Abstract
Uncaria rhynchophylla (Miq.) Miq. (Rubiaceae) is widely used as a botanical raw material for traditional Japanese and Chinese medicines. However, not all of its potentially bioactive constituents have been isolated and characterized. Herein, one new indole alkaloid triglucoside (1), nine known alkaloids (2–10) and thirteen known non-alkaloids (11–23) were isolated from the aqueous extract of Uncaria rhynchophylla hook and structurally characterized 1H and 13C NMR and high-resolution electrospray ionization mass spectrometry. The absolute configurations of isolated compounds (1, 2 and 3) were determined by the X-ray diffraction analysis of their single crystals obtained using a micro-drop crystallization technique. This technique allows single crystals to be obtained from samples as small as 50 µg, thus providing detailed structural information even on minor constituents and enabling the accurate quality monitoring of botanical raw materials more accurately.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uncaria rhynchophylla (Miq.) Miq. (Rubiaceae), an evergreen liana growing in warm climates and distributed in the southern part of the Boso peninsula in Japan and in central and southern China, is widely used as a botanical raw material for traditional Japanese and Chinese medicines. For example, a Kampo formula consisting of U. rhynchophylla hooks (Yokukansan) is used to treat neurosis, anxiety, night-time crying, and the behavioral and psychological symptoms of dementia [1]. Although the compounds contained in these hooks (alkaloids, triterpenes, phenolic acids, and flavonoids) have been extensively profiled for many decades, novel constituents continue to be discovered [2,3,4,5,6]. Given that the constant quality of traditional Japanese medicines can only be achieved when the quality of the corresponding botanical raw materials is secured, the identification of previously unreported U. rhynchophylla constituents is a matter of high practical significance. Herein, we profiled the aqueous extract of U. rhynchophylla hooks and and isolated one new alkaloid (1), nine known alkaloids (2–10), and thirteen known nonalkaloids (11–23) (Figs. 1 and 2). The structures of known compounds were determined by spectroscopic analysis (high-resolution electrospray mass spectrometry (HR-ESI–MS) and NMR spectroscopy). The absolute configuration of 1 was determined as rhynchophylloside L 11-O-β-D-glucopyranoside by single-crystal X-ray diffractometry (SC-XRD) using a micro-drop crystallization technique, the validity of which was confirmed by application to known compounds, namely rhynchophylloside G (2) [5] and vincosamide 11-O-β-D-glucopyranoside (3) [7].
Results and discussion
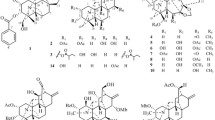
U. rhynchophylla hooks (14.25 kg) were percolated with 60% (v/v) aqueous MeOH, and the aqueous phase obtained after the evaporation of MeOH was extracted with CHCl3 to remove hydrophobic constituents and fractionated by column chromatography (DIAION HP-20, silica gel, reversed-phase C18, Sephadex LH-20, and MCI gel CHP-20P columns) to isolate one novel alkaloid (1, pale-brown powder, 68 mg), nine known alkaloids (rhynchophylloside G (2) [5], vincosamide 11-O-β-D-glucopyranoside (3) [7], vincosamide (4) [8], strictosamide (5) [9], 5b-carboxystrictosidine (6) [10], strictosidine (7) [11], cadambine (8) [11], 3a-dihydrocadambine (9) [12], and (E)-vallesiachotamine (10) [13]), and thirteen known non-alkaloids (8-epiloganic acid (11) [14], chlorogenic acid (12) [15], erigeside C (13) [16], (1S,2R,3S)-lyoniresinol-3a-O-β-glucoside (14) [17], (1R,2S,3R)-lyoniresinol-3a-O-β-glucoside (15) [17], ( +)-catechin (16) [18], (−) -epicatechin (17) [18], hyperin (18) [19], rutin (19) [20], procyanidin B1 (20) [21], procyanidin B2 (21) [21], procyanidin B3 (22) [21], and procyanidin B4 (23) [21]) (Figs. 1 and 2).
