Abstract
Purpose
Effects of aeration on distribution and release potential of organic phosphorus in sediments are of great significance. The aim of this study was to investigate effects of fine bubble aeration at the sediment‒water interface on species distributions of organic phosphorus and related microbial activities in the sediments from Nanfei River, a seriously polluted urban river in Hefei City, Anhui Province, China.
Materials and methods
A simulation experiment with a precision oxygen distribution system with fine bubble was applied and the sediments in system were taken out at intervals to test various indicators, mainly including the contents of phosphorus, species distribution of organic phosphorus, microbial biomass (MBC), and alkaline phosphatase activity (APA), as well as the number of phosphate solubilizing bacteria.
Results
The results showed that the content of dissolved organic carbon (DOC) increased in the sediments, along with the decrease of pH and the significant increase of oxidation–reduction potential (ORP). The content of total phosphorus and inorganic phosphorus in the surface sediments generally presented a downward trend when the content of organic phosphorus increased first and then decreased during aeration. The variation trends of liable organic phosphorus and moderately labile organic phosphorus were similar to organic phosphorus, while the content of non-labile organic phosphorus was slightly decreased. In addition, the MBC and APA increased. The number of organic phosphorus mineralizing bacteria (OPB) increased while that of inorganic phosphorus solubilizing bacteria (IPB) decreased, and the number of OPB was significantly correlated to APA, which corresponds to the mineralization mechanism of organic phosphorus.
Conclusions
There were two stages of phosphorus transformation in the surface sediments during the aeration treatment: the stabilization of inorganic phosphorus in the early stage and the mineralization of organic phosphorus latterly, which means an increased risk of phosphorus release into the water in the late stage. Therefore, the fine bubble aeration treatment at the sediment‒water interface applied to controlling the internal pollution of water bodies should be considered, especially focusing on the strict control of the aeration time. Overall, the present study can provide scientific guidance for in situ remediation of heavily polluted sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
It is universally accepted that phosphorus plays an extremely important role in the cycling of nutrient elements in water bodies and is a key factor for triggering eutrophication (Rydin et al. 2011; Zhu et al. 2013; Yuan et al. 2019). Sediment is regarded as a storage reservoir of phosphorus in water bodies, when external phosphorus loading pollution is effectively controlled; the release of internal phosphorus loading in sediments is a main source of phosphorus for overlying water (Golterman 2001; Shilla et al. 2009; Shan et al. 2016; Zhu et al. 2017a). Phosphorus in sediments can be divided into inorganic phosphorus (Pi) and organic phosphorus (Po) (Wang et al. 2013; Wan et al. 2020). Lots of studies mainly focused on total phosphorus and inorganic phosphorus; however, less attention has been paid to the migration and transformation of organic phosphorus (Paerl et al. 2011). In fact, organic phosphorus is an extremely crucial part of internal phosphorus loading (Worsfold et al. 2008; Liu et al. 2015). A large number of studies have shown that the relative contribution of organic phosphorus in sediments to total phosphorus can reach 80% (Dong et al. 2016), which is important for the migration of phosphorus at the sediment‒water interface. Furthermore, recent studies have drawn a conclusion that although the migration of iron and manganese combined phosphorus is the most important mechanism of phosphorus releasing in eutrophic waters, the active organic phosphorus components of sediments drove phosphorus releasing in eutrophic waters (Gonsiorczyk et al. 1998). Therefore, it is of great significance to study the content and morphological characteristics of organic phosphorus in sediments.
It was proved that microbial activities had important impacts on the releasing of phosphorus in sediments (Li et al. 2008; Ni et al. 2015). Microbes can release bounded phosphorus through processes such as metabolic reactions, extracellular release, and cell lysis, thereby affecting the geochemical cycle of phosphorus (Zhu et al. 2017b). Phosphate solubilizing bacteria (PSB) are a type of functional microorganisms that can convert insoluble and invalid phosphorus in the sediments into water-soluble available phosphorus (Rodrı́Guez and Fraga 1999). According to the different species of phosphates, phosphorus solubilizing bacteria can be categorized as organic phosphorus mineralizing bacteria (OPB) and inorganic phosphate solubilizing bacteria (IPB). OPB can promote the decomposition of organic phosphorus and releasing available phosphorus, and IPB can promote the conversion of inorganic phosphorus into soluble phosphorus (Li et al. 2018). The mineralization and degradation of organic phosphorous is mainly catalyzed by phosphatase secreted by phosphorus solubilizing bacteria. Phosphatase is an extracellular enzyme that can catalyze the hydrolysis of organic phosphorus into inorganic phosphorus, thereby improving the bioavailability of phosphorus (Zhou et al. 2008; Lü et al. 2016), in which alkaline phosphatase (ALPase) is one of the important microbial enzymes (Kobayashi et al. 1983; Ma et al. 2019).
