Abstract
Background
Propionibacterium acnes (P. acnes) has become increasingly recognized as a cause of periprosthetic joint infection (PJI).
Questions/Purposes
It is not currently known if the clinical presentation of P. acnes varies depending on the joint being infected.
Methods
We retrospectively reviewed patients infected with P. acnes after total hip, knee, and shoulder arthroplasty from two institutions. Patients were classified as having a PJI based on the Musculoskeletal Infection Society criteria and were excluded if they had a polymicrobial culture. Patient demographics, preoperative laboratory values, and microbiology data were analyzed.
Results
Eighteen knees, 12 hips, and 35 shoulders with a P. acnes PJI were identified. Median ESR was significantly higher in the knee (38.0 mm/h, IQR 18.0–58.0) and hip (33.5 mm/h, IQR 15.3–60.0) groups compared to the shoulder group (11.0 mm/h, IQR 4.5–30.5). C-reactive protein levels were higher in the knee (2.0 mg/dl, IQR 1.3–8.9) and hip (2.4 mg/dl, IQR 0.8–4.9) groups compared to the shoulder group (0.7 mg/dl, IQR 0.6–1.5). Median synovial fluid WBC was significantly higher in the knee group than shoulder group (19,950 cells/mm3, IQR 482–60,063 vs 750 cells/mm3, IQR 0–2825, respectively). Peripheral blood WBC levels were similar between groups, as was mean time of P. acnes growth in culture. Clindamycin resistance was present in all groups.
Conclusion
The manner in which a patient with P. acnes PJI presents is joint specific. Inflammatory markers were significantly higher in the knee and hip groups compared to the hip and shoulder groups, and long hold anaerobic cultures up to 14 days are necessary to accurately identify this organism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total joint arthroplasty has emerged as a successful treatment option for end-stage arthritis of many different joints. Periprosthetic joint infection (PJI) remains one of the most feared complications after an arthroplasty procedure and continues to be a major burden on the healthcare system [17]. Organism identification with focused antibiotic therapy combined with surgical intervention remains the mainstay of PJI treatment. Staphylococcus aureus is one of the most common infecting pathogens in lower extremity PJI, but other organisms such as Propionibacterium acnes (P. acnes) have garnered increased attention over the past decade.
P. acnes is a slow-growing aerotolerant Gram-positive bacillus that grows best under anaerobic conditions [27]. This organism has traditionally been hard to identify due to the requirement of long hold cultures up to 21 days for accurate identification. Recent clinical series have recognized P. acnes as the causative agent in both hip and knee PJI and is one of the leading causes of shoulder PJI [13, 26, 28–30]. P. acnes has a pathogenic secretory profile which includes lipases, proteases, and hydrolases which contribute to the virulent properties of this organism [1]. In addition, the upregulation of toll-like receptors (TLR) 2 and 4 on the macrophage in response to P. acnes is known to induce the production of pro-inflammatory cytokines such as interleukin-1 beta and tumor necrosis factor-alpha [16]. Despite this, P. acnes often presents in an indolent fashion with a limited host inflammatory response when it is the causative agent of total shoulder arthroplasty (TSA) PJI [10, 24].
The unique subacute host inflammatory response elicited by this organism during TSA PJI makes it difficult to determine preoperatively if the patient is truly infected based on routine blood work. Therefore, it is important to understand the clinical presentation of this organism during a PJI in different joints in order to give clinicians a better understanding of the signs and symptoms that are to be expected when P. acnes is the causative organism. It is not well understood if the lack of a host inflammatory response to P. acnes during a TSA PJI is primarily due to the virulence properties of the bacteria or if the joint being infected plays an important role.
The purpose of this study was to compare the clinical presentations of patients with a P. acnes total hip, knee, or shoulder PJI. We hypothesized that the clinical presentation of a P. acnes PJI will be influenced by the joint being infected and that the host inflammatory response of patients infected with P. acnes will vary depending on the infected joint. We also aimed to document the time required for cultures to be incubated as well as the antibiotic resistance profile for the P. acnes isolates found in this patient population.
