Abstract
Purpose
The escalation of nitrogen (N) deposition has resulted in phosphorus (P) limitation in alpine grasslands on the Qinghai–Tibetan Plateau (QTP). However, the impact of N deposition affects soil P transformations in alpine grasslands, and whether there is a universal pattern of N-induced soil P fraction change in terrestrial ecosystems is still not well understood.
Methods
We performed field experiments in two alpine grasslands on the QTP and a meta-analysis including 1033 records worldwide to analyze the responses of soil P fractions to N addition.
Results
We found that N addition significantly altered soil P fractions in alpine meadow, whereas it induced a minor response in alpine steppe. The N addition induced a decrease in soil inorganic phosphorus (Pi) in the alpine meadow, resulting from occluded P (i.e., C.HCl-Pt and residual-Pt). Though N addition did not change total organic P (Po) concentration, there were remarkable changes among soil organic P fractions (C.HCl-Po, NaOH-Po, and NaHCO3-Po) in the alpine meadow, with an increase in NaOH-Po but a decrease in C.HCl-Po. Soil inorganic P in the alpine meadow was associated with Ca2+ and soil pH that was also reduced by N addition. By contrast, meta-analysis results showed that N addition significantly increased the lnRR of NaOH-Pi, but decreased lnRR of C.HCl-Pt and marginally reduced lnRR of NaHCO3-Po across all terrestrial ecosystems. Among multiple environmental and experimental variables, soil pH, mean annual temperature (MAT), mean annual precipitation (MAP), N forms, and soil phosphatase activity mainly drove the response of NaHCO3-Po to N addition at the large scale. Structural equation model (SEM) further showed that soil phosphatase activity was the main direct factor controlling NaHCO3-Po response.
Conclusions
Our results suggest that soil P fractions are more sensitive to N addition in alpine meadow than in alpine steppe. The reduction of inorganic P fractions and uneven changes of organic P fractions in alpine meadow suggested that N addition may accelerate inorganic P dissolution but depress organic P mineralization. Environmental factor (e.g., MAP) and experimental variables (N rate) affected soil P fractions in response to N addition mediated by soil pH and enzymatic activities. Collectively, these findings improved our understanding of the consequences of N addition on soil organic and inorganic P transformations and predicted the trajectory of soil phosphorus fraction change under increasing N deposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil phosphorus (P) is essential but limiting nutrient for plant growth and microbial activity. Although soil contains large amounts of P, only a small fraction (less than 6% on average) can be taken up directly by plants (Yang and Post 2011). Most P in the soil cannot be immediately accessed by plants due to unavailable organic or inorganic forms. Insufficient P supply or availability in the soil results in difficulty in meeting the demand of plant growth in terrestrial ecosystems worldwide (Augusto et al. 2017). Besides the generally low concentration, poor mobility and high spatio-temporal heterogeneity of soil available P could also aggravate P limitation (Turner et al. 2012). Atmospheric P input is relatively low (0.1 kg P ha year−1) compared to N input in most global lands (Mahowald et al. 2008). Consequently, the ongoing nitrogen (N) deposition increasingly contributed to widespread P limitation to primary production and other ecological processes in global terrestrial ecosystems (Elser et al. 2007; Chen et al. 2020; Hou et al. 2020, 2021).
Anthropogenic activities such as fossil fuel combustion and chemical N fertilizers have led to a rapid increase in N deposition from the atmosphere since the industrial revolution (Penuelas et al. 2020). As the biogeochemical cycling of N and P is closely linked, increased N deposition is expected to have profound impacts on the soil P cycle (Luo et al. 2022). However, these effects have been highly debated. For example, some simulated N deposition experiments indicated N addition induced soil P limitation (Deng et al. 2017), while others suggest that long-term nitrogen loading may alleviate P limitation in terrestrial ecosystems (Chen et al. 2020). A fundamental reason for the uncertainty about ecosystem P limitation in response to N addition is the fact that the soil P pool contains several chemical forms that differ significantly in their bioavailability, behavior, and mobility in soil.
Nitrogen addition may affect the composition of soil P forms by various mechanisms. For instance, N addition may alter soil microbial activities and community structure, thereby affecting the release of organic anions to solubilize inorganic P or enzymes to mineralize organic P (Dai et al. 2019). N enrichment stimulates plant and soil microorganism demand for P in companion with incremental primary production and microbial biomass (Yang et al. 2015; Deng et al. 2016), enhancing the formation of organic P. What is more, N-induced soil acidification may dissolve mineral P and release P that could be transformed into other P forms. For example, with soil pH decreasing from 7.6 to 4.7, N addition promoted the dissolution of Ca-bound recalcitrant P to more available forms of Al- and Fe-bound P fractions and Olsen P (Wang et al. 2022a).
A few studies have reported the effects of N addition on soil P fractions till now, yet the results lack consistency. For example, N additions could decrease (Yang et al. 2015) or increase soil labile inorganic P (i.e., NaHCO3-Pi) (Chen et al. 2018a). Meanwhile, N enrichment may reduce (Fan et al. 2018) or enhance (Block et al. 2012) organic P fraction. In addition, the positive (Li et al. 2021), negative (Lu et al. 2012), and neutral (Zeng et al. 2020) influences of N addition on soil P availability have been reported in different natural ecosystems. These inconsistent results of N addition may be due to the differences in rate and form of N application and in ecosystem types. Nevertheless, N-induced P transformations among different fractions are rarely checked in field studies or by meta-analysis. The issues whether N addition can alter soil P fractions and which P form is most sensitive to N addition still remain elusive, but are urgent for understanding the coupled relation of N and P cycling.