Artificial aeration is one of in situ treatment technologies to control the release of phosphorus from sediments. Many pieces of research have explored the release and transformation of total phosphorus and inorganic phosphorus in sediments by aeration and oxygenation. For example, researches have shown that dissolved oxygen was a vital factor affecting the release of total phosphorus in sediments, and anaerobic state would accelerate the release of phosphorus in sediments. While aeration and re-oxygenation would effectively inhibit the release of phosphorus in sediments, which was mainly because aeration could increase the oxidation–reduction potential of the micro-environment at the sediment–water interface, thus enhancing phosphorus adsorption and fixation in the sediments (Roden and Wetzel 2002; Wu et al. 2014; Zou et al. 2018; Yuan et al. 2020a). However, there were few reports on the influence of the aeration process on the distribution and release potential of organic phosphorus from the sediments in heavily polluted urban rivers.
Therefore, this study developed the sediment–water interface precision air distribution system and explored the effects of fine bubble aeration on the distribution and release of the potential of organic phosphorus from the sediments in the Nanfei River, which is a heavily polluted urban river in Hefei City. We analyzed variations of different species of organic phosphorus, phosphatase, and microbial activities related to the degradation of organic phosphorus during the fine bubble aeration process in the sediments; we also discussed the microbiological mechanism which affected the release and transformation of organic phosphorus in the sediments. These would provide technical support for in situ remediation in heavily polluted urban rivers.
2 Materials and methods
2.1 Setup of simulation experiment
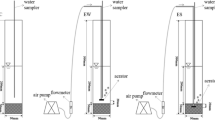
The simulation fine bubble aeration experiment was made up of a hard plexiglass water tank with length, width, and height of 1000 mm, 1000 mm, and 1500 mm, respectively. The fine bubble aeration system was placed 2 cm above the surface of the sediments which consisted of nano-aperture aeration plate (tube outer diameter 1.2 cm, bubble diameter 100–200 μm), the detailed parameters followed as the inner tube spacing of the disk of about 5 cm, and the diameter of the aeration disk of 800 mm. A long rubber hose was used to connect the aeration disk with a blower connected with adjustable flow, and a switch device was installed to switch the blower on and off. The simulation experiment is shown in Fig. 1.
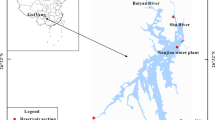
The sediment samples in the experiment were collected with a metal grabbing sampler from the main urban section of the Nanfei River, seriously polluted by industrial wastewater and urban domestic sewage. Therefore, the water quality in Nanfei River was mostly Class V or even worse than Class V of the national surface water standards. Firstly, the collected sediments and overlying water (deionized water) were thoroughly mixed, respectively, and then 20-cm-thick bottom sediments were put into each simulation equipment. Finally, the deionized water was slowly poured into the tank to make the water deep reach 80 cm. After the system was kept stable for 10 days, the fine bubble aeration treatment test was set up. The intermittent aeration was employed, with continuous aeration for 8 h every 24 h. The aeration volume was 4040 Lh−1. The whole simulation experiment was conducted for 21 days. The surface sediment samples were collected every 3 days with the quarter method at different points before each aeration, and then mixed thoroughly.
2.2 Analysis for physical and chemical parameters
Dissolved organic carbon (DOC) in the sediments was determined by the ultrapure water extraction-organic carbon analyzer method (Qi et al. 2014). The total organic carbon (TOC) was measured by the total organic carbon analyzer method, using a total organic carbon analyzer (TOC-V CPN) (Shimadzu Corporation, Japan) with a solid sampler (SSM-5000A) (Qi et al. 2014). The pH and oxidation–reduction potential (ORP) values of the sediment‒water interface were determined in real-time through a fine bubble aeration device equipped with sensors. During the aeration period, the data were recorded after opening the aeration device and before closing the device every day.
2.3 Extraction and determination of different species of organic phosphorus
After being calcined at 500 °C for 2 h in muffle furnace and then extracted by 1molL−1 HCl (16 h), the TP can be obtained by determining phosphorus concentration in the extract. Inorganic phosphorus (IP) was measured by direct extraction with 1molL−1 HCl (16 h), and then analyzed through colorimetry using the molybdate blue method, and total organic P (TOP) in the extract was calculated as the difference between TP and IP (Ivanoff et al. 1998; Liu et al. 2015).
The classification method of sediment organic phosphorus forms. The continuous extraction method of Ivanoff et al. (1998) was applied to classify the organic phosphorus in the surface sediments in the fine bubble aeration device. The organic phosphorus is divided into labile organic phosphorus (LOP), moderately labile organic phosphorus (MLOP), and non-labile organic phosphorus (NLOP) (Ivanoff et al. 1998; Lü et al. 2015). Labile organic phosphorus was initially extracted with 0.5 molL–1 NaHCO3 at pH 8.5. The extracted P (total labile P, TP) includes both OP (LOP) and inorganic P (Pi) in the supernatant. Moderately labile organic phosphorus was extracted with 1.0 molL–1HCl (including HCl-OP and Pi), followed by 0.5 molL–1NaOH. The NaOH extract was acidified with HCl until pH 0.2 to separate the non-labile fraction (humic acid-Po, HAOP) from the moderately labile fraction (fulvic acid-Po, FAOP). Finally, the highly resistant, residual organic phosphorus (Re-OP) was determined by ashing the residue from the NaOH extraction at 550 °C for 1 h, followed by dissolution in 1 molL–1 H2SO4. The moderately labile OP (MLOP) and non-labile OP (NLOP) were then calculated as the sum of HCl-OP and FAOP, and the sum of HAOP and Re-OP, respectively.