Patients and Methods
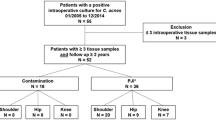
This was a multicenter retrospective review with the approval for database analysis through an expedited process from each center’s Institutional Review Board. An institutional PJI database identified patients with positive P. acnes cultures between April of 2009 and April of 2014 at one institution, and a review of all patients with a positive P. acnes culture saved in a microbiology database during the same time period was used at the second institution. All patients identified underwent a revision procedure for their PJI (Table 1). Patient demographics, comorbidities, surgical history, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), white blood cell (WBC) count, synovial fluid WBC count, synovial fluid polymononuclear (PMN) cell differential, microbiology data, and antibiotic treatment were collected (Table 1). Revision procedures were recorded. Failure of the initial treatment for infection was defined as the patient needing a subsequent surgical procedure for infection after the initial treatment. Patients were included for evaluation if they met the requirements for PJI based on the Musculoskeletal Infection Society (MSIS) criteria [23]. Patients with polymicrobial infection or a single positive P. acnes culture without additional clinical or laboratory findings consistent with PJI based on the MSIS criteria were excluded.
There were 640 patients for evaluation between the two institutions. A total of 111 patients with a positive P. acnes culture from a TKA, THA, or TSA were identified. Forty TKA patients, one hip, and eight TSA patients had a coinfecting organism and were excluded from the study. This left 18 TKA, 12 THA, and 32 TSA patients with an isolated P. acnes PJI for review.
Mean age was 62.6 ± 11.9 years (p = 0.50), and the mean was BMI 30.5 ± 7.0 kg/m2 (p = 0.53). Follow-up was 38.9 ± 16.4 months in the TKA group, 36.0 ± 17.4 months in the THA group, and 44.6 ± 19.1 months in the TSA group (p = 0.15). Seventy-four percent of patients were males (46/62) with 14 males in the TKA group, 7 in the THA group, and 25 in the TSA group. Mean Charlson-Age Comorbidity Index was similar between all groups at 2.6 ± 1.7 (p = 0.91).
Antibiotics were held prior to culture collection at the time of revision surgery. Tissue and fluid specimens were taken directly from the surgical wound using sharp dissection and placed into sterile containers without further manipulation by the surgical team. Tissue samples were sent for intraoperative histopathology routinely in the hip and knee arthroplasty groups only, with acute inflammation defined as greater than five PMN in each of five ×400 high-powered fields.
All specimens were inoculated to standard aerobic and anaerobic media; thioglycolate broth cultures were incubated anaerobically for 14 days. All P. acnes isolates were identified in either broth or agar culture. Antibiotic sensitivities were obtained on P. acnes strains for penicillin, rifampin, clindamycin, piperacillin/tazobactam, vancomycin, and ertapenem [7]. All inflammatory markers represent the labs taken within 3 weeks of the initial revision surgery. Synovial fluid cell counts and PMN cell differential were taken from either preoperative or intraoperative joint aspirations.
Descriptive statistics were used to analyze the patient demographics and antibiotic susceptibilities. Categorical variables were analyzed with a chi-square test. A non-parametric Kruskal-Wallis test was used to evaluate the differences between the three groups. Non-parametric Mann-Whitney U tests were used to analyze laboratory values that were deemed to be significant based on the Kruskal-Wallis analysis. Inflammatory laboratory values and synovial fluid cell counts were reported as the median with an interquartile range (IQR). A p value less than 0.05 was considered statistically significant.
