Abstract
Increasing the function of residual dopaminergic neurons in the nigra of PD patients is an important area of research as it may eventually compensate the loss. Although tyrosine hydroxylase (TH) is the rate-limiting enzyme in the dopamine (DA) biosynthesis pathway, there are no effective drugs/molecules to upregulate TH and increase the production of DA in nigral dopaminergic neurons. This study underlines the importance of aspirin in stimulating the expression of TH and increasing the level of DA in dopaminergic neurons. At low doses, aspirin increased the expression of TH and the production of DA in mouse MN9D dopaminergic neuronal cells. Accordingly, oral administration of aspirin increased the expression of TH in the nigra and upregulated the level of DA in striatum of normal C57/BL6 mice and aged A53T α-syn transgenic mice. Oral aspirin also improved locomotor activities of normal mice and A53T transgenic mice. While investigating mechanisms, we found the presence of cAMP response element (CRE) in the promoter of TH gene and the rapid induction of cAMP response element binding (CREB) activation by aspirin in dopaminergic neuronal cells. Aspirin treatment also increased the level of phospho-CREB in the nigra of C57/BL6 mice. The abrogation of aspirin-induced expression of TH by siRNA knockdown of CREB and the recruitment of CREB to the TH gene promoter by aspirin suggest that aspirin stimulates the transcription of TH in dopaminergic neurons via CREB. These results highlight a new property of aspirin in stimulating the TH-DA pathway, which may be beneficial in PD patients.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopaminergic neurons in the substantia nigra (SN) communicate with neurons present in the basal ganglia by releasing a particular neurotransmitter called dopamine (DA) and this interaction is responsible for fine tuning of our movements. Parkinson’s disease (PD) is the most common neurodegenerative movement disorder that is characterized by tremor, bradykinesia, rigidity, and postural instability (Olanow and Tatton 1999; Vila and Przedborski 2004). In PD, neurons of the SN degenerate, resulting in the loss of DA required for neurotransmission in the corpus striatum. It is believed that significant number of dopaminergic neurons still remain in the SN when motor signs first appear in PD patients. According to some reports (Marsden 1990; Ross et al. 2004), the remaining SN neurons could be around 50% at the onset of motor symptoms. On the other hand, some studies (Lang and Lozano 1998; Dauer and Przedborski 2003) report the presence of 30–40% SN neurons in PD patients during their first clinic visit. Therefore, although halting the progression of dopamine neuron death is one of the key goals for tackling this devastating disease, increasing the function of residual dopaminergic neurons in the nigra of early-stage PD patients is also an important area of research as it may eventually compensate the loss and reverse the disease process.
Tyrosine hydroxylase (TH) is the regulatory enzyme in catecholamine biosysthesis pathway that catalyzes the hydroxylation of tyrosine to L-DOPA, a precursor of DA. Despite intense investigations into the regulation of TH, there is no effective drug to upregulate TH and increase DA in dopaminergic neurons. Aspirin or acetylsalicylic acid is one of the most widely-used medicines in the world. Being a member of the nonsteroidal anti-inflammatory drug (NSAID) family aspirin is always used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication. It exhibits its anti-inflammatory effects by inhibiting cyclooxygenase and thereby suppressing the generation of proinflammatory molecules like prostaglandins (Vane 1971; Vane and Botting 2003). Aspirin has also been demonstrated to have beneficial effects for atherosclerosis, cardiovascular diseases and several cancers (Moyad 2001; Dai and Ge 2012; Rothwell et al. 2012; Berk et al. 2013). Recently, we have demonstrated that aspirin protects oligodendroglia, myelin-synthesizing cells in the CNS, via ciliary neurotrophic factor, implicating its possible beneficial role for remyelination (Modi et al. 2013). Moreover, we have described that low-dose aspirin increases lysosomal biogenesis and improves memory and learning in an animal model of Alzheimer’s disease (AD) via peroxisome proliferator-activated receptor α (Chandra et al. 2018; Patel et al. 2018). In this study, we tested the effect of aspirin on TH in dopaminergic neuronal cells. Here, we describe that aspirin at low doses stimulates the expression of TH and the production of dopamine in mouse MN9D dopaminergic neuronal cells via activation of CREB. Furthermore, oral administration of aspirin stimulated the level of TH in the nigra and increased the level of dopamine in the striatum of normal mice as well as aged transgenic mice expressing mutated human A53T α-syn. Accordingly low-dose aspirin also improved locomotor activities in normal as well as A53T mice.
Materials and Methods
Reagents
Cell culture materials (DMEM/F-12, L-Glutamine, Hank’s balanced salt solution, 0.05% trypsin, and antibiotic-antimycotic) were purchased from Mediatech (Washington, DC) Fetal bovine serum (FBS) was obtained from Atlas Biologicals. Aspirin and all molecular biology-grade chemicals were obtained from Sigma. Anti-TH antibody (cat# P40101–150) was obtained from PelFreez Biologicals. Anti-actin antibody (cat# ab6276) was obtained from Abcam. Anti-CBP antibody (cat# SC-369) was obtained from Santa Cruz. Anti-CREB antibody (cat# CS203204) was obtained from Millipore-Sigma. Alexa-fluor antibodies used in immunostaining were obtained from Jackson ImmunoResearch and IR-dye-labeled reagents used for immunoblotting were from Li-Cor Biosciences.
MN9D Cell Maintenance and Treatment
MN9D cells provided by Dr. Alfred Heller (University of Chicago) were cultured in high-glucose DMEM supplemented with 10% FBS and 1% antibiotic-antimycotic mixture. These cells were maintained at 37 °C in a humidified 5% CO2 incubator in their undifferentiated state and were used for experiments only below passage 20. During differentiation, cells were allowed to differentiate in neurobasal media containing 2% B27 and 1% antibiotic-antimycotic mixture for 2 d. Cells were treated with different concentrations of aspirin in either neurobasal media without B27 or serum-free DMEM.
Animals and Aspirin Treatment
A53T α-syn transgenic [B6;C3-Tg(Prnp-SNCA*A53T)83Vle/J] mice were purchased from the Jackson Lab. Animal maintenance and experiments were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use committee of the Rush University Medical Center, Chicago, IL. Nine-month-old male A53T transgenic mice were treated with low dose (2 mg/kg body wt/d) of aspirin (solubilized in 0.5% methyl cellulose) via gavage for 30d. In another set, 8–10 week old C57/BL6 male mice (Harlan Sprague Dawley, Indianapolis, IN) were also treated with aspirin similarly.
