Abstract
Core/shell nanocomposite is a very interesting structure that exhibits excellent properties such as improved thermal stability and decreased reactivity of the core nanoparticles. Core/shell nanoparticles were synthesized by means of laser ablation in ethanol without using a catalyst. The structural and optical properties of Au@LiNbO3 core/shell nanoparticles as a function of laser fluence are investigated. To fabricate the photodetector, a thin film of Au@LiNbO3 was deposited on a single crystal silicon substrate. X-ray diffraction (XRD) results show that the synthesized nanocomposite is crystalline with a rhombohedral structure and the presence of peaks related to a gold cubic structure, indicating the formation of a core/shell nanocomposite. Transmission electron microscopy (TEM) investigations confirm the formation of core/shell spherical nanoparticles, whose size depends on the laser fluence. The optical properties reveal that the optical energy gap of LiNbO3 was 4.08 eV, while the energy gap of the Au@LiNbO3 core/shell prepared at 1.3, 1.6, 2, and 2.2 J/cm2 was 3.6, 3.49, 3.4, and 3.8 eV, respectively. The optoelectronic properties of the Au@LiNbO3/Si photodetector fabricated without a buffer layer and an antireflection coating as a function of laser fluence are investigated. The optoelectronic properties show that the maximum responsivity was 0.43 A/W at 400 nm for the Au@LiNbO3/Si photodetector fabricated at 2 J/cm2. The variation of laser fluence affects the structural, optical, and electrical properties of Au@LiNbO3 core/shell. The best core/shell characteristics and photodetector were obtained at a laser energy of 2 J/cm2. The energy band diagram confirmed that the presence of Au significantly improved the photoresponse of the photodetector.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because of its outstanding physicochemical, ferroelectric, piezoelectric, and nonlinear optical capabilities, lithium niobate is one of the interesting and promising ferroelectric materials [1,2,3]. Due to these properties, it has been widely applied in a variety of fields, including optical waveguides, electro-optical modulation, holographic storage, optical parametric oscillators, etc. [4, 5]. It belongs to the space group R3c and has rhombohedral symmetry. LN’s ferroelectric phase changed to a paraelectric phase at 1200 °C. Due to its exceptional qualities, LiNbO3 has been widely used as a promising contender for “optical silicon.” Nevertheless, LN serves as a passive component and an insulator in the aforementioned applications [6,7,8]. Many of the remarkable features of LN can be utilized in semiconductor integrated devices if LN devices can be integrated onto a silicon substrate to produce an active component, combining the advantages of both LN and semiconductor materials [9,10,11]. The main challenges in fabricating an active component based on the p–n junction were the low electrical conductivity and optical absorption of LN. In an attempt to fabricate a device using LN, it is necessary to increase the electrical conductivity of LN [12,13,14]. As reported, various methods were employed to synthesize LiNbO3, for example, solution phase, sol–gel, citrate gel, electrospinning, and pulsed laser deposition [15,16,17,18]. There are many routes used to control the optical and electrical properties of LN, such as doping with different elements, for example Mg, Zn, Fe, etc. [19,20,21,22]. Several attempts to synthesize lithium niobate with a gold and silver core/shell structure have been reported. Murillo et al. [2] deposited Ag–LiNbO3 nanocomposite thin films with Ag nanoparticles (NPs) embedded into the LN matrix by the co-deposition of Ag and LN using a pulsed laser deposition (PLD) method that shows a surface plasmon resonance (SPR) in the visible range. The fabrication and characterization of a Fe-doped LiNbO3/n-Si heterojunction was demonstrated by Li et al. [23]. As reported, on solid surfaces, gold nanoparticles were assembled into 2D or 3D superlattices, nanowires, colloidal aggregates, and a core/shell in order to improve their physical and chemical properties and enable them to be efficient for many important applications such as catalysts, sensors, photonics, and surface-enhanced Raman scattering [24,25,26,27,28,29,30]. Compared to other techniques, the laser ablation of Au@LiNbO3 core/shell nanoparticles in liquid method used is promising and has several advantages including simplicity, low-cost, high purity product, fast, green synthesis, and fair control of the core–shell dimensions. [31,32,33]. The core–shell morphology give better thermal stability and decreases the reactivity.
Herein, we have demonstrated the synthesis of Au@LiNbO3 core/shell nanoparticles by two- step laser ablation in methanol at different laser fluences. The fabrication of Au@LiNbO3/Si was proposed as a simple, novel, and high-performance photodetector can used for many technological applications.