Results
Median time between the index procedure and diagnosis of infection was 18.6 months, IQR 9.2–32.0 in the TKA group; 12.7 months, IQR 4.4–39.2 in the THA group; and 7.0 months, IQR 4.0–21.0 in the TSA group (p = 0.58) (Table 1). In the TKA group, 11 patients underwent a two-stage procedure, six were treated with an irrigation and debridement, and one had a one-stage exchange. Four of the two-stage treated patients failed treatment, and three failed in the irrigation group resulting in a 62% treatment success rate. In the THA group, six underwent a two-stage exchange, five had an irrigation and debridement, and one had a one-stage exchange. Two patients failed the two-stage exchange and one failed an irrigation and debridement resulting in a 75% success rate. In the TSA group, 21 underwent a two-stage exchange, eight an irrigation and debridement, and three a one-stage exchange. One patient failed the two-stage exchange, five failed the irrigation and debridement, and one failed the one-stage exchange resulting in a 78% success rate. At the final follow-up, all patients were infection free.
The TKA and THA groups exhibited a greater elevation of inflammatory markers than the TSR group. Median ESR (normal 0–12 mm/h) was 38.0 mm/h, IQR 18.0–58.0 in the TKA group which was significantly higher than that in the TSA group (11.0 mm/h, IQR 4.5–30.5, p = 0.007) (Fig. 1), as was the median CRP (normal 0–1.0 mg/dl) (2.0 mg/dl, IQR 1.3–8.9 vs 0.7 mg/dl, IQR 0.6–1.5, respectively; p = 0.0003) (Fig. 2). THA ESR (33.5 mm/h, IQR 15.3–60.0) levels were significantly higher than the TSA group (11.0 mm/h, IQR 4.5–30.5, p = 0.01), as were the median CRP levels (Fig. 1) while CRP (THA, 2.4 mg/dl, IQR 0.8–4.9 vs TSA, 0.7 mg/dl, IQR 0.6–1.5; p = 0.02) levels were similar (Fig. 2). WBC levels (normal 4.8–10.8 K/cumm) were similar between the TKA (8.5 K/cumm, IQR 6.0–9.9), THA (6.5 K/cumm, IQR 6.0–7.8), and TSA (7.6 K/cumm, IQR 5.6–8.9) groups (p = 0.17). The median synovial fluid WBC count was significantly higher in the TKA group (19,950 cells/mm3, IQR 482–60,063) compared to that in the TSA group (750 cells/mm3, IQR 0–2825) (p = 0.02) (Table 1). THA median synovial cell counts did not reveal a statically significant difference between groups in the Kruskal-Wallis analysis (500 cells/mm3, IQR 250–77,938).
ESR levels of arthroplasty patients infected with P. acnes. Median ESR was significantly higher in the knee (38.0 mm/h, IQR 18.0–58.0, *p = 0.007) and hip (33.5 mm/h, IQR 15.3–60.0, # p = 0.01) group compared to the shoulder group (11.0 mm/h, IQR 4.5–30.5). TKA total knee arthroplasty, THA total hip arthroplasty, TSA total shoulder arthroplasty, ESR erythrocyte sedimentation rate.
CRP levels of arthroplasty patients infected with P. acnes. Median C-reactive protein levels were higher in the knee (2.0 mg/dl, IQR 1.3–8.9, *p = 0.0003) and hip (2.4 mg/dl, IQR 0.8–4.9, # p = 0.02) groups compared to the shoulder group (0.7 mg/dl, IQR 0.6–1.5). TKA total knee arthroplasty, THA total hip arthroplasty, TSA total shoulder arthroplasty, CRP C-reactive protein.
The mean time for growth of P. acnes in culture was similar between all groups (8.2 ± 3.5 days, p = 0.39) (Table 1). The number of culture samples taken at the time of surgery was similar between groups (5.0 ± 2.0 cultures, p = 0.29), as was the mean number of positive cultures obtained (2.9 ± 1.8 positive cultures, p = 0.90). This resulted in a culture positive rate for P. acnes of 51% in the TKA group, 47% in the THA group, and 67% in the TSA group. Acute intraoperative inflammation was seen in 14/17 tissue samples (82%) in the TKA group, 4/9 (44%) in the THA group. Intraoperative histopathology was not routinely available for the TSA patients.