Compound 1 was obtained as a pale-brown powder and its molecular formula was estimated as C38H48N2O20 by high resolution electrospray ionization mass spectrometry (HR-ESI–MS) at m/z 875.2698 [M + Na]+ (calculated for C38H48N2O20Na, 875.2693). The 1H, 13C and DEPT NMR spectra (Table 1, Figs. S4, S5) as well as HSQC spectra (Fig. S6) implied the presence of nineteen sp3 methines, four sp2 methines, five sp3 methylenes, six sp2 quaternary carbons, one vinyl group [δC 133.2 (C-19); δH 5.47 (1H, dt, J = 17.1, 8.6 Hz, H-19), δC 120.6 (C-18); δH 5.21 (1H, dd, J = 8.6, 1.9 Hz, H-18), and δH 5.33 (1H, dd, J = 17.1, 1.9 Hz, H-18)], two carbonyl groups [δC 162.1(C-22) and 172.9 (C-7)] and three anomeric protons [δH 4.43 (1H, d, J = 7.6 Hz, H-1′′), 4.69 (1H, d, J = 7.6 Hz, H-1′), 5.00 (1H, d, J = 7.3 Hz, H-1′′′)].
Chemical shift values in the 1H and 13C NMR spectra of 1 well resembled those of rhynchophylloside L, except for positions 10 and 12 (Table 1 and Fig. 3), while the estimated molecular formula of 1 (C38H48N2O20) corresponded to an addition of C6H10O5 to rhynchophylloside L (C32H38N2O15). The COSY and HSQC-TOCSY correlations also supported the presence of an additional spin system in 1 corresponding to an aldohexose moiety (Figs. 4, S8 and S9). The HMBC spectrum displayed long-range 1H-13C correlations from H-1′ to C-21, H-1′′ to C-2′, and from H-1′′′ to C-11, suggesting that compound 1 shared the same disaccharide chain with rhynchophylloside L at position 21 while the additional sugar is attached to the hydroxy group at C-11 (Figs. 4 and S7). However, the sugar species and their locations in 1 could not be confirmed by the extensive NMR analysis. The nine known alkaloids 2–10 and the thirteen known non alkaloids 11–23 were identified by comparing their 1H and 13C NMR spectra and MS data with those reported previously.
The absolute configurations of 1, 2, 3, 4, 8, and 10 were determined by SC-XRD (Cu Kα radiation) with a Flack parameter (Fig. 5). Given that the initial recrystallization of 1 (1 mg) from water (0.1 mL) afforded very small single crystals (Fig. S14), we reduced the volume of water and obtained better crystals using a micro-drop technique. Specifically, after compound 1 (50 µg) was charged at the center of a V-shaped vial, water (4 µL) was added, and the vial was capped. Use of V-shaped vials is preferred to keep the droplets spherical. The vial was heated to obtain a homogenous solution, with suitable single crystals of 1 subsequently obtained within a day (Fig. S15). This method was applied to grow single crystals of 2 and 3 as well as different solvent systems because absolute configurations of these compounds had been deduced from NMR and CD spectroscopic analyses in the previous reports [5, 7] while their three-dimensional structures remained elusive. The micro-drop crystallization technique allowed us to obtain the first crystallographic data of 2 and 3 only using 50 µg of materials and confirm their proposed stereochemistry were correct.
The micro-drop crystallization method developed in this study forms single crystals without oil; thus, crystallization studies are repeatable with various solvent systems in a single vial and compounds are easily recoverable after the structural analysis. Whereas state-of-the-art techniques such as the nanodrop crystallization method provides higher quality of crystals from smaller amount of molecules [22], our method is sufficient to elucidate the three-dimensional structures of rare natural products at a laboratory scale only using < 100 µg materials and a conventional XRD device, and thus does not require any expensive handling robot or specific environment.
The potential pharmacological activities of U. rhynchophylla extracts to treat Alzheimer’s disease are relevant to its inhibition of acetylcholine esterase (AChE) activities in the brain [23]. Monoterpenoid indole alkaloids such as geissoschizine methyl ether N-oxide and geissoschizine methyl ether in this plant exhibit AChE inhibitory activities [24, 25]. We examined in vitro AChE inhibitory activities of all isolated compounds, but no compound showed any inhibitory activity at 200 µM. This result is comparable with the previous report that the glycosylated derivatives of such indole alkaloids rarely show AChE inhibitory activities [5].
Conclusion
One new indole alkaloid triglucoside (1), nine known alkaloids (2–10), and thirteen known nonalkaloids (11–23) were isolated from the aqueous extract of U. rhynchophylla hooks. The typically difficult-to-obtain single crystals of alkaloid glucosides were prepared using a micro-drop crystallization method. Given the importance of knowing absolute configurations for medicinal applications, we further plan to investigate whether this method can be applied to other compounds reluctantly forming single crystals.
