Abstract
Observational studies have reported positive associations between opioid dependence and major mental disorders. However, the causal relationships and causal mechanisms between opioid dependence and mental disorders remain unknown due to potential confounding bias and reverse causality. In this study, we aim to investigate the causal associations and possible mediating mechanisms between opioid dependence and mental disorders via Mendelian randomization. Comprehensive bidirectional Mendelian randomization (MR) studies were conducted between opioid dependence and major mental disorders, including schizophrenia, bipolar disorder, major depressive disorder, panic disorder, anorexia, obsessive–compulsive disorder, post-traumatic stress disorder, and insomnia. Inverse variance weighted approach was adopted as the primary analytic method with series of sensitivity analyses. Mediation effects of chronic pain along the opioid dependence–mental disorders causal pathway were assessed by multivariate MR and two-step MR. Forward MR identified significant positive causal effects of opioid dependence on insomnia (OR = 1.03, 95% CI = (1.01, 1.05), p = 0.005), while reverse MR showed significant positive causal effects of schizophrenia on opioid dependence (OR = 1.20, 95% CI = (1.07, 1.34), p = 0.002). No significant causal associations were found between opioid dependence and other mental disorders. Neither opioid dependence on insomnia nor schizophrenia on opioid dependence causal pathway was significantly mediated by chronic pain. Higher risks of genetically predicted opioid dependence may lead to higher risks of insomnia, while higher risks of genetically predicted schizophrenia may lead to higher risks of developing opioid dependence. The majority of causal effects were acted directly rather than via chronic pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Opioid use disorder and opioid addiction remain a serious public health crisis in the USA and worldwide, with over 600 million deaths involving opioid overdose over the past two decades (Overdose, 2018). The misuse and addiction to opioids are significant drivers of shortened life expectancy and increased morbidity, including fetal death, infants born with neonatal abstinence syndrome, and transmission of infectious disease via opioid injection (Chang et al., 2019; Dowell et al., 2017; Jones, 2018; Ko et al., 2016; Peters et al., 2016). Originally prescribed for acute pain relief, opioids bind to receptors in the central and peripheral nervous systems to induce intense euphoria and could quickly develop tolerance with severe withdrawal syndromes (Shaheed et al., 2019; Degenhardt et al., 2019). Opioid dependence represents a particular transitional stage from opioid use to misuse, and several risk factors are found to be associated with opioid dependence and addiction, including low socioeconomic status, risk-taking or thrill-seeking behaviors, and history of mental disorders such as depression and anxiety (Han et al., 2015; Han et al., 2017). However, the causal relationships and biological mechanisms between opioid dependence and mental disorders remain largely unknown.
Opioid dependence often co-occurs with mental disorders. According to a national survey on drug use and health, 62% of adults with opioid use disorder in the US had a co-occurring mental illness, and 24% had a serious mental illness (Jones & McCance-Katz, 2019). With potential overlapping neurobiological basis, opioid use disorder and opioid dependence may trigger changes in the brain and increase one’s risk for developing mental disorders, and brain changes in people with mental disorders may enhance the rewarding effects of opioids misuse or dependence (Koob, 2020). In addition, patients with diagnosed mental disorders are at higher risk of opioid dependence and overdose, but on the other hand, are more likely to get opioid prescription (Feingold et al., 2018). Therefore, understanding the mechanisms of the co-occurrence and elucidating the causal directions between opioid dependence and mental disorders are of paramount importance to inform preventive strategies.
Several observational studies have reported positive associations between prescription opioid use and common mental health disorders (Davis et al., 2017; Sullivan et al., 2006; Vekaria et al., 2021). However, observational studies are subject to confounding bias and reverse causality, and directions of the causal relationships between opioid dependence and mental disorders cannot be estimated. When randomized clinical trials are not feasible due to ethical and administrative reasons, Mendelian randomization (MR), which uses genetic variants associated with the exposure at genome-wide significance level as unconfounded proxies for the exposure variable, serves as a useful tool for assessing the causal effects and directions between the exposure and outcomes of interest.
In this study, we aim to investigate the causal relationships between opioid dependence and major mental disorders, including major depressive disorder (MDD), schizophrenia (SCZ), bipolar disorder (BIP), panic disorder (PD), anorexia (ANX), obsessive–compulsive disorder (OCD), post-traumatic stress disorder (PTSD), and insomnia, using bidirectional MR design. Summary statistics were obtained from published GWAS of opioid dependence and mental disorders. Causal effects were estimated primarily using the inverse variance weighted method in two-sample MR, with robustness checks for heterogeneity and horizontal pleiotropy and other sensitivity analyses. Causal directions were assessed bidirectionally, by forward MR investigating the effects of opioid dependence on mental disorders, and reverse MR investigating the effect of mental disorders on opioid dependence. Finally, mediation effects of chronic pain along the opioid dependence on mental disorders causal pathway were assessed using multivariate MR and two-step MR methods.
Methods
Study Design
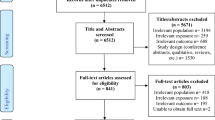
An overview of the study workflow is illustrated in Fig. 1. The study was reported according to Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR) guideline (STROBE-MR Checklist) (Skrivankova et al., 2021). All relevant GWAS had obtained ethical permissions from corresponding institutional review boards. Since our study involves only publicly available GWAS summary statistics, no ethical approval was required from the institutional review board.