In all cases, the phosphorus concentration in the extracts was determined colorimetrically by the phospho-molybdate method. The OP in the extracts was calculated by the difference between TP and Pi, and TP in all extracts was calculated with an aliquot that was digested with K2S2O8 and H2SO4. The recovery of organic P was calculated by the ratio of the sum of organic P extracted and TOP.
2.4 Analysis of microbial activities
The determination of microbial biomass carbon (MBC) method referred to the chloroform fumigation extraction method (Anderson and Domsch 1978). The Monkina media with organic phosphorus and inorganic phosphorus was used to separate, cultivate, and count OPB and IPB in sediments for determining the number of phosphate-dissolving bacteria in the sediments (Qian et al. 2010). The determination of alkaline phosphatase activity in sediments was based on the determination method of alkaline phosphatase activity in soil (Tabatabai and Bremner 1969). The method of diphenyl sodium phosphate was applied to calculate the activity of alkaline phosphatase by the rate of phenol formation during hydrolysis after adding matrix diphenyl sodium phosphate. The alkaline phosphatase activity was expressed as the number of milligrams of phenol released per hour of 1 g of dry sediments at 37 °C for 24 h, and the unit is mg (g d)−1.
2.5 Data statistical analysis
In the study, all of the analyses were performed in triplicate and the results were expressed in mean and standard error (n = 3). Origin 2018 and SPSS 19.0 statistical software were used to deal with the experimental data. All data were statistically analyzed by the ANOVA mutation analysis program. Duncan’s new multiple test method was used to analyze the significance of the difference between the microbial index data and the data of various forms of phosphorus. When P < 0.05, the difference was regarded as statistically significant. A correlation analysis was used to examine the relationships between the microbial indexes and different fractions of organic phosphorus using P-values of 0.05and 0.01.
3 Results and discussion
3.1 Impacts of fine bubble aeration on the physical and chemical conditions in surface sediments
3.1.1 Variation in TOC and DOC in sediments
The effect of fine bubble aeration on the organic matter contents of the surface sediments during the simulation experiment was investigated, and the results are shown in Fig. 2. During the fine bubble aeration, the total organic carbon (TOC) value of the surface sediments in the device showed an overall increasing trend. In the first 12 days of the aeration process, The TOC value has been on the rise. Although it has declined afterward, it has risen again in the later stage of aeration, and reached the highest value at 21 days of aeration, indicating that fine bubble aeration at the sediment–water interface can improve surface sediment TOC content. The TOC content can characterize the total amount of organic matter in the sediments, thus signifying that the rate of microbial synthesis of organic matter was greater than the rate of utilization. Meanwhile, part of the organic matter was proved to be combined with phosphorus to form organic phosphorus. In addition, the accumulation and distribution of OP in the sediments are known to be greatly influenced by organic matter, thus TOC is of great significance to the form of organic phosphorus (Müller et al. 2016). Moreover, studies have confirmed that organic matter plays a key role in retaining OP, and OP can be likely to increase with the increase of organic matter content (Zhu et al. 2015). Thus, the increased TOC may be the potential reason accounting for the accumulation of organic phosphorus at the initial stage of aeration in later experiments.
During the period of fine bubble aeration, the DOC contents of the surface sediments also exhibited an overall increasing trend (Fig. 2), which began to increase significantly after 9 days of aeration, and slightly decreased after 21 days of aeration (211.7 mg kg−1), but it was still much higher than that of the beginning stage (135.1 mg kg−1). It suggested that the fine bubble aeration at the sediment–water interface can increase the DOC content in the surface sediments. This may the result of mineralization of organic matter, which was likely to be enhanced when the microbial activity is increased under aeration (Chen et al. 2011; Preston and Basiliko 2016; Schiebel et al. 2020). DOC is an important carbon source for the growth and metabolism of microorganisms, as well as a product of microbial metabolism, can comprehensively reflect the metabolic activity of microorganisms. Notably, the previous search from Müller et al. (2016) who examined the effects of different C sources addition on the release of sediments confirmed that dissolved organic carbon can stimulate the release of phosphorus. Therefore, the increase of DOC may be responsible for the continuous decrease of total phosphorus in sediments during the aeration in later experiments.
3.1.2 The pH and ORP of the overlying water
The effect of fine bubble aeration on the pH values and ORP of the overlying water at the sediment–water interface during the simulation experiment was investigated, and the results are shown in Fig. 3. During the aeration, the pH values of the overlying water at the sediment–water interface exhibited an overall downward trend, from 8.09 at the beginning of the aeration to 7.82 at the end of the aeration. The pH value of the overlying water varied depending on metabolism of microorganisms at the sediment–water interface (Jiang et al. 2006), it was possible due to carbonates’ and organic acids’ decreased pH in the overlying water, which was produced by a large number of microorganisms growing at the surface sediments (Chen et al. 2011).