Experimental Work
Preparation of Au@LiNbO3 Core–Shell Nanoparticles
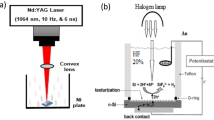
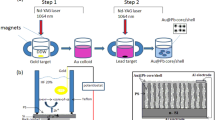
Colloidal Au nanoparticles are prepared by irradiating a high purity (99.9%) Au pellet immersed in a vessel filled with 3 ml of ethanol with 70 laser pulses of a second harmonic generation (= 532 nm) Q-switched Nd:YAG laser with a pulse duration of 7 ns and a repetition frequency of 1 Hz to synthesize Au@LiNbO3 core/shell nanoparticles. The ablation of Au nanoparticles was carried out at a laser fluence of 1.5 J/cm2. After Au colloidal preparation, a LiNbO3 wafer with an area of 1 cm2 was placed in the quartz vessel filled with Au colloid and then irradiated with 300 laser pulses at a laser fluence of 1.3, 1.6, 2, and 2.2 J/cm2. The experimental setup of the PLAL system is shown in Fig. 1a.
Characterization of Au @ LiNbO3 Core/Shell Nanoparticles
The optical absorption of colloidal Au nanoparticles, LN nanoparticles, and Au@LiNbO3 core/shell nanoparticles were measured using a UV–Vis spectrophotometer (Shimadzu UV-2550). The structural properties of the Au@LiNbO3 core/shell were investigated using an X-ray diffractometer (Panalytical X’Pert Pro). A confocal Raman spectrometer was used to record Raman spectra (HORIBA XPLORA PLUS). Transmission electron microscopy (ZEISS LEO 912) was used to examine the morphology and size of the Au@LiNbO3 core–shell.
Fabrication and Characterization of Au@LiNbO3/Si Photodetector
Fabrication of Au@LiNbO3/p-Si heterojunction photodetector was performed by depositing a thin-film Au@LiNbO3 core/shell via a spin coating route on polished (111)-oriented p-type silicon substrate. The silicon substrates used here have areas of 1 cm2 and electrical resistivity of 1–3 Ωcm. The ohmic contacts were made by the depositing of Al and In electrodes on the Au@LiNbO3 nanostructure film and silicon substrate, respectively, by using the thermal resistive technique through a metal mask. Figure 1b shows the fabrication steps of the Au@LiNbO3/Si photodetector. The current–voltage characteristics of the heterojunctions in the dark and under illumination were measured. The capacitance–voltage characteristics of the heterojunction were measured at a frequency of 200 kHz using LCR meter. The responsivity of the photodetector was measured using a monochromator (Jobin Yovn) in the spectral range of 370–1000 nm at a bias voltage of − 8 V. The power calibration of the monochromator was performed using a silicon power meter.
Results and Discussion
Figure 2 shows the XRD pattern of Au@LN core/shell nanoparticles prepared at 1.3 J/cm2, which exhibits two peaks located at 2θ = 33.05° and 61.6° which correspond to the (104) and (214) planes, respectively. The XRD pattern of the nanoparticles prepared at 1.6 J/cm2 shows the presence of eight XRD peaks located at 2θ = 33.03°, 35.9°, 41.08, 47.7°, 54.6°, 56.3°, 57.3°, and 61.7°, which correspond to (104), (110), (113), (024), (116), (122), (214), and (220) planes, respectively. While the XRD patterns of the core/shell synthesized at 2 J/cm2 have six diffraction angles at 2 = 33.04°, 35.84°, 47.7°, 54.5°, 56.6°, 57.3°, and 61.7°, corresponding to planes (104), (110), (024), (116), (122), and (214), respectively. Finally, the nanoparticles synthesized at 2.2 J/cm2 show the presence of six diffraction peaks observed at 2θ = 33.06°, 35.9°, 47.8°, 54.6°, 56.4°, 57.3°, and 61.7° corresponding to (104), (110), (024), (116), (122), and (214) planes, respectively. All the observed XRD peaks are indexed to a rhombohedral LiNbO3 according to JCPDs#20- 0631 [34,35,36]. The XRD peaks of core Au nanoparticles were observed at 2θ = 44° and 66° which correspond to (200) and (220) planes, respectively.
Figure 3 depicts the UV–Vis absorption spectra of colloidal Au@LN core and shell prepared at various laser fluences. The optical absorption spectra display a general increase with increased laser fluence, besides two groups of absorption peaks, the first one is attributed to LiNbO3 that is located at 224, 226, 227, and 231 nm for nanoparticles synthesized at 1.3, 1.6, 2, and 2.2 J/cm2 laser fluence, respectively, with the shoulder diminishing at higher laser fluence. The second peak is located at ~ 521–530 nm, which belongs to the surface plasmon resonance (SPR) of the gold nanoparticles. This peak disappeared at laser fluence of 2.2 J/cm2 This may be due to an increase in shell thickness. Increasing the LN shell thickness leads to decreasing the intensity of SPR of the Au core [37, 38]. The increase in LN concentration at higher laser fluence may be attributed to the low dipole moment of ethanol (1.69D) supporting growth via introducing extra species during the ablation, which results in a wider particle size distribution [39, 40]. Figure 4 displays the optical absorption of Au nanoparticles and the change in optical absorbance of LN nanoparticles after making the core/shell structure with Au nanoparticles.
