Abstract
Background
The efficacy of crizotinib for anaplastic lymphoma kinase (ALK)-rearranged non-small-cell lung cancer (NSCLC) patients with brain metastasis is controversial. Real-world research data are needed as further evidence.
Objective
We conducted a multicenter, retrospective study to explore how crizotinib affects the control of brain metastasis and the survival outcomes among Chinese patients.
Patients and Methods
We reviewed the medical records of unselected ALK-rearranged NSCLC patients treated with crizotinib at five hospitals in China from January 1, 2013 to November 30, 2017. Patients developing brain metastasis either before or during crizotinib treatment were included. Survival outcomes were analyzed by the Kaplan–Meier method, and prognostic factors were analyzed by multivariate Cox regression.
Results
A total of 174 patients were included in the analysis; 95 of these patients had baseline brain metastasis, while 79 patients developed brain metastasis during crizotinib treatment. Among patients with baseline brain metastasis, the median intracranial time to progression was 19.3 months (95% confidence interval [CI] 12.5–26.2) and the median overall survival (OS) was 53.4 months (95% CI not reached). A total of 135 patients experienced intracranial progression, and 94 of these patients continued crizotinib beyond progressive disease (CBPD). There was no significant difference in the median OS between patients with CBPD and without CBPD (48.3 months vs 53.4 months; p = 0.296).
Conclusions
ALK-rearranged advanced NSCLC patients with baseline brain metastasis can still achieve OS benefits from crizotinib treatment. However, patients with intracranial progression may not obtain a long-term survival benefit from continuation of CBPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this real-world study, patients with baseline brain metastasis could still obtain a long-term survival benefit from crizotinib treatment. |
Although local treatment for baseline brain metastasis significantly prolonged the time to intracranial progression, it did not provide a survival benefit. |
For patients experiencing intracranial progression, a reasonable alternative may be to switch to next-generation anaplastic lymphoma kinase (ALK) inhibitors directly. |
1 Introduction
Non–small-cell lung cancer (NSCLC) accounts for more than 80% of all cases of primary lung cancers [1]. Approximately 5% of NSCLC patients harbor anaplastic lymphoma kinase (ALK) gene rearrangements [2], and among selected populations, such as those with adenocarcinoma and those who are light smokers or have never smoked, the ALK rearrangement rate may increase to about 30% [3, 4]. Crizotinib, an oral tyrosine kinase inhibitor (TKI) of ALK, ROS1, and MET, has demonstrated potent antitumor efficacy in patients with advanced NSCLC with ALK rearrangement. In the PROFILE 1014 trial, crizotinib yielded a higher objective response rate (ORR) compared with chemotherapy (74% vs 45%; p < 0.001), prolonged progression-free survival (PFS) [median 10.9 months vs 7.0 months; hazard ratio (HR) for progression or death with crizotinib 0.45; 95% confidence interval (CI), 0.35–0.60; p < 0.001], and less toxicity [5, 6]. The median overall survival (OS) was not reached with crizotinib (95% CI 45.8-not reached) [6]. In the PROFILE 1029 trial, which focused on East Asian patients, the ORR was 87.5% with crizotinib versus 45.6% with chemotherapy (p < 0.001), and crizotinib also significantly improved PFS (median 11.1 months vs 6.8 months; HR 0.402; 95% CI 0.286–0.565; p < 0.001) [7].
Unfortunately, despite the initial impressive response to crizotinib, most ALK-rearranged NSCLC patients experience progression within 1 year, and the brain is a common site of disease progression [8]. About 30% of ALK-rearranged NSCLC patients will develop brain metastasis, while among all patients receiving crizotinib treatment—including patients with brain metastases at baseline—intracranial progression occurs in up to 70% of the patients [9, 10]. Furthermore, up to 40–50% of patients treated with crizotinib may initially develop brain metastasis before overt extracranial disease progression [11], which may result from the poor central nervous system (CNS) penetration of crizotinib [12].
For patients with baseline brain metastasis, the next-generation ALK inhibitors alectinib and brigatinib may be good alternatives. In the ALEX trial, the median PFS for patients with baseline brain metastasis was 27.7 months with alectinib versus 7.4 months with crizotinib (HR 0.35; 95% CI 0.22–0.56) [13], and the time to intracranial progression was also significantly lengthened in the alectinib group (HR 0.18; 95% CI 0.09–0.36) [14]. In the ALTA-1L trial, brigatinib significantly outperformed crizotinib in survival without intracranial disease progression among patients with baseline brain metastasis (median not reached vs 5.6 months; HR 0.27; 95% CI 0.13–0.54) [15].