During the fine bubble aeration, the ORP value of the overlying water showed an overall upward trend. The ORP value increased from 78 mV at the beginning to 178 mV at the end of the simulation experiment. It indicated that the fine bubble aeration could effectively increase the oxidation–reduction potential of the overlying water at the sediment–water interface. Many studies have shown that when the overlying water is under anaerobic reduction conditions, it would promote the release of endogenous phosphorus in the sediments, and the aerobic state will mostly inhibit its release Li et al. 2007, 2013). This phenomenon can be attributed to phosphate which can bind with Fe3+ to form Fe2(PO4)3 in the oxic and aerobic conditions. At the same time, dissolved phosphate in the overlying water can be adsorbed by Fe(OH)3 in the sediments. While in the anoxic and anaerobic system, Fe3+ is reduced to Fe2+ in a dissolved state, and the phosphorus originally combined with Fe3+can be easily released from the sediments to the overlying water (Jiang et al. 2008; Li et al. 2016). Therefore, it can be inferred that the fine bubble aeration can increase the ORP value of the overlying water at the sediment–water interface, obviously, which may have a certain effect on repressing the release of phosphorus from the sediments (Rahutomo et al. 2018).
3.2 Effects on organic phosphorus content and its fractions
3.2.1 Changes in TP, OP, and IP contents in sediments
The changes in the contents of TP, OP, and IP in the surface sediments during the fine bubble aeration at the sediment–water interface are shown in Fig. 4. The results showed that organic phosphorus was the main fraction of phosphorus in surface sediments. It can be seen from Fig. 4 that during the fine bubble aeration at the sediment–water interface, the total phosphorus in the surface sediments decreased from 548.4 mg kg−1 in the initial stage to 472.4 mg kg−1 at the end of the experiment. Although the TP content of the sediments has decreased under aeration conditions, the overall release trend was still relatively slight. These results are consistent with previous studies that phosphorus in sediments will also be released under oxygen-consuming conditions except in anaerobic conditions (Jiang et al. 2006). Jiang et al.(2008) also concluded that P release occurred in both aerobic and anoxic conditions with the presence of organisms, but P release in an anoxic environment was much greater than that in an aerobic environment in the presence of light.
We also found that the inorganic phosphorus content in the surface sediments decreased from 309.7 mg kg−1 before aeration to 371.6 mg kg−1after 21 days of fine bubble aeration at the sediment‒water interface. This result implied that part of the inorganic phosphorus might be converted into relatively stable organic phosphorus, and other parts also may be released into the overlying water during aeration. In other words, fine bubble aeration is likely to promote the relative stabilization of some inorganic phosphorus to a certain extent.
The content of organic phosphorus in the surface sediments generally increased at the early stage of aeration, while its content decreased significantly after 15 days of aeration. These results showed that during the process of the fine bubble aeration, the organic phosphorus content in the surface sediments firstly increased from 309.7 mg kg−1 at the beginning stage to 374.8 mg kg−1, and subsequently decreased to 298.8 mg kg−1at the end stage of aeration. This finding indicated that the accumulation rates of organic phosphorus were greater than the mineralization and decomposition rates at the early stage of aeration but it is opposite in the later stage of aeration. Studies have proved that organic matter usually forms strong complex organo-mineral associations with polyvalent cations (Fe3+, Al3+, and Ca2+). Moreover, the presence of the sepolyvalent cations may increase the OP adsorption capacity of the sediments by constituting a positively charged bridge between the negatively charged clay or organic sediments surfaces and the orthophosphate ions. In addition, these polyvalent ions may react with the OP to form insoluble organic phosphate salts precipitating onto the sediments’ surfaces (House and Denison 2002; Lü et al. 2016). Zhu et al. (2015) also discovered that enzymatic hydrolysis of OP was inhibited in the presence of humic acids (HA) and metal ions as the formation of Po–metal–HA. It can be speculated that Fe3+ may have increased in this research with the increase of OPR. Thus, organic phosphorus can be accumulated under the action of iron ion and organic matter. While, on the other hand, organic matter, especially dissolved organic carbon (DOC), can promote the growth of microorganisms by providing carbon sources for microorganisms including phosphate solubilizing bacteria, thus accelerating the release of phosphorus from sediments (Watts 2000; Müller et al. 2016). In conclusion, the influence of organic matter on the adsorption and release of phosphorus in sediments is complicated, which depends on the physical and chemical properties of organic matter. To explain why organic phosphorus exhibited this trend, we deduced that the adsorption and chelation of organic matter and iron ions on phosphorus were greater than the degradation of microorganisms in the early stage of aeration, but the opposite was true in the later stage of aeration. Therefore, fine bubble aeration could also promote the mineralization of organic phosphorus and increase the risk released to water bodies (Heinrich et al. 2021).
