Abstract
Hochuekkito (HET) is a Kampo prescription, used for the clinical treatment of skin diseases such as atopic dermatitis (AD), in Japan. Oral administration of HET exerts anti-allergic effects in an experimental dermatitis mice model and in patients with atopic dermatitis; however, the mechanism underlying the anti-allergic effects of HET is still unclear. Therefore, we investigated the immunopharmacological properties of the anti-allergic actions of HET using a 2,4,6-trinitrochlorobenzene (TNCB)-induced murine contact hypersensitivity (CHS) model and adoptive cell transfer experiments. Oral administration of HET (1.4 g/kg) exhibited anti-allergic effects in a TNCB-induced CHS model via activation of Tregs; this activation was observed even without antigen sensitization in donor mice. Activation was dependent on the duration of HET administration and required at least 4 days of dosing. In addition, the anti-allergic effects of HET through the activation of Tregs were not antigen specific. Flow cytometry results indicated that the proportion of CD4+CD25+Foxp3+ cells in the splenic lymphocytes increased after oral administration of HET. Therefore, oral administration of HET induced both inducible regulatory T cells (iTregs) and thymus-derived naturally occurring regulatory T cells (nTregs). Ginseng radix and Bupleuri radix were involved in the anti-allergic actions of HET through the induction and/or activation of Tregs; Bupleuri radix participated in the activation of nTregs. In conclusion, our findings suggest that HET exerts the anti-allergic effects through the induction and/or activation of Tregs. These findings elucidate the usefulness of HET as an immunomodulator.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease with symptoms that chronically fluctuate with remissions and relapses; it affects a wide range of age groups including infants, adolescents, and adults [1, 2]. The pathogenesis of AD is quite complex but involves a dysregulated immune system and a compromised skin barrier [3, 4].
Current treatment guidelines in Japan recommend a combination approach consisting of topical anti-inflammatory treatment (topical corticosteroids, topical calcineurin inhibitors, or topical Janus Kinase inhibitors), skin care, avoidance of apparent exacerbating factors, psychological counseling, and advice about daily life [2]. Recently, new agents have been developed to target pro-inflammatory cytokines including interleukin (IL)-4, IL-13, and IL-31, and their monoclonal antibodies have shown great efficacy for patients with moderate-to-severe AD [5,6,7]. Additionally, topical phosphodiesterase (PDE) 4 inhibitors and aryl hydrocarbon receptor (AhR) agonists are effective for AD skin lesion [8, 9]. However, there is no single treatment which effectively, completely cures the symptoms of AD in all patients at the present. There is a need for more therapeutic agents for the realization of personalized medicines.
In Japan, there is a desire among patients to use Kampo medicine for early symptom improvement and constitutional improvement [10, 11]. Unfortunately, there is a lack of scientific evidence for the usefulness of Kampo medicines in the treatment of AD [2] and there is a need for basic and clinical research to establish its pharmacological basis. We have previously evaluated the effectiveness of Kampo medicines used for AD treatment in Japan and clarified their anti-allergic effects and mechanisms of action [12]. Juzentaihoto (JTT), a Kampo prescription that has been used clinically for treating skin diseases such as AD, exerts anti-allergic effects by inhibiting effector T cell activation and inducing and/or activating regulatory T cells in a murine model of contact hypersensitivity (CHS) [13]. On the other hand, Orengedokuto (OGT) also showed the anti-allergic activities only by inhibiting effector T cell activation [14]. Since many other Kampo medicines have been applied to the treatment of AD, we continue evaluating the anti-allergic effects of these formulas and their mechanisms of action.
In this study, we evaluated the anti-allergic effect of Hochuekkito (HET) used clinically for the treatment of AD as well as JTT, using 2,4,6-trinitrochlorobenzene (TNCB)-induced CHS response in mice. In addition, we clarified the mechanism of its anti-allergic actions through adoptive cell transfer experiments, and we also studied the anti-allergic effects of the crude drugs that constitute HET.