The alpine grasslands (alpine meadow and alpine steppe) account for 85% of the land area in Qinghai–Tibetan Plateau (QTP) (Li et al. 2014). The QTP shows an increasing trend of N deposition in recent decades (Lu and Tian 2007; Yu et al. 2019). N enrichment shifts ecosystem nutrient limitation patterns from N limitation to NP co-limitation or P limitation in alpine grasslands. Recent studies in alpine grasslands on the QTP exhibited that P addition or NP co-addition significantly increased gross primary productivity (Wang et al. 2020, 2022b; Yang et al. 2021; Dong et al. 2022). In addition, considering that cold and dry climate inhibits the pedogenesis (Buol et al. 2011), soils in high altitude areas are generally younger than those in other regions. Hence, it likely leads to higher primary mineral P concentration and lower proportion of occluded P in QTP soils (Li et al. 2022). Organic P mineralization rate is also quite low due to the same reason (Zhou et al. 2021). Therefore, the unique climatic and soil conditions could make transformations of soil P pools in QTP different from other regions in response to increasing nitrogen deposition. However, changes in soil P fraction in the two alpine grassland ecosystems responding to N deposition remain poorly understood. Here, we experimentally address this research gap using two 8-year experimental plots established in the Tibetan Plateau. Meanwhile, to better understand N-induced transformations of soil P fractions in terrestrial ecosystems, we further combined these field evidences with a meta-analysis of N addition experiments to quantify soil P fraction responses to N additions and decipher the key driver regulating soil P fractions.
2 Materials and methods
2.1 Study site description and experimental design
These experiments were conducted in Naqu Alpine Grassland Ecosystem National Field Scientific Observation and Research Station (Kaima Station, N30° 31′–31° 55′, E91° 12′–93° 02′; 4450 m a.s.l.) and Baingoin County (N31° 26′, E90° 02′; 4678 m a.s.l.). The former is an alpine meadow of typical alpine climate with a mean annual precipitation of 400 mm and a temperature of − 2.1 °C (Wang et al. 2022b). The plant community is dominated by perennial sedges Kobresia pygmaea and Kobresia humilis that are accompanied by forb species Potentilla multifida, P. bifurca, and Aster tataricus as well as grass species Stipa aliena (Jiang et al. 2016). The latter is an alpine steppe of a frigid, subhumid, and semiarid plateau monsoon climate with an annual mean temperature of − 1.2 °C and a precipitation of 301.2 mm. The dominant plant species is Stipa purpurea, and it is accompanied by Leontopodium leontopodioide and Heteropappus bowerii (Dong et al. 2019).
Grasslands of 300 × 300 m and 100 × 100 m were fenced in Naqu alpine meadow and Baingoin alpine steppe respectively in July 2013 to conduct fertilization experiments while keeping livestock out. The same experimental design was implemented in the two sites according to a randomized complete block arrangement. There were three N levels (N0: 0 g N m−2; N1: 7.5 g N m−2; N2: 15 g N m−2) with urea (CH4ON2) as the N fertilizer. Each treatment was replicated four times. The 12 plots (5 m × 5 m dimension) were deployed randomly, each surrounded by a 2-m wide buffer zone between adjacent plots to prevent cross-contamination.
2.2 Soil sampling and analyses
Soil samples were collected at the depth of 0–10 cm in August 2020. Seven soil cores were collected randomly in each plot. These soils were fully mixed to form one bulk sample for measuring the properties. Thus, a total of 24 soil samples (two grassland types × four replications × three N levels) were separately placed in sealed plastic bags after removing plant roots and stones manually and passing through a 2-mm sieve. Then the soil samples were immediately transported to laboratory.
Soil pH was measured in a mixture of soil:water (1:2.5) with a pH meter. Soil extractable Al3+, Fe3+, and Ca2+ were determined using the methods described by Hendershot et al. (2007). Potential alkaline phosphomonoesterase activity was determined as described by Kumar and Gill (2018). Soil microbial biomass P (MBP) was measured using the chloroform fumigation-extraction method (Turner and Wright 2013).
2.3 Measurement of soil P fractions
Soil P fractions were determined using sequential extraction procedure described by Tiessen and Moir (1993), which was developed by Hedley et al. (1982). Briefly, the first extraction was conducted using 30 mL of deionized water to extract 0.5-g air-dried soil in a 50-mL centrifuge tube. After centrifuging at 25,000 g for 10 min at 0 °C, the clear supernatant was transferred to another new centrifuge tube. Then, a series of extractions of the soil remaining in the tube were applied to characterize different soil P forms with 30 mL of 0.5 M NaHCO3 (pH 8.5), 0.1 M NaOH, 1 M HCl, and hot concentrated HCl sequentially. Finally, the remaining residual soil P was extracted with 10 mL 1 M hot (80 °C) concentrated HCl (C.HCl extractable P) for 10 min.
For extracts of 0.5 M NaHCO3 (pH 8.5), 0.1 M NaOH, and hot concentrated HCl, inorganic P (Pi) concentration was estimated as the difference between total P (TP) and organic P (Po) concentration (Tiessen and Moir 1993). Soil total P concentration was the sum of all nine fractions. Specifically, NaHCO3-Po and NaHCO3-Pi are defined as the labile fractions. The NaOH-Po and NaOH-Pi are considered to be intermediately labile fractions. Other P fractions, including D.HCl-Pi, C.HCl-Po, C.HCl-Pi, and residual P, are regarded as the recalcitrant P fractions. On the basis of previous studies, many researchers have summarized the ecological significance of the nine soil P fractions in terms of their bioavailability to better understand their roles in biogeochemical cycles (Table 1).