3.2.2 Distribution characteristics of organic phosphorus components in sediments
The distribution characteristics of the different organic phosphorus components in the surface sediments during the fine bubble aeration at the sediment–water interface are shown in Fig. 5. The results showed that the order of the content of different organic phosphorus components during the fine bubble aeration was as follows: non-labile organic phosphorus (NLOP) > liable organic phosphorus (LOP) > moderately labile organic phosphorus (MLOP). We also found that non-labile organic phosphorus was the dominant organophosphorus form in the surface sediments, and the lowest one was the moderately labile organic phosphorus. This is in agreement with the research by Han et al. (2020) which found that NOP occupied the largest proportion of the various forms of organic phosphorus in sediments of the four rivers entering the lake in Hongze Lake. Bedrock et al. (1995) also pointed out that Po which is present in natural organic matter from various sources mainly appeared in the humic fractions.
The change trends of LOP and MLOP were relatively similar during fine bubble aeration at the sediment‒water interface (Fig. 5). The content of LOP increased at the initial stage of aeration, then increased obviously after 9 days of aeration. However, the content of LOP and MLOP decreased significantly after 12 and 15 days of aeration, respectively. At the end of aeration, the content of LOP decreased from 85.72 mg kg−1 before aeration to 67.89 mg kg−1, and the content of MLOP decreased from 27.76 mg kg−1 before aeration to 24.89 mg kg−1. LOP and MLOP are both organic phosphorus fractions in the sediments that were easily bioavailable, which could affect the conversion of organic phosphorus in the short term. Labile organic phosphorus mainly composed of nucleic acid, phosphate esters, and phosphorous sugar compounds. It belongs to the organic phosphorus fraction that is most easily mineralized by microbial biomass in sediments, or easily utilized by plants (Tiessen et al. 1984). Its percentage content is the key factor for the degradation of Po mineralization to Pi (Ding et al. 2013). MLOP is mainly composed of phosphate, phospholipid, phosphoprotein, calcium phytate, and magnesium (Wan et al. 2020). In addition, MLOP is the more active component in the Po, which has a certain biological effectiveness and can be hydrolyzed or mineralized under certain conditions (Wan et al. 2020). Previous studies showed that the interactions of OP with iron (III) can transform a large part of the labile and moderately labile OP forms (Zhang et al.1994; Lü et al. 2016). Schindler and Hecky (2009) also found Fe3+ can easily combine with the decomposition product phosphate of MLOP to form insoluble iron phosphate and inhibit the hydrolysis of enzymes, thereby further reducing the migration and transformation of MLOP. Therefore, the Fe3+ may play a crucial role in the stabilization of LOP and MLOP. And the rising ORP may mean that more Fe3+ have been formed, which was consistent with the results of LOP and MLOP accumulation in the early stage of aeration. While due to the degradation of microorganisms, LOP and MLOP tended to be transformed into soluble phosphate at the later stage of aeration, and then released into the overlying water (Wang et al. 2020).
The content of NLOP also showed a slight increase trend at the early stage of aeration, then decreased significantly after 12 days of aeration, and re-increased after 15 days. Eventually, the content of NLOP still decreased from 186.1 mg kg−1 to 178.9 mg kg−1at the end of aeration. This may be because a part of the NLOP is humic acid bound organic phosphorus, which has the performance of resisting microbial degradation (Zhou et al. 2010; He et al. 2011). While Lü et al. (2016) presented that the inactive organic phosphorus is only a relative chemical solubility, it can be absorbed and utilized by microorganisms and plants and still has potential biological activity under certain circumstances. In conclusion, NLOP is also the component with high content of organic phosphorus in sediments as it has low bioavailability and strong stability (Ergin et al. 1996).
The percentage of LOP and MLOP to the total organic phosphorus increased at the early stage of the simulation experiment (Fig. 5). The relative contribution of the more active part (LOP + MLOP) in the total organic phosphorus reached 52.30% at the 15th day of aeration and then it decreased. In other words, aeration would increase the proportion of (LOP + MLOP) in the early stage. However, because (LOP + MLOP) belongs to bioavailable phosphorus fractions, it is easily hydrolyzed and has a large release potential, which corresponded to the decrease in the later stage of aeration.
3.3 Effects of fine bubble aeration on the microbial activities
3.3.1 Microbial biomass carbon
Microbial biomass carbon (MBC) is an important index for microbial activity, which can reflect the total number and activity of microorganisms in sediments (Bai et al. 2020). The variation of the quantity of MBC in the surface sediments during fine bubble aeration is shown in Fig. 6. It is found that MBC showed an overall growth trend, but the growth rate was slow in the early stage and accelerated in the later stage. Consequently, the MBC increased from 245.2 mg kg−1 before aeration to 502.1 mg kg−1 after the simulation experiment. It could be speculated that aeration could increase the total amount and activity of microorganisms in sediments. MBC was found to be mainly governed by the amount of organic matter and showed a decrease in microbial biomass activity (Mao et al. 2010) with the decrease in the amount of organic matter (Fischer et al. 2002). Thus, the possible reason of increased MBC might be that aeration increased the content of TOC and DOC in the surface sediments, thus more energy and carbon sources were supplied for microbial growth, which increased microbial biomass and their activities (Boudreau 1999; Liu et al. 2014). Moreover, it was verified that the rapid increase in microbial biomass may promote the release of phosphorus (Jiang et al. 2008). Thereby, the growth trend of MBC might be responsible for the release of TP in sediments and the microbial decomposition of TOP in the later stage of aeration.