Semi-Quantitative RT-PCR Analysis
Total RNA was isolated from splenic T cells and spinal cord by using the RNeasy mini kit (Qiagen, Valencia, CA) following manufacturer’s protocol. To remove any contaminating genomic DNA, total RNA was digested with DNase. Semi-quantitative RT-PCR was carried out as described earlier (Brahmachari and Pahan 2007; Mondal et al. 2009; Roy and Pahan 2013) using a RT-PCR kit from Clontech (Mountain View, CA). Briefly, 1 μg of total RNA was reverse transcribed using oligo(dT)12–18 as primer and MMLV reverse transcriptase (Clontech) in a 20 μL reaction mixture. The resulting cDNA was appropriately-diluted, and diluted cDNA was amplified using Titanium Taq DNA polymerase and following primers. Amplified products were electrophoresed on a 1.8% agarose gels and visualized by ethidium bromide staining.
TH: Sense: 5’-CAGGACATTGGACTTGCATCTCTG-3′
Antisense: 5’-ATAGTTCCTGAGCTTGTCCTTGGC-3’
GAPDH: Sense: 5’-GGTGAAGGTCGGTGTGAACG3’
Antisense: 5’-TTGGCTCCACCCTTCAAGTG-3’
The relative expression of each gene with respect to GAPDH was measured after scanning the bands with a Fluor Chem 8800 Imaging System (Alpha Innotech, San Leandro, CA).
Real-Time PCR Analysis
The mRNA quantification was performed using the ABI-Prism7700 sequence detection system (Applied Biosystems, Foster City, CA) using SYBR Select master mix (Applied Biosystems) as described earlier (Corbett et al. 2012; Ghosh and Pahan 2012; Khasnavis et al. 2012). The mRNA expression of the targeted genes was normalized to the level of Gapdh mRNA and data was processed by the ABI Sequence Detection System 1.6 software.
Electrophoretic Mobility Shift Assay (EMSA)
MN9D neuronal cells were treated with aspirin under serum-free condition and after different min of stimulation, nuclear extracts were prepared to perform EMSA as described previously (Jana et al. 2013; Modi et al. 2013; Jana et al. 2018) with some modifications. Briefly, IRDye infrared dye end-labeled oligonucleotides containing the consensus CREB DNA-binding sequence were purchased from Licor Biosciences and nuclear extract was incubated with infrared-labeled probe in binding buffer. Samples were separated on a 6% polyacrylamide gel in 0.25× TBE buffer (Tris borate-EDTA) and analyzed by the Odyssey Infrared Imaging System (LI-COR Biosciences).
Chromatin Immunoprecipitation (ChIP) Assay
Recruitment of CREB to TH gene promoter was determined by ChIP assay as described earlier (Jana et al. 2013; Chandra et al. 2017). Briefly, MN9D neuronal cells were treated with aspirin under serum free conditions and after 30 min of stimulation, cells were fixed by adding formaldehyde (1% final concentration), and cross-linked adducts were resuspended and sonicated. ChIP was performed on the cell lysate by overnight incubation at 4 °C with 2 μg of anti-CREB, anti-CBP, or anti-RNA Polymerase II antibodies followed by incubation with protein G agarose (Santa Cruz Biotechnology) for 2 h. The beads were washed and incubated with elution buffer. To reverse the cross-linking and purify the DNA, precipitates were incubated in a 65 °C incubator overnight and digested with proteinase K. DNA samples were then purified, precipitated, and precipitates were washed with 75% ethanol, air-dried, and resuspended in TE buffer. Following primers were used for amplification of chromatin fragments of mouse TH gene.
Sense: 5′- TCC GTC GCC CTC GCT CTG TGC CCA CC -3′
Antisense: 5′- CTG GTG GTC CCG AGT TCT GTC ACC AC -3’
Immunoblotting
Western blotting was conducted as described earlier (Corbett et al. 2012; Ghosh and Pahan 2012; Modi et al. 2016). Briefly, cells were scraped in lysis buffer, transferred to microfuge tubes and spun into pellet. The supernatant was collected and analyzed for protein concentration via the Bradford method (Bio-Rad). SDS sample buffer was added to protein samples and boiled for 5 min. Denatured samples were electrophoresed on NuPAGE® Novex® 4–12% Bis-Tris gels (Invitrogen) and proteins transferred onto a nitrocellulose membrane (Bio-Rad) using the Thermo-Pierce Fast Semi-Dry Blotter. The membrane was then washed for 15 min in TBS plus Tween 20 (TBST) and blocked for 1 h in TBST containing BSA. Next, membranes were incubated at 4 °C under shaking conditions with primary antibodies followed by washing of membranes in TBST for 1 h. Membranes were then incubated in secondary antibodies for 1 h at room temperature, washed for one more hour, and visualized under the Odyssey® Infrared Imaging System (Li-COR, Lincoln, NE).
Densitometric Analysis
Protein blots were analyzed using ImageJ (NIH, Bethesda, MD) and bands were normalized to their respective β-actin loading controls. Data are representative of the average fold change with respect to control for three independent experiments.
Immunofluorescence Analysis
It was performed as described earlier (Brahmachari et al. 2009; Khasnavis and Pahan 2012). Briefly, cover slips containing 100–200 cells/mm2 were fixed with 4% paraformaldehyde followed by treatment with cold ethanol and two rinses in phosphate-buffered saline (PBS). Samples were blocked with 3% bovine serum albumin (BSA) in PBS-Tween-20 (PBST) for 30 min and incubated in PBST containing 1% BSA and primary antibodies. After three washes in PBST (15 min each), slides were further incubated with Cy2 (Jackson ImmunoResearch Laboratories, Inc.). For negative controls, a set of culture slides was incubated under similar conditions without the primary antibodies. The samples were mounted and observed under an Olympus IX81 fluorescence microscope.
Measurement of Mean Fluorescence Intensity (MFI) of TH
MFI was measured using the “measurement module” of the microsuite V Olympus software. Briefly, images were opened in their green channel in order to monitor MFI of TH in the SNpc region. After that, measurement module was opened followed by the selection of two parameters including perimeter and MFI. Perimeter was outlined with rectangular box tool and then associated MFI in that given perimeter was automatically calculated.