2.4 Literature data selection and meta-analysis
Peer-reviewed publications and dissertations before January 2023 were investigated using Web of Science, Google Scholar (http://scholar.google.com/), and China National Knowledge Infrastructure (CNKI, http://www.cnki.net) to build a compiled database. This database included the effects of N addition on soil P fractions, soil pH, soil MBP, and soil acid and alkaline phosphatase. The relevant climate conditions and fertilization practices were also included.
The criteria of selecting appropriate studies applied were as follows: (1) experimental sites must include both control and N addition treatments; (2) the values and sample sizes for the treatment and control groups were directly reported or could be extracted; (3) in order to compare the soil P fractions of different studies, we only included the uppermost soil layer that is most sensitive to nutrient input (Garcia-Palacios et al. 2015). The methods for soil P fractions in different studies are based on the Hedley method and its improved version (Hedley et al. 1982; Tiessen and Moir 1993). Based on these criteria, 30 articles, 6 dissertations, and this study, a total of 1033 records of soil P fractions and related physicochemical properties were selected. Besides direct data from tables and texts, we obtained indirectly the data by using GetData Graph Digitizer 2.25 software from figures (http://getdata-graph-digitizer.com/). In order to examine whether ecosystem type and N fertilizer form altered the response ratio of N addition, we categorized ecosystems into grassland, forest, wetland, and farmland and N application forms into NH4NO3, CO(NH2)2, and others.
2.5 Statistical analysis
First, two-way ANOVAs were used to test the effects of N level and grassland type on soil P fractions and soil properties (pH, Ca, Al, and Fe), soil microbial biomass P, and alkaline phosphatase activity at each sampling time in this study. Least significant difference (LSD) was applied to compare differences among all treatments.
Second, we used meta‐analysis to assess the influences of N addition on soil P fractions, soil pH, and soil phosphatase worldwide (Hedges et al. 1999). The effects of N addition were quantified by response ratio (RR), which was evaluated as:
with \(\overline{{X}_{\mathrm{t}}}\) and \(\overline{{X}_{\mathrm{C}}}\) as the arithmetic mean values in the treatment and the control groups respectively. The variance (ν) of \(lnRR\) was estimated as follows:
where nt and nc were the number of replicates and St and Sc were the SDs of treatment and control groups, respectively. The mean effect size (d + +) of N addition on soil fractions, soil pH, and soil phosphatase and the 95% confidence interval were calculated by mixed-effects models using the rma.mv function in the R package “metafor.” Because several papers had more than one response ratio, we also included the variable “study” as a random factor (Chen et al. 2018b). Besides, we analyzed all potential combinations of the moderator variables in a mixed‐effects meta-regression model by using the “glmulti” package in R (Calcagno and de Mazancourt 2010). The relative importance of a specific variable was calculated as the sum of Akaike weights for models that included this variable. These values can be considered the overall support for each variable across all models. A cutoff of 0.8 was set to differentiate between important and nonessential predictors (Chen et al. 2018b). Finally, the multivariable relationships between NaHCO3-Po and climatic factor such as MAT and MAP and edaphic factor such as soil pH and soil acid/alkaline phosphatase activity (soil APA) were tested using structural equation model (SEM). Testing of structure equation model was performed by using “piecewiseSEM” package (Lefcheck 2016). All statistical analyses were conducted in R version 4.1.0 (R Core Team 2020).
3 Results
3.1 Effects of N addition on alpine grassland soil properties
There was a significant effect of N addition on soil pH (Fig. 1 and Table S1), with an overall decrease in the two alpine grasslands. Soil alkaline phosphatase activity was significantly lower in N2 treatment compared to the control in the two alpine grasslands (Fig. 1 and Table S1), between which there was no difference. Soil microbial biomass P and soil exchangeable Al3+, Fe3+, or Ca2+ concentrations did not differ significantly among the N levels in either alpine grassland. However, exchangeable Al3+ and Fe3+ concentrations were significantly higher in alpine steppe than in alpine meadow, while soil microbial biomass P concentration and soil alkaline phosphatase activity were significantly higher in alpine meadow than in alpine steppe (Fig. 1 and Table S1).
Effects of N addition on soil pH (a), the activity of alkaline phosphatase (ALP) (b), and the concentration of microbial biomass P (MBP) (c), as well as extractable Ca2+ (d), Al3+ (e), and Fe3+ (f) concentrations in two alpine grasslands. Note: Error bars denote the standard error (n = 4). Different letters indicate significant difference (P < 0.05) between N addition treatments in the same alpine grassland. The graphs without letters mean no difference in means between treatments
3.2 Effects of N addition on alpine grassland soil P fractions and their percentage
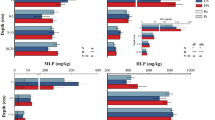
The different forms of soil P responded differently to N addition in the two alpine grassland types. In the alpine meadow, soil total P and total inorganic P significantly lower in N2 treatment than N1 and control treatments (Fig. 2). The NaHCO3-Pt and NaHCO3-Po concentration were significantly lower in N1 treatment than in N2 and control treatments. Nevertheless, C.HCl-Pt, C.HCl-Pi, and residual-Pt concentrations were significantly higher in N1 treatment than N2 and control treatments. The C.HCl-Po increased significantly while NaOH-Po decreased significantly with nitrogen level increment. Besides, resin-Pi, NaOH-Pt, NaOH-Pi, NaHCO3-Pi, and D.HCl-Pi did not change markedly among the different treatments (Fig. 3). However, N addition did not change any soil fraction significantly in the alpine steppe (Fig. 3).
