Abstract
Glial cell line-derived neurotrophic factor (GDNF) has potent neurotrophic effects and is known to promote the dopaminergic (DA) neuronal survival in cellular and animal models of Parkinson’s disease (PD). However, long-term ectopic GDNF delivery is associated with long lasting adverse side effects in PD patients. Therefore, finding safer and effective ways to elevate endogenous GDNF levels is an active area of research. This study underlines the importance of sodium benzoate (NaB), a metabolite of commonly-used spice cinnamon, a food-additive and an FDA-approved drug against hyperammonemia, in stimulating GDNF in primary mouse and human astrocytes. Presence of cAMP response element (CRE) in the Gdnf gene promoter, recruitment of CREB to the Gdnf promoter by NaB and abrogation of NaB-mediated GDNF expression by siRNA knockdown of CREB suggest that NaB induces the transcription of Gdnf via CREB. Finally, oral administration of NaB and cinnamon itself increased the level of GDNF in vivo in the substantia nigra pars compacta (SNpc) of normal as well as MPTP-intoxicated mice. Accordingly, cinnamon and NaB treatment protected tyrosine hydroxylase positive neurons in the SNpc and fibers in the striatum, normalized striatal neurotransmitters, and improved locomotor activities in MPTP-intoxicated Gfapcre mice, but not GdnfΔastro mice lacking GDNF in astrocytes. These findings highlight the importance of astroglial GDNF in cinnamon- and NaB-mediated protection of the nigrostriatum in MPTP mouse model of PD and suggest possible therapeutic potential of cinnamon and NaB in PD patients.

Cinnamon metabolite sodium benzoate (NaB) activates cAMP-response element-binding (CREB) via protein kinase A (PKA) in astrocytes. Activated CREB then binds to cAMP-response element (CRE) present in GDNF gene promoter to stimulate the transcription of GDNF in astrocytes. This astrocytic GDNF leads to nigral trophism and protects dopaminergic neurons from MPTP insult.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder. Pathologically, it is characterized by selective neuronal depletion in the substantia nigra pars compacta (SNpc) region of the brain and drastic reduction in striatal dopaminergic innervation (Kalia and Lang 2015). Another pathological hallmark for PD and dementia with Lewy Bodies (DLB) is the predominant intra-neuronal accumulation of protein aggregates [Lewy bodies (LBs) and Lewy neurites (LNs)] (Wakabayashi et al. 2007). These hallmark deposits (LBs and LNs) are primarily composed of fibril forming protein α-synuclein (α-syn), a 14 kDa presynaptic protein (Villar-Pique et al. 2016). Clinically, PD patients manifest at least two of four cardinal features namely, bradykinesia (slowness and minimal movement), rigidity, resting tremor (trembling), and an impairment of postural balance leading to disturbance of gait and falling (Lang and Lozano 1998; Jankovic 2008). To date, there is no cure for PD. Although there are medications like carbidopa, levodopa, dopamine agonists, monoamine oxidase B (MAO-B) inhibitors, Catechol-O-methyltransferase (COMT) inhibitors and surgery like deep brain stimulation, available to address some of the PD associated symptoms (Ellis and Fell 2017; Emamzadeh and Surguchov 2018), none of these can effectively halt the progression of PD. Furthermore, prolonged usage of these pharmacological and surgical interventions against PD has major issues in terms of side effects, short life span and increase in blood-brain barrier (BBB) permeability (Rascol et al. 2003; De Deurwaerdere et al. 2017). For several years, trophic factors have been pursued as potential therapeutic agents due to their ability to regulate the survival of specific neuronal populations in the central nervous system (CNS) (Aloe et al. 2012; Weissmiller and Wu 2012). One such trophic factor is glial cell-derived neurotrophic factor (GDNF), a member of the transforming growth factor beta superfamily. GDNF was first identified on the basis of its ability to support the development of embryonic DA neurons (Lin et al. 1993). Following its discovery, GDNF has been regularly shown to protect and restore mature DA neurons in the SNpc of different lesion models (Kearns and Gash 1995; Sauer et al. 1995; Winkler et al. 1996; Kirik et al. 2000). Unfortunately, ectopic GDNF delivery in humans by either intra-cerebroventricular injection or intra-striatal infusion has proven ineffective (Patel and Gill 2007). Therefore, finding a safer and more effective approach to exploit the neuro-protective effects of GDNF remains an active area of research in developing a treatment to inhibit the progression of PD.
Recently, many animal studies and clinical trials have explored the beneficial effects of cinnamon, a commonly used food spice, in Alzheimer’s disease (Modi et al. 2015b; Modi et al. 2016), diabetes (Mirfeizi et al. 2016; Santos and da Silva 2018), arthritis (Rathi et al. 2013; Shishehbor et al. 2018) and arteriosclerosis (Kang et al. 2014; Nayak et al. 2017). Cinnamon contains variety of bioactive resinous compounds, including cinnamaldehyde, cinnamyl alcohol, cinnamic acid, and numerous essential oils (Hariri and Ghiasvand 2016). In the liver, cinnamic acid is β-oxidized to benzoate that exists as sodium salt (NaB) (Abd El-Mawla et al. 2001). Interestingly, NaB is a food-additive and a FDA-approved drug for glycine encephalopathy (Neuberger et al. 2000). Being a component of Ammonul®, NaB is also a FDA-approved medication for urea cycle disorders. Recently we have shown that NaB and cinnamon protect dopaminergic neurons in a MPTP mouse model of PD (Khasnavis and Pahan 2014). Here, we describe that NaB is capable of upregulating GDNF from mouse and human astrocytes. We found that NaB treatment recruited CREB to the Gdnf gene promoter leading to its transcription. Accordingly, oral administration of NaB and cinnamon increased astroglial expression of GDNF in vivo in the nigra of normal as well as MPTP-intoxicated mice. Interestingly, NaB and cinnamon protected nigral dopaminergic neurons, preserved striatal innervation, restored striatal neurotransmitters, and improved locomotor activities in MPTP-insulted non-transgenic littermates (Gfapcre), but not astrocyte specific Gdnf conditional knockout (GdnfΔastro), mice. These results delineate an important role of astroglial GDNF in cinnamon- and NaB-mediated protection of the nigrostriatum and suggest that cinnamon and NaB may have therapeutic importance in PD.
Materials and Methods
Reagents and Antibodies
Antibodies, their applications, sources and dilutions are listed in Supplementary Table 1. Cell culture materials (DMEMF/12, antibiotic/antimycotic) were purchased from Life Technologies. Original Ceylon cinnamon (Cinnamonum verum) in ground form was obtained from Indus Organics (San Ramon, CA). Other pharmacological compounds like sodium benzoate and sodium formate were purchased from Sigma-Aldrich. All molecular biology-grade and chemicals were obtained from Sigma or Bio-Rad. IR-Dye-labeled secondary antibodies used for immunoblotting were from Li-Cor Biosciences.
Animals
Mice were maintained and experiments conducted in accordance with National Institute of Health guidelines and were approved by the Rush University Medical Center IACUC. Gdnf floxed mice [B6.129S1 (Cg)-Gdnftm1.1Neas/J, JAX stock # 014097] were bred with transgenic mice expressing the Cre enzyme under the control of the Gfap promoter [B6.Cg-Tg(Gfap-cre)73.12Mvs/J, JAX stock #012886] to generate knockout mice with Gdnf deletion in the astroglial cells (GdnfΔastro mice). The final breeding step was performed using homozygous breeding pair from F4 generation. All mice analyzed in this study were on a congenic C57BL/6 J genetic background. Genotyping of the Gdnf floxed locus and Gfapcre transgene was performed using PCR on DNA from tail biopsies and detected by primers listed in Table S2. Mice were maintained on a 14 h light, 10 h dark cycle and given a continuous supply of food and water.
MPTP Mouse Model
Mice were intoxicated with MPTP (acute) as described earlier (Ghosh et al. 2009; Khasnavis et al. 2013; Khasnavis and Pahan 2014; Chandra et al. 2017). Briefly, the mice were injected intraperitoneally (i.p.) with MPTP (20 mg/kg, Sigma-Aldrich, St. Louis, MO, United States) four times at 2-h intervals. Saline was given as controls.