Antibiotic resistance to clindamycin was observed in all groups. Seventeen percent of strains (3/18) were resistant to clindamycin in the TKA group, 17% in the THA group (2/12), and 22% in the TSA group (7/32). One strain in the TSA group had an increased mean inhibitory concentration of 3 mg/l to vancomycin and would be classified as resistant based on the European Committee on Antimicrobial Susceptibility Testing. None of the other antibiotics tested showed resistant patterns.
Discussion
P. acnes is increasingly recognized as a cause of both upper and lower extremity PJI. This is one of the first studies to compare the presentation of P. acnes PJI in different joints. Our data suggest that P. acnes shoulder PJI presents with a less prominent host peripheral inflammatory response than total knee and hip PJI. These findings have not been previously described and suggest the joint being infected may significantly influence the host inflammatory response of patients infected with P. acnes.
Although this report is one of the largest series of hip, knee, and shoulder P. acnes PJI to date, our conclusions are limited by a number of factors. The retrospective nature of this study is an inherent limitation and may be associated with a selection bias. We only included patients undergoing revision surgery for PJI and did not attempt to evaluate patients with positive P. acnes cultures that did not undergo a revision procedure. Polymicrobial P. acnes infections were also excluded, and we are unable to comment on the pathogenic role P. acnes may play in that specific scenario. We did not address surgical and medical therapies in this study since the purpose of this study was to analyze joint-specific differences in the clinical presentation of P. acnes. It is also possible these patients were seen and evaluated at varying time-points in the course of a developing PJI, which may lead to elevated inflammatory markers in some patients but not others. Finally, we did not evaluate patient outcomes because each joint has a different outcome measure tool, and it would be difficult to compare the results between the groups. Despite these limitations, we believe this report furthers our understanding of the patterns of the clinical presentation of this organism after total joint arthroplasty in different joints.
P. acnes has multiple pathogenic features that would suggest a prominent host inflammatory response can be elicited. Three distinct phylotypes of P. acnes with varying inflammatory and pathogenic properties have been identified (I–III) [18, 20]. Further subtyping has broken down type I into IA, IB, and most recently IC which possess antibiotic resistance [19]. The complete genome of Type IB P. acnes has been sequenced and has shown P. acnes possess an array of virulent features involved with the host inflammatory response including cellular adhesion molecules and extracellular degradation products [4, 5]. The type IB and II phylotypes have been most commonly associated with orthopedic implant colonization and infection, but a clear correlation between the P. acnes genomic phylotype and PJI has yet to be identified [26]. The beta-hemolytic ability of P. acnes has been suggested to be a marker of P. acnes pathogenicity, which may be connected to the tly gene and Christie-Atkins-Munch-Peterson (CAMP) factors [2, 21, 22]. In addition, some pathogenic strains of P. acnes contain a tight adhesion (Tad) locus, and a Sag gene cluster, which contributes to the hemolytic features of P. acnes, and may confer increased virulence in some strains [9]. In addition, it is well understood P. acnes—like most other bacteria causing PJI—can phenotypically transform between a free-floating planktonic phenotype and an adherent phenotype which integrates into a mature biofilm [11, 25]. The biofilm forming capabilities of P. acnes may allow the bacterium to be quiescent on the implant resulting in a limited host inflammatory response and is an important pathogenic feature of the bacteria [25].
The current report helps the treating clinician to characterize the preoperative findings of patients infected with P. acnes. It is commonly reported an infection with P. acnes in the shoulder elicits a subacute clinical response with normal to near normal peripheral inflammatory laboratory values and often-equivocal synovial fluid white blood cell counts. This report further defines the clinical presentation of knee, hip, and shoulder arthroplasty PJI with P. acnes and suggests the peripheral inflammatory response in knee and hip PJI may be more robust than TSA patients infected with P. acnes. In addition, the synovial fluid white blood cell count in the TKA patients was significantly higher than both the THA and TSA groups and may be a useful diagnostic marker for this organism during TKA PJI. It remains unclear why patients infected with P. acnes PJI present differently depending on the joint infected. One may assume that the host inflammatory presentation of P. acnes would be similar in all three joints and that the pathogenic features of the bacteria would drive the inflammatory response; however, we did not observe this. It appears the joint being infected may play a role in the systemic inflammatory response seen when P. acnes is the infecting organism and requires further investigation.