Effects of N addition on the concentration (mg/kg) of soil total P (a), total Pi (b), and total Po (c) in two alpine grasslands. Note: Error bars denote the standard error (n = 4). Different letters indicate significant difference (P < 0.05) between N addition treatments in the same alpine grassland. The graphs without letters mean no difference in means between treatments
Effects of N addition on the concentrations (mg/kg) of NaHCO3-Pt (a), NaHCO3-Pi (b), NaHCO3-Po (c), resin-Pi (d), NaOH-Pt (e), NaOH-Pi (f), NaOH-Po (g), C.HCl-Pt (h), C.HCl-Pt (i), C.HCl-Pt (j), and residual-Pt (k) in soil of two alpine grasslands. Note: Error bars denote the standard error (n = 4). Different letters indicate significant difference (P < 0.05) between N addition treatments in the same alpine grassland. The graphs without letters mean no significant difference in means between treatments
Moreover, compared with the alpine steppe, NaHCO3-Pi, NaOH-Pt, NaOH-Pi, NaOH-Po, C.HCl-Pt, and C.HCl-Pi concentrations were significantly high in alpine meadow (Fig. 3 and Table S1). We also estimated the percentages of different P fractions in soil total P in two alpine grasslands. Organic P accounted for 40.7–54.0% and inorganic P accounted for 46.0–59.3% of soil total P in alpine meadow, while the proportions were 47.3–48.6% and 51.4–52.7% in alpine steppe (Fig. S1). In alpine meadow, NaOH-Po was the primary form of soil P fractions, accounting for 23.2–29.4% of total P. The other fractions ranked as C.HCl-Pi (17.9–28.7%) > C.HCl-Po (11.9–24.9%) > residual-Pt (11.1–20.7%) > NaOH-Pi (6.5–11.8%) > NaHCO3-Pi (1.8–3.8%) > NaHCO3-Po (0.2–5.9%) > D.HCl-Pi (0.3–1.1%) > resin-Pi (0.4–0.5%) (Fig. S1). In alpine steppe, NaOH-Po was also the predominant form of soil P fractions, accounting for 26.5–28.5% of total P, followed by C.HCl-Pi (19.8–22.4%) > residual-Pt (21.2–23.2%) > C.HCl-Po (16.8–18.7%) > NaOH-Pi (4.8–6.9%) > NaHCO3-Po (2.7–3.5%) > NaHCO3-Pi (2.0–2.6%) > D.HCl-Pi (0.5%) > resin-Pi (0.4–0.5%) (Fig. S1).
3.3 Relationships between soil properties and P fractions in alpine grasslands
Correlation analysis indicated that Al3+ concentration was negatively related to soil NaOH-Pt and NaOH-Po concentrations in alpine meadow, but positively related to C.HCl-Po concentration in alpine steppe (Table 2). Fe3+ concentration was negatively and significantly correlated with NaOH-Po, but positively related to C.HCl-Po concentration in alpine meadow. Soil exchangeable Ca2+ concentration was negatively associated with C.HCl-Pt and C.HCl-Pi concentrations in alpine meadow (Table 2). Soil pH was negatively related to C.HCl-Po, while positively associated with NaOH-Pt and NaOH-Po concentrations. Soil alkaline phosphatase (ALP) activity was positively related to resin-Pi concentration. Soil microbial biomass P concentration was negatively related to resin-Pi, NaOH-Pt, NaOH-Po, and total Po concentrations in alpine meadow, but only positively associated with NaOH-Pi concentration in alpine steppe (Table 2).
3.4 Meta-analysis and controls of N addition effect on soil P fractions
The meta-analysis showed that the response of NaOH-Pi concentration was significant and positive (3.7%) but that was significantly negative for soil pH and D.HCl-P concentration, − 4.7% and − 24.4%, respectively (Fig. 4). Our results also indicated that the effect of N addition led to a marginal decrease of NaHCO3-Po concentration by 8.63% (Fig. 4). However, other fractions did not change significantly under N addition.
Effects of N addition on total P, total Pi, total Po, soil acid/alkaline phosphatase activity, soil microbial biomass P, and available P concentration (a), as well as soil P fractions (b, c). Note: Error bars represent 95% confidence intervals (CI). The effect of N addition on soil properties and soil fractions is considered significant if the 95% CI does not overlap with zero (*P < 0.05, **P < 0.01, ***P < 0.001). The gray numbers on the left represent sample sizes of observations
We explored the relative influences of multiple experimental factors, environmental parameters, and soil properties on the responses of soil P fractions to N addition. The effect of N addition on NaHCO3-Po concentration was best predicted by soil acid/alkaline phosphatase activity (soil APA), MAP, N form, MAT, and soil pH, while other variables were of minor importance (Fig. 5a). Specifically, the effect on NaHCO3-Po concentration was negatively correlated with the effect of N addition on soil APA activity or soil pH.
Model-averaged importance of the predictors for the effects of N addition on the concentrations of NaHCO3-Po (a) and NaOH-Pi (b); relationships between log response ratio (lnRR) of NaHCO3-Po concentration and lnRR of soil pH (c) and soil acid/alkaline phosphatase activity (d), mean annual temperature (MAT) (e), and mean annual precipitation (MAP) (f); effects of N addition form (NH4NO3, urea, and their mixture) on NaHCO3-Po concentration (g). Note: horizontal dashed lines represent lnRR = 0. The sample size for NaHCO3-Po and NaOH-Pi in this analysis is 42. The relative importance is based on the sum of Akaike weights derived from model selection using corrected Akaike information criteria. Climatic zone: tropical/subtropical regions, temperate and alpine zones; ecosystem: farmland, marsh, grassland, and forest. Cutoff is set at 0.8 (black dashed line) to differentiate important from nonessential predictors
The SEM analysis showed that the important controlling factors for the global variations of NaHCO3-Po concentration response were the responses of soil pH and soil APA activity (Fig. 6). The effect on NaHCO3-Po concentration correlated negatively with the effect on soil pH (standardized coefficient = − 0.279, P = 0.055) and soil APA response (standardized coefficient = -0.371, P = 0.012). The climate and N rate could influence NaHCO3-Po concentration response via the change of soil pH and soil APA. For instance, the increase of MAP reduced NaHCO3-Po concentration by increasing soil pH (standardized coefficient = − 0.156, P < 0.001) and by decreasing soil APA (standardized coefficient = 0.083, P = 0.141). Similarly, higher N rate corresponded to higher NaHCO3-Po concentration through decreasing soil pH and soil APA (standardized coefficient = 0.017, P = 0.617; standardized coefficient = 0.186, P = 0.002, respectively) (Fig. 6).
