Abstract
Background
Pazopanib is the only tyrosine kinase inhibitor approved for the treatment of patients with advanced soft tissue sarcoma (STS) who have received prior chemotherapy, but there have been limited real-world data on pazopanib for the treatment of advanced STS.
Objective
We aimed to evaluate clinical outcomes of pazopanib in patients with multiple histologic STS types in real-world settings.
Patients and Methods
We retrospectively analyzed clinical data of Korean patients with advanced STS treated with pazopanib between 2008 and 2019. Outcomes of interest included treatment response, survival according to histologic subtypes, and adverse events.
Results
The analysis included 347 STS patients. The disease control rate for all pazopanib-treated patients was 54.8% (95% confidence interval (CI) 49.5–60.0); 54 patients (15.6%) achieved a partial response and 136 (39.2%) had stable disease. Patients with alveolar soft-part sarcoma (ASPS; 90%), solitary fibrous tumor (SFT; 88.2%), synovial sarcoma (66.7%), leiomyosarcoma (61.1%), and undifferentiated pleomorphic sarcoma (59.6%) showed higher disease control rates than those with other STS subtypes. Overall, median progression-free survival (PFS) and overall survival (OS) were 5.3 months (95% CI 4.5–6.0) and 12 months (95% CI 10–14), respectively. Noticeable survival outcomes occurred in patients with ASPS and SFT, with a median PFS of 24.5 (95% CI 2.5–30.0) and 13.0 (95% CI 3.0–21.3) months, respectively. The median OS of patients with ASPS and SFT was 48 (95% CI 17–52) and 32 (95% CI 19–66) months, respectively. Adverse drug reactions occurred in 170 patients (49.0%) but were not life-threatening.
Conclusions
This real-world data analysis showed acceptable efficacy and tolerability of pazopanib in patients pretreated with cytotoxic chemotherapy for advanced STS, with favorable treatment outcomes for ASPS and SFT.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Pazopanib showed acceptable efficacy for advanced soft tissue sarcoma (STS) in a real-world setting. |
Clinical outcomes varied according to the histologic subtypes of STS. |
Remarkable outcome was observed in alveolar soft-part sarcoma and solitary fibrous tumor. |
1 Introduction
Soft tissue sarcomas (STSs), a rare and heterogeneous group of tumors with mesenchymal origin, account for approximately 1% of all malignancies worldwide [1, 2]. In Korea, about 977 patients are diagnosed with STS yearly, 45% of whom have advanced disease [3]. STS comprises more than 50 different tumor entities that exhibit considerable differences in terms of genetic alterations, pathogenesis, and clinical manifestations [4]. STS can arise from almost any site of the body, including the extremities, internal organs, or soft tissues of the trunk. Because of its rarity and heterogeneity, there have been limited advancements in the development of novel therapeutic strategies for STS compared with other cancers [5].
Surgery is the main treatment for STSs diagnosed at an early stage; however, patients with relapsed or metastatic/unresectable disease are generally incurable and receive palliative systemic therapy [2]. Anthracycline-based chemotherapy is considered the standard first-line treatment for unresectable STS [6]. Pazopanib is the only multitarget tyrosine kinase inhibitor (TKI) approved by the US FDA for the treatment of multiple subtypes of pretreated advanced STS, based on the results of a randomized phase III trial that demonstrated a significant 3-month advantage in progression-free survival (PFS) in patients with advanced STS [7]; however, the study population consisted mainly of Caucasians and the clinical efficacy and tolerability of pazopanib in Asian patients with STSs have not been fully evaluated. Although several small cohort studies and case reviews have been reported, no large-scale multicenter research has assessed the efficacy of pazopanib in Korean patients with advanced STS.
The current study investigated the clinical outcomes of 347 Korean patients with advanced STS treated with pazopanib.
2 Material and Methods
2.1 Study Population
We retrospectively collected and reviewed the clinical data of patients with advanced STS treated with pazopanib between December 2008 and April 2019 at the Asan Medical Center, Samsung Medical Center, and Yonsei University of College of Medicine, Seoul, Republic of Korea.