Materials and methods
Materials
HET is a mixture of ten constituent crude drugs purchased from Tsumura & Co. (Tokyo, Japan): 4.0 g of Astragali Radix (root of Astragalus mongholicus Bunge, Leguminosae, lot no. J05801), 4.0 g of Atractylodis Rhizoma (rhizome of Atractylodes japonica Koidzumi ex Kitamura, Compositae, lot no. K06141), 4.0 g of Ginseng Radix (root of Panax ginseng C.A. Meyer, Araliaceae, lot no. K03591), 3.0 g of Angelicae Radix (root of Angelica acutiloba Kitagawa, Umbelliferae, lot no. F26171), 1.0 g of Bupleuri Radix (root of Bupleurum falcatum Linne, Umbelliferae, lot no. L30641), 2.0 g of Zizyphi Fructus (fruit of Zizyphus jujuba Millar var. inermis Rehder, Rhamanaceae, lot no. H10411), 2.0 g of Citri Unshiu Pericarpium (peel of Citrus unshiu Marcowicz, Rutaceae, lot no. M02461), 1.5 g of Glycyrrhizae Radix (root of Glycyrrhiza uralensis Fischer, Leguminosae, lot no. F01721), 0.5 g of Cimicifugae Rhizoma (rhizome of Cimicifuga dahurica Maximowicz, Ranunculaceae, lot no. AA9131), and 0.5 g of Zingiberis Rhizoma (rhizome of Zingiber officinale Roscoe, Zingiberaceae, lot no. H12461). All crude drugs used in this study were standardized by Japanese Pharmacopeia 18th edition [15]. TNCB and olive oil were purchased from Nacalai Tesque (Kyoto, Japan). High-performance liquid chromatography (HPLC)-grade methanol, other solvents, and chemicals were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Preparation of the HET extract and crude drug extracts
A daily dose of crude drugs compounded according to the HET mixture was decocted with 600 mL of ion-exchanged and distilled water using an electric heater (HMJ-1000N; HARIO Co. Ltd., Tokyo, Japan) for 60 min. The decoction was filtered and cooled to approximately 25 °C. The filtrate was lyophilized to a powder and stored at −20 °C until further use. This extract is the daily dose administered to humans, and the yield of the HET extract was 8.65 ± 0.57 g. The hot water extracts of Ginseng radix and Bupleuri radix were also prepared similarly, and the yields of the extracts were 1.4 ± 0.03 g and 0.29 ± 0.01 g, respectively.
Three-dimensional HPLC analysis of the HET extract
Three-dimensional HPLC analysis was carried out as described previously [13, 14]. Briefly, the lyophilized HET extract powder was dissolved in distilled water (50 mg/mL) under ultrasonic conditions for a few minutes, then the solution was filtered through Millex-HP (0.45 μm, Merck Millipore, Ltd., Darmstadt, Germany). The HPLC apparatus included a PU-2089 plus pump and an MD-2018 Plus photodiode array detector (JASCO Co., Tokyo, Japan). A TSK gel ODS-80Ts column (5 μm, 4.6 mm I.D. × 150 mm, Tosoh Co., Tokyo, Japan) was used for analysis. Gradient elution with a mobile phase consisting of (A) 50 mM ammonium acetate (pH = 3.6) and (B) acetonitrile was used. The gradient elution was programmed as follows: 0–5 min, 5% (B); 5–55 min, 5%–95% (B); and 55–60 min, 95% (B). The flow rate was 1.0 mL/min and the column temperature was ambient. Peaks were identified by comparing the retention times and UV spectra of the standards. The 3D-HPLC profiles of the HET are shown in Fig. S1.
Animals
Female BALB/c mice (6 weeks old) were purchased from Japan SLC (Shizuoka, Japan). The mice were housed in standard plastic cages in an environment with controlled temperature (23 ± 1 °C) and humidity (50 ± 5%), with food (MM-3, Funabashi Farm Co. Ltd., Chiba, Japan) and water available ad libitum. Acclimatization was allowed for at least 7 days before experimentation. All experiments were performed in accordance with the guidelines of the Animal Care Committee of the Factory of Pharmacy, Meijo University (approval number: 2019PE1).
Contact hypersensitivity (CHS) model
A hapten-induced CHS model was used to evaluate the anti-allergic effects of HET as described previously [12]. Briefly, the mice were sensitized by painting their shaved abdominal skin with 100 μL of 5% TNCB in acetone. The CHS response was elicited 7 days later by painting both surfaces of the right ear of each mouse with 10 μL of 1% TNCB in olive oil. Ear thickness was measured before and 24 h after the challenge using a dial thickness gauge (G-1A; Ozaki Mfg. Co., Ltd., Tokyo Japan). Based on body surface area correction, the mouse dose is approximately 12.3 times higher than the human dose [16]; therefore, the HET dose used in the study was ten times higher than the human daily dose. HET (1.4 g/kg) was orally administered once a day for 7 days immediately after sensitization. Prednisolone (20 mg/kg) was administered once a day for 7 days immediately after sensitization as the positive control. In the control mice, mice were sensitized with 5% TNCB, and administered orally with water instead of HET. In normal group, mice were painted with acetone and administered orally with water for 7 days.
Adoptive cell transfer experiments
Adoptive cell transfer experiments were carried out as previously described [13, 14].
Since compounds that induce or activate regulatory T cells are generally not known, no appropriate reference compound was available for subsequent experiments.
Briefly, the donor mice were sensitized by painting their shaved abdominal skin with 100 μL of 5% TNCB in acetone, after which HET was orally administered as described above. Seven days after sensitization, the mice were sacrificed via cervical dislocation, and lymphocyte cell suspensions were prepared from the draining lymph nodes (dLNs; inguinal and axillary) for use in subsequent experiments. To study the effect of HET on the activation of effector T cells, lymphocytes (1 × 107 cells/mouse) were injected intravenously into naïve recipient mice, which were challenged with a topical application of 1% TNCB in olive oil after 24 h.