The optical energy gap of LiNbO3 and Au@ LiNbO3 core/shell nanoparticles was estimated using Tauc’s relationship [41,42,43]. The optical energy gap value is estimated by plotting (αhv)2 as a function of hν, and the extrapolation of the second region of the curve to the photon energy axis gives the energy gap, as shown in Fig. 5. The energy gaps of the Au@LN core–shell prepared at laser fluence of 1.3, 1.6, 2, and 2.2 J/cm2 were 3.6, 3.49, 3.4, and 3.8 eV, respectively. The energy gap of LiNbO3 was 4.12 eV, which is larger than that of the Au@LiNbO3 core/shell structure. This could be attributed to the increased particle size of Au@LN due to the presence of the core–shell structure, as shown in Fig. 6. On the other hand, increasing the energy gap with laser fluence is due to the decrease in core–shell particle size.
The TEM images of Au@LN core/shell nanoparticles prepared at various laser fluences are shown in Fig. 7. These images confirm the formation of spherical nanoparticles with core/shell morphology, since the Au core nanoparticle is surrounded by a LN shell and the size of the core–shell particle depends on the laser fluence. The particle size increases first from 15 to 23 nm as laser fluence increases from 1.3 to 1.6 J/cm2 and then decreases to 19 nm as laser fluence increases to 2.2 J/cm2, and it shows a wide range of particle distribution. This gives approval to the change in shift of the absorption edge and the change in energy gap value of the UV–Vis results. Figure 8 shows the magnified TEM images of the core/shell structure, which revealed the formation of a monodispersed core/shell with a very clear boundary between Au-core and LN-shell. The effect of laser fluence on the core size and shell thickness is shown in Fig. 9. As laser fluence increases, the core size and shell thickness increase up to the laser fluence of 2 J/cm2 and further increases in laser fluence result in decreasing the core size and shell thickness due to fragmentation process.
Figure 10 shows the SEM images of Au@LiNbO3 core/shell nanoparticles prepared at different laser fluences. These images reveal the formation of spherical nanoparticles and many, agglomerated and aggregated nanoparticles are observed.
Figure 11 shows the Raman spectra of Au@LiNbO3 core–shell nanoparticles recorded in the range of 95–1000 cm−1. For all samples except the one prepared at 1.6 J/cm2, Fig. 10 confirms the presence of seven E modes and one A Raman mode indexed as E(TO), E4, E(6TO), E(LO6), E(LO7), (E-TO8), 4A1TO, and E(LO9). This result emphasizes that all observed phonon modes in Raman spectra for synthesized samples are consistent with the vibration modes for LiNbO3 single crystal reported by other workers [44, 45]. As laser fluence increased, the peak intensity increases due to increasing the concentration of the core–shell nanoparticles. A significant enhancement in the Raman intensity may be produced after employing Au nanoparticles to LN structures due to the presence of plasmonic Au NPs, which play an essential role in the enhancement of the intensity of Raman peaks. This can originate from the local fields associated with the excitation of surface plasmon resonances by the Raman source [46, 47]. The vibration modes of the Au@LiNbO3 core/shell samples as a function of laser fluence are listed in Table 1.
Figure 12 presents the zeta potential plots of the Au@LiNbO3 core/shell synthesized at various laser fluences. The value of the zeta potential gives an indication of the potential stability of the colloidal Au@LiNbO3 core/shell. As shown in Table 2, the best value of ZP was 32 mV for the sample prepared at a laser fluence of 2.2 J/cm2, which indicates that colloidal suspensions are stable and no agglomeration and/or aggregation can be obtained. The origin of particle agglomeration is the van der Waals force and high surface energy. The nanoparticles synthesized at 2.2 J/cm2 have the highest value of ZP due to their high concentration. Ions are adsorbed on the surface of the particles, affecting the value of the isoelectric point and, as a result, increasing particle dispersion.