Patients were included in this analysis if they (1) were aged ≥ 16 years; (2) had pathologically confirmed advanced STS; (3) had failed one or more lines of chemotherapy; (4) had measurable or evaluable lesion(s) according to Response Evaluation Criteria In Solid Tumours (RECIST) version 1.1 [8]; and (5) had available clinical data and treatment records. We excluded patients with concurrent malignancies and those without available imaging studies for pazopanib response evaluation. The following baseline clinicopathological variables were reviewed: age, sex, histologic type, grade, primary tumor site, extent of metastasis, Eastern Cooperative Oncology Group (ECOG) performance status, and treatment history. The study was approved by the Asan Medical Center Institutional Review Board (IRB No. 2017-1098).
2.2 Treatment Assessment
Tumor response was evaluated using computed tomography or magnetic resonance imaging according to RECIST version 1.1 criteria. Disease control rate was defined as the percentage of patients with the best tumor response of complete response (CR), partial response (PR), or stable disease (SD). Overall survival (OS) was measured from pazopanib initiation to death from any cause, and PFS was measured from pazopanib initiation to disease progression or death from any cause with censoring of patients lost to follow-up.
2.3 Statistical Analysis
Patient demographics were summarized as numbers (percentage) and means (range) for categorical and continuous variables, respectively. Survival curves for PFS and OS were represented using the Kaplan–Meier method. Independent prognostic factors were evaluated using the Cox proportional hazards regression model. Statistically significant variables in univariate analysis were subjected to multivariable Cox proportional hazard regression models. p values < 0.05 were considered statistically significant and all reported p values were two-sided. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
3 Results
3.1 Patient Characteristics
A total of 347 patients with STS were included in the analysis. Table 1 shows their demographics and baseline characteristics. Among these patients, 170 (49.0%) were males and the median age was 51 years. The most common primary tumor site was the abdominopelvic cavity (47.8%), followed by the thorax (24.5%), extremities (21.9%), and others (5.8%). Most patients (80.7%) had an ECOG performance status of 0 or 1. Overall, 194 patients (55.9%) received only one prior line of chemotherapy, 92 (26.5%) received two lines, and 61 (17.6%) underwent three or more lines of conventional systemic therapy prior to pazopanib. Primary site involvement was found in more than half of the patients (56.5%), and 80 patients (23.1%) had liver involvement of the tumor.
Figure 1 shows the treatment regimens by lines of therapy. The combination of doxorubicin and ifosfamide (27.7%) was the most frequently used first-line chemotherapy regimen. Doxorubicin with or without other chemotherapeutic agents (25.1%) and ifosfamide-based combinations (23.6%) were also commonly used as front-line treatment.
Within the study population, the largest group of histologic subtypes was leiomyosarcoma (LMS; 95 patients, 27.4%). Undifferentiated pleomorphic sarcoma (UPS; 47 patients, 13.5%), angiosarcoma (44 patients, 12.7%), synovial sarcoma (SS; 24 patients, 6.9%), malignant peripheral nerve sheath tumor (MPNST; 20 patients, 5.8%), undifferentiated sarcoma (18 patients, 5.2%), solitary fibrous tumor (SFT, hemangiopericytoma; 17 patients, 4.9%), and alveolar soft-part sarcoma (ASPS; 10 patients, 2.9%) were also observed in this population.
3.2 Pazopanib Treatment Outcomes
Among the 347 patients, 54 (15.6%) achieved a PR, 136 (39.2%) achieved SD, and 123 (35.4%) had progressive disease (PD) as best response. Overall, the disease control rate for all patients treated with pazopanib was 54.8% (95% confidence interval (CI) 49.5–60.0). Excluding relatively rare STS subtypes (n < 10), the highest disease control rates were observed in patients with ASPS (90%) and SFT (88.2%). Patients with SS (66.7%), LMS (61.1%), and UPS (59.6%) also demonstrated relatively favorable disease control rates (Fig. 2). The best overall responses for each histologic STS subtype are shown in electronic supplementary Table A.