Cinnamon and NaB Treatment
Starting from 3 h after the last injection of MPTP, mice were treated with either cinnamon or NaB dissolved in 0.5% methylcellulose at individual doses (100 mg/kg, orally) once daily for 7 days and following behavior analysis the mice were sacrificed for biochemical studies.
Isolation of Primary Mouse Astrocytes
Astrocytes were isolated from mixed glial cultures of 2 to 3 days old mouse pups according to the procedure described earlier (Khasnavis and Pahan 2012; Modi et al. 2015a; Roy et al. 2015). Briefly, brain from pups (n > 10) were isolated and placed together in the Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) media supplemented with 10% heat-inactivated fetal bovine serum. Astroglial cells were dissociated by trituration and single cell suspension was equally plated in 4 to 5 poly-D-lysine pre-coated T-75 flasks containing complete DMEM/F12 media. On day 9, the T-75 flasks containing mixed glial cultures were washed three times with Dulbecco’s modified Eagle’s medium/F-12 and subjected to shaking at 240 rpm for 2 h at 37 °C on a rotary shaker to remove microglia. After 2 days, the shaking was repeated for 24 h for the removal of oligodendroglia and to ensure the complete removal of all non-astroglial cells. The attached cells were seeded onto new plates for further studies.
Isolation of Primary Human Astrocytes
Primary human astrocytes were prepared as described by us earlier (Brahmachari et al. 2009; Corbett et al. 2012). Briefly, 11 to 17 week old fetal brains obtained from the Human Embryology Laboratory (University of Washington, Seattle) were dissociated by trituration and trypsinization. On the 9th day, these mixed glial cultures were placed on a rotary shaker at 240 rpm at 37 °C for 2 h to remove loosely attached microglia. Then on the 11th day, flasks were shaken again at 180 rpm at 37 °C for 18 h to remove oligodendroglia. The attached cells remaining were primarily astrocytes. These cells were trypsinized and sub cultured in complete media at 37 °C with 5% CO2 in air to yield more viable and healthy cells. More than 98% of the cells obtained by this method were found to be positive for GFAP, a marker for astrocytes.
Assay of GDNF by ELISA
Level of GDNF was monitored in cell supernatants and tissue homogenates by high-sensitivity ELISA kit (Promega, Madison, WI) as described before (Villadiego et al. 2005), following manufacturer’s protocol.
Semi-Quantitative RT-PCR
Total RNA was isolated from the primary astrocytes using Quick-RNA™ MiniPrep kit (ZYMO RESEARCH Inc, Catalog Nos. R1054 & R1055) following the manufacturer’s protocol. Isolated total RNA was digested with DNase and RT-PCR was carried out as described earlier (Corbett et al. 2013) using the RT-PCR kit from Clontech and the primers listed in Supplementary Table 2. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used to ascertain that an equivalent amount of cDNA was synthesized from different samples.
Real-Time PCR
Real-time PCR analysis was performed in the ABI-Prism7700 sequence detection system (Applied Biosystems) as described earlier (Corbett et al. 2013). Complementary DNA (cDNA) was created using TaqMan Universal Master Mix and amplified with SYBR Green-conjugated PCR master mix (Applied Biosystems) and the primers listed in Supplementary Table 2. Data were processed by the ABI Sequence Detection System 1.6 software and analyzed by the relative 2-ΔΔCT method.
Immunoblotting and Densitometric Analyses
For whole cell and tissue lysates, samples were homogenized in RIPA buffer containing protease and phosphatase inhibitors (Sigma), rotated end over end for 30 min at 4 °C and centrifuged for 10 min at 15,000 g. The supernatant was aliquoted and stored at −80 °C until use. Protein concentrations were determined using a NanoDrop 2000 (Thermo Fisher), and 15–30 μg sample was heat-denatured and resolved on 12% or 15% polyacrylamide-SDS gels in MES buffer (50 mM MES, 50 mM Tris base, 0.1% SDS, 1 mM EDTA, pH 7.3) or 1X SDS Running Buffer. Proteins were transferred to 0.45 μm nitrocellulose membranes in Towbin Buffer (25 mM Tris, 192 mM glycine, 20% (w/v) methanol) under wet conditions (40 V for 120 mins). Membranes were blocked for 1 h with blocking buffer (Li-Cor), incubated with primary antibodies (Supplementary Table 1) overnight at 4 °C under shaking conditions, washed, incubated with IR-dye labeled secondary antibodies (1:5000; Li-Cor) for 45 min at room temperature, washed and visualized with the Odyssey Infrared Imaging System (Li-Cor). Blots were converted to binary, analyzed using Image J software and normalized to the loading control (β-actin).
Immunofluorescence Staining
We performed immunofluorescence staining using procedure described earlier (Patel et al. 2018). Briefly, After NaB treatment, primary mice or human astrocytes were washed three times with 1X PBS, fixed in 4% paraformaldehyde for 10 min or with chilled methanol overnight, washed again with 1X PBS and incubated first with monoclonal or polyclonal primary antibodies (Supplementary Table 1) and then with the Cy2 or Cy5 conjugated secondary antibodies. Following secondary antibody incubation, coverslips were rinsed in 1X PBS, mounted on slides in Fluormount (Sigma) and imaged using an Olympus BX41 fluorescent microscope equipped with a Hamamatsu ORCA-03G camera.
Immunohistochemistry (IHC)
Following NaB or Cinnamon treatment, the mice were anesthetized and intracardially perfused with 1X PBS followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer, pH 7.4. The brains were post fixed in PFA overnight at 4 °C, and on next day were stored in phosphate buffer containing 30% sucrose at 4 °C. Coronal striatal and nigral sections were cut and saved in serial order at −20 °C until immunostained.
IHC, Using Fluorescence Microscopy
Free-floating brain sections were analyzed using immunofluorescence microscopy following procedure mentioned earlier (Ghosh et al. 2007, 2009). Briefly, samples were incubated in PBS containing 0.05% Tween 20 (PBST) and 10% sucrose for 3 h and then 30% sucrose overnight at 4 °C. Brain was then embedded in O.C.T (Tissue Tech) at −80 °C, and processed for conventional cryosectioning. Free floating sections (40 μm) were treated with cold ethanol (−20 °C) followed by two rinses in PBS, blocking with 3% bovine serum albumin in PBST and double labeling with antibodies (Supplementary Table 1). After three washes in PBST, sections were further incubated with Cy2 and Cy5 (Jackson ImmunoResearch Laboratories, Inc.). The samples were mounted and observed under the Olympus BX41 fluorescent microscope equipped with a Hamamatsu ORCA-03G camera.
IHC, Using Light Microscopy
Free floating sections from the striatum (thickness; 40 μm) and ventral midbrain (thickness; 40 μm) were stained using standard immuno-histochemical procedures as described earlier (Ghosh et al. 2007, 2009). Briefly, after quenching with 3% hydrogen peroxide (H2O2) and 10% methanol for 5 min sections were pre-incubated in 2% normal goat serum (NGS; Vector Laboratories, Burlingame, CA) and 0.3% Triton X-100 for 60 min followed by incubation with rabbit anti-TH polyclonal antibody (Supplementary Table 1) overnight at 40C, followed by incubation for 2 h with the biotinylated goat anti-rabbit antibody (BA1000, 1:200, Vector Laboratories) the next day. Vectastain Elite ABC peroxidase kit (Vector Laboratories) was used for visualization using 0.06% diaminobenzidine and H2O2. The sections were mounted on gelatin/chrome-coated slides, air-dried, dehydrated, cleared and mounted using Fluormount (Sigma). Histological images for the figures were generated using bright light microscope [Olympus microscope (BX61) attached to a Nikon digital camera DXM1200]. Quantitation of striatal TH immunostaining was performed as described earlier (Ghosh et al. 2007, 2009) and striatal optical density measurements that reflect dopaminergic fiber innervation were determined by image J analysis.