The majority of patients in our study were men (74%), which is consistent with previously described literature [8, 24]. P. acnes density is highest around the shoulder, but is also not an infrequently identified organism around the knee and hip [12]. P. acnes is known to live in the pilosebaceous glands, and may inoculate the wound at the time of surgery, or cause delayed PJI through a hematogenous route as suggested in one preclinical model [1, 3]. It has been previously shown P. acnes is able to recolonize the surgical skin edges within 30 to 80 min, which may represent a mechanism of infection that has been previously under recognized [14]. Males with an increased density of hair follicles around their surgical wound may be at increased risk of a P. acnes infection, but this has not been evaluated as a major risk factor for P. acnes PJI in both upper and lower extremity surgery [24].
We noted P. acnes antibiotic resistance to clindamycin in all three arthroplasty populations, which is consistent with previous reports [7, 15]. This further demonstrates the necessity of organism identification with an associated antibiotic susceptibility profile. Although the majority of P. acnes strains analyzed have been sensitive to penicillin, it is imperative one does not assume this organism is pan-sensitive. In addition to clindamycin resistance, we found one strain with an increased mean inhibitory concentration to vancomycin. An antibiotic treatment plan tailored to the bacterium’s sensitivity profile is necessary. Due to these findings, we currently advise caution with the use of clindamycin in penicillin-allergic patients when P. acnes is suspected and antibiotic sensitivities are not yet available.
The mean time to growth of P. acnes was similar in all three arthroplasty groups. The time to growth and the probability of P. acnes being a true pathogen have been investigated. The mean time to growth in our study was 8.2 days which is consistent with the previous literature [6, 24]. The increased recognition of P. acnes in the upper extremity has resulted in many institutions to hold their upper extremity cultures for 14–21 days. This may not necessarily be the case for lower extremity arthroplasty cultures unless the lab is specifically directed to hold them for an extended time. It is the senior author’s institutional standard to hold all anaerobic cultures for 14 days due to the increased recognition of this organism in both upper and lower extremity arthroplasty.
P. acnes is a potentially under recognized cause of total joint arthroplasty infection. Extended culture incubation for at least 14 days improves the ability to detect this organism, and should be utilized if P. acnes is suspected as the causative organism in both upper and lower extremity arthroplasty surgery. The clinical presentation of P. acnes PJI is joint specific with shoulder arthroplasty patients presenting in a more subacute fashion, and patients with a THA or TKA PJI presenting more overtly with more elevated inflammatory markers. Clindamycin antibiotic resistance was present in all three arthroplasty groups, and further highlights the importance of organism identification with susceptibility testing.
References
Achermann Y, Goldstein EJ, Coenye T, et al. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev. 2014; 27(3): 419.
Achermann Y, Tran B, Kang M, et al. Immunoproteomic identification of in vivo-produced propionibacterium acnes proteins in a rabbit biofilm infection model. Clin Vaccine Immunol. 2015; 22(5): 467.
Blomgren G, Lundquist H, Nord CE, et al. Late anaerobic haematogenous infection of experimental total joint replacement. A study in the rabbit using Propionibacterium acnes. J Bone Joint Surg Br. 1981; 63B(4): 614.
Bruggemann H. Insights in the pathogenic potential of Propionibacterium acnes from its complete genome. Semin Cutan Med Surg. 2005; 24(2): 67.
Bruggemann H, Henne A, Hoster F, et al. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science. 2004; 305(5684): 671.
Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011; 49(7): 2490.
Crane JK, Hohman DW, Nodzo SR, et al. Antimicrobial susceptibility of Propionibacterium acnes isolates from shoulder surgery. Antimicrob Agents Chemother. 2013; 57(7): 3424.
Dodson CC, Craig EV, Cordasco FA, et al. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elbow Surg. 2010; 19(2): 303.
Fitz-Gibbon S, Tomida S, Chiu BH, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013; 133(9): 2152.