Disease control rate for pazopanib treatment. ASPS alveolar soft-part sarcoma, SFT solitary fibrous tumor, SS synovial sarcoma, LMS leiomyosarcoma, UPS undifferentiated pleomorphic sarcoma, AS angiosarcoma, MPNST malignant peripheral nerve sheath tumor, US undifferentiated sarcoma, PR partial response, SD stable disease
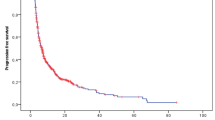
Survival analysis showed a median overall PFS of 5.3 months (95% CI 4.5–6.0). The median PFS in patients with ASPS, SFT, angiosarcoma, LMS, UPS, and SS was 24.5, 13.0, 6.3, 6.0, 5.8, and 5.5 months, respectively. The median OS of the 347 patients was 12 months (95% CI 10–14), and the median OS for patients with ASPS, SFT, LMS, and SS was 48, 32, 16, and 14 months, respectively. Notably, patients with ASPS and SFT achieved outstanding survival outcomes compared with patients with other subtypes (Table 2; Figs. 3, 4).
Kaplan–Meier analysis of progression-free survival according to histologic subtypes. LMS leiomyosarcoma, SS synovial sarcoma, UPS undifferentiated pleomorphic sarcoma, AS angiosarcoma, MPNST malignant peripheral nerve sheath tumor, ASPS alveolar soft-part sarcoma, SFT solitary fibrous tumor, US undifferentiated sarcoma
Kaplan–Meier analysis of overall survival according to histologic subtypes. LMS leiomyosarcoma, SS synovial sarcoma, UPS undifferentiated pleomorphic sarcoma, AS angiosarcoma, MPNST malignant peripheral nerve sheath tumor, ASPS alveolar soft-part sarcoma, SFT solitary fibrous tumor, US undifferentiated sarcoma
A Cox univariate analysis showed that unfavorable prognostic factors for survival in patients with advanced STS who underwent pazopanib treatment were male sex and poor ECOG performance status (≥ 2). After adjusting for confounding variables by multivariate Cox regression analysis, poor ECOG performance status (≥ 2) and the number of lines of previous chemotherapy (three or more) were significant poor prognostic factors for patient survival (Table 3).
3.3 Dose and Toxicity Profile of Pazopanib Treatment
The daily average dose of pazopanib was approximately 700 mg among the study population. The mean relative dose intensity was 83.4% and the mean starting dose was 717 mg daily. Most patients (72.6%) started at a daily dose of 800 mg, but 48 patients (13.8%) started at daily doses ≤ 50% of the standard recommended dose of 800 mg/day (Table 4); 113 patients (32.6%) experienced at least one dose modification.
Adverse events occurred in 170 patients (49.0%). The most common toxicities were diarrhea (77 patients, 22.2%), nausea (75 patients, 21.6%), and tumor pain (68 patients, 19.6%). Relatively rare adverse effects were also reported, including pneumothorax (9 patients, 2.6%), deep vein thrombosis (4 patients, 1.2%), pulmonary embolism (2 patients, 0.6%), and heart failure (2 patients, 0.6%); however, none of these adverse events were grade 4 or 5 according to the Common Terminology Criteria of Adverse Events (CTCAE) version 4.0. Grade 3 adverse events were observed in only 2.3% of patients (Table 5).
4 Discussion
The current multicenter, large-scale analysis of real-world data demonstrated that pazopanib is effective and can be administered safely in patients pretreated with cytotoxic chemotherapy for advanced STS. Additionally, our results showed that the efficacy of pazopanib treatment can vary according to the histologic STS subtype.
The PALETTE phase III clinical trial showed that pazopanib significantly increased the median PFS compared with placebo (4.6 vs. 1.6 months; hazard ratio [HR] 0.31, 95% CI 0.24–0.40; p < 0.0001). The median OS with pazopanib treatment in this trial was 12.5 months (95% CI 10.6–14.8). In terms of efficacy, our study results showed similar survival outcomes as those of the PALETTE trial. The median PFS and OS in all 347 patients were 5.3 months (95% CI 4.5–6.0) and 12 months (95% CI 10–14), respectively, in the present study. These results are also concordant with other previously published data [9,10,11,12].