Alectinib, which was not available in China until September 2018, is still much less affordable than crizotinib, and brigatinib is still not available in China. Hence, how to help ALK-rearranged NSCLC patients with brain metastasis achieve optimal survival benefits from a reasonable treatment strategy is an important issue that is being researched extensively. In order to reveal treatment patterns and survival outcomes for these patients in a real-world setting, we conducted a large-sample, multicenter, retrospective study to explore the efficacy of crizotinib in patients with ALK-rearranged NSCLC with brain metastasis in the Chinese population and to identify prognostic factors.
2 Patients and Methods
2.1 Patients
We retrospectively investigated patients with advanced ALK-positive NSCLC who had received crizotinib treatment at five hospitals in China from January 1, 2013 to November 30, 2017 and who developed brain metastasis either before or during crizotinib treatment. The study was approved by the Ethics Committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (approval no. 18-082/1660). Because of the retrospective nature of this analysis, informed consent from the patients was not required.
All patients meeting the following criteria were included in this analysis: (1) a diagnosis of histologically or cytologically verified locally advanced, recurrent, or metastatic NSCLC; (2) ALK rearrangement; (3) age 18 years or older; (4) administration of crizotinib for at least 21 days; and (5) the presence of a brain metastasis. A positive ALK status was determined by Ventana anti-ALK (D5F3) immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), real-time reverse transcription polymerase chain reaction (RT-PCR), or next-generation sequencing (NGS). Brain metastasis was diagnosed by enhanced magnetic resonance imaging (MRI).
2.2 Data Collection and Follow-up
The clinical data extraction from medical records and the follow-up telephone calls were performed by clinicians at each center; the information was then sent to the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences for data assembly, validation, and analysis. The response to crizotinib was evaluated by regular imaging examinations, in accordance with the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1). Survival outcomes were collected from the initiation of crizotinib treatment to the patient’s death or the end of the study at January 31, 2019.
2.3 Statistical Analysis
The Kaplan–Meier method was applied to estimate the time to progression (TTP), PFS, and OS. Factors influencing survival outcomes were assessed with proportional hazard models (multivariate Cox regression). For all tests, two-sided p values of < 0.05 were considered statistically significant. All analyses were performed using SPSS® version 25.0 (SPSS, Inc., Chicago, IL, USA).
3 Results
3.1 Demographic and Clinical Characteristics of Patients
A total of 174 patients from five hospitals in various geographic regions were included in this study, among whom 95 patients harbored baseline brain metastasis at the initiation of crizotinib treatment and 79 patients developed brain metastasis during crizotinib treatment. The demographic and clinical characteristics of these patients are summarized in Table 1.
Among patients with baseline brain metastases before crizotinib treatment, the median age was 50.2 years (range 24.3–76.5 years). The proportions of male and female patients were relatively balanced. All patients in this group were diagnosed with adenocarcinoma, and most of them were non-smokers (70.5%), had stage IV disease (95.8%), and had Eastern Cooperative Oncology Group performance status (ECOG PS) scores of 0–1 (91.6%). A total of 66 (69.5%) patients had > 3 brain metastases lesions, and 79 (83.2%) had extracranial metastases. Fifty-three patients (55.8%) received crizotinib as first-line treatment, and more than half of the patients received local treatment for brain metastases, including surgery, whole-brain radiation therapy (WBRT), and stereotactic radiosurgery (SRS).
Among patients who developed brain metastases during crizotinib treatment, the median age was 49.8 years (range 20.4–82.5 years). As with patients with baseline brain metastases, most of this group were non-smokers (72.2%) and had ECOG PS scores of 0–1 (94.9%), stage IV disease (87.3%), and a histological diagnosis of adenocarcinoma (97.5%). More than half of these patients received crizotinib as first-line treatment.