Immunohistochemistry
Mice were anesthetized with ketamine-xylazine injectables and perfused with PBS followed by dissection of half brain from each mouse for biochemical assays and half for immunohistochemistry (Ghosh et al. 2007; Khasnavis and Pahan 2014). Briefly, half brains were incubated in 4% paraformaldehyde (w/v) followed by 30% sucrose overnight at 4 °C. Brains were then embedded, sectioned (30 μm thick), and processed for tyrosine hydroxylase (TH) staining as described previously (Benner et al. 2004; Ghosh et al. 2007).
HPLC Analysis for Measurement of Dopamine and its Metabolite Levels
Levels of dopamine, DOPAC (3, 4-dihydroxyphenylacetic acid) and HVA (homovanillic acid) were quantified in striatum and supernatants of MN9D cells as described earlier (Dasgupta et al. 2003; Benner et al. 2004; Roy et al. 2006; Ghosh et al. 2007; Khasnavis et al. 2014). Briefly, mice were sacrificed by cervical dislocation after treatment and their striata were collected and immediately frozen in dry ice and stored at -80 °C until analysis. On the day of the analysis, tissues were sonicated in 0.2 M perchloric acid containing isoproterenol and resulting homogenates were centrifuged at 20,000 x g for 15 min at 4 °C. After pH adjustment and filtration, 10 μl of supernatant was injected onto a Eicompak SC-3ODS column (Complete Stand-Alone HPLC-ECD System EiCOMHTEC-500 from JM Science Inc., Grand Island, NY) and analyzed following manufacturer’s protocol. While analyzing supernatants of MN9D cells, 10 μl supernatant was directly injected onto a Eicompak SC-3ODS column.
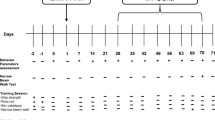
Behavioral Analyses
After treatment, animals were placed individually into an open field arena made of plexiglass cage [42 cm (L) × 42 cm (W) × 32 cm (H)]. In this procedure, the open field environment is much larger than that of the home cage, and is unfamiliar to the animal. Therefore, in the beginning of experiment animals were allowed to adapt with the environment for 5 min. After that, animal’s open field behavior was recorded. Each animal was monitored for three times with 5 min acquisition and 5 min of interval time. Motor activity was monitored in the Ethovision XT 13.0 Open Field Activity System (Noldus). In this system, the plexiglass cage is closely monitored by a computer-assisted camera that continuously tracks the animal’s movement. Test cage was cleaned with alcohol after replacing each animal. In the open-field experiment, various parameters, such as horizontal activity, total distance, number of movements, movement time, rest time, stereotypy counts, rearing, mean distance, meantime, center distance, and center time, were measured from collected videos accordingly. In rotarod, animals were allowed to run on a motorized backward moving platform (Med associates Inc) at a constant speed of 24 rpm with cut-off time 5 min. To eliminate stress and fatigues, mice were given a 5-min rest interval. Each animal was recorded three times.
Statistical Analysis
Statistical analyses were performed with Student’s t test (for two-group comparisons) and one-way ANOVA, followed by Tukey’s multiple-comparison tests, as appropriate (for multiple groups comparison), using Prism 7 (GraphPad Software). Data represented as mean ± SD or mean ± SEM as stated in figure legends. A level of p < .05 was considered statistically significant.
Results
Stimulation of TH and Dopamine Synthesis by Aspirin in Mouse MN9D Dopaminergic Neuronal Cells
To determine whether aspirin, a commonly-used drug, could be used to stimulate TH, MN9D dopaminergic neuronal cells were treated with different concentrations of aspirin for 2 h under serum-free conditions. It is evident from RT-PCR (Fig. 1a) and real-time PCR (Fig. 1b) that aspirin dose-dependently stimulated the mRNA expression of TH in MN9D cells. Although aspirin was not effective in inducing the expression of TH mRNA at a concentration of 0.25 μM, significant increase in TH mRNA was seen at 0.5 μM aspirin with the highest increase observed at 2 μM aspirin (Fig. 1a, b). However, fold induction of TH mRNA decreased at higher concentrations of aspirin and we did not find any induction at 10 μM aspirin (Fig. 1a, b). Therefore, we carried out a time-dependent study using 2 μM aspirin and found that aspirin was capable of upregulating TH mRNA significantly starting from 30 min of incubation with maximum stimulation observed at 2 h followed by a decrease in subsequent hours of incubation (Fig. 1c, d).
Dose- and time-dependent induction of tyrosine hydroxylase (TH) in mouse MN9D neuronal cells by aspirin. a Mouse MN9D neuronal cells were treated with different concentrations of aspirin under serum-free conditions for 2 h followed by monitoring the mRNA expression of TH by semi-quantitative RT-PCR a and real-time PCR b. Cells were treated with 2 μM aspirin for different time periods under serum-free condition followed by monitoring the mRNA expression of TH by semi-quantitative RT-PCR c and real-time PCR d. Results are mean ± SD of at least three independent experiments. ***p < 0.001 vs control; ns, not significant. One-way ANOVA with Tukey’s multiple comparison test shows that both dose- (B) or time- (C) dependent increase in TH mRNA by aspirin is significant (p < 0.0001). Cells were treated with different concentrations of aspirin under serum-free conditions for 2 h followed by monitoring the protein level of TH by Western blot e. Bands were scanned and values (TH/actin) presented as relative to control f. Results are mean ± SD of three different experiments. *p < 0.05 & **p < 0.01 vs control; ns, not significant. One-way ANOVA with Tukey’s multiple comparison test shows that dose-dependent increase in TH protein by aspirin is significant (p < 0.01). Cells were treated with 2 μM aspirin for 2 h followed by double-label immunofluorescence with antibodies against TH and actin g. Mean fluorescence intensity (MFI) of TH and actin was calculated in 20 different cells h and presented as MFI-TH/MFI-actin. Results are mean ± SEM of 20 different cells per group. *p < 0.05 & ***p < 0.001 vs control. i Cells were treated with 2 μM aspirin for different time periods followed by measuring the level of DA, DOPAC and HVA in supernatants by HPLC. Results are mean ± SD of at least three independent experiments. ***p < 0.001 vs control; ns, not significant. One-way ANOVA with Tukey’s multiple comparison test shows that time-dependent increase in DA by aspirin is significant (p < 0.0001)
Next, we examined the effect of aspirin on the induction of TH protein in MN9D neuronal cells. Similar to the increase in TH mRNA, aspirin significantly increased the level of TH protein and maximum increase seen at 2 μM aspirin (Fig. 1e, f & Supplementary Fig. 1). To confirm these results further, aspirin-treated MN9D cells were double-labeled for TH and actin (Fig. 1g) followed by analysis of mean fluorescence intensity (MFI) (Fig. 1h). Similar to Western blot results, aspirin treatment increased the level of TH without modulating the status of actin (Fig. 1g, h). Together, these results suggest that aspirin is capable of augmenting the expression of both TH mRNA and protein in MN9D cells.