3.3.2 Variation in number of phosphate solubilizing bacteria
The variation of the number of phosphorus solubilizing bacteria in the surface sediments during the aeration is shown in Fig. 7. The total number of OPB in the surface sediments varied from 1.06 × 105 to 6.55 × 105 cells g−1 (dry sediments), and the IPB varied from 1.28 × 104 to 4.56 × 104 cell g−1 (dry sediments). Organic phosphorus mineralizing bacteria in the surface sediments were significantly larger than inorganic phosphorus solubilizing bacteria, which indicated that organic phosphorus mineralizing bacteria had a certain advantage in phosphorus solubilizing bacteria during the fine bubble aeration.
The results showed that the number of IPB decreased overall during aeration, and the number of IPB decreased significantly at the initial stage. Although it increased after 12 days of aeration, then it decreased from 4.56 × 104 cells g−1 (dry sediments) to 2.08 × 104 cells g−1 (dry sediments) at the end of aeration. In contrast, the number of OPB showed an increase trend overall during the aeration period. It just declined slightly at the initial stage, but increased significantly after 3 days of aeration, and reached the highest value at 15 days after aeration. Although the number of OPB had declined again afterward, it eventually increased from 1.98 × 105 cells g−1 (dry sediments) to 5.27 × 105 cells g−1 (dry sediments) at the end of aeration as compared to the initial stage. This may be because the phosphorus release at the initial stage was dominated by IPB. As the aeration process extended, the number of inorganic phosphorus decreased while the organic phosphorus increased, resulting in the inhibition of inorganic phosphorus solubilizing bacteria (Li et al. 2018). In addition, as the oxidation–reduction potential (ORP) of the sediment–water interface increased, inorganic phosphorus may be inactivated and stabilized, which decreased the number of IPB. The change of OPB number was similar to the variation of total organic phosphorus, with the increase of organic phosphorus content during aeration. In other words, when the substrate organic phosphorus content was sufficient, OPB could be fully grown (Zhou et al. 2011). Therefore, at the later period of aeration, the decline of organic phosphorus may also be one of the reasons for the decrease of OPB.
3.3.3 Activity of alkaline phosphatase
The change of alkaline phosphatase activity (APA) during aeration at the sediment–water interface is shown in Fig. 8. We observed a rapid increase in APA from 24.12 mg (kg h)−1 at the initial stage to 69.45 mg (kg h) −1 at the end of aeration. This indicated that aeration at the sediment‒water interface significantly improved the activity of alkaline phosphatase in the surface sediments, which was similar to the previous results (Chen et al. 2011). Yuan et al. (2020b) also reported that higher APA values were detected under both oxic and anaerobic conditions in the near-surface layer of sediments where higher Po concentrations occurred, while there existed a higher APA under oxygen-enriched conditions relative to the low oxygen conditions. This could be explained that organic phosphorus could induce phosphatase activity, thereby causing the increase of the APA with the increase of organic phosphorus content during aeration (Zhou et al. 2008). APA plays a key role in organic phosphorus mineralization, which is a necessary factor for the conversion of organic phosphorus to inorganic phosphorus (Zhu et al. 2016), so it could be inferred that the mineralization of organic phosphorus occurred at this time, which was also consistent with the aforementioned decrease in the content of organic phosphorus during the middle and late stage of aeration.
Organic phosphorus mineralizing bacteria (OPB) produces alkaline phosphatase during the process of organophosphate mineralization (Liu et al. 2017). Therefore, the alkaline phosphatase activity was expected to be related to the number of organic phosphorus mineralizing bacteria theoretically. According to our results, the number of OPB during aeration was roughly increasing. The correlation coefficient (r) between the number of OPB and APA was 0.862 (P < 0.01); it revealed that the increase of the number of OPB during aeration may also lead to the increase of APA.
Similarly, the correlation coefficient (r) between DOC and APA is 0.994 (P < 0.01), which suggested that DOC contents were significantly positively correlated with alkaline phosphatase activity. These results implied that the increase of DOC may lead to the increase of the number of bacteria related to phosphorus transformation, thus enhancing the activity of alkaline phosphatase (Torres et al. 2017). And the results may also be explained by a mechanism of phosphatase induction by organic matter, as the total organic carbon content correlated significantly with APA (Su et al. 2005). Additionally, APA showed a significant negative correlation with the IP contents (P < 0.05). This was because microorganisms tend to produce more phosphatase with the lack of IP in sediments, and excessive labile IP could repress the synthesis of APA (Wang et al. 2016). Therefore, the decreased inorganic phosphorus of the sediments during aeration also had a certain effect on the increase of APA.