Experimental
General experimental procedures
1D and 2D NMR spectra were recorded on a Bruker AVANCE NEO 600 (1H: 600 MHz, 13C: 150 MHz) spectrometer and JEOL ECA-600 (1H: 600 MHz, 13C: 150 MHz), using TMS as an internal standard. Chemical Shifts (δ) are presented in ppm and coupling constants (J) in Hz. HR-ESI–MS experiments were acquired using an Orbitrap Exploris 120 mass spectrometer (Thermo Fisher Scientific). Single-crystal X-ray diffraction data were acquired on a Rigaku XtaLAB Synergy-R diffractometer using Cu Ka radiation. Optical rotation value was recorded on a JASCO P-2200 polarimeter. UV spectra was obtained with a JASCO V-750 spectrophotometer. IR spectra was obtained with a JASCO FT/IR-4600 spectrophotometer. Circular dichroism was measured on JASCO J-1100 spectrometer. Powdered DIAION (Mitsubishi Chemical Co., Japan), Sephadex LH-20 (Amersham Pharmacia Biotech AB, Uppsala, Sweden), MCI gel CHP20P (Mitsubishi Chemical Co., Japan), silica gel 60 (Merck, Darmstadt, Germany) and ODS-A-HG (YMC, Kyoto, Japan, 50 µm) were used for column chromatography (CC). Silica gel GF254 plates (Merck; 0.25 mm in thickness) were used for TLC analysis.
Plant material
The hook-bearing stems of Uncaria rhynchophylla (Miq.) Miq. were purchased from Santai Country Yuanhui Commerce and Trade Co., Ltd. in Sichuan, China. A voucher specimen (THS102306) was deposited in the herbarium of Tsumura Co., Ltd. in Japan.
Isolation of new compound 1
The dried hook-bearing stems of U. rhynchophylla (14.25 kg) were percolated with 60% MeOH in H2O (101.5 L). After concentration of MeOH, the crude extract in water was partitioned with CHCl3 to obtain the CHCl3-soluble extract and the water-soluble extract. After concentration of the aqueous phase, the crude extract was subjected to DIAION HP-20 eluting successively with 60% MeOH in H2O and MeOH to obtain two fractions (60 M, DM2).
60 M was fractionated by Sephadex LH-20 CC (10–100% MeOH in H2O) to afford fractions 60M1 (61.82 g), 60M2 (21.26 g), 60M3 (115.54 g). Further separation of 60M1 (61.82 g) by ODS CC (10–100% MeOH in H2O) to obtain five fractions 60M11, 60M12, 60M13, 60M14 and 60M15. 60M13 was chromatographed on silica gel CC (CHCl3/MeOH/H2O, 40:10:1–6:4:1, v/v, successively) to obtain fractions 60M131, 60M132 and 60M133. Purification of 60M133 on ODS CC (15–16% MeCN in 50 mM NH4Ac aq.) and MCI gel CHP-20P (H2O to MeOH) afford compound 1 (68 mg). The isolation scheme of other known compounds is summarized in supplementary Fig. S13.
Spectroscopic data of rhynchophylloside L 11-O-β-D-glucopyranoside (1)
Pale-brown powder; [α]D20 –99 (c = 0.1, MeOH); UV λmax (MeOH) nm (log ɛ): 247 (4.71), 253 (4.71), 310 (4.07), 321 (4.06); CD λmax (MeOH) nm (Δɛ): 224 (+ 10.47), 252 (–11.09), 294 (+ 1.02); IR (KBr) cm–1: 3398, 2923, 2877, 1635, 1604, 1574, 1454, 1250, 1076, 505; 1H and 13C NMR (600 and 150 MHz, respectively, DMSO-d6), see Table 1; positive ion HR-ESI–MS, m/z 875.2698 [M + Na]+ (calculated for C38H48N2O20Na, 875.2693).