Flowchart of the overall study design. Eight mental disorders include schizophrenia (SCZ), bipolar disorder (BIP), major depressive disorder (MDD), panic disorder (PD), anorexia (ANX), obsessive–compulsive disorder (OCD), post-traumatic stress disorder (PTSD), and insomnia. Assumption of Mendelian randomization (MR) include the following: (1) instrumental variables are strongly associated with exposures (i.e., the relevance assumption); (2) instrumental variables should not be associated with confounders (i.e., the independence assumption); (3) instrumental variables should influence the outcomes only through the exposures (i.e., the exclusion restriction assumption). GWAS, genome-wide association study; CAUSE, causal analysis using summary effect; RAPS, robust adjusted profile score; MR, Mendelian randomization
Data Source
Summary statistics were obtained from the GWAS of opioid dependence (1303 cases and 455,045 controls) (Jiang et al., 2021), MDD (135,458 cases and 344,901 controls) (Wray et al., 2018), SCZ (53,386 cases and 77,258 controls) (Trubetskoy et al., 2022), BIP (41,917 cases and 371,549 controls) (Mullins et al., 2021), PD (2147 cases and 7760 controls) (Forstner et al., 2021), ANX (16,992 cases and 55,525 controls) (Watson et al., 2019), PTSD (23,212 cases and 151,447 controls) (Nievergelt et al., 2019), OCD (2688 cases and 7037 controls) (Arnold et al., 2018), insomnia (593,724 cases and 1771,286 controls) (Watanabe et al., 2022), and chronic pain (152 cases and 456,196 controls) (Jiang et al., 2021). All participants included in the analyses were of European ancestry. Opioid dependence, insomnia, and chronic pain’s GWAS are from UK Biobank (UKB), and other 7 mental disorders’ GWAS are from Psychiatric Genomics Consortium (PGC). Detailed description of data sources is provided in Table 1. Table S17 showed the baseline characteristic of cohorts in UKB. Table S18—Table S24 showed the country, cases number, and controls number of each PGC cohort.
Since weak instrument bias and inflation of Type I error rate could result from partially overlapped samples of the exposure and outcome GWAS (Burgess et al., 2016), we additionally checked the sample overlap between participants in the GWAS of opioid dependence and those in the GWAS of mental disorders. Since GWAS of insomnia involved participants from the UK Biobank, we additionally assessed the amount of bias using sensitivity analysis to account for sample overlap with opioid dependence. GWAS of the remaining mental disorders involve cohorts from the Psychiatric Genomics Consortium and did not have sample overlap with opioid dependence.
Selection of Genetic Instruments
For opioid dependence, a p value threshold of 1e-5 was used for selecting instrumental SNPs since no enough valid instrumental SNPs could be obtained using p value threshold of 5e-8 or 5e-6. SNPs were then clumped by linkage disequilibrium (LD) < 0.001 within a 100 kb window for independence. When instrumental SNPs were not present in outcome GWAS, we used SNPs in outcome GWAS with high LD (> 0.8) to replace. Palindromic SNPs with intermediate allele frequencies were removed. This resulted in 9 valid instrumental SNPs for opioid dependence (Table S5). Although using relatively relaxed p value thresholds could increase the statistical power by using larger numbers of genetic instruments, it could potentially lead to weak instrument bias (Pierce & Burgess, 2013). Therefore, we further tested the strength of association between these 9 instrumental SNPs and phenotypic opioid dependence using F statistics, where an F value greater than 10 indicates strong association between instrumental SNPs and phenotypic opioid dependence (Pierce et al., 2011). Robust adjusted profile score MR (MR-RAPS) was also conducted to test weak instrument bias (SCORE, 2018).
Similarly in the reverse MR, instrumental SNPs were identified for each mental disorder. Following the suggestions of previous studies, a p value threshold 5e-8 of was used to select instrumental SNPs for SCZ, BIP, and ANX, while a threshold of 5e-6 was used for MDD, PD, PTSD, OCD, and insomnia due to insufficient number of instrumental SNPs under more stringent p value thresholds (Gage et al., 2018; Choi et al., 2019; Rosoff et al., 2021). After clumping by LD, we obtained 43, 146, 51, 13, 6, 19, 13, and 13 instrumental SNPs for MDD, SCZ, BIP, PD, ANX, PTSD, OCD, and insomnia, respectively. Detailed information about individual instrumental SNP for each mental disorder were provided in Table S6-S13. Again, the strength of association between instrumental SNPs and each mental disorder when using relaxed p value threshold was assessed by F statistics and MR-RAPS.
Statistical Analyses
For forward MR, univariate two-sample MR was performed between opioid dependence and each mental disorder, with Bonferroni correction for multiple comparisons. Inverse variance weighted MR was used as the primary analytic approach when heterogeneity and horizontal pleiotropy did not exist. Cochran Q statistic and MR pleiotropy residual sum and outlier (MR-PRESSO) / MR-Egger tests were used to assess heterogeneity and horizontal pleiotropy, respectively (Burgess & Thompson, 2017; Huedo-Medina et al., 2006; Verbanck et al., 2018). In case of heterogeneity, results from weighted median method (which only requires 50% of the weight to come from valid genetic instruments) were adopted, and in case of horizontal pleiotropy, results from MR-Egger regression models (which gives consistent estimates when 100% of the genetic instruments were invalid) were adopted. We also conducted leave-one-SNP-out analysis to check whether results were driven by individual genetic instrument. For reverse MR, univariate two-sample MR was performed between each mental disorder and opioid dependence following the same analytic strategy. MR-CAUSE analysis, which accounts for both correlated and uncorrelated pleiotropy between exposure and outcome variables, was conducted as a sensitivity analysis when significant causal effect was obtained between opioid dependence and mental disorders.