Structural equation model (SEM) revealing the influences of mean annual precipitation (MAP), N rate, response of soil pH, and soil acid/alkaline phosphatase activity (soil APA) on the response of NaHCO3-Po concentration. Note: The solid lines refer to significant relationships, whereas the dashed lines refer to non-significant relationships. Asterisks indicate different significance levels (*P < 0.05; **P < 0.01; ***P < 0.001)
4 Discussion
In this study, we combined two manipulative field experiments and a meta-analysis to examine the responses of soil P fractions to N addition. Our results showed that N addition reduced total P and total Pi concentrations and changed soil Pi (i.e., C.HCl-Pi and residual-Pt) and Po (i.e., NaCOH3-Po, NaOH-Po, and C.HCl-Po) fractions in alpine meadow. However, different from field experiments, meta-analysis found that soil Pi (i.e., D.HCl-P and NaOH-Pi) and Po (i.e., NaCOH3-Po) fractions based on large-scale results were shifted by N addition (Fig. 3). Although N addition had no consistent effects from field experiments to global analysis, N-induced changed soil P fractions implied that P limitation may be alleviated with increasing N loading. Both field experiments and global analysis highlighted, to some extent, the importance of soil pH and phosphatase in determining soil P fractions. Besides, field experiments emphasized that extractable cations (i.e., Ca2+) played an important role in mediating soil P fractions, while meta-analysis indicated that environmental factor (e.g., MAP) and experimental variables (N rate) were also the key predictors of soil NaCOH3-Po concentration. Below, we discussed potential mechanisms for soil P fractions to N addition from alpine region to global terrestrial ecosystems.
4.1 Differential responses of soil P fractions to N addition
Our results demonstrated that N1 treatment significantly increased occluded P (C.HCl-Pt) but reduced labile P concentration (NaHCO3-Pt), as well as N2 treatment reduced occluded P (residual-Pt) in alpine meadow (Fig. 3). Compared with field experiments, the decrease in labile Po (NaHCO3-Po) concentration was marginally significant but no significant change in occluded P concentration with N addition in meta-analysis (Fig. 4). Labile Pi is weakly adsorbed to Fe and Al at mineral surfaces, while labile Po can be easily adsorbed on the soil surface and readily mineralizable through phosphatase from plants and microbes (Hou et al. 2018b). With increasing N input into ecosystem, higher plant growth and P demand would stimulate the release of phosphatase to mineralize more labile Po and supply more available P. At the same time, plants can also secrete root exudates, including carbon sources to exchange P nutrient with soil bacteria coding phoD or phoC gene that produce phosphatase (Luo et al. 2022). Under these circumstances, soil microorganisms and plants can replenish labile Po through organic P mineralization (Chen and Moorhead 2022). However, N-induced soil acidification may reduce plant and microbial P demand by restraining their growth and activity, which may decrease release of phosphatase to soil. Soil phosphatase activity suppressed by lowering soil pH could slow down organic P mineralization. Furthermore, reductions in soil pH with increasing N loading could also affect the persistence and buildup of organic P indirectly by mobilizing P from secondary minerals of aluminum, calcium, and iron in soil (Hou et al. 2018c). This might explain why changes of labile Po (NaHCO3-Po) concentration with soil phosphatase activity or soil pH showed a tendency to decrease in N1 and then increase in N2 treatment.
As the most recalcitrant P fractions physically encapsulated by secondary minerals (Cross and Schlesinger 1995), occluded P included about 15% organic P and 85% inorganic P (Hedley et al. 1982). Short period or low level of N addition would generally stimulate plant growth (76.87 vs. 87.16 g m−2 of the two treatments, data not shown) and enhance P demand, resulting in the reduction in labile P. Besides, N-induced soil acidification likely facilitated the turnover of more labile Pi to occluded P (Yao et al. 2022), which to some extent caused the increase of occluded P concentration. However, long-term or high-level N input aggravated soil acidification and suppressed plant growth and microbial activity, which decreased P uptake and retention of plant and microbes, as well as accelerated solubilization of Pi and caused occluded P concentration to increase in alpine meadow. These reasons could explain the variations of soil occluded P pool in field experiments or meta-analysis.
Different from field experiments, our meta-analysis also showed N-induced reductions in soil primary mineral P (D.HCl-P) concentration but an increase in secondary mineral P (NaOH-Pi) concentration (Figs. 4 and S3), implying that N addition significantly changed soil P fractions in terrestrial ecosystems. Primary mineral P (D.HCl-P) was mainly regulated by soil pH in natural terrestrial ecosystems (Hou et al. 2018a). Thus, N-induced soil acidification can well explain the reduction of primary mineral P. It is possible that primary mineral P was much less stable in acidic soil (Helfenstein et al. 2020). The data of our meta-analysis were mainly from subtropical and tropical forests with high MAP where soil acidification was more serious under increasing N loading. N-induced soil acidification could further result in dissolution of mineral phases and release exchangeable cations (Al3+, Fe3+, or Ca2+) to form Fe and Al oxides or carbonate that are easily precipitated or adsorbed by phosphate (Fernandez et al. 2003). Incremental Al/Fe oxide with N input can account for the increase of secondary mineral P (NaOH-Pi) concentration. Therefore, an increase in NaOH-Pi concentration but a decrease in D.HCl-P concentration may be closely related to N-induced soil acidification and changes of exchangeable cations.