Several studies have examined the efficacy and tolerability of pazopanib for the treatment of advanced STS in Asian patients. Yoo et al. studied 43 Korean patients and suggested that pazopanib seemed to have antitumor activity in patients who had been heavily pretreated for metastatic STS [9]. Another retrospective study that included 156 Japanese patients with relapsed STS reported acceptable survival outcomes. The researchers in this Japanese study also proposed differences in the clinical benefit of pazopanib among histologic STS types [10]. However, the relatively small sample sizes in these previous reports prevented profound investigations of the relationship between tumor histology and treatment outcomes. To our knowledge, our study is the largest retrospective study to assess the outcomes of pazopanib for the treatment of patients with various subtypes of advanced STS.
The results of the present study showed that the pazopanib treatment response and clinical outcomes varied according to STS histologic subtypes. In our study population, 54 patients (15.6%) achieved PR and 136 (39.2%) had SD, corresponding to a disease control rate of 54.8% and objective response rate of 15.6%. Patients with ASPS (90%), SFT (88.2%), SS (66.7%), LMS (61.1%), UPS (59.6%), and epithelioid sarcoma (57.1%) had satisfactory disease control rates following pazopanib treatment. In terms of objective response rate, pazopanib demonstrated acceptable efficacy in patients with advanced SFT (35.3%), SS (33.3%), and ASPS (30.0%). Better survival outcomes were observed in patients with ASPS, SFT, LMS, and SS than in patients with other types of STS.
Pazopanib showed remarkable efficacy in patients with advanced ASPS and SFT. ASPS is a rare STS subtype representing < 1% of all STSs [13]. Given the extreme rarity of the disease, available clinical data are not sufficient, although ASPS has greater metastatic potential and poorer long-term outcomes than other STSs. Several reports have suggested its sensitivity to the effect of vascular endothelial growth factor receptor (VEGFR)-predominant TKIs [14,15,16,17]. A retrospective review of data of 44 patients with advanced ASPS by Stacchiotti et al. confirmed the activity of pazopanib in the treatment of advanced ASPS, with a 27% overall response rate by RECIST, a median PFS of 13.6 months, and 59% of patients progression-free at 12 months [16]. In our study, the median PFS of 10 patients with ASPS was 24.5 months and the overall response rate was 30%. In a recently published randomized phase II trial, cediranib, a TKI with a similar spectrum of activity, was also shown to have significant clinical activity in this disease [18]. The results of this trial provide the strongest evidence of the effectiveness of TKIs in ASPS patients. The utility of angiogenesis inhibitors such as VEGFR blockers, including pazopanib or cediranib can be explained by the fact that ASPS cells use lactate as an energy source, with consequent upregulation of hypoxia-inducible factor (HIF)-1α and VEGF, resulting in angiogenesis, cell proliferation, metastasis, and myogenic differentiation [18,19,20,21].
SFT, another rare subtype of STS, has limited responsiveness to cytotoxic chemotherapy [22,23,24]. For this reason, several researchers have investigated targeted therapies for the treatment of patients with advanced SFT, and multiple TKIs, including pazopanib, have been studied for the treatment of SFT [25,26,27]. Most recently, the results of a phase II trial evaluating pazopanib for the treatment of advanced SFT have been reported [28]. Of 35 evaluable patients, 18 (51%) had achieved PR, 9 (26%) had SD, and 8 (23%) had PD. The median PFS of all patients was 5.57 months (95% CI 4.29–6.84). Our findings regarding the outstanding efficacy of pazopanib therapy in patients with SFT are in line with these recent results.
The spectrum of adverse events was generally consistent with the known safety profile of pazopanib; however, fewer adverse events were reported in the current study than in the PALETTE trial. In particular, grade 4 and 5 adverse events were not observed and grade 3 adverse events occurred in 2.3% of patients. This finding may be related to lower relative dose intensity compared with the PALETTE trial and the retrospective nature of the present study as not all adverse events might be completely documented in the medical records.
Clinical research for STS is somewhat challenging because of its low incidence and heterogeneity [29]. Although we included a considerable number of patients with STS, the study population was not sufficiently large to identify a significant association between tumor histology and clinical outcomes in patients receiving pazopanib treatment. In addition, given the retrospective nature of this study design, the results might be affected by selection or recall biases. Nevertheless, our study demonstrated the efficacy and tolerability of pazopanib for the treatment of advanced STS in real-world settings. The outcomes from these real-world data provide valuable insight into the effectiveness and safety of the drug in clinical practice.