Mediation effects of chronic pain along the mental disorder on opioid dependence causal pathway was evaluated using both multivariate MR and two-step MR analysis (Carter et al., 2021). Specifically, to estimate the mediation effects of chronic pain along the mental disorder on opioid dependence causal pathway, both mental disorder and chronic pain were included as the exposure variables in multivariate MR, while effects of mental disorder on chronic pain and effects of chronic pain on opioid dependence were successively estimated and multiplied in two-step MR analysis.
Causal effects between opioid dependence and mental disorders are quantified by odds ratio (ORs), which represent the risk for mental disorders per one unit increase in log odds of opioid dependence (forward MR), or the risk for opioid dependence per one unit increase in log odds of mental disorders (reverse MR). Both point estimates and 95% confidence intervals (CI) were reported. For consistent estimation of the effects and directions between opioid dependence and mental disorders, only results with consistent magnitude and direction across multiple MR analytic strategies are considered. Bonferroni corrected p value threshold < 0.05/8 (across 8 mental disorders) was used for statistical significance. All two-sample MR analyses were performed using the “TwoSampleMR” and “MRPRESSO” package in R (version 4.0.3).
Results
Causal Effects of Opioid Dependence on Mental Disorders
As shown in Fig. 2, using 9 valid instrumental SNPs strongly associated with opioid dependence, univariate forward MR analysis showed significant positive causal effects of opioid dependence on insomnia via inverse variance weighted approach (OR = 1.03, 95% CI = (1.01, 1.05), p = 0.0053). Specifically, higher probabilities of opioid dependence were causally related to higher risks of insomnia. Cochran’s Q statistics did not show evidence of heterogeneity (p = 0.26), and neither MR-PRESSO nor MR-Egger test intercept tests showed evidence of horizontal pleiotropy (p = 0.61 for MR-PRESSO, p = 0.81 for MR-Egger). Scatter plot and funnel plot are shown in Figure S1 and Figure S2. F statistics showed strong association between genetic instruments of opioid dependence and phenotypic opioid dependence (F value = 11,338). Results from the MR-CAUSE model suggested that causal model of opioid dependence on insomnia outperformed both the null and sharing model (Table 2).
Causal effects of opioid dependence on mental disorders assessed by forward MR. Boxes represent point estimates of the causal effects, and error bars represent 95% confidence intervals. Methods in bold represent appropriate MR analytical approach after testing for assumptions of heterogeneity and horizontal pleiotropy. OR, odds ratio; CI, confidence interval; IVW, inverse variance weighted; PRESSO, pleiotropy residual sum and outlier; RAPS, robust adjusted profile score; ANX, anorexia; BIP, bipolar disorder; MDD, major depressive disorder; OCD, obsessive–compulsive disorder; PD, panic disorder; PTSD, post-traumatic stress disorder; SCZ, schizophrenia
Since the GWAS sample of insomnia involved UK Biobank participants and overlapped with opioid dependence, we assessed the risk of bias using sensitivity analysis. No significant risk of bias or inflation of Type I error was observed for the causal effects of opioid dependence on insomnia (bias < 0.001, regardless of overlap proportion, Table S14). Further, causal effects of opioid dependence on insomnia were consistent in terms of directions and magnitude across multiple MR approaches. Leave-one-SNP-out analyses did not show that results were driven by individual SNPs (Table S3).
Causal Effects of Mental Disorders on Opioid Dependence
Having observed significant causal effects of opioid dependence on insomnia, we conducted reverse MR analysis investigating the causal effects of mental disorders on opioid dependence. As shown in Fig. 3, using 146 valid instrumental SNPs, univariate MR analysis showed significant positive effects of schizophrenia on opioid dependence (OR = 1.20, 95% CI = (1.07, 1.34), p = 0.002) via inverse variance weighted approach. Specifically, higher risks of genetically predicted schizophrenia would lead to higher chances of developing opioid dependence. Cochran’s Q statistics did not show any evidence of heterogeneity (p = 0.23), and neither MR-PRESSO / MR-Egger test intercept tests showed evidence of horizontal pleiotropy (p = 0.22 for MR-PRESSO, p = 0.45 for MR-Egger). Scatter plot and funnel plot are shown in Figure S3 and Figure S4. F statistics showed strong association between genetic instruments of schizophrenia and phenotypic schizophrenia (F value = 295).
Causal effects of mental disorders on opioid dependence assessed by reverse MR. Boxes represent point estimates of the causal effects, and error bars represent 95% confidence intervals. Methods in bold represent appropriate MR analytical approach after testing for assumptions of heterogeneity and horizontal pleiotropy. OR, odds ratio; CI, confidence interval; IVW, inverse variance weighted; PRESSO, pleiotropy residual sum and outlier; RAPS, robust adjusted profile score; ANX, anorexia; BIP, bipolar disorder; MDD, major depressive disorder; OCD, obsessive–compulsive disorder; PD, panic disorder; PTSD, post-traumatic stress disorder; SCZ, schizophrenia
Results of the MR-CAUSE analysis suggested that causal model of schizophrenia on opioid dependence outperformed both the null and sharing model (Table 2). Further, causal effects of schizophrenia on opioid dependence were consistent in terms of directions and magnitude across multiple MR approaches. Leave-one-SNP-out analyses did not show that results were driven by any individual SNPs (Table S4).