Although these transformations among labile P, moderately labile P, and occluded P concentration under different N input conditions may replenish available P and consequently relieve P limitation with N addition, these P turnover processes may increase risk of P leaching losses. Given that total P and total Pi concentration also decreased under N2 treatment (Fig. 2), it is likely that the mobilization of P accelerated soil P removal by means of plant uptake and leaching losses (Tran et al. 2020). Differing from soil Pi, soil total Po in either grassland was not affected significantly by N addition (Fig. 3c), which, in agreement with previous research (Zhang et al. 2020; Wang et al. 2022a), suggested that mineralization of soil organic P was depressed in the present study. The probable reason was that light degradation of litter and mineralization of Po together with plant P uptake make soil Po concentration reach a dynamic balance (Kritzler and Johnson 2009). Despite non-significant changes in soil total Po concentration in alpine meadow, large variation among soil organic P fractions (NaCOH3-Po, NaOH-Po, C.HCl-Po) could clearly occur in response to N addition (Fig. 3). Besides N-induced shift in soil phosphatase activity could regulate Po transformation, soil pH indirectly affects the forms of organic P in both absolute concentration and relative proportion by affecting metal ions (Hou et al. 2018c).
4.2 Factors influencing on soil P fractions
Despite the ratio of soil C:N and N:P in alpine meadow has not been significantly altered by N addition (Table S2), recent meta-analysis showed that N addition increased significantly the ratio of MBN:MBP, as well as the ratio of shoot C:N and N:P in grasslands (Xu et al. 2022). The variations in the C:N:P ratios of plant and microbes indicate that with the increasing N loading, the ecosystem may gradually shift from N limitation to P limitation in alpine meadow. The stoichiometric ratio of C, N, and P in alpine meadow was a possible reason for the changed NaHCO3-Pt through affecting plant growth and microbial metabolism. The significant relationships between labile P (NaHCO3-Pt) concentration and the ratio of soil C:N and N:P supported the above assumptions. Our results also demonstrated significant relationships between soil pH or Ca2+ concentration and occluded P (C.HCl-Pt, C.HCl-Po, and C.HCl-Pi) concentration (Table 2), which indicated that N addition promoted transformation occluded P to labile P by decreasing soil pH and changing extractable Ca2+ concentration in alpine meadow. Previous studies suggested that changes in metal cations played an important role in the solubilization of occluded P (Hou et al. 2018a, b, c). Wang et al. (2022a) found that N addition promoted the dissolution of recalcitrant Ca-bound P to occluded P by decreasing soil pH. These results were consistent with those of this study and suggested the critical role of soil pH or Ca2+ concentration in soil P transformation of alpine meadow. Besides, as enzyme cofactors, exchangeable Ca2+ could influence genes abundance such as phoD, phoX, and βPPhy in soil microbial communities, which potentially changed P fractions (Neal and Glendining 2019) and deserved further research.
Although N-induced responses were negatively correlated with soil phosphatase activity and soil pH (Fig. 5), N addition (rate) drove change of labile Po (NaHCO3-Po) concentration primarily by modification of soil phosphatase activity but not soil pH at large spatial scale (Figs. 6 and S3), which highlighted that biotic factors played a vital role in P mineralization owing to the ability of producing soil phosphatase by plants and soil microbes. Soil microorganisms themselves can replenish soil labile P through Po mineralization, P release during microbial turnover, and Pi dissolution (Li et al. 2022). At the species level, plants have evolved a set of traits involving physiology, root morphology, and symbiotic mycorrhizal relations to increase P acquisition and relieve P limitation (Ding et al. 2021). Higher plant species diversity tended to result in higher soil phosphatase activity at community level and mineralize more Po to promote plant growth (Chen et al. 2022). In addition, direction and magnitude of soil P transformation among various fractions may differ with soil acidity-alkalinity and buffering capacity. In acidic soil, N-induced soil acidification likely facilitated the turnover of more labile Pi to occluded P (Yao et al. 2022). In alkaline soil with excessive N input, the main transformation may be from recalcitrant Ca-bound Pi to moderately occluded (e.g., Al- and Fe-bound) P (Wang et al. 2022a). Hence, the mechanisms underlying the interactions among plants and microbes in soil P cycling deserve further research, especially P transformations processes linking phosphorus-acquisition strategies using more soil types. Overall, though our SEM model did not include any soil Pi fraction, the crucial role that soil pH played in soil phosphorus cycle involving dissolution of Pi and Po mineralization processes could not be neglected. Higher MAP tended to aggravate soil acidification with N addition (Fig. 6), suggesting that weaker mineralization of Po but stronger Pi dissolution may occur with increasing N deposition in terrestrial ecosystems. Therefore, soil phosphatase-dominated mineralization and soil pH-dominated dissolution processes were essential to meet the enhanced P demand and maintain the balance of soil labile P in the various ecosystems.
5 Conclusion
Collectively, our conclusions could be summarized as follows: (1) N addition stimulated solubilization of Pi in alpine meadow rather than in alpine steppe; (2) soil inorganic P in the alpine meadow was associated with Ca2+ and soil pH; (3) meta-analysis showed that N addition decreased soil pH, labile Po (NaHCO3-Po), and primary mineral P (D.HCl-Pi) concentration but increased secondary mineral P (NaOH-Pi) concentration; (4) SEM model indicated soil pH and soil phosphatase drove P mineralization. Overall, these findings suggested that P transformation processes is closely linked to biotic factors (plant and microbial phosphorus-acquisition strategies). Further experimental studies are needed to target essential factors and interactions so as to conclusively determine complex mechanism regarding ecosystem P limitations with increasing nitrogen deposition.