3.4 Correlation analysis of microbial indicators and organic phosphorus components
The data of microbial indicators and various fractions of phosphorus were analyzed, and the results are shown in Table 1. We discovered that there was a significant correlation among the microbial indicators. Notably, APA was significantly positively correlated with the number of OPB, which was in accord with the research of Zhou et al. (2011). This can be due to the fact that OPB can produce alkaline phosphatase, which hydrolyzes phosphate ester and other organic phosphorus into bioavailable phosphate (Zhou et al. 2008; Mudryk et al. 2015). Therefore, APA was supposed to be correlated with the content of organophosphorus in the sediments. However, there were no obvious correlations between the organic phosphorus and various microbial indicators (except IPB). The possible reason for this might be that the sediment environment of Nanfei River is a long-term stable system, and the sediment environment in the aeration device may not have reached equilibrium. On the other hand, aeration changed the pH value and increased the content of organic matter, as well as the oxidation–reduction potential at the sediment‒water interface, thus causing changes in the chemical balance at the sediment‒water interface (Song et al. 2020). The changes in the form and content of organic phosphorus may also be related to other chemical processes, not only affected by microorganisms.
4 Conclusions
In this study, a simulation experiment with a precision oxygen distribution system with nanopore was conducted to study the effect of fine bubble aeration on the sediment–water interface on species distributions of organic phosphorus and related microbial activities in the sediments. The results indicated that aeration can increase the organic matter content and the redox potential in the surface sediments. Conversely, the pH value of the overlying water at the sediment‒water interface presented a general decreasing trend. The content of total phosphorus and inorganic phosphorus in the surface sediments generally showed a declining trend, indicating that fine bubble aeration may accelerate the relative stabilization of inorganic phosphorus or the release of overlying water. While the content of organic phosphorus increased firstly and then decreased during aeration, revealing that the mineralization and decomposition rate of organic phosphorus by microorganisms in the later stage of aeration was greater than the accumulation rate of organic phosphorus. The findings suggest that fine bubble aeration may also promote the mineralization of organic phosphorus and increase the risk of its release to water bodies. Moreover, the variation trend of LOP and MLOP was similar to TOP, increasing firstly and then declining. Meanwhile, the content of NLOP has slightly decreased compared with that before aeration, which is mainly due to the ability of NLOP to resist microbial degradation, but it still has potential biological activity.
The MBC and alkaline phosphatase activities were significantly increased in the surface sediments during aeration, revealing that the total amount and activity of microorganisms related to the conversion of organic phosphorus could be increased in the surface sediments. The number of IPB decreased overall, while the number of OPB increased overall. The trend of the two changes was roughly opposite, and there was a significant negative correlation between them. The number of OPB was significantly positively correlated with alkaline phosphatase activity, which is associated with the mechanism of organophosphate mineralization. There was a significant correlation among the microbial indicators in the surface sediments, but no obvious correlation between the organic phosphorus and the microbial indicators except IPB, which may be caused by other chemical processes in the surface sediments. As the changes in the form and content of phosphorus may also be related to other chemical processes, its mineralization and fixation may not only be affected by microorganisms. Therefore, the relationship between the physiochemical parameters and microbial indicators requires a further study to reveal the specific mechanisms of OP mineralization in sediments. Thus, we may come to a conclusion that short-term aeration may be more beneficial to the internal P management than long-term aeration. Although a considerable further effort is needed to examine the effects of aeration on distributions of organic phosphorus forms and related microbial activity on the sediment‒water interface, present findings can still provide a theoretical basis and technical support for in situ remediation of heavily polluted river sediments with aeration at the sediment‒water interface.
References
Anderson JPE, Domsch KH (1978) Mineralization of bacteria and fungi in chloroform-fumigated soils. Soil Biol Biochem 10:207–213
Bai J, Yu L, Ye XF, Yu ZB, Guan YN, Li XW, Cui BS, Liu XH (2020) Organic phosphorus mineralization characteristics in sediments from the coastal salt marshes of a Chinese delta under simulated tidal cycles. J Soils Sediments 20:513–523
Bedrock CN, Cheshire MV, Chudek JA, Fraser AR, Goodman BA, Shand CA (1995) Effect of pH on precipitation of humic acid from peat and mineral soils on the distribution of phosphorus forms in humic and fulvic acid fractions. Commun Soil Sci Plan 26:1411–1425