5 Conclusions
This large-scale, real-world data analysis evaluated the treatment outcome of pazopanib in patients with pretreated advanced STS. Pazopanib had acceptable efficacy and tolerability for the treatment of advanced STS, and noticeable differences were observed in the activity of pazopanib therapy among STS subtypes. A remarkable response to pazopanib was observed in patients with ASPS and SFT. Further prospective studies for each STS subtype and biomarker investigation for pazopanib are strongly recommended.
References
Skubitz KM, D’Adamo DR. Sarcoma. Mayo Clin Proc. 2007;82(11):1409–32.
Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353(7):701–11.
Kim HS, Nam CM, Jang SY, Choi SK, Han M, Kim S, et al. Characteristics and treatment patterns of patients with advanced soft tissue sarcoma in Korea. Cancer Res Treat. 2019;51(4):1380–91.
Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schöffski P, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC study 62043). J Clin Oncol. 2009;27(19):3126–32.
Lee DY, Staddon AP, Shabason JE, Sebro R. Phase I and phase II clinical trials in sarcoma: Implications for drug discovery and development. Cancer Med. 2019;8(2):585–92.
Sleijfer S, Quali M, van Glabbeke M, Krarup-Hansen A, Rodenhuis S, Le Cesne A, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur J Cancer. 2010;46(1):72–83.
van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–86.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 11). Eur J Cancer. 2009;45(2):228–47.
Yoo KH, Kim HS, Lee SJ, Park SH, Kim SJ, Kim SH, et al. Efficacy of pazopanib monotherapy in patients who had been heavily pretreated for metastatic soft tissue sarcoma: a retrospective case series. BMC Cancer. 2015;15:154.
Nakamura T, Matsumine A, Kawai A, Araki N, Goto T, Yonemoto T, et al. The clinical outcome of pazopanib treatment in Japanese patients with relapsed soft tissue sarcoma: a Japanese Musculoskeletal Oncology Group (JMOG) study. Cancer. 2016;122(9):1408–16.
Gelderblom H, Judson IR, Benson C, Merimsky O, Grignani G, Katz D, et al. Treatment patterns and clinical outcomes with pazopanib in patients with advanced soft tissue sarcomas in a compassionate use setting: results of the SPIRE study. Acta Oncol. 2017;56(12):1769–75.
Kim JH, Park HS, Heo SJ, Kim SK, Han JW, Shin KH, et al. Differences in the efficacies of pazopanib and gemcitabine/docetaxel as second-line treatments for metastatic soft tissue sarcoma. Oncology. 2019;96(2):59–69.
Jaber OI, Kirby PA. Alveolar soft part sarcoma. Arch Pathol Lab Med. 2015;139(11):1459–62.
Kummar S, Allen D, Monks A, Polley EC, Hose CD, Ivy SP, et al. Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol. 2013;31(18):2296–302.
Stacchiotti S, Negri T, Zaffaroni N, Palassini E, Morosi C, Brich S, et al. Sunitinib in advanced alveolar soft part sarcoma: evidence of a direct antitumor effect. Ann Oncol. 2011;22(7):1682–90.
Stacchiotti S, Mir O, Le Cesne A, Vincenzi B, Fedenko A, Maki RG, et al. Activity of pazopanib and trabectedin in advanced alveolar soft part sarcoma. Oncologist. 2018;23(1):62–70.
Kim M, Kim TM, Keam B, Kim YJ, Paeng JC, Moon KC, et al. A phase II trial of pazopanib in patients with metastatic alveolar soft part sarcoma. Oncologist. 2019;24(1):20–e29.
Judson I, Morden JP, Kilburn L, Leahy M, Benson C, Bhadri V, et al. Cediranib in patients with alveolar soft-part sarcoma (CASPS): double-blind, placebo-controlled, randomised, phase 2 trial. Lancet Oncol. 2019;20(7):1023–34.
Paoluzzi L, Maki RG. Diagnosis, prognosis, and treatment of alveolar soft-part sarcoma: a review. JAMA Oncol. 2019;5(2):254–60.