Promoter Mapping and Chromatin Immunoprecipitation (ChIP) Assay
The mouse Gdnf promoter was analyzed for predicted transcription factor binding sites, using MatInspector online software (Cartharius et al. 2005) with the matrix threshold set at 0.80. The primer sets (Supplementary Table 2) that can amplify fragments flanking the CRE binding element in the mouse Gdnf promoter, were then designed upstream and downstream of transcription factor binding sites to amplify immunoprecipitated DNA. Chromatin was prepared and immunoprecipitated as described earlier (Roy et al. 2013; Corbett et al. 2015). Briefly, NaB (250 μM) treated mouse primary astrocytes were fixed with formaldehyde (1.42% final volume) and quenched with 125 mM glycine. The astroglial cells were pelleted and lysed in IP buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 5 mM EDTA, Nonidet P-40 (0.5% v/v), Triton X-100 (1.0% v/v). After one wash with 1.0 ml of IP buffer, the pellet was re-suspended in 1 ml of IP buffer and sonicated. Sheared chromatin was split into two fractions (one to be used as Input). The remaining fraction was incubated overnight under rotation at 4 °C with antibodies listed in Supplementary Table 1, followed by incubation with protein G-agarose (Santa Cruz Biotechnology) for 2 h at 4 °C under rotation. Beads were then washed five times with cold IP buffer, and a total of 50 μl of 10% Chelex (10 g/100 ml H2O) was added directly to the washed protein G beads and vortexed. After 10 min boiling, the Chelex/protein G bead suspension was allowed to cool to room temperature. Proteinase K (100 μg/ml) was then added, and the beads were incubated for 30 min at 55 °C while shaking, followed by another round of boiling for 10 min. The suspension was centrifuged, and the supernatant was collected and used directly as a template in PCR using ChIP primers listed in Supplementary Table 2.
Rotarod Test
The motor coordination of mice was measured on the Rotarod apparatus (ENV-576 M; Med-associates Inc.), using protocol described earlier (Ghosh et al. 2007, 2009). Briefly, mice were transported (within their home cage) to acclimate to the testing room for 1 h prior to trial. Before acquisition, the parameters of the Rotarod system quipped with automatic fall detector such as start speed and acceleration were carefully checked before acquisition. Each mouse was placed on the confined section of the rod and trial was initiated with a smooth increase in speed from 4 rpm to 40 rpm for 5 min. If the mouse did not fall from the rod, it was removed from the rod after 5 min. The latency to fall was measured in seconds and used for the analysis.
Open Field Test
Open Field test was performed as described earlier (Ghosh et al. 2007, 2009) with slight modifications and used to assess spontaneous exploratory activity and stress-related behaviors in open environment. Briefly, each mouse was allowed to freely explore an open field arena for 5 min. The testing apparatus was a classic open field (i.e., a wooden floor square arena, 40 × 40 cm, with walls 30 cm high). A video camera (Basler Gen I Cam - Basler acA 1300–60) connected to a Noldus computer system was placed above the box. Each mouse was placed individually on the center of the arena and the locomotor activity and stereotypical behaviors like rearing and grooming were monitored for 5 min using live video tracking system (Noldus System). The central area was arbitrarily defined as a square of 20 × 20 cm (half the total area).
Statistical Analysis
Statistical analysis was conducted, using Graph Pad Prism 7.0c software. Unless otherwise stated, one-way or two-way ANOVA followed by Bonferroni’s post-hoc analyses was performed to determine the significance of differences among multiple experimental groups. Student’s t test was used when the significance of differences was determined between two groups. Data were expressed as mean ± standard error (SEM) or mean ± standard deviation (SD), and values with P < 0.05 were considered statistically significant.
Results
Cinnamon Metabolite Sodium Benzoate (NaB) Upregulates GDNF Expression in Mouse and Human Primary Astrocytes
GDNF is important for the survival, maintenance and regeneration of dopaminergic neuronal populations in the adult brain. Depletion of GDNF along with other neurotrophic factors like nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) has been linked with disease pathology noticed in several neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease and Huntington’s diseases (Allen et al. 2013). Astrocytes, most abundant cell type within the central nervous system (CNS), perform variety of tasks, from axon guidance and synaptic maintenance, to the regulation of the BBB and blood flow. Primarily, astrocytes are involved in the production of a host of neurotrophic factors, including GDNF and BDNF, which support neuronal development, plasticity and survival (Koyama 2002; Seifert et al. 2006). Since cinnamon cannot be metabolized in astrocytes, cells were treated with increasing doses of NaB, a major metabolite of cinnamon, to examine the expression of GDNF. We used sodium formate (NaFO) as a negative control for NaB. As evident from semi-quantitative RT-PCR (Fig. 1a) and real-time PCR (Fig. 1b), NaB treatment led to marked increase in the Gdnf mRNA expression in mouse astrocytes with 250 μM dose showing maximum effect. However, NaFO failed to induce the Gdnf mRNA expression (Fig. 1a and b). Time course study shows that NaB (250 μM) upregulated Gdnf mRNA expression in mouse astrocytes with maximum induction seen at 4 h of treatment (Fig. 1c and d). Next, we monitored the induction of GDNF protein following NaB treatment by immunoblotting and immunofluorescence analyses. Consistent to the upregulation of Gdnf mRNA, NaB, but not NaFO, increased GDNF protein levels in the mouse astrocytes within 8 h of stimulation (Fig. 1e, f and g). For raw blots, please see Supplementary Fig. 1. We also investigated if NaB could elicit the similar effect in human astrocytes. As appeared from Fig. 2a-b, NaB, but not NaFO, increased the level of Gdnf mRNA in a time-dependent manner in primary human astrocytes with maximum upregulation seen at either 4 h or 6 h of stimulation. Dose-dependent study showed that NaB was capable of upregulating Gdnf mRNA in human astrocytes at a dose of 50 μM (Fig. 2c). However, maximum increase was seen at a dose of 250 μM or higher (Fig. 2c). Being a neurotrophic factor, since GDNF is released from GDNF-expressing cells, we measured the level of GDNF in supernatants of human astrocytes by ELISA. Consistent to mRNA expression, NaB dose-dependently increased the production of GDNF (Fig. 2d). Again, the maximum increase in GDNF production was seen at a dose of 250 μM NaB (Fig. 2d). Immunofluorescence analysis of NaB-treated human astrocytes also confirmed this finding (Fig. 2e). However, in all the cases, NaFO remained unable to induce the expression of either Gdnf mRNA (Fig. 2a-c) or GDNF protein (Fig. 2d-e). Together, these results suggest that NaB is capable of stimulating the GDNF expression in both mouse and human astrocytes.