SC-XRD analysis of 1, 2, 3, 4, 8 and 10
Compound 1 (50 µg) was placed into V-type vial and added 4 µL of water. After caped vial, the suspension was dissolved by heating. The suitable single crystals of compound 1 was crystalized within a day. (C38H48N2O20)·6(H2O), M = 960.88, crystal size 0.142 × 0.013 × 0.008 mm3, orthorhombic, space group P212121, a = 6.9177(1) Å, b = 21.6692(3) Å, c = 28.7263(5) Å, V = 4306.10(11) Å 3, Z = 4, α = β = γ = 90°, density (calcd) = 1.482 g·cm–3, F(000) = 2040.0, reflections collected/unique 64,432/8602 (Rint = 0.0467), final R indices (I > 2σ (I)) R1 = 0.0476, wR2 = 0.1043, goodness of fit = 1.149, Flack parameter = 0.01(5). Crystallographic data for compound 1 have been deposited with the Cambridge Crystallographic Data Center (CCDC 2258683).
Compound 2 (50 µg) was placed into V-type vial and added 10 µL of water/ethanol/acetonitrile. After caped vial, the suspension was dissolved by heating. The suitable single crystals of compound 2 was crystalized within a day. (C38H50N2O19)·2(H2O), M = 874.83, crystal size 0.19 × 0.04 × 0.03 mm3, orthorhombic, space group P212121, a = 5.8287(2) Å, b = 19.4840(4) Å, c = 38.4044(10) Å, V = 4361.4(2) Å 3, Z = 4, α = β = γ = 90°, density (calcd) = 1.332 g·cm–3, F(000) = 1856.0, reflections collected/unique 37,841/8418 (Rint = 0.0458), final R indices (I > 2σ (I)) R1 = 0.0557, wR2 = 0.1514, goodness of fit = 1.125, Flack parameter = 0.09(7). Crystallographic data for 2 have been deposited with the Cambridge Crystallographic Data Center (CCDC 2264009).
Compound 3 (50 µg) was placed into V-type vial and added 10 µL of water/acetonitrile. After caped vial, the suspension was dissolved by heating. The suitable single crystals of compound 3 was crystalized within a day. C32H40N2O14, M = 676.66, crystal size 0.05 × 0.03 × 0.01 mm3, monoclinic, space group P21, a = 16.8414(6) Å, b = 5.9860(2) Å, c = 18.8582(6) Å, V = 1881.77(11) Å 3, Z = 2, α = γ = 90°, β = 98.187(3)°, density (calcd) = 1.194 g·cm–3, F(000) = 716.0, reflections collected/unique 23,514/7326 (Rint = 0.0507), final R indices (I > 2σ (I)) R1 = 0.0607, wR2 = 0.1521, goodness of fit = 1.019, Flack parameter = − 0.2(2). Crystallographic data for 3 have been deposited with the Cambridge Crystallographic Data Center (CCDC 2263997).
Compound 4 (50 µg) was placed into V-type vial and added 10 µL of ethanol. After caped vial, the suspension was dissolved by heating. The suitable single crystals of compound 4 was crystalized within a day. (C26H30N2O8)·(C2H6O), M = 544.59, crystal size 0.25 × 0.02 × 0.014 mm3, monoclinic, space group P21, a = 8.2300(2) Å, b = 5.85790(10) Å, c = 27.3883(6) Å, V = 1307.99(5) Å 3, Z = 2, α = γ = 90°, β = 97.862(2), density (calcd) = 1.383 g·cm–3, F(000) = 580.0, reflections collected/unique 26,131/5286 (Rint = 0.0484), final R indices (I > 2σ (I)) R1 = 0.0469, wR2 = 0.1305, goodness of fit = 1.097, Flack parameter = 0.00(10). Crystallographic data for 4 have been deposited with the Cambridge Crystallographic Data Center (CCDC 2263990).
Compound 8 was recrystallized from methanol. 2(C27H32N2O10)·8(H2O), M = 1233.22, crystal size 0.12 × 0.05 × 0.03 mm3, orthorhombic, space group P212121, a = 12.7952(4) Å, b = 19.1742(9) Å, c = 24.2979(9) Å, V = 5961.2(4) Å 3, Z = 4, α = β = γ = 90°, density (calcd) = 1.374 g·cm–3, F(000) = 2624.0, reflections collected/unique 39,208/11453 (Rint = 0.0697), final R indices (I > 2σ (I)) R1 = 0.0790, wR2 = 0.1902, goodness of fit = 1.176, Flack parameter = 0.04(11). Crystallographic data for 8 have been deposited with the Cambridge Crystallographic Data Center (CCDC 2259276).