Figure 13 illustrates the dark forward and reverse current–voltage characteristics of p-Au@LiNbO3 NPs/p-Si heterojunction at room temperature. It is clearly seen that the samples exhibited rectifying properties, indicating the formation of diode-like devices. The highest rectification factor was found for heterojunction prepared at 2 J/cm2. As is obvious, the forward current increases as the bias voltage increases due to decreasing the depletion region, and the turn-on voltage was found to depend on the laser fluence. For heterojunctions prepared at 1.3 J/cm2 and 1.6 J/cm2, the turn-on voltage is less than 2 V, while it is greater than 2 V for samples prepared at 2 and 2.2 J/cm2. This can be justified as a result of decreasing the electrical resistivity of the Au@LiNbO3 core–shell as well as the dominance of diffusion current. The reverse current increases slightly for all samples with increasing bias voltage, particularly at voltages greater than 2 V. The forward current of the heterojunction prepared at 2 and 2.2 J/cm2 is larger than the other fabricated heterojunctions due to the decreasing of the electrical resistivity of the nanoparticles.
The ideality factor β of the heterojunction could be extracted from diode equation [50]
where K is the Boltzmann constant, q is the electron charge, and Is is the saturation current. The saturation current was determined from the Ln (If) versus Vf plot, where If is the forward current and Vf is the voltage at forward bias as shown in Fig. 14.
The ideality factors of the p-Au@LiNbO3/p-Si heterojunction devices fabricated at laser fluences of 1.3, 1.6, 2, and 2.2 J/cm2 were 6.5, 3.9, 2.0, and 5.3, respectively. The large value of the ideality factor of the heterojunction indicates deviation from the ideal diode, suggesting the presence of defects like trapping, series resistance, and surface states arising from agglomeration and aggregation of the nanoparticles [51, 52]. The value of the ideality factor indicates that the heterojunction prepared at 2 J/cm2 has the best junction characteristics. By using thermionic emission of current–voltage characteristics of the Au@LN/Si heterojunction, the barrier height Φ can be given by [53]:
where A is the area of the heterojunction and A* is the Richardson constant. The values of the barrier height of the Au@LN NPs/Si heterojunctions fabricated at laser fluences of 1.3, 1.6, 2, and 2.2 J/cm2 were 0.84, 0.83, 0.85, and 0.82 eV, respectively. The capacitance voltage (C-V) characteristics of the heterojunctions are depicted in Fig. 15.The linear relationship indicates that the fabricated hetero-junctions are abrupt in nature. The value of the built-in-potential Vbi can be calculated by extrapolating the linear part to C−2 = 0. The values of the built-in potential were 0.6, 0.5, 0.4, and 0.8 V for the heterojunctions prepared at 1.3, 1.6, 2, and 2.2/cm2, respectively. As shown in Fig. 15, the capacitance of all heterojunctions is inversely proportional to the reverse bias voltage. The reduction in the device capacitance with bias voltage resulted from the widening of the depletion layer [54]. This properly gives an indication of the behavior of the charge transition between the junction sides [55]. Increasing the capacitance of the junction with laser fluence can be attributed to increasing the hole concentration of the core–shell nanoparticles. The linearity characteristics of the photodetectors are shown in Fig. 16. The photocurrent is plotted versus light intensity. This figure gives information about the linear dynamic range (LDR). The LDR is given by the following equation:
where Pmax is the maximum amount of light that illuminated the photodetector and NEP is the noise equivalent power. The values of LDR estimated from Fig. 16 of the fabricated photodetector fabricated at 1.6, 2, and 2.2 J/cm2 were 59, 152, and 61 dB, respectively.
Figure 17 shows the effect of laser fluence on the spectral responsivity Rλ of the p-Au@LiNbO3/p-Si photodetectors measured at reverse bias of − 8 V. A clear peak of response was observed at 399 nm for all heterojunctions, which can be indexed to the absorption edge of the Au@LiNbO3 core–shell and a second peak appeared at 850 nm which attributed to the silicon substrate [56][57]. The maximum responsivity was 0.43 A/W at 400 nm for the photodetector fabricated with 2 J/cm2. Furthermore, a small peak appeared at 600 nm for the photodetector fabricated, which may be attributed to the SPR effect of the Au core. It is clearly noticed that It is clear that the photodetector’s responsivity is dependent on laser fluence, which can be attributed to the increasing shell thickness with laser fluence. Increasing the shell thickness improves the responsiveness in the visible region. On the other hand, an increase in the electrical resistivity of the nanoparticles leads to an extension of the depletion region toward the Au@LiNbO3 and, in turn, enhances the short wavelength responsivity. The specific detectivity of the photodetector was calculated from the following equation:
where A is the sensitive area of the photodetector, P is the light power, e is the electron charge, Id is the dark current of the photodetector, and Iph is the photocurrent of the photodetector. The specific detectivity D* of the photodetectors was determined as a function of laser fluence, as shown in Fig. 18. The maximum D* was 1.32 × 1012 and 1.08 × 1012 Jones at 380 nm and 850 nm, respectively, for Au @ Au@LN/p-Si photodetector fabricated at 2 J/cm2. The presence of Au nanoparticles is critical in reducing e–h recombination caused by the induction of a high electric field region and increasing photocurrent of the photodetector [58, 59]. The external quantum efficiency EQE of the photodetector can be defined is the ratio of incident number of photons to number of generated electrons. It can be determined from the following relationship:
The value of EQE at 400 nm for the photodetectors prepared at laser fluences of 1.3, 1.6, 2, and 2.2 J/cm2 was estimated from Eq. (5) and found to be 0.33, 0.48, 1.3, and 1.13, respectively, at a bias voltage of − 8 V. As shown, the value of EQE is larger than unity, which can be explained as follows: the width of the depletion region widens as the reverse bias voltage is applied, suggesting that the device is fully depleted at this voltage, resulting in efficient photogenerated carrier collection [60]. Furthermore, the applied bias voltage improves charge injections and forms free carriers, which absorb light photons and increase quantum efficiency dramatically through the photomultiplication effect. The figures of merit of the fabricated photodetector have been compared with other heterojunction-based silicon photodetectors as depicted in Table 3.