Sodium benzoate stimulates the expression of GDNF in primary mouse astrocytes. Astrocytes isolated from 2 to 3 d old mouse pups were treated with increasing concentrations of NaB (μM) for 4 h followed by Gdnf mRNA analysis through semi-quantitative RT-PCR (a) and real-time PCR (b). Results are mean ± SD of three independent experiments; aP < 0.01 vs. control or bP < 0.05 vs. control. Sodium formate (NaFO 500 μM) was used as a negative control for NaB. We also treated WT mouse astrocytes with NaB (250 μM) for various durations, followed by measurement of the Gdnf mRNA expression by semi-quantitative RT-PCR (c) and real-time PCR (d). Results are mean ± SD of three independent experiments; aP < 0.001 vs. control, bP < 0.01 vs. control and cP < 0.05 vs. control. After 8 h of NaB treatment, GDNF protein levels were monitored in astroglial cells by immunoblotting (e) and densitometric analyses (f). Densitometric values of GDNF were normalized with respect to β actin and then compared with control. Sodium formate (NaFO 500 μM) was used as a negative control for NaB. Results are mean ± SD of three independent experiments; aP < 0.01 vs. control; bP < 0.05 vs. control. The GDNF protein levels were also corroborated by double labeling the NaB treated- WT astrocytes with antibodies against GFAP and GDNF (g). DAPI was used to visualize the nucleus in astrocytes. Scale bar 20 μm
Sodium benzoate stimulates the expression of GDNF in primary human astrocytes. Astrocytes isolated from human fetal brain tissues were treated with NaB (250 μM) for different time periods followed by Gdnf mRNA analysis using semi-quantitative RT-PCR (a) and real-time PCR (b). NaFO (500 μM) was used as a negative control for NaB. Results are mean ± SD of three independent experiments; aP < 0.01 vs. control, bP < 0.05 vs. control. Next, we performed dose dependent analyses with increasing concentrations of NaB (μM) for 4 h in isolated human astroglial cells using NaFO (500 μM) as a negative control for NaB. Following NaB and NaFO treatment, Gdnf mRNA levels were analyzed by semi-quantitative RT-PCR (c). Similarly, following 8 h treatment with increasing NaB dosage (μM) and NaFO (500 μM) as negative control, we monitored GDNF protein levels in supernatants obtained from NaB and NaFO treated human astroglial cells by ELISA (d). Results are mean ± SD of three independent experiments; aP < 0.01 vs. control, bP < 0.05 vs control. GDNF protein levels were also analyzed by double labeling NaB treated human fetal astrocytes with antibodies against GFAP and GDNF (e). Scale bar 20 μm
Involvement of CREB in NaB-Mediated Upregulation of GDNF in Astrocytes
Next, we elucidated the underlying molecular mechanism by which NaB increases Gdnf transcription in astrocytes. To determine putative binding sites for transcription factors (TFs) the mGdnf promoter was analyzed using MatInspector program (Genomatix). Based on this analysis, predicted binding sites for several TFs including Cyclic AMP response element (CRE)-binding protein (CREB) was identified near transcription start site (TSS) in the mGdnf gene promotor (Fig. 3a). CREB is a 43 kDa protein and a member of the leucine zipper family of transcription factors, which regulates gene transcription by binding to CRE, a cis-acting enhancer element in the regulatory region of various genes. CREB upon activation promotes target gene activation in part by means of recruitment of the co-activators namely CREB-binding protein (CBP) and/or p300 (Goodman and Smolik 2000). Earlier we have shown that NaB is capable of inducing the activation of CREB in astrocytes (Modi et al. 2015a) and neurons (Modi et al. 2016). Therefore, here, we examined if CREB was involved in the transcription of Gdnf in NaB-treated astrocytes. Using ChIP assay, we examined the recruitment of CREB to the Gdnf promoter. CREB antibody was used to immunoprecipitate formaldehyde cross-linked protein-chromatin complexes from NaB-treated or control (untreated) mouse primary astrocytes and the resulting immunoprecipitated DNA was amplified by PCR. Parallel reactions were also set up using antibodies against CBP and p300. We used RNA pol II as positive control and IgG as negative control for transcription. Notably, we found enhanced recruitment of CREB, CBP and RNA Pol II to the Gdnf promoter in NaB-treated mouse astrocytes as compared to untreated cells (Fig. 3b). On the other hand, anti-p300 antibodies and IgG failed to precipitate CREB-binding Gdnf promoter fragments from control and NaB-treated astroglial chromatin, suggesting that CBP, but not p300, is acting here as coactivator. Consistently, real time PCR analyses also demonstrated a significant increase in CREB, CBP and RNA Pol II recruitment, but not p300, to the CRE of the Gdnf promoter in NaB-treated astrocytes as compared with untreated astrocytes (Fig. 3c). Next, we examined the effects of CREB knockdown to further confirm the involvement of CREB in the sequence of events leading to Gdnf transcription in human astrocytes. Accordingly, we employed CREB siRNA to investigate the role of CREB in NaB-mediated expression of Gdnf mRNA. Following NaB treatment, we observed marked upregulation of Gdnf mRNA expression in control siRNA-transfected, but not CREB siRNA-transfected, human astrocytes (Fig. 3d and e). Together these results indicate that NaB increases the transcription of Gdnf in astrocytes via CREB.
NaB mediated- GDNF upregulation involves transcriptional activation of CREB in mice and human astrocytes. We performed sequence analysis of mGdnf promoter with the Genomatix MatInspector program. a Cartoon depicting consensus frequencies represented by sequence logo of putative CRE binding site located upstream of the transcription start site in mouse Gdnf promoter sequence. b – c ChIP analysis monitoring the recruitment of CREB and associated co-activators to the indicated position on mGdnf promoter using RT-PCR (b) and Real time PCR (c) analyses. Briefly, WT primary mice astrocytes were treated with NaB (250 μM) for 2 h under serum-free condition and isolated cross-linked chromatin containing specific DNA-protein complexes were immunoprecipitated using antibodies specific to CREB, CBP, RNA Pol II and IgG. Eluted DNA was PCR amplified using two of mGdnf promoter primer sets as indicated in Table S2. All values are corrected for input DNA and are relative to control. Significance of the mean among groups was analyzed through one-way ANOVA [F (9, 30) = 9.8134; P < 0.001] followed by Bonferroni’s post hoc test and results were represented as mean ± SD; aP < 0.001 vs. control or bP < 0.01 vs. control. Human astrocytes were transfected with CREB-siRNA and control siRNA for 48 h, followed by 4 h of NaB (250uM) treatment. The specificity of siRNA knockdown on the Gdnf mRNA expression was monitored in NaB treated human astrocytes by semi-quantitative RT-PCR (d) and Real-time PCR (e) analyses. Results are mean ± S.D. of three different experiments. aP < 0.001 vs. control; bP < 0.001 vs. NaB
Oral Administration of NaB and Cinnamon Increases the Level of GDNF In Vivo in the Substantia Nigra Pars Compacta (SNpc) of Normal as Well as MPTP-Intoxicated Mice
Since, we noticed the marked up-regulation of GDNF expression in both mouse and human astrocytes, we next examined if NaB can replicate the similar effect in vivo in the brain. Consistent with our cell culture data, we noticed marked increase in Gdnf mRNA levels (Fig. 4a and b) in the SNpc of male C57/BL6 mice, following 10d of oral administration of NaB. Further, as evident from ELISA, NaB also increased the level of GDNF protein in the SNpc of male C57/BL6 mice (Fig. 4c). Previously, we have demonstrated that oral administration of ground cinnamon (Cinnamonum verum powder) is capable of increasing the level of NaB in serum and brain of mice (Jana et al. 2013). Accordingly, we next examined if cinnamon by itself was capable of increasing the levels of GDNF in the brain. Interestingly, after 10 days of feeding, Cinnamonum verum powder markedly increased the GDNF mRNA expression (Fig. 4d) and GDNF protein level (Fig. 4e and f) in the SNpc of male C57/BL6 mice.
Oral administration of NaB and cinnamon increases the level of glial derived neurotrophic factor in vivo in the substantia nigra pars compacta (SNpc) of mice. Six to eight week old male C57/BL6 mice (n = 5 in each group) received increasing amounts of NaB (mg/kg body weight/d) orally via gavage for 10 days. NaFO (50 mg/kg body weight/d) was used as a negative control for NaB. Following NaB or NaFO oral feeding, we monitored the Gdnf mRNA levels by semi-quantitative RTPCR (a) and real-time PCR (b) and GDNF protein levels by ELISA (c) in the SNpc. Results are means ± SE of five mice per group; aP < 0.01 vs. control. Similarly, C57/BL6 mice aged 6 to 8 weeks (n = 5 in each group) received increasing amounts of cinnamon (Cinnamonum verum powder) via gavage and after 10 d of feeding, Gdnf mRNA expression was monitored in the SNpc by semi-quantitative RT-PCR (d). GDNF protein levels in the SNpc of cinnamon-fed mice were monitored by ELISA (e). Data are means ± SEM of five mice per group; aP < 0.01 vs. control, bP < 0.05 vs. control. GDNF protein levels were also analyzed by double labeling of nigral sections harvested from cinnamon-fed C57/BL6 mice with antibodies against GFAP and GDNF (f). DAPI was used to visualize nucleus. Scale bar = 50 μm. For each experimental analysis, control mice received only vehicle (0.5% methylcellulose)
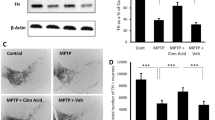
Next, we tested the trophic efficacy of NaB and cinnamon in acute MPTP mouse model, one the most widely used animal models of PD employing toxins (Meredith and Rademacher 2011). MPTP mouse model mimics many aspects of the disease and more importantly, provides investigators a platforms for testing symptomatic and neuroprotective drugs. As evident from GDNF Western blot, after MPTP intoxication, the level of GDNF decreased in the SNpc (Fig. 5a-b). However, oral administration of NaB and cinnamon increased the level of GDNF in the nigra of MPTP-intoxicated mice (Fig. 5a-b). Next, we examined the cellular source of increased GDNF levels in the CNS by double labeling nigral sections for GFAP and GDNF. Notably, compared to MPTP intoxicated mice, nigral section harvested from both NaB and cinnamon fed MPTP intoxicated mice showed increased GDNF signals aggregation around and within the cytoplasm of GFAP expressing astrocytes (Fig. 5c). Further, through quantification of co-localized cells (Fig. 5d), we noticed that MPTP insult decreased the number of GDNF+GFAP+ cells in the nigra as compared to control mice (Fig. 5d). However, oral treatment of NaB and cinnamon significantly increased number of GDNF+GFAP+ cells as compared to MPTP-intoxicated group (Fig. 5d). These results indicate that NaB and cinnamon both can stimulate astrocytes to synthesize GDNF in the nigral region of both control and MPTP-intoxicated mice. We also analyzed the TH protein levels in the SNpc of MPTP-intoxicated mice following NaB and cinnamon treatment. Similar to our earlier report (Khasnavis and Pahan 2014), we also noticed a significant increase in the nigral TH protein levels (Fig. 5e and g) in both NaB- and cinnamon-fed MPTP-intoxicated mice as compared to vehicle-treated MPTP-intoxicated mice. For raw blots, please see Supplementary Fig. 1.