3.2 Efficacy of Crizotinib in Patients with Baseline Brain Metastasis
For the 95 patients who had baseline brain metastasis, the median follow-up time was 35.1 months. The intracranial ORR of patients who did not receive local treatment was 13.6%, and the intracranial disease control rate (DCR) was 90.9%. Fifty-six patients (58.9%) experienced intracranial progression, and the median intracranial TTP (icTTP) was 19.3 months (95% CI 12.5–26.2) (Fig. 1a).
Kaplan–Meier estimate of the intracranial time to progression (icTTP) and overall survival (OS) from the initiation of crizotinib treatment among patients with baseline brain metastasis: a icTTP; b OS; c IcTTP stratified by with or without local treatment for baseline brain metastasis; d OS stratified by with or without local treatment for baseline brain metastasis. CI confidence interval
Eighty-one patients (85.3%) experienced disease progression. The median PFS was 11.5 months (95% CI 9.2–13.9 months). After initial progression, 34 patients (42.0%) continued to receive crizotinib; six patients (7.4%) received a combination of crizotinib and chemotherapy/bevacizumab; 18 patients (22.2%) received next-generation ALK inhibitors; and six patients (7.4%) received chemotherapy. Seventeen patients (21.0%) died within 1 month after progression. The median OS was 53.4 months (95% CI not reached) (Fig. 1b), and the 1-, 2-, 3-, 4- and 5-year OS rates were 87.4, 74.1, 59.7, 53.3 and 49.2%, respectively.
When age (< 65 vs ≥ 65 years), sex, number of brain metastasis (≤ 3 vs > 3), the presence of extracranial disease, line of crizotinib treatment (first vs ≥ second line), and local treatment of baseline brain metastasis (with vs without) were included in a multivariate Cox regression analysis, we found that local treatment for baseline brain metastasis significantly prolonged the icTTP for these patients (without local treatment vs with local treatment: HR 1.93; 95% CI 1.10–3.37; p = 0.021).The median icTTP values for patients with and without local treatment were 27.4 months (95% CI 15.1–39.8) and 11.9 months (95% CI 2.6–21.2), respectively (p = 0.025) (Fig. 1c). However, when these variables were included in a multivariate Cox regression analysis for OS, local treatment for baseline brain metastasis did not influence the OS (without local treatment vs with treatment: HR 1.44; 95% CI 0.70–2.97; p = 0.328), but male patients experienced a significantly longer OS than female patients (female vs male: HR 2.24; 95% CI 1.12–4.49; p = 0.023). The median OS for patients without local treatment was not reached, while the median OS for patients with local treatment was 53.4 months (95% CI not reached) (p = 0.695) (Fig. 1d).
3.3 Efficacy of Crizotinib in Patients Developing Brain Metastasis During Treatment
The median follow-up time for these 79 patients was 39.5 months. The median OS from initiation of crizotinib treatment was 42.3 months (95% CI 23.8-60.8) [Fig. 2a]. The 1-, 2-, 3-, 4- and 5-year OS rates were 87.3, 68.1, 52.2, 48.0 and 40.2%, respectively. The median OS after brain metastasis had occurred was 28.5 months (95% CI 8.4–48.6) [Fig. 2b].
Kaplan–Meier estimate of overall survival (OS) among patients developing brain metastasis during crizotinib treatment: a OS from the initiation of crizotinib treatment; b OS after brain metastasis; c OS after brain metastasis stratified by with or without local treatment after brain metastasis; d OS from initiation of crizotinib treatment stratified by with or without extracranial progression. CI confidence interval
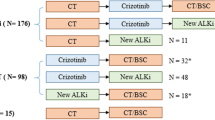
After brain metastasis had developed, 57 patients (72.2%) continued to receive crizotinib; three patients received a combination of crizotinib and chemotherapy/bevacizumab; 11 patients (13.9%) switched to next-generation ALK inhibitors, and eight patients (10.1%) received other treatments such as chemotherapy. Five of the patients who continued on crizotinib treatment also had extracranial progression. Only one of the 11 patients that switched to next-generation ALK inhibitors received local brain metastasis treatment, although six of these patients only experienced intracranial progression. There was no significant difference in the median OS after progression between patients with and without local brain metastasis treatment (28.5 months vs 24.1 months, respectively; p = 0.432) (Fig. 2c).