An energy band diagram has been constructed to understand the rectifying properties of the fabricated device, as shown in Fig. 19. The electron affinity of silicon is 4.05 eV, and its energy gap is 1.1 eV at room temperature, and the work function of Au is 5 eV. Crystalline Au@LN deposited using the LAL method is usually Li ion deficient. Its band gap at optimum laser fluence is found to be 3.8 eV. LN’s electron affinity is currently thought to be unaffected by impurity levels, with a value of 1.1 eV [65]. The band offsets of LN/Si heterojunction wee estimated and found to be ΔEC = χSi − χLN = 4.05 − 1.1 = 2.95 eV and Δ EV = χSi + EgSi − ( χLN + EgLN) = 4.05 + 1.12 − (1.1 + 3.8) = 0.27 eV. It is noteworthy that the electron jumps from Au to LN to substitute for the missing electron via the recombination process.
Conclusion
We have successfully synthesized an Au@LiNbO3 core/shell structure using pulsed laser ablation in ethanol without using catalyst. The variation of laser fluence affects the structural, optical, and electrical properties of Au@LiNbO3 core/shell. The presence of peaks related to LiNbO3 and Au in the XRD data revealed that the product has a polycrystalline structure and confirmed the formation of the core/shell structure. The TEM investigation confirmed the formation of core/shell nanoparticles with a spherical shape, with the gold core surrounded by a LiNbO3 shell. The laser fluence was found to increase shell thickness. The current–voltage properties of Au@LiNbO3/Si confirmed the formation of a good heterojunction with a rectification factor that depends on the laser fluence. The parameters of an isotype Au@LiNbO3/Si heterojunction photodetector as a function of laser fluence were investigated. The spectral responsivity results revealed that the fabricated photodetectors have two peaks of response, specifically located at 399 and 50 nm. The maximum responsivity was 0.43A/W at 399 nm for the photodetector fabricated with a laser fluence of 2 J/cm2. Increasing the responsivity in the visible region through the insertion of a buffer layer and selection the optimum preparation conditions is underway. The findings of this work confirm that the characteristics of core/shell synthesized in an ethanol medium are better than those obtained in distilled water due to the lower particle agglomeration.
Data Availability
No applicable.