In addition to schizophrenia, we observed nominal significant causal effects of MDD (OR = 1.33, 95% CI = (1.02, 1.72), p = 0.0323) on opioid dependence using 43 valid instrumental SNPs. Specifically, higher risks of genetically predicted MDD would lead to higher risks of opioid dependence at nominal significant level. However, these results became non-significant after Bonferroni correction for multiple comparisons.
Mediating Effects of Chronic Pain Along the Opioid Dependence on Mental Disorder Causal Pathway
To investigate whether causal effects of opioid dependence on insomnia and causal effects of schizophrenia on opioid dependence are mediated by chronic pain, we conducted both multivariate MR and two-step MR analyses. As shown in Table 3, both multivariate MR and two-step MR analyses suggest that opioid dependence effect insomnia via the direct pathway and independent of chronic pain. What is more, genetically predicted risk of schizophrenia impacts opioid dependence via a direct route independent of chronic pain. Detailed results of total effects, direct effects, and indirect effects can be found in Table S15 and Table S16.
Discussion
In this study, we comprehensively investigated the causal relationships between opioid dependence and multiple mental disorders using bidirectional MR design. Despite high genetic correlation and strong pleiotropy between various mental disorders (Amare et al., 2020; Cardno & Owen, 2014), our results suggested a high degree of variability regarding their causal patterns with opioid dependence. Our analyses provide statistical evidence for a positive causal effect of opioid dependence on insomnia, and a positive causal effect of schizophrenia on opioid dependence. By dissecting the total effects of schizophrenia on opioid dependence (and of opioid dependence on insomnia), we also found that the majority of causal effects are acted directly from schizophrenia to opioid dependence (and from opioid dependence to insomnia), compared to those mediated through chronic pain.
Associations between opioid use and sleep disorders have been reported in many literatures, suggesting that both are linked to brain regions involved in reward processing. Opioids intake could increase daytime sleepiness and decrease sleep latency, while disrupting night sleep and augmenting night awakening due to acute withdrawal effects (Roth, 2009; Schierenbeck et al., 2008). Studies also showed dose-dependent effects of opioids on total sleep, sleep efficiency, and sleep disruptions (Kay et al., 1981; Moore & Kelz, 2009). While the initiation and continuation of opioid use have been known to reduce rapid eye movement (REM) sleep without inducing sleep arousal, chronic use and addiction to opioids were found to elevate REM sleep with pronounced alteration in slow-wave sleep, wakefulness, and arousal (Wang & Teichtahl, 2007; Shaw et al., 2005; Hartwell et al., 2014). In addition to chronic use of opioids, acute opioid withdrawal could also contribute to sleep disturbances by elonging sleep latency and decreasing total sleep and REM sleep time (Schierenbeck et al., 2008), which act as risk factors for substance relapse (Hartwell et al., 2014; Morgan et al., 2010; Asaad et al., 2011; Dijkstra et al., 2008; Haack et al., 2020).
Consistent with many observational studies, we found positive causal effects of opioid dependence on insomnia (Battle, 2013; Dolsen & Harvey, 2017; Serdarevic et al., 2017; Tran et al., 2009). A randomized controlled trial showed that using buprenorphine, which has been approved for prescription opioid detoxification since 2002, 2 patients with a 4-week buprenorphine taper demonstrated remarkable sleep improvement (Dunn et al., 2015). On the contrary, a prospective longitudinal study led by Nordmann et al. showed that methadone maintenance treatment had no effect on sleep disturbance, suggesting that sleep disturbances are not a cause r obstacle to the initiation or continuation of methadone maintenance treatment (Nordmann et al., 2016). All these findings support our conclusion that there exists positive causal effect of opioid dependence on insomnia, not the other way around. Considering the complicated relationships between substance addiction and sleep and great amount of confounding bias from observational studies, there has been no published study investigating or claiming the causal relationships between opioid dependence and insomnia. Our findings thus stand as strong evidence with important therapeutic implications on sleep disturbances.
Epidemiologic studies have shown that rates of substance abuse remained higher in patients with psychological disorders than in the general population (Regier et al., 1990). Although studies found lower prevalence of opioid abuse among patients with schizophrenia compared to those with major depressive disorders and bipolar disorder (Chiappelli et al., 2018; Farrell et al., 2002), substantial evidence suggested that co-occurring opioid use disorder in schizophrenia patients was associated with worsened clinical outcomes, treatment non-compliance, and poor overall quality of life (Sayers et al., 2005; Li et al., 2020; Clark et al., 2019; Watkins et al., 2019; Hjorthøj et al., 2018; Shekhar, 2019). A meta-analysis of placebo-controlled clinical trials of opioid antagonists (such as naloxone, naltrexone, nalmefene, and buprenorphine) in schizophrenia patients showed that opioid antagonists are associated with a significant reduction in the positive, negative, total, and general symptoms of schizophrenia (Clark et al., 2020).