The function of TH is to synthesize dopamine (DA) and therefore, to understand the functional significance of aspirin-mediated TH upregulation, we measured the level of DA from the supernatant of aspirin-treated and untreated neuronal cells by HPLC. Although aspirin remained unable to increase DA within 30 min of stimulation, significant elevation of DA was observed at 1 h of treatment with highest DA release seen at 2 h (Fig. 1i). As expected, aspirin-induced DA release was parallel to TH protein upregulation (Fig. 1e, f). However, we did not find increase in DOPAC and HVA, metabolites of DA, at 1 h and 2 h of incubation (Fig. 1i). The level of DOPAC was higher at 4 h (Fig. 1i), which could be due to degradation of DA.
Next, we examined if aspirin could increase the expression of TH and upregulate the production of dopamine in differentiated MN9D neuronal cells. As evident from Fig. 2a, b and Supplementary Fig. 2, aspirin rapidly increased the expression TH protein in differentiated MN9D neuronal cells. This was also supported by double-label immunofluorescence analysis of TH and β-actin (Fig. 2c, d). Similar to that observed in undifferentiated cells (Fig. 1i), aspirin treatment also increased the production of dopamine in differentiated MN9D neuronal cells and the highest increase was observed at 2 h of stimulation (Fig. 2e).
Increase in TH and dopamine in differentiated mouse MN9D neuronal cells by aspirin. a Mouse MN9D neuronal cells were allowed to differentiate in neurobasal media containing B27 for 2 d following treatment with 2 μM aspirin for different time periods in neurobasal media without B27. The protein level of TH was monitored by Western blot (A). Bands were scanned and values (TH/actin) presented as relative to control b. Results are mean ± SD of different experiments. *p < 0.05 & ***p < 0.001 vs control; ns, not significant. Cells were treated with 2 μM aspirin for 2 h followed by double-label immunofluorescence with antibodies against TH and actin c. Mean fluorescence intensity (MFI) of TH and actin was calculated in 20 different cells d and presented as MFI-TH/MFI-actin. Results are mean ± SEM of 20 different cells per group. ***p < 0.001 vs control; ns, not significant. E) Cells were treated with 2 μM aspirin for different time periods followed by measuring the level of DA, DOPAC and HVA in supernatants by HPLC. Results are mean ± SD of at least three independent experiments. *p < 0.05 & ***p < 0.001 vs control; ns, not significant. One-way ANOVA with Tukey’s multiple comparison test shows that time-dependent increase in DA by aspirin is significant (p < 0.0001)
Oral Administration of Aspirin Increases the Level of TH in the Nigra of C57/BL6 and A53T α-Syn Transgenic Mice
Since aspirin increased the expression of TH in MN9D neuronal cells, we next examined if oral administration of aspirin upregulated the level of TH in vivo in the nigra of normal C57/BL6 mice. Mice were treated with aspirin at a dose of 2 mg/kg body wt/d for 30 d followed by monitoring the level of TH in the nigra by Western blot and immunohistochemistry. Western blot of nigral extracts showed significant increase in TH protein by aspirin treatment (Fig. 3a, b & Supplementary Fig. 3). Immunofluorescence analysis (Fig. 3c) followed by MFI quantification (Fig. 3d) also showed increase in TH in the nigra of aspirin-treated C57/BL6 mice. Accordingly, TH DAB staining followed by monitoring optical density of TH also indicated nigral upregulation of TH by aspirin treatment.
Oral administration of aspirin increases the level of TH in vivo in the nigra of C57/BL6 mice. Male C57/BL6 mice (n = 5 per group) were treated with aspirin (2 mg/kg body wt/d) mixed in 0.5% methylcellulose orally via gavage. Control mice received 0.5% methylcellulose as vehicle. After 30 d of treatment, the level of TH was monitored in the SNpc by Western blot a. Actin was run as loading control. Bands were scanned and values (TH/actin) presented as relative to control b. Results are mean ± SEM of five mice per group. ***p < 0.001 (= 5.28 × 10−4) vs control by two-tailed paired t-tests. The level of TH was monitored in ventral midbrain sections by immunofluorescence c. MFI of TH d was calculated in two nigral sections of each of five mice per group. Results are mean ± SEM of five mice per group. ***p < 0.001 (=1 × 10−7) vs control by two-tailed paired t-tests. The level of TH was monitored in ventral midbrain sections by DAB immunostaining e. Optical density of TH f was calculated in two nigral sections of each of five mice per group. Results are mean ± SEM of five mice per group. *p < 0.05 (= 0.012632) vs control by two-tailed paired t-tests
Neuropathological hallmarks of PD are the presence of intracytoplasmic inclusions containing α-synuclein (α-syn) and the demise of dopaminergic neurons in the nigra (Surmeier et al. 2017). Since A53T α-synuclein transgenic (A53T-Tg) mice are widely used to study α-syn pathology, we examined if aspirin treatment could also increase the expression of TH in the nigra of A53T-Tg mice. Similar to that observed in C57/BL6 mice (Fig. 3), Western blot (Fig. 4a, b & Supplementary Fig. 4), immunofluorescence (Fig. 4c, d) and DAB immunostaining (Fig. 4e, f) results showed that oral aspirin treatment was capable of increasing the level of TH in the nigra of A53T-Tg mice.