To evaluate the effect of HET on the induction and/or activation of regulatory T cells, lymphocytes (1 × 107) were injected intravenously into naïve recipient mice that were sensitized 24 h after injection, then challenged after 7 days later. The ear thickness was measured before and after the challenge. The vehicle (Hank’s buffered salt solution, HBSS)-injected recipient mice served as the vehicle-negative control group.
MACS
The lymphocytes, prepared as mentioned in the previous section, were sorted on an MS separation column (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions; they were separated into CD4-positive and CD4-negative fractions. The cells of the CD4-positive fraction were separated into CD25-positive and -negative fractions. The number of cells adoptively transferred to recipient mice was quantitatively determined using flow cytometry as follows: 1 × 107 cells/mouse for whole cells, 5 × 106 cells/mouse for CD4− cell fraction, 5 × 106 cells/mouse for CD4+CD25− cell fraction, and 5 × 105 cells/mouse for CD4+CD25+ cell fraction.
Flow cytometry
The mice were orally administered HET or water (three mice each) for 7 days. Spleen cells were prepared by mincing and tapping the spleen fragments on a 100 μm nylon mesh (Cell Strainer, Falcon, Corning, NY, USA) in cold Hank’s buffered salt solution (pH = 7.2, HBSS) with 2% fetal calf serum. Erythrocytes were depleted by treating with 0.83% ammonium chloride-Tris buffer. Cells were washed twice with 2% HBSS; 2 × 106 cells were aliquoted into each round tube. To determine cell viability, the cells were stained using Fixable Viability Stain 575 V (BD Biosciences, Franklin Lakes, NJ, USA) for 15 min at 25 °C in the dark. To block Fc receptors and minimize nonspecific binding, the cells were re-suspended in staining buffer containing anti-mouse CD16/32 antibody (clone: 2.4G2, Tonbo Biosciences, San Diego, CA, USA) for 10 min at 4 °C. For phenotypic characterization of Treg cells, cells were stained with CD4-FITC (clone: GK1.5, BD Biosciences), CD25-PE (clone: PC61, BD Biosciences), and glucocorticoid-induced tumor necrosis factor receptor family-related gene (GITR)-BV421 (clone: DTA-1, BD Biosciences) in 2% FBS/HBSS for 30 min at 4 °C in the dark. Fixation, permeabilization, and intracellular/nuclear staining with Foxp3-Alexa Fluor®647 (clone: MF23, BD Biosciences) were performed using the transcription factor buffer set (BD Biosciences) according to the manufacturer’s instructions. Flow cytometry was performed on an LSR Fortessa X20 (BD Biosciences); 5 × 105 cells were counted for each sample. Gating strategy for analysis is shown in Fig. S2. Data were analyzed using FACS Diva software (BD Biosciences).
Statistical analysis
Results are presented as mean ± standard error of the mean (SEM). Differences among groups were evaluated using analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons. Statistical analyses were performed using GraphPad Prism version 8.
Results
The effect of HET on a TNCB-induced murine CHS model
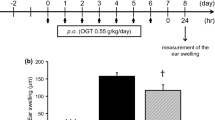
There are some reports that HET exerts the anti-allergic effects on the hapten-induced allergic dermatitis induced by single or multiple application of TNCB [17,18,19]. We first confirmed the anti-allergic effect of HET on the TNCB-induced CHS response in mice, using prednisolone (PSL) as the positive control. Oral administration of HET (1.4 g/kg) significantly suppressed ear swelling 24 h after the topical application of 1% TNCB, at a level comparable with the oral administration of PSL (Fig. 1a, b).
Anti-allergic effects of HET on TNCB-induced contact hypersensitivity (CHS) response in mice. a Experimental protocol. b Ear swelling 24 h after the topical application of 1% TNCB on the ear. N, normal group; CTRL, control group; HET, Hochuekkito group; PSL, prednisolone group. Each column represents the mean ± SEM of five mice. ***p < 0.001, **p < 0.01 vs. the control group using ANOVA with Bonferroni correction for multiple comparisons
Adoptive cell transfer experiments were performed to understand the mechanism of anti-allergic action of HET in the TNCB-induced CHS response as described previously [13].
Lymphocyte cell suspensions isolated from the regional lymph nodes of the skin (the inguinal and axillary lymph nodes) were obtained from mice sensitized with 5% TNCB on the abdomen, and orally administered HET or water for 7 days. The lymphocytes were then injected into naïve BALB/c mice. The recipient mice were challenged via topical application of 1% TNCB on the ear after 24 h to study the effect of HET on Teff activation (Fig. 2a). Ear swelling was observed in control recipient mice administered water; this was not observed in the vehicle recipient mice, which were injected with buffer instead of lymphocytes. This was indicative of Teff activation. The suppression of ear swelling was not observed in HET-treated mice (Fig. 2b), suggesting that HET has an anti-allergic effect, which is independent of Teff activation.