References
Rüter CE, Brüske D, Suntsov S, Kip D (2020) Investigation of ytterbium incorporation in lithium niobate for active waveguide devices. Appl Sci 10:2189
Huang J, Zhang D, Qi Z, Zhang B, Wang H (2021) Hybrid Ag–LiNbO3 nanocomposite thin films with tailorable optical properties. Nanoscale Adv 3:1121–1126
Meriche F et al (2010) Fabrication and investigation of 1D and 2D structures in LiNbO3 thin films by pulsed laser ablation. Opt Mater (Amst) 32:1427–1434
Fakhri MA, AbdulRazzaq MJ, Alwahib AA, Muttlak WH (2020) Theoretical study of a pure LinbO3/Quartz waveguide coated gold nanorods using supercontinuum laser source. Opt Mater 109:110363
Jeong IK, Park S (2011) Correlated thermal motion in ferroelectric LiNbO3 studied using neutron total scattering and a rietveld analysis. J Korean Phys Soc 59:2756–2759
Taleb SM, Fakhri MA, Adnanm SA (2020) Optical Investigations of Nanophotonic LiNbO3 Films Deposited by Pulsed Laser Deposition Method, Defect and Diffusion. Forum 398:16–22
Meriche F et al (2007) Micro structuring of LiNbO3 by using nanosecond pulsed laser ablation. Appl Surf Sci 254:1327–1331
Taleb SM, Fakhri MA, Adnan SA (2019) Physical investigations of nanophotonic LiNbO3 films for photonic applications. J Ovonic Res 15(4):261–269
Lee SH, Noh TW (1998) Nonlinear optical properties of LiNbO3/Al2O3 films epitaxially grown by pulsed laser deposition. Integr Ferroelectr 20:25–37
Balestrino G et al (2001) Epitaxial LiNbO3 thin films grown by pulsed laser deposition for optical waveguides. Appl Phys Lett 78:1204–1206
Fakhri MA, Al-Douri Y, Bouhemadou A, Ameri M (2017) Structural and optical properties of nanophotonic LiNbO 3 under stirrer time effect. J Opt Commun 39(3):297–306
Qi Y, Li Y (2020) Integrated lithium niobate photonics. Nanophotonics 9:1287–1320
Desiatov B, Lončar M (2019) Silicon photodetector for integrated lithium niobate photonics. Appl Phys Lett 115:12
Al-Douri Y, Fakhri MA, Badi N, Voon CH (2018) Effect of stirring time on the structural parameters of nanophotonic LiNbO3 deposited by spin-coating technique. Optik 156:886–890
Wang X, Ye Z, He J, Cao L, Zhao B (2004) The role of SiO2 buffer layer in the growth of highly textured LiNbO3 thin film upon SiO2/Si by pulsed laser deposition. Mater Lett 58(200):3597–3600
Fakhri MA, Al-Douri Y, Hashim U, Salim ET, Prakash D, Verma KD (2015) Optical investigation of nanophotonic lithium niobate-based optical waveguide. Appl Phys B: Lasers Opt 121(1):107–116
Kakehi Y, Okamoto A, Sakurai Y, Nishikawa Y, Yotsuya T, Ogawa S (2001) Epitaxial growth of LiNbO3 thin films using pulsed laser deposition. Appl Surf Sci 169–170:560–563
Fakhri MA, Al-Douri Y, Salim ET, Hashim U, Yusof Y, Choo EB, Salim ZT, Jurn YN (2016) Structural properties and surface morphology analysis of nanophotonic LINBO3. ARPN J Eng Appl Sci 11(8):4974–4978
Taylor P, Klose F, Wöhlecke M, Kapphan S (1989) (Uv-excited luminescence of LiNbO3 and LiNbO3 : Mg. Ferroelectrics 92:181–187
Tumuluri A, Raju KCJ (2014) Luminescence of LiNbO3 polycrystalline ceramics: effect of Sc2O3 and Lu2O3 doping. Ceram Int 40:3371–3377
Shih WC, Wang TL, Sun XY, Wu MS (2008) Growth of c-axis-oriented LiNbO3 films on ZnO/SiO2/Si substrate by pulsed laser deposition for surface acoustic wave applications. Jpn J Appl Phys 47:4056–4405
Fakhri MA, Hashim U, Salim ET, Salim ZT (2016) Preparation and charactrization of photonic LiNbO3generated from mixing of new raw materials using spry pyrolysis method. J Mater Sci: Mater Electron 27(12):13105–13112
Li W et al (2019) Fabrication and characteristics of heavily Fe-doped LiNbO3/Si heterojunction. Materials (Basel) 12:1–7
Zhang J, Li Li, Xiao Z, Liu Di, Wang S, Zhang J, Hao Y, Zhang W (2016) Hollow sphere TiO2–ZrO2 prepared by self-assembly with polystyrene colloidal template for both photocatalytic degradation and H2 evolution from water splitting. ACS Sustain Chem Eng 4:2037–2046
Zhang M, Ngo TH, Rabiah NI, Otanicar TP, Phelan PE, Swaminathan R, Dai LL (2014) Core–shell and asymmetric polystyrene–gold composite particles via one-step pickering emulsion polymerization. Langmuir 30(201):75–82