Oral administration of aspirin increases the level of TH in vivo in the nigra ofA53T-Tgmice. Male A53T-Tg mice (n = 5 per group) were treated with aspirin (2 mg/kg body wt/d) mixed in 0.5% methylcellulose orally via gavage. Control mice received 0.5% methylcellulose as vehicle. After 30 d of treatment, the level of TH was monitored in the SNpc by Western blot a. Actin was run as loading control. Bands were scanned and values (TH/actin) presented as relative to control b. Results are mean ± SEM of five mice per group. ***p < 0.001 (= 1.865 × 10−4) vs control by two-tailed paired t-tests. The level of TH was monitored in ventral midbrain sections by immunofluorescence c. MFI of TH d was calculated in two nigral sections of each of five mice per group. ***p < 0.001 (= 4.69 × 10−9) vs control by two-tailed paired t-tests. The level of TH was monitored in ventral midbrain sections by DAB immunostaining e. Optical density of TH f was calculated in two nigral sections of each of five A53T-Tg mice per group. Results are mean ± SEM of five mice per group. **p < 0.01 (= 0.00245) vs control by two-tailed paired t-tests
Oral Administration of Aspirin Increases the Density of TH Fibers in the Striatum of C57/BL6 and A53T α-Syn Transgenic Mice
Striatum is the main recipient of nigral fibers and therefore, in PD, neuronal degeneration involving the SNpc results in denervation of dopaminergic terminals in the striatum. Since aspirin increased the level of TH in dopaminergic neurons in the nigra, we examined if aspirin treatment could upregulate TH fibers in the striatum. Similar to increase in TH in cell bodies in the nigra by aspirin treatment (Figs. 3 and 4), we observed greater level of TH fibers in the striatum of aspirin-treated C57/BL6 mice as compared to untreated mice (Fig. 5a, b). Accordingly, the density of TH fibers also increased in the striatum of A53T-Tg mice upon aspirin treatment (Fig. 5c, d).
Oral administration of aspirin increases the density of TH fibers in vivo in the striatum of C57/BL6 andA53T-Tgmice. Male C57/BL6 (a & b) and A53T-Tg (c & d) mice (n = 5 per group) were treated with aspirin (2 mg/kg body wt/d) mixed in 0.5% methylcellulose orally via gavage. Control mice received 0.5% methylcellulose as vehicle. After 30 d of treatment, striatal sections were immunostained for TH (A, C57/BL6; C, A53T-Tg). Optical density of TH fibers was quantified in two striatal sections of each of five mice per group (B, C57/BL6; D, A53T-Tg). Results are mean ± SEM of five mice per group. *p < 0.05 (= 0.03206) vs control (B); **p < 0.01 (= 0.00124) vs control (D) by two-tailed paired t-tests
Oral Administration of Aspirin Increases the Level of Dopamine in the Striatum of C57/BL6 and A53T α-Syn Transgenic Mice
Next, to determine whether aspirin treatment also leads to biochemical improvement in the striatum, we quantified levels of neurotransmitters in striata after 30 d of aspirin treatment. Aspirin treatment led to significant increase in striatal dopamine (Fig. 6a) and DOPAC (Fig. 6b), but not and HVA (Fig. 6c) as compared with striata of vehicle-treated C57/BL6 mice. Therefore, we examined if aspirin treatment could also increase neurotransmitters in the striatum of A53T-Tg mice. Similar to that found in C57/BL6 mice, aspirin treatment also elevated the level of dopamine (Fig. 6d) and DOPAC (Fig. 6e), but not and HVA (Fig. 6f), in the striatum of A53T-Tg mice.
Oral administration of aspirin increases the level of DA in vivo in the striatum of C57/BL6 andA53T-Tgmice. Male C57/BL6 (a-c) and A53T-Tg (d-f) mice (n = 5 per group) were treated with aspirin (2 mg/kg body wt/d) mixed in 0.5% methylcellulose orally via gavage. Control mice received 0.5% methylcellulose as vehicle. After 30 d of treatment, levels of DA (A & D), DOPAC (B & E) and HVA (c & f) were measured in striatum by HPLC. Results are mean ± SEM of five mice per group. **p < 0.01 (= 0.00102) vs control (A);**p < 0.01 (= 0.00914) vs control (B);**p < 0.01 (= 0.00744) vs control (D);*p < 0.05 (= 0.04617) vs control (E);*p < 0.05 (= 0.04054) vs control by two-tailed paired t-tests. NS, not significant
Oral Administration of Aspirin Improves Locomotor Activities of C57/BL6 and A53T α-Syn Transgenic Mice
In order to examine whether aspirin treatment leads to only structural and neurotransmitter enhancement but also functional improvements, we monitored locomotor activities. Consistent to that observed in case of nigral neurons, striatal fibers and striatal neurotransmitters, oral administration of aspirin increased overall movement (Fig. 7a), distance traveled (Fig. 7c), velocity (Fig. 7d), and rearing (Fig. 7e) in C57/BL6 mice. Similarly aspirin treatment also improved locomotor activities in A53T-Tg mice as evidenced by track plot (Fig. 7b), distance traveled (Fig. 7f), velocity (Fig. 7g) and rearing (Fig. 7h).
Oral administration of aspirin improves locomotor activities in C57/BL6 andA53T-Tgmice. Male C57/BL6 (a, c-e) and A53T-Tg (b, f-h) mice (n = 5 per group) were treated with aspirin (2 mg/kg body wt/d) mixed in 0.5% methylcellulose orally via gavage. Control mice received 0.5% methylcellulose as vehicle. After 30 d of treatment, locomotor activities were monitored (A & B, track plot; C & F, distance traveled; D & G, velocity; E & H, rearing). Results are mean ± SEM of five mice per group. **p < 0.01 (= 0.00518) vs control (C);**p < 0.01 (= 0.00312) vs control (D);*p < 0.05 (= 0.03509) vs control (E);*p < 0.05 (= 0.01560) vs control (F);**p < 0.01 (= 0.00197) vs control by two-tailed paired t-tests. NS, not significant
Aspirin Stimulates the Expression of TH in Dopaminergic Neuronal Cells Via CREB
Given the evidence that aspirin can stimulate TH expression and DA synthesis, we intended to characterize the upstream factors that can regulate TH. Therefore, we analyzed the promoter of TH gene via MatInspector tool and found the presence of a consensus cAMP response element (CRE) (Fig. 8a). This prompted us to investigate the role of CREB in aspirind-mediated upregulation of TH. At first, we examined if aspirin was capable of inducing the activation of CREB in neuronal cells. As evident from Fig. 8b, aspirin treatment rapidly induced the phosphorylation of CREB with maximum phosphorylation observed at 15 or 30 min. However, phosphorylation of CREB decreased afterwards (Fig. 8b). In contrast, aspirin was unable to increase the level of total CREB (Fig. 8b). Quantification of phospho-CREB and total CREB also confirmed these finding (Fig. 8c). To further confirm this result, we performed EMSA in order to monitor DNA-binding activity of CREB in MN9D neuronal cells. As evident from Fig. 8d, aspirin treatment led to time-dependent increase in DNA-binding activity of CREB with induction observed as early as 15 min. These results suggest that aspirin is capable of inducing the activation of CREB in MN9D neuronal cells. Since aspirin treatment increased the expression of TH in vivo in the nigra of mice, we also monitored the level of phospho-CREB and total CREB in the nigra of aspirin-treated mice. Similar to MN9D neuronal cells, aspirin treatment also increased the level of phospho-CREB, but not total CREB, in the nigra of C57/BL6 mice (Fig. 8e, f). For raw blots, please see Supplementary Fig. 5. These results were also supported by immunofluorescence analyses of nigral sections for phospho-CREB (Fig. 8g, h) and total CREB (Fig. 8i, j).