Numerous hypotheses have been proposed to explain the possible mechanisms through which schizophrenia interplay with substance use. The cumulative risk factor model posits that individuals with schizophrenia have increased propensity to develop substance abuse due to the accumulation of cognitive, social, educational, and vocational deficits (Mueser et al., 1990), while the self-medication hypothesis suggests that patients with schizophrenia develop substance abuse to lessen the schizophrenic symptoms or side effects related to antipsychotic treatments (Khantzian, 1997). Psaman et al. also revealed new risk loci of lifetime cannabis use and showed evidence for a causal positive influence of schizophrenia risk on cannabis use (Pasman et al., 2018). Our findings of a positive causal effect of schizophrenia on opioid dependence support the primary addiction hypothesis (also called reward deficiency model), which argues that shared neuropathology of schizophrenia and substance addiction involving dopaminergic and glutamatergic regulation of the mesolimbic pathway leads to higher risks of comorbid schizophrenia and substance addiction (Chambers et al., 2001). Results from epidemiologic studies demonstrated inconsistent conclusions regarding the direction of causal effects between psychotic disorders and substance use (Abdel-Baki et al., 2017; Barkus, 2016; Gage & Munafò, 2015; Kendler et al., 2015). Our findings thus provide an evidence-based conclusion that genetic determinants of schizophrenia, especially within neural circuits related to reward processing, place patients at increased vulnerability to opioid dependence. Further, people with substance use disorders often experience comorbid chronic physical health conditions including chronic pain, cancer, and cardiovascular diseases (Garland et al., 2013; Schulte & Hser, 2013). An estimated 10% of chronic pain patients reported misuse of prescription opioids (Garland et al., 2013). Although chronic pain and related emotional distress could increase the risk of opioid dependence via dysregulating the reward circuitry, our results suggested that the majority causal effects of schizophrenia (and of opioid dependence) are likely to be implemented directly on the mesolimbic system via shared genetic architecture.
Unlike observational investigations, our study avoids potential confounding bias by utilizing a two-sample Mendelian randomization design that relies on summary statistics obtained from large-scale population cohorts. Multiple sensitivity analyses were conducted to robustly check the assumptions required for Mendelian randomization and ensure the validity of both the analytical approaches and results. To our knowledge, this is the first study comprehensively investigating the causal relationships between opioid dependence and major mental disorders. With that being said, we also need to acknowledge potential limitations of the study. Firstly, although we evaluated the associational strength of instrumental SNPs with opioid dependence using F statistics and replicated the MR analyses using MR-RAPS, the use of a relatively relaxed p value threshold in selecting the instrumental SNPs for opioid dependence could lead to weak instrumental bias. Secondly, GWAS of opioid dependence, OCD, and PD have relatively small sample sizes, which could lead to decreased statistical power in detecting a meaningful significant signal. Future research involving larger population cohorts are needed to elucidate the causal relationships between opioid dependence and OCD/PD. Thirdly, participant samples overlapped between GWAS of opioid dependence and insomnia, which could potentially lead to weak instruments bias and inflation of Type I error rate. However, results from sensitivity analyses suggest that both bias and inflation of Type I error rate should be kept at relatively negligible level. Finally, since our analyses are restricted to GWAS samples of European ancestry, our conclusions cannot be genialized to the general populations.
Data Availability
The GWAS summary data of opioid dependence is available at https://www.ebi.ac.uk/gwas/studies/GCST90043725. The GWAS summary data of 8 mental disorders are available at https://figshare.com/articles/dataset/an2019/14671980; https://figshare.com/articles/dataset/bip2021_noUKBB/22564402; https://figshare.com/articles/dataset/MDD2_MDD2018_GWAS_sumstats_w_o_UKBB/21655784; https://figshare.com/articles/dataset/ocd2018/14672103; https://figshare.com/articles/dataset/panic2019/16602218; https://figshare.com/articles/dataset/ptsd2019/14672133; https://figshare.com/articles/dataset/scz2022/19426775; https://research.23andme.com/collaborate/#publication. The GWAS summary data of chronic pain is available at https://www.ebi.ac.uk/gwas/studies/GCST90043740. Ethics statement All relevant GWAS had obtained ethical permissions from corresponding institutional review boards. Since our study involves only publicly available GWAS summary statistics, no ethical approval was required from the institutional review board.
References
Abdel-Baki, A., Ouellet-Plamondon, C., Salvat, É., Grar, K., & Potvin, S. (2017). Symptomatic and functional outcomes of substance use disorder persistence 2 years after admission to a first-episode psychosis program. Psychiatry Research, 247, 113–119.
Amare, A. T., Vaez, A., Hsu, Y. H., Direk, N., Kamali, Z., Howard, D. M., ... & Hartman, C. A. (2020). Bivariate genome-wide association analyses of the broad depression phenotype combined with major depressive disorder, bipolar disorder or schizophrenia reveal eight novel genetic loci for depression. Molecular psychiatry, 25(7), 1420–1429.
Arnold, P. D., Askland, K. D., Barlassina, C., Bellodi, L., Bienvenu, O. J., Black, D., ... & Zai, G. (2018). Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Molecular psychiatry, 23(5), 1181–1181.
Asaad, T. A., Ghanem, M. H., Samee, A. M. A., & El-Habiby, M. M. (2011). Sleep profile in patients with chronic opioid abuse: A polysomnographic evaluation in an Egyptian sample. Addictive Disorders & Their Treatment, 10(1), 21–28.
Barkus, E. (2016). High-potency cannabis increases the risk of psychosis. BMJ Ment Health, 19(2), 54–54.
Battle, D. E. (2013). Diagnostic and statistical manual of mental disorders (DSM). In Codas, 25(2), 191–192.
Burgess, S., & Thompson, S. G. (2017). Interpreting findings from Mendelian randomization using the MR-Egger method. European Journal of Epidemiology, 32, 377–389.
Burgess, S., Davies, N. M., & Thompson, S. G. (2016). Bias due to participant overlap in two-sample Mendelian randomization. Genetic Epidemiology, 40(7), 597–608.