Compound 10 was recrystallized from acetone. C21H22N2O3, M = 350.40, crystal size 0.11 × 0.06 × 0.04 mm3, orthorhombic, space group P212121, a = 7.13640(10) Å, b = 9.8793(2) Å, c = 25.4299(5) Å, V = 1792.88(6) Å 3, Z = 4, α = β = γ = 90°, density (calcd) = 1.298 g·cm–3, F(000) = 744.0, reflections collected/unique 17,419/3672 (Rint = 0.0306), final R indices (I > 2σ (I)) R1 = 0.0339, wR2 = 0.0954, goodness of fit = 0.810, Flack parameter = 0.07(9). Crystallographic data for 10 have been deposited with the Cambridge Crystallographic Data Center (CCDC 2255637).
AChE Inhibitory Assay
The AChE inhibitory activities of isolated compounds were examined based on Ellman’s method [26] using Amplite® Colorimetric Acetylcholinesterase Assay Kit (AAT Bioquest, Inc.). Briefly, 10 µL of the tested compound solution dissolved in H2O with or without 4% DMSO was mixed with 30 µL of the Assay Buffer and 10 µL of acetylcholinesterase solution (200 mU/mL in the Assay Buffer) on a 96-well microplate. Neostigmine bromide was used as a positive control. After incubation at room temperature for 20 min to facilitate binding of compounds to AChE, 50 µL of the Working Solution containing 5,5′-dithiobis(2-nitrobenzoic acid) and acetylthiocholine iodide was added. After 30 min incubation, the absorbance of each well was recorded at a wavelength of 405 nm. Following the previous report [5], the AChE inhibitory activity was calculated as follows: inhibition % = (C − S)/ (C − B) × 100% (C, the absorbance of control; S, the absorbance of sample solution; and B, the absorbance of blank).
References
Tabuchi M, Yamaguchi T, Lizuka S, Imamura S, Ikarashi Y, Kase Y (2009) Ameliorative effects of yokukansan, a traditional Japanese medicine, on learning and non-cognitive disturbances in the Tg2576 mouse model of alzheimer’s disease. J Ethnopharmacol 122:157–162
Song LL, Wang Y, Xu CB, Lei XQ, Guo QL, Shi JG (2022) Minor monoterpene derivatives from an aqueous extract of the hook-bearing stem of Uncaria rhynchophylla. J Asian Nat Prod Res 24:432–444
Li RF, Zhu CG, Xu CB, Guo QL, Shi JG (2021) Minor alkaloids from an aqueous extract of the hook-bearing stem of Uncaria rhynchophylla. J Asian Nat Prod Res 23:513–526
Li RF, Guo QL, Zhu CG, Xu CB, Wei YZ, Cai J, Wang Y, Sun H, Zhang TT, Shi JG (2021) Minor triterpenes from an aqueous extract of the hook-bearing stem of Uncaria rhynchophylla. J Asian Nat Prod Res 23:307–317
Guo Q, Si X, Shi Y, Yang H, Liu X, Liang H, Tu P, Zhang Q (2019) Glucoconjugated monoterpene indole alkaloids from Uncaria rhynchophylla. J Nat Prod 82:3288–3301
Guo Q, Yang H, Liu X, Si X, Liang H, Tu P, Zhang Q (2018) New zwitterionic monoterpene indole alkaloids from Uncaria rhynchophylla. Fitoterapia 127:47–55
Wang P, Luo J, Wang XB, Fan BY, Kong LY (2015) New indole glucosides as biosynthetic intermediates of camptothecin from the fruits of Camptotheca acuminata. Fitoterapia 103:1–8
Aimi N, Shito T, Fukushima K, Itai Y, Aoyama C, Kunisawa K, Sakai S, Hagiwara J, Yamasaki K (1982) Studies on plants containing indole alkaloids. VIII indole alkaloid glycosides and other constituents of the leaves of Uncaria rhynchophylla MIQ. Chem Pharm Bull 30:4046–4051
Patty-Lukats A, Kocsis A, Szabo LF, Podanyi B (1999) Configurative correlation and conformational analysis of strictosidine and vincoside derivatives. J Nat Prod 62:1492–1499
Aimi N, Seki H, Sakai S (1992) Synthesis of lyaloside, a prototypal β-carboline gluco indole alkaloid in rubiaceous plants. Chem Pharm Bull 40:2588–2590
Handa SS, Borris RP, Cordell GA, Phillipson JD (1983) NMR spectral analysis of cadambine from anthocephalus chinensis. J Nat Prod 46:325–330
Sakamoto J, Umeda Y, Rakumitsu K, Sumimoto M, Ishikawa H (2020) Total synthesis of (–)-strictosidine and related indole alkaloid glycosides. Angew Chem Int Ed 59:13414–13422