Aspirin induces the activation of CREB in MN9D neuronal cells and in vivo in the nigra of C57/BL6 mice. a Position of CRE in the mouse TH gene promoter. MN9D neuronal cells were treated with 2 μM aspirin for different min under serum-free conditions followed by monitoring the level of phospho-CREB (pCREB) by Western blot b. Graph represents densitometric analysis of pCREB protein levels normalized to total CREB (loading control) c. Results are mean ± SD of three independent experiments. **p < 0.01 vs control; *p < 0.05 vs control. d Cells were incubated with 2 μM aspirin for different min under serum-free conditions followed by monitoring the DNS-binding activity of CREB by EMSA. e Male C57/BL6 mice (n = 5 per group) were treated with aspirin (2 mg/kg body wt/d) mixed in 0.5% methylcellulose orally via gavage. Control mice received 0.5% methylcellulose as vehicle. After 7 d of treatment, the level of phospho-CREB and total CREB was monitored in the SNpc by Western blot. f Bands were scanned and values (P-CREB/CREB) presented as relative to control. Results are mean ± SEM of five mice per group. ***p < 0.001 vs control. Nigral sections were double-labeled for either TH & P-CREB g or TH & CREB (I). Mean fluorescence intensities (MFI) of P-CREB (H) and CREB (J) were calculated in two sections (one image per section) of each of five mice per group. **p < 0.01 vs control (H); NS, not significant (J)
Next, to investigate the role of CREB in aspirin-mediated expression of TH, we employed CREB siRNA. Decrease in CREB protein expression in MN9D cells by CREB siRNA, but not control siRNA, indicates the specificity of CREB siRNA (Fig. 9a, b). Stimulation of TH protein expression by aspirin in control siRNA-transfected cells, but not CREB siRNA-transfected cells (Fig. 9c, d), suggest that aspirin increases the expression of TH in neuronal cells via CREB. For raw blots, please see Supplementary Fig. 6.
Aspirin increases the transcription of TH in MN9D neuronal cells via CREB. a Cells were transfected with either control or CREB siRNA. Forty-eight h after transfection, level of CREB was monitored by Western blot. b Graph represents densitometric analysis of CREB protein levels normalized to β-Actin (loading control). Results are mean ± SD of three independent experiments. **p < 0.01 vs control (no siRNA). c Cells were transfected with either control siRNA or CREB siRNA. Forty-eight h after transfection, cells were treated with 2 μM aspirin for 2 h under serum-free condition followed by monitoring the level of TH by Western Blot. d Graph represents densitometric analysis of TH protein levels normalized to β-Actin (loading control). Results are mean ± SD of three independent experiments. **p < 0.01 vs control; *p < 0.05 vs control-siRNA. Cells were treated with 2 μM aspirin for 30 min in serum free media. Then immunoprecipitated chromatin fragments were amplified by semi-quantitative e and quantitative f PCR as described under “Materials and Methods”. Results are the mean ± SD of three separate experiments. ***p < 0.001 vs control
Aspirin Induces the Recruitment of CREB to the Promoters of TH Gene in Mouse MN9D Dopaminergic Neuronal Cells
Next, to understand whether CREB was directly involved in the transcription of TH gene, we examined the recruitment of CREB to TH gene promoter by ChIP assay. Cells were treated with different doses of aspirin for 30 min and after immunoprecipitation of aspirin-treated neuronal chromatin fragments using antibodies against CREB, we were also able to amplify 170 bp fragment from the TH promoter (Fig. 9e, f). Because CREB-binding protein (CBP), a histone acetyl transferase, plays an important role in multiple CREB-mediated transcriptional activities, we investigated if CBP was also involved in aspirin-induced transcription of the TH gene. As evident from PCR (Fig. 9e) and real-time PCR (Fig. 9f), aspirin treatment induced the recruitment of CBP to the TH gene promoter. Consistent to the recruitment of CREB and CBP to the CRE, aspirin was able to recruit RNA polymerase to the TH gene promoter (Fig. 9e, f). These results are specific as no amplification product was observed in any of the immuno-precipitates obtained with control IgG (Fig. 9e, f). These results demonstrate that aspirin treatment is capable of stimulating the recruitment of CREB and CBP to TH gene promoter in dopaminergic neuronal cells.
Discussion
Increasing TH in the nigra and augmenting DA in the striatum may have therapeutic importance in PD. To date, the FDA has approved a very few drugs for the treatment of PD-related pathologies. Therefore, delineating new drugs that can stimulate DA production in the brain of PD patients is an active area of research. Aspirin is one of the most widely-used therapeutics in the world. The essential medicines list (EML) of the World Health Organization (WHO) contains detailed information of the most efficacious, safe and cost-effective drugs that are needed for basic healthcare. It is important to mention that the WHO has continued to have aspirin on its Model EML (20th edition, 2017) (WHO 2017). Here, we demonstrate that low dose of aspirin upregulates TH and increases DA production in dopaminergic neuronal cells. In normal mice, aspirin treatment increased the expression of TH in the nigra and the level of DA in striatum. Similarly, after oral administration, low dose of aspirin also elevated nigral TH and increased striatal DA in A53T-Tg mice. Accordingly, aspirin treatment also improved locomotor activities in normal mice as well as in A53T-Tg mice. Therefore, our study indicates that low-dose aspirin may be a therapeutic approach for treatment of early stage PD when significant number of dopaminergic neurons is still present.