Wang DP, Zeng HC (2009) Multifunctional roles of TiO2 nanoparticles for architecture of complex core−shells and hollow spheres of SiO2−TiO2−polyaniline system. Chem Mater 21:4811–4823
Mei Y, Lu Y, Polzer F, Ballauff M, Drechsler M (2007) Catalytic activity of palladium nanoparticles encapsulated in spherical polyelectrolyte brushes and core−shell microgels. Chem Mater 19:1062–1069
Salim ZT, Hashim U, Arshad MM, Fakhri MA, Salim ET (2017) Zinc oxide flakes-corolla lobes like nano combined structure for SAW applications. Mater Res Bull 86:215–219
Richter J et al (2014) Core-shell potassium niobate nanowires for enhanced nonlinear optical effects. Nanoscale 6:5200–5207
Nichols WT, Sasaki T, Koshizaki N (2006) Laser ablation of a platinum target in water. I. Ablation Mechanisms. J Appl Phys 100:114911
Fakhri MA, Salim ET, Hashim U, Abdulwahhab AW, Salim ZT (2017) Annealing temperature effect on structural and morphological properties of nano photonic LiNbO3. J Mater Sci: Mater Electron 28(22):16728–16735
Ismail RA, Mousa AM, Amin MH (2018) Synthesis of hybrid Au@PbI2 core-shell nanoparticles by pulsed laser ablation in ethanol. Mater Res Express 29:1–16
Khalil I, Chou CM, Tsai KL, Hsu S, Yehye WA, Hsiao VKS (2019) Gold nanofilm-coated porous silicon as surface-enhanced Raman Scattering substrate. Appl Sci 9:1–12
Fakhri MA, Wahid MH, Badr BA, Kadhim SM, Salim ET, Hashim U, Salim ZT (2007) Enhancement of Lithium Niobate nanophotonic structures via spin-coating technique for optical waveguides application. Eur Phys J 162(7):01004
Son JW, Orlov SS, Phillips B, Hesselink L (2006) Pulsed laser deposition of single phase LiNbO3 thin film waveguides. J Electroceramics 17:591–595
Fakhri MA, Wahid MH, Kadhim SM, Badr BA, Salim ET, Hashim U, Salim ZT (2017) The structure and optical properties of lithium niobate grown on quartz for photonics application. Eur Phys J Conf 162:01005
Sun D, Zhang G, Huang J, Wang H, Li Q (2014) Plant-mediated fabrication and surface enhanced raman property of flower-like Au@Pd nanoparticles. Materials (Basel) 7:1360–1369
Adnan SA, Tawfiq ZH, Fakhri MA (2020) Gold Nanoparticles in Liquid Based on Photonic Crystal Fiber PCF for Sensors Application Defect Diffus Forum 398:23–28
Chen Y, Yeh C (2002) Laser ablation method : Use of surfactants to form the dispersed Ag nanoparticles. Colloids Surf A: Physicochem Eng Asp 197:133–139
Ali HS, Fakhri MA (2020) An overview of Au & photonic crystal fiber of sensors. Mater Sci Forum 1002:282–289
Ismail RA, Habubi NF, Abbod MM (2016) Preparation of high-sensitivity In2S3/Si heterojunction photodetector by chemical spray pyrolysis. Opt Quant Electron 48:455. https://doi.org/10.1007/s11082-016-0725-5
Kobayashi Y et al (2017) “Light scattering from polar and pseudoscalar modes in doped LiNbO3 and LiTaO3 monocrystals humidity dependence of surface resistances of LiNbO3 and LiTaO3 single crystals. J Phys Conf Ser 918:012017
Jawad M, Ismail R, Yahea K (2011) Preparation of nanocrystalline Cu2O thin film by pulsed laser deposition. Mater Sci: Mater Electron 22:1244–1247
Mohanty D, Chaubey GS, Yourdkhani A, Adireddy S, Caruntu G, Wiley JB (2012) Synthesis and piezoelectric response of cubic and spherical LiNbO 3 nanocrystals. RSC Adv 2:1913–1916
Fakhri MA, Salim ET, Wahid MH, Hashim U, Salim ZT (2018) Optical investigations and optical constant of nano lithium niobate deposited by spray pyrolysis technique with injection of Li2CO3 and Nb2O5 as raw materials. J Mater Sci: Mater Electron 29(11):9200–9208
Santulli AC, Zhou H, Berweger S, Raschke MB, Sutter E, Wong SS (2010) Synthesis of single-crystalline one-dimensional LiNbO3 nanowires. CrystEngComm 12:2675–2678
Fakhri MA, Salim ET, Abdulwahhab AW, Hashim U, Salim ZT (2018) Optical properties of micro and nano LiNbO3thin film prepared by spin coating. Opt Laser Technol 103:226–232
Shandilya S, Sharma A, Tomar M, Gupta V (2012) Optical properties of the c-axis oriented LiNbO3 thin film. Thin Solid Films 520:2142–2146
Fakhri MA, Salim ET, Wahid MHA, Abdulwahhab AW, Salim ZT, Hashim U (2019) Heat treatment assisted-spin coating for LiNbO3 films preparation: Their physical properties. J Phys Chem Solids 131:180–188