Frangiamore SJ, Saleh A, Kovac MF, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015; 97(1): 63.
Furustrand Tafin U, Corvec S, Betrisey B, et al. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2012; 56(4): 1885.
Grice EA, Kong HH, Conlan S, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009; 324(5931): 1190.
Holmberg A, Lood R, Morgelin M, et al. Biofilm formation by Propionibacterium acnes is a characteristic of invasive isolates. Clin Microbiol Infect. 2009; 15(8): 787.
Johnston DH, Fairclough JA, Brown EM, et al. Rate of bacterial recolonization of the skin after preparation: four methods compared. Br J Surg. 1987; 74(1): 64.
Khassebaf J, Hellmark B, Davidsson S, et al. Antibiotic susceptibility of Propionibacterium acnes isolated from orthopaedic implant-associated infections. Anaerobe. 2015; 32: 57.
Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002; 169(3): 1535.
Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007; 89(Suppl 3): 144.
McDowell A, Gao A, Barnard E, et al. A novel multilocus sequence typing scheme for the opportunistic pathogen Propionibacterium acnes and characterization of type I cell surface-associated antigens. Microbiology. 2011; 157(Pt 7): 1990.
McDowell A, Hunyadkurti J, Horvath B, et al. Draft genome sequence of an antibiotic-resistant Propionibacterium acnes strain, PRP-38, from the novel type IC cluster. J Bacteriol. 2012; 194(12): 3260.
McDowell A, Perry AL, Lambert PA, et al. A new phylogenetic group of Propionibacterium acnes. J Med Microbiol. 2008; 57(Pt 2): 218.
Nakatsuji T, Tang DC, Zhang L, et al. Propionibacterium acnes CAMP factor and host acid sphingomyelinase contribute to bacterial virulence: potential targets for inflammatory acne treatment. PLoS One. 2011; 6(4): e14797.
Nodzo SR, Hohman DW, Crane JK, et al. Hemolysis as a clinical marker for propionibacterium acnes orthopedic infection. Am J Orthop (Belle Mead NJ). 2014; 43(5): E93.
Parvizi J. New definition for periprosthetic joint infection. Am J Orthop (Belle Mead NJ). 2011; 40(12): 614.
Pottinger P, Butler-Wu S, Neradilek MB, et al. Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Joint Surg Am. 2012; 94(22): 2075.
Ramage G, Tunney MM, Patrick S, et al. Formation of Propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials. 2003; 24(19): 3221.
Sampedro MF, Piper KE, McDowell A, et al. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis. 2009; 64(2): 138.
Singh JA, Sperling JW, Schleck C, et al. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012; 21(11): 1534.
Tunney MM, Patrick S, Gorman SP, et al. Improved detection of infection in hip replacements. A currently underestimated problem. J Bone Joint Surg Br. 1998; 80(4): 568.
Tunney MM, Ramage G, Patrick S, et al. Antimicrobial susceptibility of bacteria isolated from orthopedic implants following revision hip surgery. Antimicrob Agents Chemother. 1998; 42(11): 3002.
Zeller V, Ghorbani A, Strady C, et al. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007; 55(2): 119.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Scott R. Nodzo, MD, K. Keely Boyle, MD, Samrath Bhimani, BS, and Andy O. Miller, MD have declared that they have no conflict of interest. Geoffrey H. Westrich, MD, reports personal fees from Stryker, DJ Orthopaedics, and Mallinckrodt Pharmaceuticals, other from Exactech, outside the work. Thomas R. Duquin, MD, reports personal fees from Zimmer Biomet, outside the work.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed Consent
Informed consent was waived from all patients for being included in the study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Additional information
Level of Evidence: Prognostic Study Level III
Rights and permissions
About this article
Cite this article
Nodzo, S.R., Boyle, K.K., Bhimani, S. et al. Propionibacterium acnes Host Inflammatory Response During Periprosthetic Infection Is Joint Specific. HSS Jrnl 13, 159–164 (2017). https://doi.org/10.1007/s11420-016-9528-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11420-016-9528-2