5 References
Augusto L, Achat DL, Jonard M et al (2017) Soil parent material-a major driver of plant nutrient limitations in terrestrial ecosystems. Glob Chang Biol 23:3808–3824
Block CE, Knoepp JD, Fraterrigo JM et al (2012) Interactive effects of disturbance and nitrogen availability on phosphorus dynamics of southern Appalachian forests. Biogeochemistry 112:329–342
Buol SW, Southard RJ, Graham RC et al (2011) Soil genesis and classification. John Wiley & Sons
Calcagno V, de Mazancourt C (2010) glmulti: an R package for easy automated model selection with (generalized) linear models. J Stat Softw 34:1–29
Chen H, Chen ML, Li DJ et al (2018a) Responses of soil phosphorus availability to nitrogen addition in a legume and a non-legume plantation. Geoderma 322:12–21
Chen J, Luo YQ, Garcia-Palacios P et al (2018b) Differential responses of carbon-degrading enzyme activities to warming: implications for soil respiration. Glob Chang Biol 24:4816–4826
Chen J, van Groenigen KJ, Hungate BA et al (2020) Long-term nitrogen loading alleviates phosphorus limitation in terrestrial ecosystems. Glob Chang Biol 26:5077–5086
Chen J, Moorhead DL (2022) Progressively decreased nitrogen-stimulation of soil phosphatase activity with long-term nitrogen addition. Appl Soil Ecol 169:104213
Chen XL, Chen HYH, Chang SX et al (2022) Meta-analysis shows that plant mixtures increase soil phosphorus availability and plant productivity in diverse ecosystems. Nat Ecol Evol 6:1112–1121
Cross AF, Schlesinger WH (1995) A literature review and evaluation of the Hedley fractionation: applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 64:197–214
Dai ZM, Liu GF, Chen HH et al (2019) Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J 14:757–770
Deng M, Liu L, Sun Z et al (2016) Increased phosphate uptake but not resorption alleviates phosphorus deficiency induced by nitrogen deposition in temperate Larix principis-rupprechtii plantations. New Phytol 212:1019–1029
Deng Q, Hui DF, Dennis S et al (2017) Responses of terrestrial ecosystem phosphorus cycling to nitrogen addition: a meta-analysis. Global Ecol Biogeogr 26:713–728
Ding WL, Cong WF, Lambers H (2021) Plant phosphorus-acquisition and -use strategies affect soil carbon cycling. Trends Ecol Evol 36:899–906
Dong JF, Che RX, Jia SG et al (2019) Responses of ammonia-oxidizing archaea and bacteria to nitrogen and phosphorus amendments in an alpine steppe. Eur J Soil Sci 71:940–954
Dong JF, Cui XY, Niu HS et al (2022) Effects of nitrogen addition on plant properties and microbiomes under high phosphorus addition level in the alpine steppe. Front Plant Sci 13:89436
Elser JJ, Bracken MES, Cleland EE et al (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Fan YX, Lin F, Yang LM et al (2018) Decreased soil organic P fraction associated with ectomycorrhizal fungal activity to meet increased P demand under N application in a subtropical forest ecosystem. Biol Fert Soils 54:149–161
Fernandez IJ, Rustad LE, Norton SA et al (2003) Experimental acidification causes soil base-cation depletion at the Bear Brook Watershed in Maine. Soil Sci Soc Am J 67:1909–1919
Garcia-Palacios P, Vandegehuchte ML, Shaw EA et al (2015) Are there links between responses of soil microbes and ecosystem functioning to elevated CO2, N deposition and warming? A global perspective. Glob Change Biol 21:1590–1600
Hedges LV, Gurevitch J, Curtis PS et al (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J 46:970–976
Helfenstein J, Pistocchi C, Oberson A et al (2020) Estimates of mean residence times of phosphorus in commonly considered inorganic soil phosphorus pools. Biogeosciences 17:441–454
Hendershot WH, Lalande H, Duquette M (2007) Ion exchange and exchangeable cations. Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton, FL, USA, pp 197–206
Hou EQ, Chen CR, Luo YQ et al (2018a) Effects of climate on soil phosphorus cycle and availability in natural terrestrial ecosystems. Glob Chang Biol 24:3344–3356
Hou EQ, Tan X, Heenan M et al (2018b) A global dataset of plant available and unavailable phosphorus in natural soils derived by Hedley method. Sci Data 5:180166
Hou EQ, Wen DZ, Kuang YW et al (2018c) Soil pH predominantly controls the forms of organic phosphorus in topsoils under natural broadleaved forests along a 2500 km latitudinal gradient. Geoderma 315:65–74
Hou EQ, Luo YQ, Kuang YW et al (2020) Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat Commun 11:637
Hou EQ, Wen DZ, Jiang LF et al (2021) Latitudinal patterns of terrestrial phosphorus limitation over the globe. Ecol Lett 24:1420–1431
Jiang LL, Wang SP, Pang Z et al (2016) Grazing modifies inorganic and organic nitrogen uptake by coexisting plant species in alpine grassland. Biol Fert Soils 52:211–221
Kritzler UH, Johnson D (2009) Mineralisation of carbon and plant uptake of phosphorus from microbially-derived organic matter in response to 19 years simulated nitrogen deposition. Plant Soil 326:311–319
Kumar V, Gill KD (2018) To estimate the activity of alkaline phosphatase in serum. In: Basic concepts in clinical biochemistry: a practical guide. Springer, Singapore
Lefcheck JS (2016) piecewiseSEM: piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol Evol 7:573–579