When age (< 65 vs ≥ 65 years), sex, line of crizotinib treatment (first vs ≥ second line), local brain metastasis treatment after progression (with vs without), continuation of crizotinib after brain metastasis, and extracranial progression status were included in a multivariate Cox regression analysis, we found that with extracranial progression (with extracranial progression vs without extracranial progression: HR 2.87; 95% CI 1.20–6.87; p = 0.018) and age ≥ 65 (< 65 vs ≥ 65 years: HR 0.38; 95% CI 0.15–0.99; p = 0.048) were both risk factors for this group of patients. We further calculated the OS between patients with and without extracranial progression. The OS of patients with extracranial progression was 21.9 months (95% CI 13.3–30.4), and that of patients without extracranial progression was 56.2 months (95% CI not reached) (Fig. 2d).
3.4 Efficacy of First-Line or Second- or Later-Line Crizotinib Treatment
A total of 100 patients with brain metastasis received crizotinib as first-line treatment, among whom 53 (53.0%) harbored baseline brain metastasis. The median OS of these patients was 45.0 months (95% CI 27.8–62.1) (Fig. 3a). When age (< 65 vs ≥ 65 years), sex, baseline brain metastasis status, and local brain metastasis treatment status were included in a multivariate Cox regression analysis, we found that sex significantly influenced the OS (female vs male: HR 1.93; 95% CI 1.02–3.65; p = 0.042). The median OS of patients with local brain metastasis treatment was 34.6 months (95% CI 27.3–41.9), and that of patients without local brain metastasis treatment was 56.2 months (95% CI 22.6–89.8) (p = 0.281) (Fig. 3b). Intracranial progression status was not included in the multivariate Cox regression analysis, because patients without intracranial progression were all still alive at the end of the study period. In the first-line treatment setting, the median OS of patients with intracranial progression was significantly different from that of patients without (p = 0.030).
Kaplan–Meier estimate of overall survival (OS) from the initiation of crizotinib treatment for patients receiving crizotinib as first-line or second- or later-line therapy: a OS of first-line therapy patients; b OS of first-line therapy patients stratified by with or without local brain metastasis treatment; c OS of ≥ second-line therapy patients; d OS of ≥ second-line therapy patients stratified by with or without local brain metastasis treatment. CI confidence interval
Seventy-four patients received crizotinib as second- or later-line treatment, among whom 42 (56.8%) harbored baseline brain metastasis and 69 (93.2%) experienced progression. The median OS of patients receiving crizotinib as second- or later-line treatment was 53.4 months (95% CI not reached) (Fig. 3c). When age (< 65 vs ≥ 65 years), sex, baseline brain metastasis status, and local brain metastasis treatment status were included in a multivariate Cox regression analysis, we found that without local brain metastasis treatment (without vs with local treatment: HR 2.68; 95 CI 1.26–5.72; p = 0.010) was an independent risk factor for this group of patients. The median OS of patients with local brain metastasis treatment was not reached, while that of patients without local brain metastasis treatment was 12.5 months (95% CI 0.0–31.5) (p = 0.007) (Fig. 3d). Intracranial progression status was not included in the multivariate Cox regression analysis, because patients without intracranial progression in the second- or later-line treatment setting were all still alive at the end of the study period. In addition, the median OS for patients with intracranial progression was also significantly different from that for patients without (p = 0.002).
3.5 Efficacy of Crizotinib for Patients Continuing Crizotinib Beyond Progressive Disease
A total of 135 patients experienced intracranial progression as defined by RECIST 1.1, among whom 94 patients continued crizotinib beyond progressive disease (CBPD). Nine patients were excluded from the analysis due to combination with other systemic agents. Among patients continuing CBPD, 76 out of 85 patients experienced only intracranial progression and 55 out of 85 patients received local brain metastasis treatment after progression. Among the 41 patients who discontinued crizotinib, 28 received next-generation ALK inhibitors. There was no significant difference in median OS between patients with CBPD and without CBPD (48.3 months vs 53.4 months, respectively; p = 0.296) (Fig. 4a).
