Motzer R, Porta C, Alekseev B, Rha SY, Choueiri TK, Mendez-Vidal MJ, et al. Health-related quality-of-life outcomes in patients with advanced renal cell carcinoma treated with lenvatinib plus pembrolizumab or everolimus versus sunitinib (CLEAR): a randomised, phase 3 study. Lancet Oncol. 2022;23:768–80.
Rzeniewicz K, Larkin J, Menzies AM, Turajlic S. Immunotherapy use outside clinical trial populations: never say never? Ann Oncol. 2021;32:866–80.
Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123:1904–11.
Kawashima A, Takayama H, Arai Y, Tanigawa G, Nin M, Kajikawa J, et al. One-month relative dose intensity of not less than 50% predicts favourable progression-free survival in sorafenib therapy for advanced renal cell carcinoma in Japanese patients. Eur J Cancer. 2011;47:1521–6.
Iwamoto K, Ishihara H, Takagi T, Kondo T, Yoshida K, Iizuka J, et al. Evaluation of relative dose intensity during the early phase of first-line sunitinib treatment using a 2-week-on/1-week-off regimen for metastatic renal cell carcinoma. Med Oncol. 2018;35:78.
Ishihara H, Takagi T, Kondo T, Iwamoto K, Tachibana H, Yoshida K, et al. Decreased relative dose intensity during the early phase of treatment impacts the therapeutic efficacy of sunitinib in metastatic renal cell carcinoma. Jpn J Clin Oncol. 2018;48:667–72.
Ishihara H, Takagi T, Kondo T, Homma C, Tachibana H, Fukuda H, et al. Association between immune-related adverse events and prognosis in patients with metastatic renal cell carcinoma treated with nivolumab. Urol Oncol. 2019;37(355):e21–9.
Ikeda T, Ishihara H, Nemoto Y, Tachibana H, Fukuda H, Yoshida K, et al. Prognostic impact of immune-related adverse events in metastatic renal cell carcinoma treated with nivolumab plus ipilimumab. Urol Oncol. 2021;39(735):e9-16.
Motzer RJ, Escudier B, McDermott DF, Arén Frontera O, Melichar B, Powles T, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer. 2020;8: e000891.
Albiges L, Guegan J, Le Formal A, Verkarre V, Rioux-Leclercq N, Sibony M, et al. MET is a potential target across all papillary renal cell carcinomas: result from a large molecular study of pRCC with CGH array and matching gene expression array. Clin Cancer Res. 2014;20:3411–21.
Recondo G, Che J, Jänne PA, Awad MM. Targeting MET dysregulation in cancer. Cancer Discov. 2020;10:922–34.
Pal SK, Tangen C, Thompson IM Jr, Balzer-Haas N, George DJ, Heng DYC, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet. 2021;397:695–703.
Lee CH, Voss MH, Carlo MI, Chen YB, Zucker M, Knezevic A, et al. Phase II trial of cabozantinib plus nivolumab in patients with non-clear-cell renal cell carcinoma and genomic correlates. J Clin Oncol. 2022;40:2333–41.
Hayami N, Ubara Y, Okaneya T, Fujii T, Nagashima Y, Ohashi K. Outcome of renal cell carcinoma in patients on dialysis compared to non-dialysis patients. Semin Dial. 2020;33(4):316–21.
Vitale MG, Baldessari C, Milella M, Buti S, Militello AM, Di Girolamo S, et al. Immunotherapy in dialysis-dependent cancer patients: our experience in patients with metastatic renal cell carcinoma and a review of the literature. Clin Genitourin Cancer. 2019;17:e903–8.
Tachibana H, Kondo T, Ishihara H, Takagi T, Tanabe K. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma and end-stage renal disease at 2 centers. Clin Genitourin Cancer. 2019;17:e772–8.
Tomita Y, Kondo T, Kimura G, Inoue T, Wakumoto Y, Yao M, et al. Nivolumab plus ipilimumab versus sunitinib in previously untreated advanced renal-cell carcinoma: analysis of Japanese patients in CheckMate 214 with extended follow-up. Jpn J Clin Oncol. 2020;50:12–9.
Uemura M, Tomita Y, Miyake H, Hatakeyama S, Kanayama HO, Numakura K, et al. Avelumab plus axitinib vs sunitinib for advanced renal cell carcinoma: Japanese subgroup analysis from JAVELIN Renal 101. Cancer Sci. 2020;111:907–23.
Tamada S, Kondoh C, Matsubara N, Mizuno R, Kimura G, Anai S, et al. Pembrolizumab plus axitinib versus sunitinib in metastatic renal cell carcinoma: outcomes of Japanese patients enrolled in the randomized, phase III, open-label KEYNOTE-426 study. Int J Clin Oncol. 2022;27:154–64.
Acknowledgements
The authors thank Ms. Nobuko Hata (Department of Urology, Tokyo Women’s Medical University) for her secretarial efforts.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflicts of interest/competing interests
Toshio Takagi received honoraria from BristolMyers Squibb and Ono Pharmaceutical. Tsunenori Kondo received honoraria from Pfizer, Novartis, Bristol-Myers Squibb, and Ono Pharmaceutical. Hiroki Ishihara, Yuki Nemoto, Kazutaka Nakamura, Hidekazu Tachibana, Takashi Ikeda, Hironori Fukuda, Kazuhiko Yoshida, Hirohito Kobayashi, Junpei Iizuka, Hiroaki Shimmura, and Yasunobu Hashimoto have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The study protocol was approved by the Institutional Ethics Review Board of each institution (Tokyo Women’s Medical University, Tokyo Women’s Medical University Adachi Medical Center, Saiseikai Kawaguchi General Hospital, Saiseikai Kazo Hospital, and Jyoban Hospital; ID: 2020-0009). The present study was performed according to the guidelines of the 1964 Declaration of Helsinki and its later amendments. Owing to the retrospective observational nature of this study, the need for informed consent was waived.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to this study’s conception and design, data collection, and analysis. The first draft of the manuscript was written by HI and all authors commented on the previous drafts of the manuscript. All authors read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ishihara, H., Nemoto, Y., Nakamura, K. et al. Comparison of Outcomes Between Therapeutic Combinations Based on Immune Checkpoint Inhibitors or Tyrosine Kinase Inhibitor Monotherapy for First-Line Therapy of Patients with Advanced Renal Cell Carcinoma Outside of Clinical Trials: A Real-World Retrospective Multi-Institutional Study. Targ Oncol 18, 209–220 (2023). https://doi.org/10.1007/s11523-023-00956-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-00956-8