Zhu D et al (2009) The origin of the high diode-ideality factors in GaInN/GaN multiple quantum well light-emitting diodes. Appl Phys Lett 94:2–4
Mazari H, Benamara Z, Ameur K, Benseddik N, Bonnaud O, Olier R (2009) Influence of Ru 3 + ions at Al / GaAs interface on Schottky diodes. Int J Nanoelectron Mater 2:147–156
Ismail RA, Fadhil FA (2014) Effect of electric field on the properties of bismuth oxide nanoparticles prepared by laser ablation in water. J Mater Sci: Mater Electron 25:1435–1440. https://doi.org/10.1007/s10854-014-1747-z
Bengal W (2012) Schottky barrier of Al on chemically grown n-CdSe nanoparticles. Satyajit Saha a, Amit manna 22:77–83
Ismail RA (2009) Characteristics of P-Cu2O/N-Si heterojunction photodiode made by rapid thermal oxidation. J SemicondTechnology Sci 9:51–54
Macleod BA et al (2012) Built-in potential in conjugated polymer diodes with changing anode work function: interfacial states and deviation from the Schottky − Mott limit. J Phys Chem Lett 3:1202–1207
Ismail RA et al (2018) Preparation of low cost n-ZnO/MgO/p-Si heterojunction photodetector by laser ablation in liquid and spray pyrolysis. Materi Res Express 5.5:055018
Ismail RA, Hamoudi WK, Abbas HF (2018) Synthesis of Au nanoparticles–decorated CdS nanowires via laser ablation in liquid for optoelectronic applications. Appl Phys A 124:683
Mousa A, Ismail RA, Amin M (2019) Hybrid p-Au@PbI2/n-Si heterojunction photodetector prepared by pulsed laser ablation in liquid. Optik 183:933–941
Ismail RA, Abdul-Hamed RS (2017) Laser ablation of Au–CuO core–shell nanocomposite in water for optoelectronic devices. Mater Res Express 4:125020
Faisal AD, Ismail RA, Khalef WK et al (2020) Synthesis of ZnO nanorods on a silicon substrate via hydrothermal route for optoelectronic applications. Opt Quant Electron 52:212. https://doi.org/10.1007/s11082-020-02329-1
Yao J, Shao J, Wang Y, Zhao Z, Yang G (2015) Ultra-broadband and high response of the Bi2Te3–Si heterojunction and its application as a photodetector at room temperature in harsh working environments. Nanoscale 7:12535–12541
Ismai R, Hassan K, Abdulrazaq O, Abode H (2007) Optoelectronic properties of CdTe/Si heterojunction prepared by pulsed Nd:YAG-laser deposition technique. Mater Sci Semicond Process 10:19–23
Gunasekaran S, Thangaraju D, Marnadu R et al (2021) Photosensitive activity of fabricated core-shell composite nanostructured p-CuO@CuS/n-Si diode for photodetection applications. Sens Actuator A Phys 317:112373
Mousa AM, Ismail RA, Amin MH (2019) Hybrid p-Au@PbI2/n-Si heterojunction photodetector prepared by pulsed laser ablation in liquid. Optik - Int J for Light Electron Opt 183:933–941
Li W, Cui J, Zheng D, Wang W, Wang S, Song S, Liu H, Kong Y, Xu J (2019) Fabrication and characteristics of heavily Fe-doped LiNbO3/Si heterojunction. Materials 12:2659
Acknowledgements
The authors would like to thank the University of Technology-Iraq for the logistic support this work.
Author information
Authors and Affiliations
Contributions
Conceptualization: Raid A. Ismail and Evan T. Salim; methodology: Evan T. Salim and Marwa S. Alwazny; validation: Raid A. Ismail, Evan T. Salim, and Marwa S. Alwazny; formal analysis: Raid A. Ismail, Evan T. Salim, and Marwa S. Alwazny; investigation: Raid A. Ismail, Evan T. Salim, and Marwa S. Alwazny; resources: Raid A. Ismail, Evan T. Salim, and Marwa S. Alwazny; data curation: Raid A. Ismail, Evan T. Salim, and Marwa S. Alwazny; writing—original draft preparation: Marwa S. Alwazny; writing—review and editing: Raid A. Ismail and Evan T. Salim; visualization: Raid A. Ismail and Evan T. Salim; supervision: Raid A. Ismail and Evan T. Salim; project administration: Raid A. Ismail and Evan T. Salim; funding acquisition: Raid A. Ismail, Evan T. Salim, and Marwa S. Alwazny. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ismail, R.A., Salim, E.T. & Alwazny, M.S. Nanosecond Laser Ablation of Au@LiNbO3 Core–Shell Nanoparticles in Ethanol: Properties and Application in Optoelectronic Devices. Plasmonics 18, 561–576 (2023). https://doi.org/10.1007/s11468-022-01780-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-022-01780-y