Kaplan–Meier estimate of overall survival (OS) from the initiation of crizotinib treatment for patients with or without continuation of crizotinib beyond progressive disease (CBPD): a OS of patients experiencing intracranial progression stratified by with or without CBPD; b OS of patients experiencing intracranial progression stratified by with or without extracranial progression. CI confidence interval
When age (< 65 vs ≥ 65 years), sex, local treatment after intracranial progression, line of crizotinib treatment, extracranial progression status, and continuation of crizotinib treatment were included in a multivariate Cox regression analysis, we found that extracranial progression was an independent risk factor for OS (with extracranial progression vs without: HR 2.04; 95% CI 1.02–4.08; p = 0.043). Patients with only intracranial progression had a significantly longer median OS than those with extracranial progression (not reached vs 24.7 months, respectively; p = 0.009) (Fig. 4b).
4 Discussion
With a poor cerebrospinal fluid (CSF)-to-plasma ratio of 0.0026 [12], the efficacy of crizotinib for patients with brain metastasis remains controversial, as has been shown in most case reports or subset analyses of clinical trials. To our knowledge, our research is the largest multicenter, retrospective, real-world study of the efficacy of crizotinib in patients with advanced ALK-rearranged NSCLC with brain metastasis in China.
A retrospective pooled analysis of the PROFILE 1005 and 1007 trials [9] showed that intracranial ORR and DCR at 12 weeks were 18% and 56%, respectively, among patients with previously untreated brain metastasis, while the intracranial ORR and DCR at 12 weeks were 33% and 62%, respectively, among patients who had previously undergone cranial radiotherapy. The median icTTP was 7 and 13.2 months for patients with untreated and treated brain metastases, respectively. The PROFILE 1014 trial showed an improved icTTP of 15.7 months (95% CI 10.0-not reached) and an intracranial DCR of 85% (95% CI 70–94) at 12 weeks among patients with treated brain metastasis [16].
Our study showed a relatively lower intracranial ORR of 13.6% and a slightly higher DCR of 90.9%, which may result from the fact that many brain metastasis lesions were not measurable in our study cohort and could only be assessed as stable disease in accordance with RECIST 1.1. The median PFS in our study was similar to the outcomes reported in previous clinical trials, but the median icTTP in our study was relatively longer. The proportion of patients receiving local treatment for baseline brain metastasis may partly explain this phenomenon, as according to the multivariate Cox regression analysis, local treatment for baseline brain metastasis significantly prolonged the icTTP. Our study revealed that local treatment for baseline brain metastasis provided no OS benefit, and this result is in accordance with the findings of Pacheco et al. [17]. These investigators defined all progressing lesions treated with local ablative therapy while patients continued to receive crizotinib as oligoprogressive disease (OPD) [17]. They performed a multivariate analysis which revealed that local ablative therapy for OPD while continuing crizotinib treatment did not significantly prolong OS (HR 0.58; 95% CI 0.28–1.20; p = 0.143).
Although we were not able to compare the OS of patients with baseline brain metastasis in our study with the OS of the whole population due to the immature OS of the PROFILE 1014 trial patients (median OS of 53.4 months and 1- and 4-year OS rates of 87.4% and 53.3% in our study vs 83.5% and 56.6%, respectively, among the whole population in the PROFILE 1014 trial [6]), we further confirmed that patients with baseline brain metastasis before crizotinib treatment could still obtain an OS benefit from crizotinib treatment.
For patients without brain metastasis, because of the significantly improved PFS and the advantage of controlling intracranial progression [13], it may be better to choose alectinib as first-line treatment. However, for patients already receiving crizotinib and developing new brain metastases during crizotinib treatment or for patients with baseline brain metastasis and developing intracranial progression during crizotinib treatment, whether to switch to next-generation ALK inhibitors or to continue with crizotinib plus brain radiotherapy still remains debatable.
Our study has demonstrated that for all patients with intracranial progression, continuation of CBPD did not achieve a significant OS benefit. This result is reasonable, even though most patients continuing to receive crizotinib also received local treatment for brain metastasis in our study. However, neither local treatment for baseline brain metastasis nor local treatment after intracranial progression significantly influenced OS according to the multivariate Cox regression analysis. We further confirmed by multivariate Cox regression analysis that instead of CBPD, it was the extracranial progression status that influenced the OS. Since many patients not receiving CBPD received next-generation ALK inhibitors, this result indicates that for patients with intracranial progression, it might be a reasonable alternative to switch to a next-generation ALK inhibitor directly. Further research is needed to optimize treatment selection in this setting.