Effect of HET on effector T cell (Teff) activation and induction/activation of regulatory T cells (Tregs) in TNCB-induced CHS response. a Experimental protocol. b Ear swelling 24 h after the topical application of 1% TNCB on the ear to evaluate the effects of HET on the effector T cell activation. N, normal group; Vehicle, vehicle-negative control group; CTRL, control group; HET, Hochuekkito group. Each column represents the mean ± SEM of five mice. c Ear swelling 24 h after the topical application of 1% TNCB on the ear to evaluate the effects of HET on the induction and/or activation of regulatory T cells. N, normal group; Vehicle, vehicle-negative control group; CTRL, control group; HET, Hochuekkito group. Each column represents the mean ± SEM of six mice. ***p < 0.001, **p < 0.01 vs. the control group using ANOVA with Bonferroni correction for multiple comparisons. See the “Materials and Methods” section for details
Lymphocyte cell suspensions were injected into naïve BALB/c recipient mice, sensitized with 5% TNCB on their pre-shaved abdomen after 24 h. TNCB (1%) was applied to the right ear 7 days after sensitization, to induce CHS response and investigate the induction or activation of Tregs. Ear swelling was suppressed significantly in the HET recipient group (Fig. 2c), suggesting that oral administration of HET induced and/or activated Tregs.
The induction and/or activation of Tregs by HET was observed without sensitization of donor mice with 5% TNCB (Fig. 3a, b). These results suggest that the Tregs induced and/or activated by HET could be both naturally occurring regulatory T cells (nTregs) and inducible regulatory T cells (iTregs) (Fig. 2c, 3b).
Effect of HET on induction/activation of regulatory T cells (Tregs) in TNCB-induced CHS response without sensitization with 5% TNCB in donor mice. a Experimental protocol. b Ear swelling 24 h after the topical application of 1% TNCB on the ear. N, normal group; Vehicle, vehicle-negative control group; CTRL, control group; HET, Hochuekkito group. Each column represents the mean ± SEM of five mice. ***p < 0.001, **p < 0.01 vs. the control group using ANOVA with Bonferroni correction for multiple comparisons. See the “Materials and Methods” section for details
We examined the influence of the administration period of HET on the activation of nTregs. Adoptive cell transfer experiments were conducted in donor mice by changing the administration period of HET to 1, 4, and 7 days without antigen sensitization (Fig. 4a). Ear swelling was suppressed in a time-dependent manner following HET administration (Fig. 4b). Therefore, we further investigated the properties of Tregs induced and activated by HET.
Effect of different administration periods of HET on the induction and/or activation of regulatory T cells (Tregs) in TNCB-induced CHS response without sensitization with 5% TNCB in donor mice. a Experimental protocol. b Ear swelling 24 h after the topical application of 1% TNCB on the ear. N, normal group; Vehicle, vehicle-negative control group; CTRL, control group; HET, Hochuekkito group. Each column represents the mean ± SEM of 3 to 5 mice. ***p < 0.001, **p < 0.01 vs. the control group using ANOVA with Bonferroni correction for multiple comparisons. See the “Materials and Methods” section for details
Characterization of Tregs induced and activated by oral administration of HET
We examined the antigen dependency of Tregs induced or activated by oral administration of HET (Fig. 5a). The suppression of ear swelling in recipient mice induced by oxazolone (OXZ) was observed only in the absence of antigen sensitization with 5% TNCB in donor mice administered HET for 7 days (Fig. 5b, c). This indicated the possibility that oral administration of HET activates nTregs.
Antigen specificity of regulatory T cells induced and/or activated by oral administration of HET. a Experimental protocol. b, c Ear swelling 48 h after the topical application of 0.5% oxazolone (OXZ) on the ear. In donor mice (b) and (c), HET and distilled water were administered orally once every day to the treated group and the control (CTRL) group, respectively, starting immediately after they were sensitized with 5% TNCB. After 7 days, lymphocytes were obtained from the dLNs and injected into naïve recipient mice. The lymphocytes obtained from the control donor mice and HET-treated donor mice were injected intravenously into the CTRL group and the HET group, respectively. HBSS alone was injected intravenously in the vehicle mice. All mice, except those in the normal (N) group, were sensitized through the application of 0.5% OXZ on shaved abdomens. 24 h after cell transfer and then challenged on their right ears with 0.5% OXZ, after 7 days. Ear swelling was measured 48 h after the challenge. Each column represents the mean ± SEM of 3–5 mice. ***p < 0.001 vs. the control group using ANOVA with Bonferroni correction for multiple comparisons. †p < 0.05 vs. the control group using ANOVA with Bonferroni correction for the selected two group comparisons
We characterized the Treg subsets induced and activated by oral administration of HET. We fractionated the dLNs lymphocytes using magnetic-activated cell sorting (MACS) to CD4−, CD4+CD25−, and CD4+CD25+ cell fractions and performed adoptive cell transfer experiments (Fig. 6a). The CD4+CD25+ cell fraction significantly suppressed ear swelling, indicating that the CD4+CD25+ fraction mainly contained Tregs induced and activated by oral administration of HET (Fig. 6b). To confirm that oral administration of HET activated nTregs, we analyzed the cell composition in splenic lymphocytes obtained from mice administered HET for 7 days without antigen sensitization. Flow cytometry analysis using well-defined Treg markers such as CD4, CD25, Foxp3, and GITR revealed that oral administration of HET significantly increased the percentage of CD4+CD25+Foxp3+ cells, CD4+CD25+GITR+ cells, and CD4+CD25+Foxp3+GITR+ cells (Fig. S2 and Fig. 7).