Mechanisms by which the TH gene is upregulated are poorly understood. We analyzed the TH gene promotor using the Genomatix Software Suite and found one cAMP response element (CRE) between 67 and 88 base pairs upstream of the TH open reading frame. Although aspirin is well established for its anti-inflammatory properties and as an inhibitor of nuclear factor-kappa B (NF-κB) (Vane 1971; Kopp and Ghosh 1994), we did not find any NF-κB response element in the promoter of TH gene. Earlier we have reported that aspirin induces the activation of CREB in astrocytes and thereby upregulates the transcription of CNTF (Modi et al. 2013). Therefore, we were prompted to investigate whether CREB was required for aspirin-mediated upregulation of TH in neuronal cells. Activation of CREB by aspirin alone, abrogation of aspirin-mediated TH upregulation by siRNA knockdown of CREB, and the recruitment of CREB and CREB-binding protein (CBP) to the CRE of the TH gene promoter by aspirin treatment suggest that aspirin employs CREB for the transcription of TH. In addition to the upregulation of TH, activation of CREB may participate in multiple cellular processes in the brain (Saura and Cardinaux 2017). For example, neurotrophic factors including BDNF and GDNF that may have beneficial effects for the nigrostriatum are controlled by the CREB pathway (Zhang et al. 2012; Jana et al. 2013; Roy et al. 2015). Moreover, CREB is considered as the master regulator of memory and learning (Roy and Pahan 2015; Belgacem and Borodinsky 2017). These findings highlight the wide applicability and therapeutic potential of aspirin-mediated activation of CREB in PD and other neurological diseases.
At present, there is no effective therapy available to prevent or halt the progression of PD. Sinemet (Carbidopa-Levodopa) is the most widely-used drug for PD however, after a few years of treatment, patients develop dyskinesia. Inhibitors of the enzyme catechol-O-methyl transferase (COMT) are sometimes used to extend the half-life of levodopa. Dopamine receptor agonists that have a longer half-life than levodopa are also used for PD patients. However, these medications provide only limited symptomatic relief. Deep brain stimulation (DBS) is definitely an alternative to different drugs, although a minority of PD patients meets the strict criteria for DBS surgery. There are several advantages of using aspirin over the available PD therapeutics. Aspirin is widely used as a medication and is available over the counter. Multiple epidemiological studies have pointed towards a beneficial role of aspirin in different neurodegenerative disorders (Nilsson et al. 2003; Kern et al. 2012). Aspirin is much more economical and readily available than other PD medications. According to Aubin et al. (Aubin et al. 1998), aspirin protects against MPTP-induced DA depletion in mice. Aspirin is also well established for its anti-inflammatory properties and as an inhibition of nuclear factor-kappa B (NF-κB) (Vane 1971; Kopp and Ghosh 1994). PD is known to have a prominent inflammatory component and we have demonstrated that selective inhibition of NF-κB activation by NEMO-binding peptides leads to protection of the nigrostriatum in hemiparkinsonian mice (Ghosh et al. 2007) and monkey (Mondal et al. 2012). Hence, although we did not address this issue in the present study, it is likely that aspirin also controls the inflammation in A53T-Tg mice and thereby helps in attenuation of the pathology. Although one study did not find any reduction in PD incidence among aspirin users (Gagne and Power 2010), we must remember that people without aspirin use are more likely to die of cardiovascular disease than those who use aspirin, and death from congestive heart failure precludes diagnosis of PD that would have developed later in life. Therefore, the relationship between aspirin use and PD could be noncausal, and further research is needed to establish such association.
In summary, we have delineated that aspirin, a widely used medication, augments the level of TH and increases DA production in dopaminergic neurons. We also show that oral administration of low-dose aspirin stimulates the TH-DA pathway and improves locomotor activities in control as well as A53T-Tg mice.
References
Aubin N, Curet O, Deffois A, Carter C (1998) Aspirin and salicylate protect against MPTP-induced dopamine depletion in mice. J Neurochem 71:1635–1642
Belgacem YH, Borodinsky LN (2017) CREB at the crossroads of activity-dependent regulation of nervous system development and function. Adv Exp Med Biol 1015:19–39
Benner EJ, Mosley RL, Destache CJ, Lewis TB, Jackson-Lewis V, Gorantla S, Nemachek C, Green SR, Przedborski S, Gendelman HE (2004) Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A 101:9435–9440
Berk M, Dean O, Drexhage H, McNeil JJ, Moylan S, O'Neil A, Davey CG, Sanna L, Maes M (2013) Aspirin: a review of its neurobiological properties and therapeutic potential for mental illness. BMC Med 11:74
Brahmachari S, Pahan K (2007) Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J Immunol 179:275–283
Brahmachari S, Jana A, Pahan K (2009) Sodium benzoate, a metabolite of cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. J Immunol 183:5917–5927
Chandra G, Kundu M, Rangasamy SB, Dasarathy S, Ghosh S, Watson R, Pahan K (2017) Increase in Mitochondrial Biogenesis in Neuronal Cells by RNS60, a Physically-Modified Saline, via Phosphatidylinositol 3-Kinase-Mediated Upregulation of PGC1alpha. J NeuroImmune Pharmacol
Chandra S, Jana M, Pahan K (2018) Aspirin induces Lysosomal biogenesis and attenuates amyloid plaque pathology in a mouse model of Alzheimer's disease via PPARalpha. J Neurosci 38:6682–6699
Corbett GT, Roy A, Pahan K (2012) Gemfibrozil, a lipid-lowering drug, upregulates IL-1 receptor antagonist in mouse cortical neurons: implications for neuronal self-defense. J Immunol 189:1002–1013
Dai Y, Ge J (2012) Clinical use of aspirin in treatment and prevention of cardiovascular disease. Thrombosis 2012:245037
Dasgupta S, Jana M, Liu X, Pahan K (2003) Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells. J Biol Chem 278:22424–22431
Dauer W, Przedborski S (2003) Parkinson's disease: mechanisms and models. Neuron 39:889–909