de Carvalho Junior AR, Vieira IJC, de Carvalho MG, Braz-Filho R, Lima MAS, Ferreira RO, Maria EJ, de Oliveira DB (2017) 13C-NMR spectral data of alkaloids isolated from psychotria species (Rubiceae). Molecules 22:103–124
Yoshizawa F, Deyama T, Takizawa N, Usmanghani K, Ahmad M (1990) The constituents of cistanche tubulosa (SCHRENK) HOOK. f. II. : isolation and structures of a new phenylethanoid glycoside and a new neolignan glycoside. Chem Pharm Bull 38:1927–1930
Han T, Li H, Zhang Q, Zheng H, Qin L (2006) New thiazinediones and other components from Xanthium strumarium. Chem Nat Compd 42:567–570
Klick S, Herrmann K (1988) Glucosides and glucose esters of hydroxybenzoic acids in plants. Phytochemistry 27:2177–2180
Sun G, Zhang X, Xu XD, Yang JS, Lv LX, Zhong M (2012) The isolation and structure identification of four lignan’s stereoisomers from Uncaria sinensis (oliv.) Havil. J Med Plants Res 6:2200–2205
Chao PM, Kuo YH, Lin YS, Chen CH, Chen SW, Kuo YH (2010) The metabolic benefits of polygonum hypoleucum ohwi in hepG2 cells and wistar rats under lipogenic stress. J Agric Food Chem 58:5174–5180
Kato K, Ninomiya M, Tanaka K, Koketsu M (2016) Effects of functional groups and sugar composition of quercetin derivatives on their radical scavenging properties. J Nat Prod 79:1808–1814
Li YL, Li J, Wang NL, Yao XS (2008) Flavonoids and a new polyacetylene from bidens parviflora willd. Molecules 13:1931–1941
Mohri Y, Sagehasi M, Yamada T, Hattori Y, Morimura K, Kamo T, Hirota M, Makabe H (2007) An efficient synthesis of procyanidins. rare earth metal lewis acid catalyzed equimolar condensation of catechin and epicatechin. Tetrahedron Lett 48:5891–5894
Tyler AR, Ragbirsingh R, McMonagle CJ, Waddell PG, Heaps SE, Steed JW, Thaw P, Hall MJ, Probert MR (2020) Encapsulated nanodroplet crystallization of organic-soluble small molecules. Chem 7:1755–1765
Ahmad MZ, Ahmad J, Amin S, Rahman M, Anwar M, Mallick N, Ahmad FJ, Rahman Z, Kamal MA, Akhter S (2014) Role of nanomedicines in delivery of anti-acetylcholinesterase compounds to the brain in alzheimer’s disease. CNS Neurol Disord Drug Targets 13:1315–1324
Jiang WW, Su J, Wu XD, He J, Peng LY, Cheng X, Zhao QS (2015) Geissoschizine methyl ether N-oxide, a new alkaloid with antiacetylcholinesterase activity from Uncaria rhynchophylla. Nat Prod Res 29:842–847
Yang ZD, Duan DZ, Du J, Yang MJ, Li S, Yao XJ (2012) Geissoschizine methyl ether, a corynanthean-type indol alkaloid from Uncaria rhynchophylla as a potential acetylcholinesterase inhibitor. Nat Prod Res 26:22–28
Ellman G, Courteny K, Anderes V, Featherstone R (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Acknowledgements
I would like to thank Mr. Satoshi Matsumiya (Chiba University) for technical assistance for ECD spectrometry. We would also like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (No. 22H05124 and 19H05652 to M.Y.) from MEXT.
Author information
Authors and Affiliations
Contributions
YK conceived the idea of the study. YK developed the experimental analysis plan. YK, RS and HN conducted experiments. YK, RS, RA, and KA discussed analytic data. LS contributed to correcting plant materials. YK drafted the original manuscript. MY supervised the conduct of this study. All authors reviewed the manuscript draft and revised it critically on intellectual content. All authors approved the final version of the manuscript to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koseki, Y., Nishimura, H., Asano, R. et al. Isolation of new indole alkaloid triglucoside from the aqueous extract of Uncaria rhynchophylla. J Nat Med (2024). https://doi.org/10.1007/s11418-024-01836-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11418-024-01836-9