Characterization of regulatory T cells induced and/or activated by oral administration of HET using magnetic-activated cell sorting (MACS). a Experimental protocol. b Ear swelling 24 h after the topical application of 1% TNCB on the ear. N, normal group; Vehicle, vehicle-negative control group; CTRL, control group; HET, Hochuekkito group. Each column represents the mean ± SEM of 4–6 mice. ***p < 0.001, *p < 0.05 vs. the control group using ANOVA with Bonferroni correction for multiple comparisons. †p < 0.05 vs. the control group using ANOVA with Bonferroni correction for the selected two group comparisons. See the “Materials and Methods” section for details
Characterization of regulatory T cells activated by oral administration of HET using flow cytometry. a CD25 and Foxp3 expressions in CD4 + cells. b CD25 and GITR expressions in CD4 + cells. c CD25 and Foxp3 expressions in CD4 + GITR + cells. d Comparison of CD4, CD25, Foxp3, and GITR expression in the spleen obtained from control (CTRL) and HET-treated mice. Each column represents the mean ± SEM of three samples. **p < 0.01 vs. the control group using student’s t test. See the “Materials and Methods” section for details
The effects of Ginseng radix and Bupleuri radix on the induction and activation of Tregs in a murine CHS model
To identify the active crude drugs, among the constituents of HET that induce and/or activate Tregs, we studied the effects of 10 crude drugs on a murine CHS model and identified the crude drugs that are active against CHS. Oral administration of the hot water extracts of Ginseng radix significantly suppressed ear swellings in a murine CHS model (Fig. 8a). Adoptive cell transfer experiments revealed that the hot water extracts of Ginseng Radix exerted anti-allergic actions via induction and/or activation of Tregs in CHS without suppression of Teff activation (Fig. 8b, c).
Effects of hot water extracts of Ginseng radix (GR) on a murine CHS model (a) and effects of GR extracts on the effector T cell (Teff) activation (b) and the induction and/or activation of regulatory T cells (Tregs) (c). N, normal group; CTRL, control group; Vehicle, vehicle-negative control group; GR, hot water extracts Ginseng radix (233 mg/kg, p.o.) group. Each column represents the mean ± SEM of 3–5 mice. ***p < 0.001, **p < 0.01 vs. the control group using ANOVA with Bonferroni correction for multiple comparisons. See the “Materials and Methods” section for details
In addition, the hot water extracts of Bupleuri radix exerted anti-allergic effects in a murine CHS model through the induction and/or activation of Tregs (Fig. 9a, b, c).
Effects of hot water extracts of Bupleuri radix (BR) on a murine CHS model (a) and effects of GR extracts on the effector T cell (Teff) activation (b) and the induction and/or activation of regulatory T cells (Tregs) (c). N, normal group; CTRL, control group; Vehicle, vehicle-negative control group; BR, the hot water extract of Bupleuri radix (48 mg/kg, p.o.) group. Each column represents the mean ± SEM of 3–5 mice. ***p < 0.001 vs. the control group using ANOVA with Bonferroni correction for multiple comparisons. †p < 0.05 vs. the control group using ANOVA with Bonferroni correction for the selected two group comparisons. See the “Materials and Methods” section for details
The hot water extracts of Ginseng radix and Bupleuri radix suppressed ear swelling by induction and/or activation of CD4 + CD25 + cell fractions in adoptive transfer experiments, as observed using MACS technology (Fig. S3).
We performed adoptive cell transfer experiments without antigen sensitization in donor mice; the hot water extract of Ginseng radix activated iTregs and the hot water extract of Bupleuri radix activated both iTregs and nTregs (Fig. 10a, b). In addition, activation of nTreg by hot water extract of Bupleuri radix was dependent on the duration of administration, requiring at least 4 days of dosing like HET (Fig. S4).
Effects of hot water extracts of Ginseng radix and Bupleuri radix on the induction and/or activation of regulatory T cells (Tregs) without antigen sensitization in donor mice. a Experimental protocol. b Ear swelling 24 h after the topical application of 1% TNCB on the ear. N, normal group; Vehicle, vehicle-negative control group; CTRL, control group; GR, the hot water extracts of Ginseng radix group; BR, the hot water extracts of Bupleuri radix group. Each column represents the mean ± SEM of 3–5 mice. ***p < 0.001, **p < 0.01 vs. the control group using ANOVA with Bonferroni correction for multiple comparisons. See the “Materials and Methods” section for details
Discussion
In the present study, we evaluated the anti-allergic effects of HET on a murine model of CHS and clarified the mechanism underlying these effects using adoptive cell transfer experiments. We demonstrated that HET exerted the anti-allergic actions via the induction and/or activation of Tregs.