Gagne JJ, Power MC (2010) Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology 74:995–1002
Ghosh A, Pahan K (2012) Gemfibrozil, a lipid-lowering drug, induces suppressor of cytokine signaling 3 in glial cells: implications for neurodegenerative disorders. J Biol Chem 287:27189–27203
Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K (2007) Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A 104:18754–18759
Jana A, Modi KK, Roy A, Anderson JA, van Breemen RB, Pahan K (2013) Up-regulation of neurotrophic factors by cinnamon and its metabolite sodium benzoate: therapeutic implications for neurodegenerative disorders. J NeuroImmune Pharmacol 8:739–755
Jana M, Ghosh S, Pahan K (2018) Upregulation of myelin gene expression by a physically-modified saline via phosphatidylinositol 3-kinase-mediated activation of CREB: implications for multiple sclerosis. Neurochem Res 43:407–419
Kern S, Skoog I, Ostling S, Kern J, Borjesson-Hanson A (2012) Does low-dose acetylsalicylic acid prevent cognitive decline in women with high cardiovascular risk? A 5-year follow-up of a non-demented population-based cohort of Swedish elderly women. BMJ Open 2:e001288
Khasnavis S, Pahan K (2012) Sodium benzoate, a metabolite of cinnamon and a food additive, upregulates neuroprotective Parkinson disease protein DJ-1 in astrocytes and neurons. J NeuroImmune Pharmacol 7:424–435
Khasnavis S, Pahan K (2014) Cinnamon treatment upregulates neuroprotective proteins Parkin and DJ-1 and protects dopaminergic neurons in a mouse model of Parkinson's disease. J NeuroImmune Pharmacol 9:569–581
Khasnavis S, Jana A, Roy A, Mazumder M, Bhushan B, Wood T, Ghosh S, Watson R, Pahan K (2012) Suppression of nuclear factor-kappaB activation and inflammation in microglia by physically modified saline. J Biol Chem 287:29529–29542
Khasnavis S, Roy A, Ghosh S, Watson R, Pahan K (2014) Protection of dopaminergic neurons in a mouse model of Parkinson's disease by a physically-modified saline containing charge-stabilized nanobubbles. J NeuroImmune Pharmacol 9:218–232
Kopp E, Ghosh S (1994) Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 265:956–959
Lang AE, Lozano AM (1998) Parkinson's disease. First of two parts. N Engl J Med 339:1044–1053
Marsden CD (1990) Parkinson's disease. Lancet 335:948–952
Modi KK, Sendtner M, Pahan K (2013) Up-regulation of ciliary neurotrophic factor in astrocytes by aspirin: implications for remyelination in multiple sclerosis. J Biol Chem 288:18533–18545
Modi KK, Rangasamy SB, Dasarathi S, Roy A, Pahan K (2016) Cinnamon converts poor learning mice to good learners: implications for memory improvement. J NeuroImmune Pharmacol 11:693–707
Mondal S, Roy A, Pahan K (2009) Functional blocking monoclonal antibodies against IL-12p40 homodimer inhibit adoptive transfer of experimental allergic encephalomyelitis. J Immunol 182:5013–5023
Mondal S, Roy A, Jana A, Ghosh S, Kordower JH, Pahan K (2012) Testing NF-kappaB-based therapy in hemiparkinsonian monkeys. J NeuroImmune Pharmacol 7:544–556
Moyad MA (2001) An introduction to aspirin, NSAids, and COX-2 inhibitors for the primary prevention of cardiovascular events and cancer and their potential preventive role in bladder carcinogenesis: part II. Semin Urol Oncol 19:306–316
Nilsson SE, Johansson B, Takkinen S, Berg S, Zarit S, McClearn G, Melander A (2003) Does aspirin protect against Alzheimer's dementia? A study in a Swedish population-based sample aged > or =80 years. Eur J Clin Pharmacol 59:313–319
Olanow CW, Tatton WG (1999) Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci 22:123–144
Patel D, Roy A, Kundu M, Jana M, Luan CH, Gonzalez FJ, Pahan K (2018) Aspirin binds to PPARalpha to stimulate hippocampal plasticity and protect memory. Proc Natl Acad Sci U S A 115:E7408–E7417
Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D, Hardman J, Launer L, Masaki K, Tanner CM, White LR (2004) Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Ann Neurol 56:532–539
Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z (2012) Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 379:1591–1601
Roy A, Pahan K (2013) Myelin basic protein-primed T helper 2 cells suppress microglial activation via AlphaVBeta3 integrin: implications for multiple sclerosis. J Clin Cell Immunol 7:158
Roy A, Pahan K (2015) PPARalpha signaling in the hippocampus: crosstalk between fat and memory. J NeuroImmune Pharmacol 10:30–34
Roy A, Fung YK, Liu X, Pahan K (2006) Up-regulation of microglial CD11b expression by nitric oxide. J Biol Chem 281:14971–14980
Roy A, Jana M, Kundu M, Corbett GT, Rangaswamy SB, Mishra RK, Luan CH, Gonzalez FJ, Pahan K (2015) HMG-CoA Reductase inhibitors bind to PPARalpha to Upregulate Neurotrophin expression in the brain and improve memory in mice. Cell Metab 22:253–265
Saura CA, Cardinaux JR (2017) Emerging roles of CREB-regulated transcription Coactivators in brain physiology and pathology. Trends Neurosci 40:720–733
Surmeier DJ, Obeso JA, Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18:101–113
Vane JR (1971) Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 231:232–235
Vane JR, Botting RM (2003) The mechanism of action of aspirin. Thromb Res 110:255–258
Vila M, Przedborski S (2004) Genetic clues to the pathogenesis of Parkinson's disease. Nat Med 10(Suppl):S58–S62
WHO (2017) 20th Model List of Essential Medicine. In: http://www.who.int/medicines/publications/essentialmedicines/20th_EML2017_FINAL_amendedAug2017.pdf?ua=1, pp 2,25,36,50
Zhang F, Lu YF, Wu Q, Liu J, Shi JS (2012) Resveratrol promotes neurotrophic factor release from astroglia. Exp Biol Med (Maywood) 237:943–948
Acknowledgements
This study was supported by merit awards from Department of Veteran Affairs (I01BX002174 and I01BX003033) and a grant (NS083054) from NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
None.
Electronic supplementary material
ESM 1
(PDF 1105 kb)
Rights and permissions
About this article
Cite this article
Rangasamy, S.B., Dasarathi, S., Pahan, P. et al. Low-Dose Aspirin Upregulates Tyrosine Hydroxylase and Increases Dopamine Production in Dopaminergic Neurons: Implications for Parkinson’s Disease. J Neuroimmune Pharmacol 14, 173–187 (2019). https://doi.org/10.1007/s11481-018-9808-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-018-9808-3