Stockwin LH, Vistica DT, Kenney S, Schrump DS, Butcher DO, Raffeld M, et al. Gene expression profiling of alveolar soft-part sarcoma (ASPS). BMC Cancer. 2009;9:22.
Goodwin ML, Jin H, Straessler K, Smith-Fry K, Zhu JF, Monument MJ, et al. Modeling alveolar soft part sarcomagenesis in the mouse: a role for lactate in the tumor microenvironment. Cancer Cell. 2014;26(6):851–62.
Park MS, Araujo DM. New insights into the hemangiopericytoma/solitary fibrous tumor spectrum of tumors. Curr Opin Oncol. 2009;21(4):327–31.
Levard A, Derbel O, Méeus P, Ranchère D, Ray-Coquard I, Blay JY, et al. Outcome of patients with advanced solitary fibrous tumors: the Centre Léon Bérard experience. BMC Cancer. 2013;13:109.
Park MS, Ravi V, Conley A, Patel SR, Trent JC, Lev DC, et al. The role of chemotherapy in advanced solitary fibrous tumors: a retrospective analysis. Clin Sarcoma Res. 2013;3(1):7.
Stacchiotti S, Negri T, Libertini M, Palassini E, Marrari A, De Troia B, et al. Sunitinib malate in solitary fibrous tumor (SFT). Ann Oncol. 2012;23(12):3171–9.
Maruzzo M, Martin-Liberal J, Messiou C, Miah A, Thway K, Alvarado R, et al. Pazopanib as first line treatment for solitary fibrous tumours: the Royal Marsden Hospital experience. Clin Sarcoma Res. 2015;5:5.
Park MS, Patel SR, Ludwig JA, Trent JC, Conrad CA, Lazar AJ, et al. Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumor. Cancer. 2011;117(21):4939–47.
Martin-Broto J, Stacchiotti S, Lopez-Pousa A, Redondo A, Bernabeu D, de Alava E, et al. Pazopanib for treatment of advanced malignant and dedifferentiated solitary fibrous tumour: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2019;20(1):134–44.
Schöffski P, Cornillie J, Wozniak A, Li H, Hompes D. Soft tissue sarcoma: an update on systemic treatment options for patients with advanced disease. Oncol Res Treat. 2014;37(6):355–62.
Author information
Authors and Affiliations
Contributions
Study concept: Jeong Eun Kim and Tae Won Kim. Study design: Jeong Eun Kim. Data acquisition: Jeong Eun Kim, Jung Yong Hong, Jee Hung Kim, Hyo Song Kim, and Jin-Hee Ahn. Quality control of the data and algorithms: Jeong Eun Kim. Data analysis and interpretation: Jeong Eun Kim, Ji Sung Lee, and Chung Ryul Oh. Statistical analysis: Ji Sung Lee. Article preparation: Chung Ryul Oh and Jeong Eun Kim. Article editing: Chung Ryul Oh. Article review: Jeong Eun Kim, Jung Yong Hong, Jee Hung Kim, and Hyo Song Kim.
Corresponding author
Ethics declarations
Funding
This work was funded by a 2017 cancer research support project from the Korea Foundation for Cancer Research (CB-2017-B-2), and was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number HI18C2383).
Conflict of interest
Chung Ryul Oh, Jung Yong Hong, Jee Hung Kim, Ji Sung Lee, Hyo Song Kim, Tae Won Kim, Jin-Hee Ahn, and Jeong Eun Kim declare they have no conflicts of interest that might be relevant to the contents of this manuscript.
Consent for publication
The authors declare that the manuscript has not been published previously, in whole or in part, and is not under consideration for publication elsewhere. All authors have approved the manuscript and consent to its publication.
Ethics approval
This study was approved by the Asan Medical Center Institutional Review Board (IRB No. 2017-1098).
Availability of data and material
The data contain potentially identifiable patient information and cannot be shared publicly due to ethical and legal restrictions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oh, C.R., Hong, J.Y., Kim, J.H. et al. Real-World Outcomes of Pazopanib Treatment in Korean Patients with Advanced Soft Tissue Sarcoma: A Multicenter Retrospective Cohort Study. Targ Oncol 15, 485–493 (2020). https://doi.org/10.1007/s11523-020-00731-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-020-00731-z