HET is used traditionally to treat fatigue, loss of appetite, and anorexia in patients with various diseases [20,21,22]. It has been reported that HET performs various immunopharmacological functions in the treatment of AD through basic and clinical studies [17,18,19], [23,24,25,26,27]. Unfortunately, the mechanism underlying the anti-allergic action of HET is still unclear. In a series of studies on the anti-allergic effects of Kampo medicines used for the treatment of AD in clinical practice, we have already shown that JTT has a two-way mechanism of action: suppression of Teff activation and induction and/or activation of iTregs [13]. JTT and HET are both prescriptions used as “hozai” (any formula that reinforces the body and restores depletion of qi and/or blood) to restore physical strength during and after illness, but there are differences between them. JTT is used for both “qi” deficiency and “blood” deficiency, while HET is used mainly for “qi” deficiency. Since both formulations have been reported to have immunomodulatory actions [28, 29], we thought that they may have similar anti-allergic properties in a murine CHS responses.
Oral administration of HET exerted anti-allergic effects in a murine CHS model only by inducting and/or activating of Tregs, while JTT has been shown to exert anti-allergic actions from two direction by inhibiting Teff activation and inducing and/or activating Tregs. The CD4+CD25+ cell fractions were involved in the anti-allergic effects of HET. HET not only induced and activated iTregs but also activated nTregs; this was elucidated through adoptive cell transfer experiments in which conditions such as the presence or absence of antigen sensitization and antigen specificity were changed. There was an increase in the number of cells that possessed the typical phenotype of nTregs, such as CD4+CD25+Foxp3+ and CD4+CD25+Foxp3+GITR+ cells, as observed in splenocytes obtained from mice administered HET for 7 days without antigen sensitization. We investigated cytokine production induced by the addition of anti-CD3ε and anti-CD28 monoclonal antibodies (T cell receptor stimuli) in the dLNs obtained from mice sensitized with 5% TNCB; however, we did not detect any changes in IFN-γ, TGF-β, and IL-10 production with the oral administration of HET (data not shown). Therefore, oral administration of HET may activate nTregs, rather than induce and/or activate iTregs.
Oral administration of HET suppresses chronic contact hypersensitivity induced by repeated cutaneous application of TNCB in BALB/c mice, possibly through the suppression of serum IgE and IgG1 and IL-4 in the inflamed skin [17]. Oral administration of HET inhibits ear swelling and reduces the serum IgE levels induced by repeated local exposure to mite antigens (Dermatophagoides farinae crude extract) in NC/Nga mice [18]. In addition, it inhibits the increase in the mRNA expression of IL-4 and IFN-γ in the dLNs and in the inflamed skin lesions. HET may reverse the Th1/Th2 imbalance caused by multiple sensitizations with antigen; however, the exertion of anti-allergic effects of HET through induction and/or activation of Tregs was not reported.
Since induction and activation of Tregs is a therapeutic strategy for autoimmune and allergic disorders [30, 31], the clinical application of traditional medicines, such as Kampo medicines, which show immunomodulatory effects via regulatory T cells, is promising. The hot water extracts of Ginseng radix and Bupleuri radix exerted anti-allergic effects in a TNCB-induced murine CHS model through induction and/or activation of Tregs, suggesting that the hot water extracts of Bupleuri radix activated iTregs and nTregs, while the hot water extracts of Ginseng radix activated only iTreg. Hot water extracts of Korean Red Ginseng, made by first steaming fresh ginseng and then drying it, exerts anti-allergic effects against TNCB-induced contact dermatitis in NC/Nga mice [32]. However, this is the first report that hot water extracts of Ginseng radix and Bupleuri radix exert anti-allergic effects by inducing and activating Tregs. The anti-inflammatory and anti-allergic activities of the hot water extracts of Ginseng radix or Bupleuri radix could be attributed to ginsenosides and saikosaponins. Therefore, it is necessary to isolate and identify the active ingredients of Ginseng radix and Bupleuri radix.
In the Japanese guidelines for the treatment of AD, the usefulness of Kampo medicines has not been denied, but at this point, there is no opinion on the usefulness of specific prescriptions, and they should be administered by physicians who are skilled in Kampo medicine [2]. A series of studies revealed that Kampo medicines showing anti-allergic effects in a murine CHS models have different mechanisms of action. Namely, JTT and HET, which are used in the subacute and chronic phases as “hozai”, showed anti-allergic effects through induction and activation of Tregs, while OGT, which is used in the acute phase and has strong anti-inflammatory effects as “seinetsuzai” (any cooling formula that is used for heat patterns), showed anti-allergic effects through suppression of Teff activation. We believe that these findings will help in the selection of prescriptions in the treatment of AD with Kampo medicine.
In conclusion, HET exerts anti-allergic effects by inducing and/or activating nTregs and iTregs. An existing traditional medicine, such as HET, could be used as a ready-to-use medicine for inflammatory skin diseases such as AD. The active ingredients of HET will be identified through future studies to understand its immunomodulatory actions at the molecular level.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary material files.