Oral treatment of NaB and cinnamon renders astroglial GDNF mediated neurotrophic effect following MPTP induced neurodegeneration. 6–8 wk. old C57/BL6 mice (n = 5) were insulted with MPTP (20 mg/kg body wt/inj, four i.p injections at every 2-h interval). After 3 h of last MPTP injection, mice in each group were fed with NaB (100 mg/kg body wt/d) or cinnamon (100 mg/kg body wt/d) via oral gavage for 7 days. A control group without MPTP intoxication was also included. Following NaB or cinnamon treatment, GDNF protein levels were analyzed in the nigral homogenates from each group by immunoblot analysis (a). β actin was run as loading control. b Densitometric analysis of GDNF protein was performed using image J and densitometric values of GDNF were normalized with respect to β actin and presented as relative to control group. Results are mean ± SEM of mice (n = 5) per group. aP < 0.001 vs. MPTP, bP < 0.01 vs. MPTP. We also analyzed the GDNF protein expression by double labeling nigral sections from NaB or cinnamon fed C57/BL6 mice with GFAP and GDNF (c). Scale bar 50 μm. To confirm the co-localization of GFAP with GDNF in nigral region following NaB or cinnamon treatment, we quantified number of GFAP+ and GDNF+ cells per mm2 of nigral region using 2 sections from each of five mice per group (d). One-way ANOVA [F(3,36) = 8.401; p < 0.001] followed by Bonferroni’s post hoc test was applied to assess the significance of the mean among groups and represented as mean ± SEM; aP < 0.001 vs. MPTP or bP < 0.01 vs. MPTP. Similarly, immunoblot analysis of TH protein (e) in nigral lysates of MPTP intoxicated wt mice treated with NaB or cinnamon was performed followed by densitometric analyses (f). β actin was blotted as a housekeeping protein. Results are mean ± SEM of mice (n = 5) per group. aP < 0.01 vs. MPTP. Protein levels of TH were also analyzed by double labeling nigral sections from NaB or cinnamon fed C57/BL6 mice with GFAP and TH (g). Scale bar 50 μm
Astroglial Deletion of Gdnf Abrogated NaB- and Cinnamon-Mediated Recovery against MPTP-Induced Neurodegeneration
Since both NaB and cinnamon treatment enhanced astrocytic Gdnf expression in the nigra of MPTP-intoxicated mice, we next investigated the role of astrocytic Gdnf in NaB- and cinnamon-mediated dopaminergic neuroprotection. To address our hypothesis, we used the transgenic mice generated through cre-lox recombination technique that lacked astrocyte specific Gdnf (GdnfΔastro mice) (Fig. 6a and b). Age-matched Gfapcre mice served as controls. Both Gfapcre and GdnfΔastro groups were MPTP-intoxicated and were treated with either NaB or cinnamon starting from 3 h after last MPTP injection, for 7 days. In each group, saline-treated mice that did not receive MPTP served as controls for MPTP insults. Following, NaB or cinnamon treatment, mice from both the groups were transcardially perfused with saline followed by 4% paraformaldehyde, their brains were removed, cryosectioned, and immunostained for expression of TH, the rate-limiting enzyme in dopamine synthesis. As expected, MPTP intoxication led to significant loss of SNpc TH-positive neurons in both Gfapcre and GdnfΔastro mice (Fig. 6c) compared to their respective saline-injected controls. Interestingly, MPTP-intoxicated Gfapcre mice treated with either NaB or cinnamon, showed significant improvement in nigral TH-positive neurons (Fig. 6c). In contrast, no such protective effects were seen in MPTP-intoxicated GdnfΔastro mice by either NaB or cinnamon (Fig. 6c). We further corroborated this observation by analyzing TH immunoblotting of nigral homogenates obtained from mice of both Gfapcre (Fig. 6d and e) and GdnfΔastro groups (Fig. 6f and g). For raw blots, please see Supplementary Fig. 1. Similar to the loss of nigral TH neurons, MPTP intoxication led to significant reduction of striatal TH optical densities in both Gfapcre and GdnfΔastro mice (Fig. 7a) compared with their respective saline-injected controls. Further, NaB- and cinnamon-fed MPTP-intoxicated Gfapcre mice exhibited marked protection of striatal TH fibers (Fig. 7a and b). In contrast, NaB or cinnamon treatment failed to produce similar protection of striatal TH fibers in MPTP-intoxicated GdnfΔastro mice (Fig. 7a and b). To determine whether NaB or cinnamon may also protect against biochemical deficits caused by MPTP intoxication, we next assessed the levels of dopamine and two of its metabolites, 3, 4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) in the striata. As expected, compared to sham intoxication (saline injection), MPTP intoxication led to a characteristic significant diminution in striatal levels of dopamine and it’s metabolites in both Gfapcre mice and GdnfΔastro mice (Fig. 7c-e). However, MPTP-intoxicated Gfapcre mice that received either NaB or cinnamon showed marked protection against MPTP-induced loss in the striatal dopamine, DOPAC, and HVA (Fig. 7c-e). In contrast, such protection was not seen in case of NaB- or cinnamon-fed MPTP-intoxicated GdnfΔastro mice (Fig. 7c-e). Together, these results indicate that NaB and cinnamon protect the nigrostriatum in MPTP mouse model of PD via astrocytic GDNF.