Cardno, A. G., & Owen, M. J. (2014). Genetic relationships between schizophrenia, bipolar disorder, and schizoaffective disorder. Schizophrenia Bulletin, 40(3), 504–515.
Carter, A. R., Sanderson, E., Hammerton, G., Richmond, R. C., Davey Smith, G., Heron, J., ... & Howe, L. D. (2021). Mendelian randomisation for mediation analysis: Current methods and challenges for implementation. European journal of epidemiology, 36(5), 465–478.
Chambers, R. A., Krystal, J. H., & Self, D. W. (2001). A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological Psychiatry, 50(2), 71–83.
Chang, K. C., Lee, K. Y., Lu, T. H., Hwang, J. S., Lin, C. N., Ting, S. Y., ... & Wang, J. D. (2019). Opioid agonist treatment reduces losses in quality of life and quality-adjusted life expectancy in heroin users: Evidence from real world data. Drug and alcohol dependence, 201, 197–204.
Chiappelli, J., Chen, S., Hackman, A., & Hong, L. E. (2018). Evidence for differential opioid use disorder in schizophrenia in an addiction treatment population. Schizophrenia Research, 194, 26–31.
Choi, K. W., Chen, C. Y., Stein, M. B., Klimentidis, Y. C., Wang, M. J., Koenen, K. C., & Smoller, J. W. (2019). Assessment of bidirectional relationships between physical activity and depression among adults: A 2-sample mendelian randomization study. JAMA Psychiatry, 76(4), 399–408.
Clark, S. D., & Abi-Dargham, A. (2019). The role of dynorphin and the kappa opioid receptor in the symptomatology of schizophrenia: A review of the evidence. Biological Psychiatry, 86(7), 502–511.
Clark, S. D., Van Snellenberg, J. X., Lawson, J. M., & Abi-Dargham, A. (2020). Opioid antagonists are associated with a reduction in the symptoms of schizophrenia: A meta-analysis of controlled trials. Neuropsychopharmacology, 45(11), 1860–1869.
Davis, M. A., Lin, L. A., Liu, H., & Sites, B. D. (2017). Prescription opioid use among adults with mental health disorders in the United States. The Journal of the American Board of Family Medicine, 30(4), 407–417.
Degenhardt, L., Grebely, J., Stone, J., Hickman, M., Vickerman, P., Marshall, B. D., ... & Larney, S. (2019). Global patterns of opioid use and dependence: Harms to populations, interventions, and future action. The Lancet, 394(10208), 1560–1579.
Dijkstra, B. A., De Jong, C. A., Krabbe, P. F., & van der Staak, C. P. (2008). Prediction of abstinence in opioid-dependent patients. Journal of Addiction Medicine, 2(4), 194–201.
Dolsen, E. A., & Harvey, A. G. (2017). Life-time history of insomnia and hypersomnia symptoms as correlates of alcohol, cocaine and heroin use and relapse among adults seeking substance use treatment in the United States from 1991 to 1994. Addiction, 112(6), 1104–1111.
Dowell, D., Arias, E., Kochanek, K., Anderson, R., Guy, G. P., Losby, J. L., & Baldwin, G. (2017). Contribution of opioid-involved poisoning to the change in life expectancy in the United States, 2000–2015. JAMA, 318(11), 1065–1067.
Dunn, K. E., Saulsgiver, K. A., Miller, M. E., Nuzzo, P. A., & Sigmon, S. C. (2015). Characterizing opioid withdrawal during double-blind buprenorphine detoxification. Drug and Alcohol Dependence, 151, 47–55.
Farrell, M., Boys, A., Bebbington, P., Brugha, T., Coid, J., Jenkins, R., ... & Taylor, C. (2002). Psychosis and drug dependence: Results from a national survey of prisoners. The British Journal of Psychiatry, 181(5), 393–398.
Feingold, D., Brill, S., Goor-Aryeh, I., Delayahu, Y., & Lev-Ran, S. (2018). The association between severity of depression and prescription opioid misuse among chronic pain patients with and without anxiety: A cross-sectional study. Journal of Affective Disorders, 235, 293–302.
Forstner, A. J., Awasthi, S., Wolf, C., Maron, E., Erhardt, A., Czamara, D., ... & Schumacher, J. (2021). Genome-wide association study of panic disorder reveals genetic overlap with neuroticism and depression. Molecular psychiatry, 26(8), 4179–4190.
Gage, S. H., & Munafò, M. R. (2015). Rethinking the association between smoking and schizophrenia. The Lancet Psychiatry, 2(2), 118–119.
Gage, S. H., Bowden, J., Davey Smith, G., & Munafò, M. R. (2018). Investigating causality in associations between education and smoking: A two-sample Mendelian randomization study. International Journal of Epidemiology, 47(4), 1131–1140.
Garland, E. L., Froeliger, B., Zeidan, F., Partin, K., & Howard, M. O. (2013). The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neuroscience & Biobehavioral Reviews, 37(10), 2597–2607.
Haack, M., Simpson, N., Sethna, N., Kaur, S., & Mullington, J. (2020). Sleep deficiency and chronic pain: Potential underlying mechanisms and clinical implications. Neuropsychopharmacology, 45(1), 205–216.
Han, B., Compton, W. M., Jones, C. M., & Cai, R. (2015). Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003–2013. JAMA, 314(14), 1468–1478.