References
Miyano K, Tsunemi Y (2021) Current treatments for atopic dermatitis in Japan. J Dermatol 48:140–151
Saeki H, Huya Y, Furuta AH, Ichiyama S, Katsunuma T, Katoh N, Tanaka A, Tsunemi Y, Nakahara T, Nagao M, Narita M, Hide M, Fujisawa T, Futamura M, Masuda K, Matsubara T, Murota H, Yamamoto-Hanada K (2022) English version of clinical practice guidelines for the management of atopic dermatitis 2021. J Dermatol 49:e315–e375
Bieber T (2008) Atopic dermatitis N Eng J Med 358:1483–1494
Kabashima K (2013) New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci 70:3–11
Kamata M, Tada Y (2021) A literature review of real-world effectiveness and safety of dupilumab for atopic dermatitis. JID Innovations 1:100042. https://doi.org/10.1016/j.xjidi.2021.100042
Nakajima S, Tie D, Nomura T, Kabashima K (2021) Novel pathogenesis of atopic dermatitis from the view of cytokines in mice and humans. Cytokine 148:1555664. https://doi.org/10.1016/j.cyto.2021.155664
Tominaga M, Takamori K (2022) Peripheral itch sensitization in atopic dermatitis. Allergol Int 71:265–277
Saeki H, Imamura T, Yokota D, Tsubouchi H (2022) Difamilast ointment in Japanese adult and pediatric patients with atopic dermatitis: a phase III, long-term, open-label study. Dermatol Ther 12:1589–1601
Vu YH, Hashimoto-Hachiya A, Takemura M, Yumine A, Mitamura Y, Nakahara T, Furue M, Tsuji G (2020) IL-24 negatively regulates keratinocyte differentiation induced by tapinarof, an aryl hydrocarbon receptor modulator: implication in the treatment of atopic dermatitis. Int J Mol Sci 21:9412. https://doi.org/10.3390/ijms21249412
Nakahara T, Takemoto S, Houzawa H, Nakayama M (2022) Desire for alternative treatment options in patients with atopic dermatitis in Japan: results of a web-based cross-sectional study (AD-JOIN study). Dermatol Ther 12:1383–1396
Shimizu T (2013) Efficacy of kampo medicine in treating atopic dermatitis: an overview. Evid Based Complement Altern Med. https://doi.org/10.1155/2013/260235
Nose M, Sakushima J, Harada D, Ogihara Y (1999) Comparison of immunopharmacological actions of 8 kinds of Kampo-hozais clinically used in atopic dermatitis on delayed-type hypersensitivity in mice. Biol Pharm Bull 22:48–54
Tsuge A, Yonekura S, Watanabe S, Kurosaki Y, Hisaka S, Nose M (2022) Juzentaihoto exerts anti-allergic effects by inhibiting effector T-cell activation and inducing and/or activating regulatory T cells in a murine model of contact hypersensitivity. Int Arch Allergy Immunol 183:1–13
Tsuge A, Watanabe A, Kodama Y, Hisaka S, Nose M (2022) Orengedokuto exerts anti-allergic effects via inhibition of effector T cell activation in a murine model of contact hypersensitivity. J Nat Med 76:144–151
The Ministry of Health, Labour and Welfare of Japan (2021) The Japanese pharmacopeia 18th ed., Crude drugs and related drugs. The Ministry og Health, Labour and Welfare of Japan, Tokyo. https://www.mhlw.go.jp/content/11120000/000904450.pdf
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7:27–31
Nakada T, Watanabe K, Matsumoto T, Santa K, Triizuka K, Hanawa T (2002) Effect of orally administered Hochu-ekki-to, a Japanese herbal medicine, on contact hypersensitivity caused by repeated application of antigen. Int Immunopharmacol 2:910–911
Gao XK, Fuseda K, Shibata T, Tanaka H, Inagaki N, Nagai H (2005) Kampo medicines for mite antigen-induced allergic dermatitis in NC/Nga mice. eCAM 2:191–199
Yamashita H, Makino T, Inagaki N, Nose M, Mizukami H (2013) Assessment of relief from pruritus due to Kampo medicines by using murine models of atopic dermatitis. J Trad Med 30:114–123
Wang XQ, Takahashi T, Zhu SJ, Moriya J, Saegusa S, Yamakawa J, Kusaka K, Itoh T, Kanda T (2004) Effect of hochu-ekki-to (TJ-41), a Japanese herbal medicine, on daily activity in a murine model of chronic fatigue syndrome. eCAM 1:203–206
Jeong JS, Ryu BH, Kim JS, Park JW, Choi WC, Yoon SW (2010) Bojungikki-Tang for cancer-related fatigue: a pilot randomized clinical trial. Integr Cancer Ther 9:331–338
He Q, Sawada M, Yamasaki A, Akazawa S, Furuta H, Uenishi H, Meng X, Nakahashi T, Ishigaki Y, Moriya J (2020) Neuroinflammation, oxidative stress, and the treatment with Kampo medicine. Biol Pharm Bull 43:110–115
Kobayashi H, Mizuno N, Kutsuna H, Teramae H, Ueoku S, Onoyama J, Yamanaka K, Fujita N, Ishii M (2003) Hochu-ekki-to suppresses development of dermatitis and elevation of serum IgE level in NC/Nga mice. Drug Exp Clin Res 29:81–84