Oral treatment of NaB and cinnamon rescues loss of dopaminergic neurons in the nigra of MPTP-intoxicated non-Tg, but notGdnfΔastro, mice. As shown in schematic representation (a), age matched Gfapcre and Gdnf floxed mice were bred together to produce littermates consisted of four categories namely, Wild type (WT), Heterozygous (Het), non-transgenic (Gfapcre) and astrocyte specific Gdnf conditional knockout mice (GdnfΔastro). Genomic DNA isolated from tails of littermates born to breeding pairs made from F1 generation were analyzed by RT PCR (b) using primer set against Gfapcre and Gdnf floxed alleles (Table S2). 6–8 week old Gfapcre mice (n = 6) or GdnfΔastro mice (n = 6) were insulted with MPTP (20 mg/kg body wt/inj, four i.p injections at every 2-h interval). After 3 h of the last MPTP injection, mice in each group were fed with NaB (100 mg/kg body wt/d) or cinnamon (100 mg/kg body wt/d) via oral gavage for 7 days. A control group without MPTP intoxication was also included. Following NaB or cinnamon treatment, 3 sections spanning from different SNpc regions of each mice/group (n = 6) were stained for TH (C). Scale bar, 20 μm. To check the TH protein levels, nigral homogenates from GdnfΔastro mice and age matched non-transgenic littermates (Gfapcre) were immunoblotted with TH (D-G). β actin was run as loading control. Bands were scanned and presented as relative to control. Results are mean ± SEM of mice (n = 6) per group. aP < 0.001 vs. MPTP or bP < 0.01 vs. MPTP. Abbreviations: SNpc, substantia nigra pars compacta; VTA, ventral tegmental area
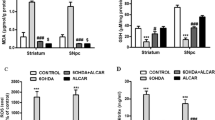
Oral treatment of NaB and cinnamon rescues loss of TH fibers and neurotransmitters in the striatum of MPTP-intoxicated non-Tg, but notGdnfΔastro, mice. 6–8 wk. old GdnfΔastro mice (n = 6) or age matched non-transgenic littermates (Gfapcre mice; n = 6) were insulted with MPTP (20 mg/kg body wt/inj; four i.p injections at every 2-h interval). After 3 h of the last MPTP injection, mice in each group were fed with (100 mg/kg body wt/d) or cinnamon (100 mg/kg body wt/d) via oral gavage for 7 days. A control group without MPTP intoxication was also included in each group. Following NaB or cinnamon, striatal sections from each group were stained for TH (a) followed by quantification of TH+ fibers using image J software (b). Scale bar, 10 μm. Statistical analyses for fiber density of TH+ fibers were performed in 2 sections per mice for (n = 5) per group using two-way ANOVA considering genotype [F(1,72) = 14.78; P < 0.001] and treatment [F(3,72) = 33.53; P < 0.001] as two independent variables. Interaction statistics between two independent variables were calculated as well [F(3,72) = 5.135; P = 0.003 (P < 0.01)]. Bonferroni’s post hoc test was applied to assess the significance of the mean; aP < 0.001 vs. MPTP. Concentrations of dopamine (c), DOPAC (d), and HVA (e) were measured in the striatum by HPLC. Results were analyzed using two-way ANOVA for n = 6 mice/group with genotype and treatment as two independent effectors, generating descriptive statistics for dopamine: [interaction effect – F(3,40) = 5.286; P = 0.004 (P < 0.01), treatment effect – F(3,40) = 21.33; P < 0.001 and genotype effect – F(1,40) = 30.58; P < 0.001]; for DOPAC: [interaction effect – F(3,40) = 1.579; P = 0.210 (P > 0.05), treatment effect – F(3,40) = 10.05; (P < 0.001) and genotype effect – F(1,40) = 9.048; (P < 0.001)] and for HVA: [interaction effect - F(3,40) = 1.497; P = 0.230 (P > 0.05); treatment effect - F(3,40) = 6.861; P < 0.001 and genotype effect - F(1,40) = 4.717; P = 0.036 (<0.05)]. Bonferroni’s post hoc test was applied to assess the significance of the mean among groups and represented as mean ± SEM; aP < 0.001 vs. MPTP; bP < 0.01 vs. MPTP or cP < 0.05 vs. MPTP. Arrow indicate anterior commissure. Abbreviations: CC, corpus callosum, CPu, caudate putamen, NAc, nucleus accumbens, S, septum
NaB and Cinnamon Alleviate Motor Deficits in a MPTP Mouse Model of PD Via Astrocytic Gdnf
To evaluate the therapeutic capabilities of NaB and Cinnamon in vivo, we again used the MPTP mouse model of PD that lacked astrocyte specific Gdnf (GdnfΔastro mice) with their age matched control littermates (Gfapcre mice) and treated these mice with either NaB or cinnamon following experimental timeline illustrated in Fig. 8a. First, we evaluated the effect of NaB and cinnamon treatment on locomotor coordination of MPTP-intoxicated mice using rotarod apparatus. In comparison to saline-treated mice, after MPTP intoxication, both Gfapcre and GdnfΔastro mice exhibited significantly reduced locomotor performance on rotarod (Fig. 8b). However, NaB and cinnamon treatment improved rotarod performance in MPTP-intoxicated Gfapcre mice, but not MPTP-intoxicated GdnfΔastro mice (Fig. 8b). Next, we tracked their locomotor activity (Fig. 8c) and associated stereotypic behaviors like rearing and grooming frequency (Fig. 8d and e) in the open field arena using the NOLDUS tracking software. As expected, following MPTP intoxication, significant reduction in rearing frequency was noticed in both Gfapcre and GdnfΔastro groups when compared to saline-treated mice (Fig. 8d). However, following NaB or cinnamon treatment, MPTP-intoxicated Gfapcre mice, but not MPTP intoxicated- GdnfΔastro mice, displayed increase in rearing frequency (Fig. 8d). Similar results were seen in case of grooming frequency (Fig. 8e). Together, these data suggest that NaB and cinnamon improve the motor function in MPTP mouse model of PD through astrocytic Gdnf.
Oral treatment of NaB and cinnamon improved motor functions in MPTP-intoxicated age-matched non-Tg, but not inGdnfΔastro, mice. a Schematic representation of MPTP intoxication and Nab or cinnamon oral treatment for age matched Gfapcre mice and Gdnf floxed mice (n = 6 mice/group). After 3 h of the last MPTP insult (20 mg/kg body wt/inj; four i.p injections at every 2-h interval) mice in each group were fed with NaB (100 mg/kg body wt/d) or cinnamon (100 mg/kg body wt/d) via oral gavage for 7 days. A control group without MPTP intoxication was also included. On 7 d of MPTP intoxication, mice were tested for their motor function by using apparatus such as Rotarod (b). Two-way ANOVA analysis followed by Bonferroni’s post hoc test; showed statistical significance represented as mean ± SEM; aP < 0.01 or bP < 0.05 vs. MPTP, for interaction effect – F(3,40) = 3.622; P = 0.021, treatment effect – F(3,40) = 17.22; P < 0.001 and genotype effect – F(1,40) = 44.71; P < 0.001. Using Noldus software and open field arena, we also recorded mice movement in open field arena (c) followed by tracking complex motor activity parameters that include grooming frequency (d), and rearing frequency (e). Results were analyzed using two-way ANOVA with genotype and treatment as two independent factors, generating descriptive statistics for rearing frequency [interaction effect – F(3,40) = 1.794; P = 0.164 (P > 0.05), treatment effect – F(3,40) = 18.52; P < 0.001 and genotype effect – F(1,40) = 18.35; P < 0.001] and for grooming frequency [interaction effect - F(3,40) = 1.200; P = 0.322 (P > 0.05); treatment effect - F(3,40) = 13.80; P < 0.001 and genotype effect - F(1,40) = 6.635; P = 0.014 (P < 0.05)]. Following two-way ANOVA, Bonferroni’s post hoc test was also applied to assess the significance of the mean among groups and represented as mean ± SEM; aP < 0.01 vs. MPTP or bP < 0.05 vs. MPTP
Discussion
Parkinson’s disease is the most common neurodegenerative movement disorder, with median age-standardized annual incidence rates in high-income countries like United States is of 14 per 100,000 people in the total population, and 160 per 100,000 people aged 65 years or older (Hirtz et al. 2007). Until now, the treatment of PD remains symptomatic and has not been established to provide a disease-modifying effect. Therefore, finding pharmacological or physical interventions aimed towards disease-modifying effects in PD patients are of major potential medical relevance. It is well known that rescuing and restoration of damaged neuronal population in PD is mediated through nigral trophic factor GDNF. Accordingly, to date, a number of clinical trials have been undertaken to study the delivery of GDNF, directly to the PD brain, but they yielded mixed results. The problem may be due to the site and method of delivery, namely the distribution of GDNF after different injection or infusion techniques (Nutt et al. 2003; Lang et al. 2006; Morrison et al. 2007; Slevin et al. 2007). In treatments utilizing cell-based delivery systems or gene therapy applied with viral vectors, over-expression of the Gdnf gene and protein could promote cell viability in the dopaminergic midbrain neurons and induce symptomatic improvement of rats with 6-OHDA lesions and of monkeys treated with MPTP (Kong et al. 2008; Andereggen et al. 2009). However these therapies still have critical issues regarding their clinical applications due to questions about the efficacy and stability of gene transfer and possibility of immune responses. While there have been efforts to overcome the problems in delivering cell or gene therapy through neurosurgery, increasing attention is now being paid to strategies that induce the expression of endogenous trophic factors or enhance their signaling as alternative therapeutic options for PD (Saavedra et al. 2008).