Han, B., Jones, C. M., Blanco, C., & Compton, W. M. (2017). National trends in and correlates of nonmedical use of prescription stimulants, nonmedical use frequency, and use disorders. The Journal of Clinical Psychiatry, 78(9), 21718.
Hartwell, E. E., Pfeifer, J. G., McCauley, J. L., Moran-Santa Maria, M., & Back, S. E. (2014). Sleep disturbances and pain among individuals with prescription opioid dependence. Addictive Behaviors, 39(10), 1537–1542.
Hjorthøj, C., Albert, N., & Nordentoft, M. (2018). Association of substance use disorders with conversion from schizotypal disorder to schizophrenia. JAMA Psychiatry, 75(7), 733–739.
Huedo-Medina, T. B., Sánchez-Meca, J., Marín-Martínez, F., & Botella, J. (2006). Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods, 11(2), 193.
Jiang, L., Zheng, Z., Fang, H., & Yang, J. (2021). A generalized linear mixed model association tool for biobank-scale data. Nature Genetics, 53(11), 1616–1621.
Jones, C. M. (2018). Reprint of trends and key correlates of prescription opioid injection misuse in the United States. Addictive Behaviors, 86, 24–31.
Jones, C. M., & McCance-Katz, E. F. (2019). Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug and Alcohol Dependence, 197, 78–82.
Kay, D. C., Pickworth, W. B., & Neider, G. L. (1981). Morphine-like insomnia from heroin in nondependent human addicts. British Journal of Clinical Pharmacology, 11(2), 159–169.
Kendler, K. S., Lönn, S. L., Sundquist, J., & Sundquist, K. (2015). Smoking and schizophrenia in population cohorts of Swedish women and men: A prospective co-relative control study. American Journal of Psychiatry, 172(11), 1092–1100.
Khantzian, E. J. (1997). The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry, 4(5), 231–244.
Ko, J. Y., Patrick, S. W., Tong, V. T., Patel, R., Lind, J. N., & Barfield, W. D. (2016). Incidence of neonatal abstinence syndrome—28 states, 1999–2013. Morbidity and Mortality Weekly Report, 65(31), 799–802.
Koob, G. F. (2020). Neurobiology of opioid addiction: Opponent process, hyperkatifeia, and negative reinforcement. Biological Psychiatry, 87(1), 44–53.
Li, K. J., Chen, A., & DeLisi, L. E. (2020). Opioid use and schizophrenia. Current Opinion in Psychiatry, 33(3), 219–224.
Moore, J. T., & Kelz, M. B. (2009). Opiates, sleep, and pain: The adenosinergic link. The Journal of the American Society of Anesthesiologists, 111(6), 1175–1176.
Morgan, P. T., Pace-Schott, E., Pittman, B., Stickgold, R., & Malison, R. T. (2010). Normalizing effects of modafinil on sleep in chronic cocaine users. American Journal of Psychiatry, 167(3), 331–340.
Mueser, K. T., Yarnold, P. R., Levinson, D. F., Singh, H., Bellack, A. S., Kee, K., ... & Yadalam, K. G. (1990). Prevalence of substance abuse in schizophrenia: Demographic and clinical correlates. Schizophrenia bulletin, 16(1), 31–56.
Mullins, N., Forstner, A. J., O’Connell, K. S., Coombes, B., Coleman, J. R., Qiao, Z., ... & Potash, J. B. (2021). Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nature genetics, 53(6), 817–829.
Nievergelt, C. M., Maihofer, A. X., Klengel, T., Atkinson, E. G., Chen, C. Y., Choi, K. W., ... & Stevens, J. S. (2019). International meta-analysis of PTSD genome-wide association studies identifies sex-and ancestry-specific genetic risk loci. Nature communications, 10(1), 4558.
Nordmann, S., Lions, C., Vilotitch, A., Michel, L., Mora, M., Spire, B., ... & ANRS Methaville study group. (2016). A prospective, longitudinal study of sleep disturbance and comorbidity in opiate dependence (the ANRS Methaville study). Psychopharmacology, 233, 1203–1213.
Overdose, O. (2018). Understanding the epidemic. Centers for Disease Control and Prevention: Atlanta.
Pasman, J. A., Verweij, K. J., Gerring, Z., Stringer, S., Sanchez-Roige, S., Treur, J. L., ... & Vink, J. M. (2018). GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal effect of schizophrenia liability. Nature neuroscience, 21(9), 1161–1170.
Peters, P. J., Pontones, P., Hoover, K. W., Patel, M. R., Galang, R. R., Shields, J., ... & Duwve, J. M. (2016). HIV infection linked to injection use of oxymorphone in Indiana, 2014–2015. New England Journal of Medicine, 375(3), 229–239.
Pierce, B. L., & Burgess, S. (2013). Efficient design for Mendelian randomization studies: Subsample and 2-sample instrumental variable estimators. American Journal of Epidemiology, 178(7), 1177–1184.
Pierce, B. L., Ahsan, H., & VanderWeele, T. J. (2011). Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. International Journal of Epidemiology, 40(3), 740–752.
Regier, D. A., Farmer, M. E., Rae, D. S., Locke, B. Z., Keith, S. J., Judd, L. L., & Goodwin, F. K. (1990). Comorbidity of mental disorders with alcohol and other drug abuse: Results from the Epidemiologic Catchment Area (ECA) study. JAMA, 264(19), 2511–2518.