Our current study has several limitations. First, as a retrospective study, recall bias may have been introduced. Additionally, since next-generation ALK inhibitors have not been widely available in China, few patients were able to receive these drugs. We were not able to analyze the impact of subsequent treatment with next-generation ALK inhibitors after crizotinib treatment, which requires further investigation in the future.
5 Conclusions
Patients with advanced ALK-rearranged NSCLC with baseline brain metastasis can still benefit from crizotinib treatment. Local treatment for baseline brain metastasis can prolong the time to intracranial progression, but it does not result in a survival benefit. Patients with intracranial progression may not achieve a long-term survival benefit from the continuation of CBPD.
References
The National Comprehensive Cancer Network (NCCN): Clinical Practice Guidelines in Oncology: non-small cell lung cancer, 2018; version 3. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp-nscl.
Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. New Engl J Med. 2010;363(18):1693–703. https://doi.org/10.1056/NEJMoa1006448.
Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–53. https://doi.org/10.1200/jco.2009.22.6993.
Sun JM, Lira M, Pandya K, et al. Clinical characteristics associated with ALK rearrangements in never-smokers with pulmonary adenocarcinoma. Lung Cancer (Amsterdam, Netherlands). 2014;83(2):259–64. https://doi.org/10.1016/j.lungcan.2013.11.009.
Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. New Engl J Med. 2014;371(23):2167–77. https://doi.org/10.1056/NEJMoa1408440.
Solomon BJ, Kim DW, Wu YL, et al. Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. J Clin Oncol. 2018;36(22):2251–8. https://doi.org/10.1200/jco.2017.77.4794.
Wu YL, Lu S, Lu Y, et al. Results of PROFILE 1029, a phase III comparison of first-line crizotinib versus chemotherapy in East Asian patients with ALK-positive advanced non-small cell lung cancer. J Thor Oncol. 2018;13(10):1539–48. https://doi.org/10.1016/j.jtho.2018.06.012.
Dagogo-Jack I, Gill CM, Cahill DP, Santagata S, Brastianos PK. Treatment of brain metastases in the modern genomic era. Pharmacol Ther. 2017;170:64–72. https://doi.org/10.1016/j.pharmthera.2016.10.011.
Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–8. https://doi.org/10.1200/jco.2014.59.0539.
Wardak Z, Choy H. Improving treatment options for brain metastases from ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34(34):4064–5. https://doi.org/10.1200/jco.2016.69.9587.
Rusthoven CG, Doebele RC. Management of brain metastases in ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34(24):2814–9. https://doi.org/10.1200/jco.2016.67.2410.
Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–5. https://doi.org/10.1200/jco.2010.34.1313.
Camidge DR, Peters S, Mok T, et al. Updated efficacy and safety data from the global phase III ALEX study of alectinib (ALC) vs crizotinib (CZ) in untreated advanced ALK + NSCLC. J Clin Oncol. 2018;36(suppl): abstr 9043.
Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK +) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. 2018;29(11):2214–22. https://doi.org/10.1093/annonc/mdy405.
Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. New Engl J Med. 2018;379(21):2027–39. https://doi.org/10.1056/NEJMoa1810171.
Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK-positive non-small-cell lung cancer: results from PROFILE 1014. J Clin Oncol. 2016;34(24):2858–65. https://doi.org/10.1200/jco.2015.63.5888.
Pacheco JM, Gao D, Smith D, et al. Natural history and factors associated with overall survival in stage IV ALK-rearranged non-small cell lung cancer. J Thorac Oncol. 2019;14(4):691–700. https://doi.org/10.1016/j.jtho.2018.12.014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by Pfizer investigator funding.
Conflict of interest
Junling Li has recived Pfizer investigator funding. Puyuan Xing, Shouzheng Wang, Qiang Wang, Di Ma, Xuezhi Hao, Mengzhao Wang, Yan Wang, Li Shan, Tao Xin, Li Liang, Hongge Liang, Yang Du, and Zhaohui Zhang declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Rights and permissions
About this article
Cite this article
Xing, P., Wang, S., Wang, Q. et al. Efficacy of Crizotinib for Advanced ALK-Rearranged Non-Small-Cell Lung Cancer Patients with Brain Metastasis: A Multicenter, Retrospective Study in China. Targ Oncol 14, 325–333 (2019). https://doi.org/10.1007/s11523-019-00637-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-019-00637-5