Although few approaches are there to increase GDNF, here we introduce a widely-used natural product to augment this nigral trophic factor in vivo in the brain. Cinnamon, a commonly used spice and flavoring agent, is used by people on a daily basis. It is being used since medieval times to treat variety of disorders including arthritis, coughing, hoarseness, sore throats, etc. As a matter of fact, even in today’s modern era, cinnamon is still an extensively researched natural product owing to its therapeutic properties (Rathi et al. 2013; Kang et al. 2014; Modi et al. 2015b; Mirfeizi et al. 2016; Modi et al. 2016; Nayak et al. 2017; Santos and da Silva 2018; Shishehbor et al. 2018). After oral intake, cinnamon is metabolized into NaB, a FDA-approved drug against hyperglycinemia and urea cycle disorders. NaB is also a component of Ammonul®, a FDA-approved drug used in the treatment for hepatic metabolic defects associated with hyperammonemia such as urea cycle disorder in children. We have found that cinnamon and NaB have immunomodulatory properties to protect mice from experimental allergic encephalomyelitis, an animal model of multiple sclerosis (Brahmachari and Pahan 2007). Cinnamon and NaB are capable of upregulating neuroprotective molecules (Parkin and DJ-1) and protecting the nigrostriatum in MPTP mouse model of PD (Khasnavis and Pahan 2014). Cinnamon and NaB also inhibits the activation of p21rac to reduce NADPH oxidase-mediated production of reactive oxygen species (Modi et al. 2015b). Here we describe that NaB is also capable of upregulating GDNF in astrocytes. While NaB increased the expression of Gdnf mRNA and the production of GDNF protein in mouse and human astrocytes, NaFO, a compound with similar structure to NaB without the aromatic ring, remained unable to stimulate GDNF, indicating the specificity of the effect.
Signaling mechanisms for driving the upregulation of GDNF in astrocytes are poorly understood. We analyzed the Gdnf promoter using the Genomatix Software Suite and found binding sites for several transcription factors such as AP1, CREB, C/EBPβ, NF-κB, etc. However, earlier we have seen that NaB is anti-inflammatory and that NaB inhibits the activation of NF-κB and the expression of different proinflammatory molecules in glial cells (Brahmachari et al. 2009). On the other hand, we have described that NaB is capable of inducing the activation of CREB in both astrocytes (Modi et al. 2015a) and neurons (Modi et al. 2016) via protein kinase A. Therefore, we investigated a role of CREB in NaB-mediated upregulation of GDNF in astrocytes. Recruitment of CREB and CREB-binding protein (CBP), an important co-activator, to the CRE of the Gdnf promoter in NaB-treated astrocytes as compared to untreated astrocytes and abrogation of the capability of NaB to stimulate the transcription of Gdnf gene by siRNA knockdown of CREB suggest that NaB upregulates the level of GDNF in astrocytes via CREB.
While many drugs show GDNF-upregulating effect in cell culture models, very few of these display efficacy in vivo in the SNpc. Therefore, we examined whether NaB and cinnamon were able to increase GDNF in vivo in the SNpc of control as well MPTP-challenged mice. Interestingly, level of GDNF was much less in the SNpc of MPTP-intoxicated mice than control mice suggesting that Parkinsonian toxicity reduces the level of GDNF in the nigra. Earlier we described that after oral administration of cinnamon to mice, NaB is detected in both serum and brain (Jana et al. 2013). Accordingly, oral treatment of NaB and cinnamon increased the level of astrocytic GDNF in the nigra of MPTP-insulted mice. Next, to delineate the role of astrocytic GDNF in NaB- and cinnamon-mediated protection of the nigrostriatum in MPTP mouse model of PD, we used cre-flox recombination protocol to selectively delete GDNF in astrocytes. Interestingly, specific deletion of GDNF from astrocytes abrogated the protective effect of NaB and cinnamon on dopaminergic neurons, striatal fibers, neurotransmitters, and locomotor activities in MPTP mouse model of PD. During PD and other neurodegenerative disorders, while neurons die, usually glial cells such as astroglia do not die, but undergo activation and gliosis. Moreover, astrocytes are major cell type in the CNS, indicating that any contribution to nigral trophic effect from astrocytes would be significant. Therefore, it makes sense to utilize astrocytes for the protection of dopaminergic neurons in a neurodegenerative nigra.
In summary, cinnamon metabolite NaB increases the expression of GDNF in astrocytes via CREB and that oral administration of NaB and cinnamon protects dopaminergic neurons, restores striatal innervation, preserves striatal neurotransmitters, and improves locomotor activities in MPTP mouse model of PD via astroglial GDNF. Although the in vitro situation of mouse and human astrocytes in culture and in vivo condition of nigral astrocytes in 8–10 week old MPTP-intoxicated mice do not truly resemble the in vivo situation of astrocytes in the nigra of PD patients, our results suggest that upregulation of astroglial GDNF by oral NaB and cinnamon may have therapeutic importance in PD and other neurodegenerative disorders.
References
Abd El-Mawla AM, Schmidt W, Beerhues L (2001) Cinnamic acid is a precursor of benzoic acids in cell cultures of Hypericum androsaemum L. but not in cell cultures of Centaurium erythraea RAFN. Planta 212:288–293
Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK (2013) GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther 138:155–175
Aloe L, Rocco ML, Bianchi P, Manni L (2012) Nerve growth factor: from the early discoveries to the potential clinical use. J Transl Med 10:239
Andereggen L, Meyer M, Guzman R, Ducray AD, Widmer HR (2009) Effects of GDNF pretreatment on function and survival of transplanted fetal ventral mesencephalic cells in the 6-OHDA rat model of Parkinson's disease. Brain Res 1276:39–49
Brahmachari S, Pahan K (2007) Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J Immunol 179:275–283
Brahmachari S, Jana A, Pahan K (2009) Sodium benzoate, a metabolite of cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. J Immunol 183:5917–5927
Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T (2005) MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21:2933–2942
Chandra G, Roy A, Rangasamy SB, Pahan K (2017) Induction of adaptive immunity leads to Nigrostriatal disease progression in MPTP mouse model of Parkinson's disease. J Immunol 198:4312–4326
Corbett GT, Roy A, Pahan K (2012) Gemfibrozil, a lipid-lowering drug, upregulates IL-1 receptor antagonist in mouse cortical neurons: implications for neuronal self-defense. J Immunol 189:1002–1013
Corbett GT, Roy A, Pahan K (2013) Sodium phenylbutyrate enhances astrocytic neurotrophin synthesis via protein kinase C (PKC)-mediated activation of cAMP-response element-binding protein (CREB): implications for Alzheimer disease therapy. J Biol Chem 288:8299–8312
Corbett GT, Gonzalez FJ, Pahan K (2015) Activation of peroxisome proliferator-activated receptor alpha stimulates ADAM10-mediated proteolysis of APP. Proc Natl Acad Sci U S A 112:8445–8450
De Deurwaerdere P, Di Giovanni G, Millan MJ (2017) Expanding the repertoire of L-DOPA's actions: a comprehensive review of its functional neurochemistry. Prog Neurobiol 151:57–100
Ellis JM, Fell MJ (2017) Current approaches to the treatment of Parkinson's disease. Bioorg Med Chem Lett 27:4247–4255
Emamzadeh FN, Surguchov A (2018) Parkinson's disease: biomarkers, treatment, and risk factors. Front Neurosci 12:612
Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K (2007) Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A 104:18754–18759
Ghosh A, Roy A, Matras J, Brahmachari S, Gendelman HE, Pahan K (2009) Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson's disease. J Neurosci 29:13543–13556