Rosoff, D. B., Smith, G. D., & Lohoff, F. W. (2021). Prescription opioid use and risk for major depressive disorder and anxiety and stress-related disorders: A multivariable Mendelian randomization analysis. JAMA Psychiatry, 78(2), 151–160.
Roth, T. (2009). Does effective management of sleep disorders reduce substance dependence? Drugs, 69, 65–75.
Sayers, S. L., Campbell, E. C., Kondrich, J., Mann, S. C., Cornish, J., O’Brien, C., & Caroff, S. N. (2005). Cocaine abuse in schizophrenic patients treated with olanzapine versus haloperidol. The Journal of Nervous and Mental Disease, 193(6), 379–386.
Schierenbeck, T., Riemann, D., Berger, M., & Hornyak, M. (2008). Effect of illicit recreational drugs upon sleep: Cocaine, ecstasy and marijuana. Sleep Medicine Reviews, 12(5), 381–389.
Schulte, M. T., & Hser, Y. I. (2013). Substance use and associated health conditions throughout the lifespan. Public Health Reviews, 35(2), 1–27.
SCORE, U. R. A. P. (2018). Statistical inference in two-sample summary-data mendelian randomization using robust adjusted profile score. arXiv preprint arXiv:1801.09652.
Serdarevic, M., Osborne, V., Striley, C. W., & Cottler, L. B. (2017). The association between insomnia and prescription opioid use: Results from a community sample in Northeast Florida. Sleep Health, 3(5), 368–372.
Shaheed, C. A., McLachlan, A. J., & Maher, C. G. (2019). Rethinking “long term” opioid therapy. Bmj, 367.
Shaw, I. R., Lavigne, G., Mayer, P., & Choinière, M. (2005). Acute intravenous administration of morphine perturbs sleep architecture in healthy pain-free young adults: A preliminary study. Sleep, 28(6), 677–682.
Shekhar, A. (2019). Role of kappa opioid receptors in symptoms of schizophrenia: What is the neurobiology? Biological Psychiatry, 86(7), 494–496.
Skrivankova, V. W., Richmond, R. C., Woolf, B. A., Yarmolinsky, J., Davies, N. M., Swanson, S. A., ... & Richards, J. B. (2021). Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: The STROBE-MR statement. Jama, 326(16), 1614–1621.
Sullivan, M. D., Edlund, M. J., Zhang, L., Unützer, J., & Wells, K. B. (2006). Association between mental health disorders, problem drug use, and regular prescription opioid use. Archives of Internal Medicine, 166(19), 2087–2093.
Tran, A., Fuller, J. M., Wong, K. K., Krass, I., Grunstein, R., & Saini, B. (2009). The development of a sleep disorder screening program in Australian community pharmacies. Pharmacy World & Science, 31, 473–480.
Trubetskoy, V., Pardiñas, A. F., Qi, T., Panagiotaropoulou, G., Awasthi, S., Bigdeli, T. B., ... & Lazzeroni, L. C. (2022). Map** genomic loci implicates genes and synaptic biology in schizophrenia. Nature, 604(7906), 502–508.
Vekaria, V., Bose, B., Murphy, S. M., Avery, J., Alexopoulos, G., & Pathak, J. (2021). Association of co-occurring opioid or other substance use disorders with increased healthcare utilization in patients with depression. Translational Psychiatry, 11(1), 265.
Verbanck, M., Chen, C. Y., Neale, B., & Do, R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nature Genetics, 50(5), 693–698.
Wang, D., & Teichtahl, H. (2007). Opioids, sleep architecture and sleep-disordered breathing. Sleep Medicine Reviews, 11(1), 35–46.
Watanabe, K., Jansen, P. R., Savage, J. E., Nandakumar, P., Wang, X., Hinds, D. A., ... & Posthuma, D. (2022). Genome-wide meta-analysis of insomnia prioritizes genes associated with metabolic and psychiatric pathways. Nature genetics, 54(8), 1125–1132.
Watkins, A., John, A., Bradshaw, C., Jones, J., & Jones, M. (2019). Schizophrenia in high risk opioid users: A short communication on an autopsy study. Psychiatry Research, 276, 112–114.
Watson, H. J., Yilmaz, Z., Thornton, L. M., Hübel, C., Coleman, J. R., Gaspar, H. A., ... & Seitz, J. (2019). Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nature genetics, 51(8), 1207–1214.
Wray, N. R., Ripke, S., Mattheisen, M., Trzaskowski, M., Byrne, E. M., Abdellaoui, A., ... & Viktorin, A. (2018). Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nature genetics, 50(5), 668–681.
Acknowledgements
The authors thank the National Natural Science Foundation of China and Shanghai Science and Technology Commission for supporting this study.
Funding
This work is supported by the Young Scientists Fund of the National Natural Science Foundation of China (Grant No. 82304241) and the General Projects of Shanghai Science and Technology Commission (Grant No. 21ZR1405000).
Author information
Authors and Affiliations
Contributions
XL contributed to the conception and design of the study. YH and LQ participated in the assessments and data extraction processes. YH, RS, and HD performed the statistical analyses. XL and YH discussed the results and wrote the original draft of the manuscript. XL performed the review and editing. XL supervised the project and contributed to the funding acquisition. All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the finalized manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Y., Qian, L., Shi, R. et al. Understanding the Causal Relationships Between Opioid Dependence and Risk of Mental Disorders: A Comprehensive Two-Sample Mendelian Randomization Study. Int J Ment Health Addiction (2024). https://doi.org/10.1007/s11469-024-01315-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s11469-024-01315-y