Li Q, Lv JH, Peng CH et al (2021) Nitrogen-addition accelerates phosphorus cycling and changes phosphorus use strategy in a subtropical Moso bamboo forest. Environ Res Lett 16:024023
Li YY, Dong SK, Wen L et al (2014) Soil carbon and nitrogen pools and their relationship to plant and soil dynamics of degraded and artificially restored grasslands of the Qinghai-Tibetan Plateau. Geoderma 213:178–184
Li ZL, Zhang DY, Peng YF et al (2022) Divergent drivers of various topsoil phosphorus fractions across Tibetan alpine grasslands. J Geophys Res-Biogeo 127:e2022JG006795
Lu CQ, Tian HQ (2007) Spatial and temporal patterns of nitrogen deposition in China: synthesis of observational data. J Geophys Res-Biogeo-Atmos 112:D22S05
Lu XK, Mo JM, Gilliam FS et al (2012) Nitrogen addition shapes soil phosphorus availability in two reforested tropical forests in Southern China. Biotropica 44:302–311
Luo M, Moorhead DL, Ochoa-Hueso R et al (2022) Nitrogen loading enhances phosphorus limitation in terrestrial ecosystems with implications for soil carbon cycling. Funct Ecol 36:2845–2858
Mahowald N, Jickells TD, Baker AR et al (2008) Global distribution of atmospheric phosphorus sources, concentrations and deposition rates, and anthropogenic impacts. Global Biogeochem Cy 22:GB4026
Neal AL, Glendining MJ (2019) Calcium exerts a strong influence upon phosphohydrolase gene abundance and phylogenetic diversity in soil. Soil Biol Biochemistry 139:107613
Penuelas J, Janssens IA, Ciais P et al (2020) Anthropogenic global shifts in biospheric N and P concentrations and ratios and their impacts on biodiversity, ecosystem productivity, food security, and human health. Glob Chang Biol 26:1962–1985
Team RC (2020) R: a language and environment for statistical computing, version 4.1.0. Vienna, Austria: R Found Stat Comput 2013
Tiessen H, Moir JO (1993) Characterization of available P by sequential extraction. soil sampling and methods of analysis. Lewis Publishers, Boca Raton 75–86
Tran CTK, Watts-Williams SJ, Smernik RJ et al (2020) Effects of plant roots and arbuscular mycorrhizas on soil phosphorus leaching. Sci Total Environ 722:137847
Turner BL, Lambers H, Condron LM et al (2012) Soil microbial biomass and the fate of phosphorus during long-term ecosystem development. Plant Soil 367:225–234
Turner BL, Wright SJ (2013) The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry 117:115–130
Wang DJ, Zhou HK, Yao BQ et al (2020) Effects of nutrient addition on degraded alpine grasslands of the Qinghai-Tibetan Plateau: a meta-analysis. Agr Ecosyst Environ 301:106970
Wang RZ, Yang JJ, Liu HY (2022a) Nitrogen enrichment buffers phosphorus limitation by mobilizing mineral-bound soil phosphorus in grasslands. Ecology 103:e3616
Wang YF, Jiang LL, Wang ZS et al (2022b) Phosphorus enrichment increased community stability by increasing asynchrony and dominant species stability in alpine meadow of Qinghai‐Tibet Plateau. J Geophys Res-Biogeo 127:e2022bJG006819
Xu H, Qu Q, Li G et al (2022) Impact of nitrogen addition on plant-soil-enzyme C-N–P stoichiometry and microbial nutrient limitation. Soil Biol Biochemistry 170:108714
Yang GB, Peng YF, Abbott BW et al (2021) Phosphorus rather than nitrogen regulates ecosystem carbon dynamics after permafrost thaw. Glob Chang Biol 27:5818–5830
Yang K, Zhu JJ, Gu JC et al (2015) Changes in soil phosphorus fractions after 9 years of continuous nitrogen addition in a Larix gmelinii plantation. Ann Forest Sci 72:435–442
Yang X, Post WM (2011) Phosphorus transformations as a function of pedogenesis: a synthesis of soil phosphorus data using Hedley fractionation method. Biogeosciences 8:2907–2916
Yao XY, Hui DF, Hou EQ et al (2022) Differential responses and mechanistic controls of soil phosphorus transformation in Eucalyptus plantations with N fertilization and introduced N2-fixing tree species. New Phytol 237:2039–2053
Yu GR, Jia YL, He NP et al (2019) Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat Geosci 12:424–429
Zeng QX, Zeng XM, Lin KM et al (2020) Responses of soil phosphorus fractions and microorganisms to nitrogen application in a subtropical Phyllostachys pubescen forest. J Appl Ecol 31:753–760
Zhang HZ, Shi LL, Fu SL et al (2020) Effects of nitrogen deposition and increased precipitation on soil phosphorus dynamics in a temperate forest. Geoderma 380:114650
Zhou J, Li XL, Peng F et al (2021) Mobilization of soil phosphate after 8 years of warming is linked to plant phosphorus-acquisition strategies in an alpine meadow on the Qinghai-Tibetan Plateau. Glob Chang Biol 27:6578–6591
Acknowledgements
We thank for all the researchers whose data were included in this meta-analysis.
Funding
This work was supported by the National Natural Science Foundation of China (42041005, 31971530).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Caixian Tang
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, W., Jiang, L., Li, T. et al. Unraveling the consequences of nitrogen addition on soil phosphorus fractions in alpine grasslands: insights from field experiments and global analysis. J Soils Sediments 23, 3721–3734 (2023). https://doi.org/10.1007/s11368-023-03639-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-023-03639-z