Goodman RH, Smolik S (2000) CBP/p300 in cell growth, transformation, and development. Genes Dev 14:1553–1577
Hariri M, Ghiasvand R (2016) Cinnamon and chronic diseases. Adv Exp Med Biol 929:1–24
Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R (2007) How common are the "common" neurologic disorders? Neurology 68:326–337
Jana A, Modi KK, Roy A, Anderson JA, van Breemen RB, Pahan K (2013) Up-regulation of neurotrophic factors by cinnamon and its metabolite sodium benzoate: therapeutic implications for neurodegenerative disorders. J NeuroImmune Pharmacol 8:739–755
Jankovic J (2008) Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79:368–376
Kalia LV, Lang AE (2015) Parkinson's disease. Lancet 386:896–912
Kang H, Park SH, Yun JM, Nam TG, Kim YE, Kim DO, Kim YJ (2014) Effect of cinnamon water extract on monocyte-to-macrophage differentiation and scavenger receptor activity. BMC Complement Altern Med 14:90
Kearns CM, Gash DM (1995) GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res 672:104–111
Khasnavis S, Pahan K (2012) Sodium benzoate, a metabolite of cinnamon and a food additive, upregulates neuroprotective Parkinson disease protein DJ-1 in astrocytes and neurons. J NeuroImmune Pharmacol 7:424–435
Khasnavis S, Pahan K (2014) Cinnamon treatment upregulates neuroprotective proteins Parkin and DJ-1 and protects dopaminergic neurons in a mouse model of Parkinson's disease. J NeuroImmune Pharmacol 9:569–581
Khasnavis S, Roy A, Ghosh S, Watson R, Pahan K (2013) Protection of dopaminergic neurons in a mouse model of Parkinson's disease by a physically-modified saline containing charge-stabilized nanobubbles. J NeuroImmune Pharmacol 9:218–232
Kirik D, Rosenblad C, Bjorklund A, Mandel RJ (2000) Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson's model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci 20:4686–4700
Kong XY, Cai Z, Pan L, Zhang L, Shu J, Dong YL, Yang N, Li Q, Huang XJ, Zuo PP (2008) Transplantation of human amniotic cells exerts neuroprotection in MPTP-induced Parkinson disease mice. Brain Res 1205:108–115
Koyama Y (2002) Functional alterations of astroglia on brain pathologies and their intracellular mechanisms. Nihon Yakurigaku Zasshi 119:135–143
Lang AE, Lozano AM (1998) Parkinson's disease. First of two parts. N Engl J Med 339:1044–1053
Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VGF, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M (2006) Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59:459–466
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130–1132
Meredith GE, Rademacher DJ (2011) MPTP mouse models of Parkinson's disease: an update. J Park Dis 1:19–33
Mirfeizi M, Mehdizadeh Tourzani Z, Mirfeizi SZ, Asghari Jafarabadi M, Rezvani HR, Afzali M (2016) Controlling type 2 diabetes mellitus with herbal medicines: a triple-blind randomized clinical trial of efficacy and safety. J Diabetes 8:647–656
Modi KK, Jana M, Mondal S, Pahan K (2015a) Sodium benzoate, a metabolite of cinnamon and a food additive, Upregulates Ciliary Neurotrophic factor in astrocytes and Oligodendrocytes. Neurochem Res 40:2333–2347
Modi KK, Roy A, Brahmachari S, Rangasamy SB, Pahan K (2015b) Cinnamon and its metabolite sodium benzoate attenuate the activation of p21rac and protect memory and learning in an animal model of Alzheimer's disease. PLoS One 10:e0130398
Modi KK, Rangasamy SB, Dasarathi S, Roy A, Pahan K (2016) Cinnamon converts poor learning mice to good learners: implications for memory improvement. J NeuroImmune Pharmacol 11:693–707
Morrison PF, Lonser RR, Oldfield EH (2007) Convective delivery of glial cell line-derived neurotrophic factor in the human putamen. J Neurosurg 107:74–83
Nayak IN, Chinta R, Jetti R (2017) Anti-atherosclerotic potential of aqueous extract of Cinnamomum Zeylanicum bark against glucocorticoid induced atherosclerosis in Wistar rats. J Clin Diagn Res 11:FC19–FC23
Neuberger JM, Schweitzer S, Rolland MO, Burghard R (2000) Effect of sodium benzoate in the treatment of atypical nonketotic hyperglycinaemia. J Inherit Metab Dis 23:22–26
Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr., Lozano AM, Penn RD, Simpson RK, Jr., Stacy M, Wooten GF, factor IGSGIiGcl-dn (2003) Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 60:69–73
Patel NK, Gill SS (2007) GDNF delivery for Parkinson's disease. Acta Neurochir Suppl 97:135–154
Patel D, Roy A, Kundu M, Jana M, Luan CH, Gonzalez FJ, Pahan K (2018) Aspirin binds to PPARalpha to stimulate hippocampal plasticity and protect memory. Proc Natl Acad Sci U S A 115:E7408–E7417
Rascol O, Payoux P, Ory F, Ferreira JJ, Brefel-Courbon C, Montastruc JL (2003) Limitations of current Parkinson's disease therapy. Ann Neurol 53(Suppl 3):S3–S12 discussion S12-15
Rathi B, Bodhankar S, Mohan V, Thakurdesai P (2013) Ameliorative effects of a Polyphenolic fraction of Cinnamomum zeylanicum L. bark in animal models of inflammation and arthritis. Sci Pharm 81:567–589
Roy A, Jana M, Corbett GT, Ramaswamy S, Kordower JH, Gonzalez FJ, Pahan K (2013) Regulation of cyclic AMP response element binding and hippocampal plasticity-related genes by peroxisome proliferator-activated receptor alpha. Cell Rep 4:724–737
Roy A, Jana M, Kundu M, Corbett GT, Rangaswamy SB, Mishra RK, Luan CH, Gonzalez FJ, Pahan K (2015) HMG-CoA Reductase inhibitors bind to PPARalpha to Upregulate Neurotrophin expression in the brain and improve memory in mice. Cell Metab 22:253–265
Saavedra A, Baltazar G, Duarte EP (2008) Driving GDNF expression: the green and the red traffic lights. Prog Neurobiol 86:186–215
Santos HO, da Silva GAR (2018) To what extent does cinnamon administration improve the glycemic and lipid profiles? Clin Nutr ESPEN 27:1–9
Sauer H, Rosenblad C, Bjorklund A (1995) Glial cell line-derived neurotrophic factor but not transforming growth factor beta 3 prevents delayed degeneration of nigral dopaminergic neurons following striatal 6-hydroxydopamine lesion. Proc Natl Acad Sci U S A 92:8935–8939
Seifert G, Schilling K, Steinhauser C (2006) Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci 7:194–206
Shishehbor F, Rezaeyan Safar M, Rajaei E, Haghighizadeh MH (2018) Cinnamon consumption improves clinical symptoms and inflammatory markers in women with rheumatoid arthritis. J Am Coll Nutr:1–6
Slevin JT, Gash DM, Smith CD, Gerhardt GA, Kryscio R, Chebrolu H, Walton A, Wagner R, Young AB (2007) Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year of treatment and 1 year of withdrawal. J Neurosurg 106:614–620
Villadiego J, Mendez-Ferrer S, Valdes-Sanchez T, Silos-Santiago I, Farinas I, Lopez-Barneo J, Toledo-Aral JJ (2005) Selective glial cell line-derived neurotrophic factor production in adult dopaminergic carotid body cells in situ and after intrastriatal transplantation. J Neurosci 25:4091–4098
Villar-Pique A, Lopes da Fonseca T, Outeiro TF (2016) Structure, function and toxicity of alpha-synuclein: the Bermuda triangle in synucleinopathies. J Neurochem 139(Suppl 1):240–255
Wakabayashi K, Tanji K, Mori F, Takahashi H (2007) The Lewy body in Parkinson's disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology 27:494–506
Weissmiller AM, Wu C (2012) Current advances in using neurotrophic factors to treat neurodegenerative disorders. Transl Neurodegener 1:14
Winkler C, Sauer H, Lee CS, Bjorklund A (1996) Short-term GDNF treatment provides long-term rescue of lesioned nigral dopaminergic neurons in a rat model of Parkinson's disease. J Neurosci 16:7206–7215
Acknowledgements
This study was supported by a merit award from Veteran Affairs (I01BX003033) and a grant (NS083054) from NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 212 kb)
Rights and permissions
About this article
Cite this article
Patel, D., Jana, A., Roy, A. et al. Cinnamon and its Metabolite Protect the Nigrostriatum in a Mouse Model of Parkinson’s Disease Via Astrocytic GDNF. J Neuroimmune Pharmacol 14, 503–518 (2019). https://doi.org/10.1007/s11481-019-09855-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-019-09855-0