Kobayashi H, Takahashi K, Mizuno N, Kutsuna H, Ishii M (2004) An alternative approach to atopic dermatitis. Part I—Case-series presentation. eCAM 1:49–62
Kobayashi H, Takahashi K, Mizuno N, Kutsuna H, Ishii M (2004) An alternative approach to atopic dermatitis: Part II—Summary of cases and discussion. eCAM 1:145–155
Kobayashi H, Ishii M, Takeuchi S, Tanaka Y, Shintani T, Yamatodani A, Kusunoki T, Furue M (2010) Efficacy and safety of a traditional herbal medicine, Hochu-ekki-to in the long-term management of kikyo (delicate constitution) patients with atopic dermatitis: a 6 month, multicenter, double-blind, randomized, placebo-controlled study. eCAM 7:367–373
Jeong MK, Kim TE, Kim A, Jung J, Son MJ (2019) The herbal drug, Bu-Zhong-Yi-Qi-Tang, for the treatment of atopic dermatitis. Medicine 98:1–4
Gao X, Yanaka H, Inagaki N, Teramachi H, Tsuchiya T, Nagai H (2006) Immunopharmacological studies on the effects of Juzentaihoto and Hochuekkito on experimental autoimmune encephalomyelitis in rats. J Trad Med 23:196–202
Kiyomi A, Matsuda A, Nara M, Yamazaki K, Imai S, Sugiura M (2021) Immunological differences in human peripheral blood mononuclear cells treated with traditional Japanese herbal medicines Hochuekkito, Juzentaihoto, and Ninjin’yoeito from different pharmaceutical companies. eCAM. Doi: https://doi.org/10.1155/2021/7605057
Jiang S, Lechier RI (2006) CD4+CD25+ regulatory T-cell therapy for allergy, autoimmune disease and transplant rejection. Inflamm Allrgy Drug Targets 5:239–242
Miyara M, Wing K, Sakaguchi S (2009) Therapeutic approaches to allergy and autoimmunity based on FoxP3+ regulatory T-cell activation and expansion. J Allergy Clin Immunol 123:749–755
Lee JH, Cho SH (2011) Korean red ginseng extract ameliorates skin lesions in NC/Nga mice: an atopic dermatitis model. J Ethnopharmacol 133:810–817
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11418_2023_1683_MOESM1_ESM.pdf
Supplementary file1 Fig. S1 3D-HPLC profile of hochuekkito (HET). Fig. S2 Gating strategy for analysis of CD4+CD25+FoxP3+, CD4+CD25+GITR+, and GITR+CD25+Foxp3+ cells in splenic lymphocytes administered by HET. (1) Whole cells were fractionated by side scatter (SSCs) and forward scatter (FSCc) to lymphocytes. (2, 3) Debris and doublets were discriminated. (4) Single cells were then fractionated by Fixable viability stain 575 V into living cells. (5) Living cells were fractionated by CD4. (6) CD4+ populations were then fractionated by Foxp3 and CD25 to analyze the population of CD25+ and Foxp3+ cells. (7) CD4+ populations were then fractionated by GITR and CD25 to analyze the population of GITR+ and CD25+ cells. (8) Alternatively, living cells were fractionated by CD4 and GITR to analyze the population of CD4+ and GITR+ cells. (9) CD4+ and GITR+ cells were fractionated by CD25 and Foxp3 to analyze the population of CD4+CD25+GITR+Foxp3+ cells. Fig. S3 Characterization of regulatory T cells induced and/or activated by oral administration of hots water extracts of Ginseng radix (a) and Bupleuri radix (b) using magnetic-activated cell sorting. Each column represents the mean ± SEM of 3 to 5 mice. *** p < 0.001, * p < 0.05 vs. the control group using ANOVA with Bonferroni correction for multiple comparisons. †† p < 0.01 vs. the control group using ANOVA with Bonferroni correction for the selected two group. See the Materials and Methods section for details. Fig. S4 Effect of different administration periods of hot water extract of Bupleuri radix on the induction and/or activation of regulatory T cells (Tregs) in TNCB-induced CHS response without sensitization with 5% TNCB in donor mice. (a) Experimental protocol. (b) Ear swelling 24 h after the topical application of 1% TNCB on the ear. N, normal group; Vehicle, vehicle-negative control group; CTRL, control group; BR, hot water extract of Bupleuri radix group. Each column represents the mean ± SEM of 3 to 5 mice. ***p < 0.001vs. the control group using ANOVA with Bonferroni correction for multiple comparisons. † p < 0.05 vs. the control group using ANOVA with Bonferroni correction for the selected two group. See the Materials and Methods section for details (PDF 572 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsuge, A., Chiba, S., Yagura, Y. et al. Hochuekkito exerts the anti-allergic effects via activating regulatory T cells in a murine model of contact hypersensitivity. J Nat Med 77, 352–362 (2023). https://doi.org/10.1007/s11418-023-01683-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-023-01683-0