Boudreau BP (1999) A theoretical investigation of the organic carbon-microbial biomass relation in muddy sediments. Aquat Microb Ecol 17:181–189
Chen JJ, Lu SY, Zhao YK, Wang W, Huang MS (2011) Effects of overlying water aeration on phosphorus fractions and alkaline phosphatase activity in surface sediment. J Environ Sci 23:206–211
Ding SM, Xu D, Bai XL, Yao XC, Fan CX, Zhang CX (2013) Speciation of organic phosphorus in a sediment profile of Lake Taihu: chemical forms and their transformation. J Environ Sci 25:637–644
Dong DP, Zhang TX, Zhang DY, Wang QY, Li DF, Wang GX (2016) Characteristics of organic phosphorus fractions in the sediments of the black water aggregation in Lake Taihu. Environ Sci 37:4194–4202 (in Chinese)
Ergin M, Gaines A, Galletti GC, Chiavari G, Fabbri D, Yücesoy-Eryilmaz F (1996) Early diagenesis of organic matter in recent Black Sea sediments: characterization and source assessment. Appl Geochem 11:711–720
Fischer H, Wanner SC, Pusch M (2002) Bacterial abundance and production in river sediments as related to the biochemical composition of particulate organic matter (POM). Biogeochemistry 61:37–55
Golterman HL (2001) Fractionation and bioavailability of phosphates in lacustrine sediments: a review. Limnetica 20:15–29
Gonsiorczyk T, Casper P, Koschel R (1998) Phosphorus-binding forms in the sediment of an oligotrophic and an eutrophic hard water lake of the Baltic Lake District (Germany). Water Sci Technol 37:51–58
Han N, Yuan XY, Zhou HH, Zhu H, Xiong YT, Chen YZ (2020) Distribution characteristics of organic phosphorus in the sediments of rivers entering the Lake Hongze and the effects of exogenous substances on their fraction transformation. J Lake Sci 32:665–675 (in Chinese)
He Z, Olk DC, Cade-Menun BJ (2011) Forms and lability of phosphorus in humic acid fractions of Hord silt loam soil. Soil Sci Soc Am J 75:1712–1722
Heinrich L, Rothe M, Braun B, Hupfer M (2021) Transformation of redox-sensitive to redox-stable iron-bound phosphorus in anoxic lake sediments under laboratory conditions. Water Res 189:116609
House WA, Denison FH (2002) Total phosphorus content of river sediments in relationship to calcium, iron and organic matter concentrations. Sci Total Environ 282:341–351
Ivanoff DB, Reddy KR, Robinson S (1998) Chemical fractionation of organic phosphorus in selected Histosols1. Soil Sci 163:36–45
Jiang X, Jin XC, Yao Y, Li L, Wu FC (2006) Effects of oxygen on the release and distribution of phosphorus in the sediments under the light condition. Environ Pollut 141:482–487
Jiang X, Jin XC, Yao Y, Li LH, Wu FC (2008) Effects of biological activity, light, temperature and oxygen on phosphorus release processes at the sediment and water interface of Taihu Lake, China. Water Res 42:2251–2259
Kobayashi K, Matsui M, Haraguchi H, Fuwa K (1983) Identification of alkaline phosphatase in sea water. J Inorg Biochem 18:41–47
Li B, Ding SM, Fan CX, Zhong JC, Zhang L, Yin HB (2008) Distributions of nitrogen and phosphorus in interstitial waters in the sediments of Fubao Bay in Lake Dianchi and their relationships with the activities of microbe and alkaline phosphatase in the surface sediments. J Lake Sci 20:420–427 (in Chinese)
Li H, Song CL, Cao XY, Zhou YY (2016) The phosphorus release pathways and their mechanisms driven by organic carbon and nitrogen in sediments of eutrophic shallow lakes. Sci Total Environ 572:280–288
Li HF, Li ZJ, Qu JH, Tian HL, Yang XH (2018) Combined effects of phosphate-solubilizing bacterium XMT-5 (Rhizobium sp.) and submerged macrophyte Ceratophyllumdemersum on phosphorus release in eutrophic lake sediments. Environ Sci Pollut R 25:18990–19000
Li HY, Liu L, Li MY, Zhang XR (2013) Effects of pH, temperature, dissolved oxygen, and flow rate on phosphorus release processes at the sediment and water interface in storm sewer. J Anal Methods Chem 2013:104316
Li QM, Wen Z, Wang XX, Zhou YY, Yang H, Ji GL (2007) Phosphorus in interstitial water induced by redox potential in sediment of Dianchi Lake, China. Pedosphere 17:739–746
Liu K, Ni ZK, Wang SR, Ni CY (2015) Distribution characteristics of phosphorus in sediments at different altitudes of Poyang Lake. China Environ Sci 35:856–861 (in Chinese)
Liu WX, Jiang L, Hu SJ, Li LH, Liu LL, Wan SQ (2014) Decoupling of soil microbes and plants with increasing anthropogenic nitrogen inputs in a temperate steppe. Soil Biol Biochem 72:116–122
Liu YQ, Cao XY, Li H, Zhou ZJ, Wang SY, Wang ZC, Song CL, Zhou YY (2017) Distribution of phosphorus-solubilizing bacteria in relation to fractionation and sorption behaviors of phosphorus in sediment of the Three Gorges Reservoir. Environ Sci Pollut Res 24:17679–17687
Lü C, He J, Zuo L, Vogt RD, Zhu L, Zhou B, Mohr CW, Guan R, Wang W, Yan D (2016) Processes and their explanatory factors governing distribution of organic phosphorous pools in lake sediments. Chemosphere 145:125–134
Lü CW, He J, Zhou B, Vogt RD, Guan R, Wang WY, Zuo L, Yan DH (2015) Distribution characteristics of organic phosphorus in sediments from Lake Hulun, China. Environ Sci-Proc Imp 17:1851–1858
Ma J, Wang PF, Ren LX, Wang X, Paerl HW (2019) Using alkaline phosphatase activity as a supplemental index to optimize predicting algal blooms in phosphorus-deficient lakes: a case study of Lake Taihu, China. Ecol Indic 103:698–712
Mao HF, He J, LüCW LiangY, Liu HL, Wang FJ (2010) Correlation between microbial biomass and organic carbon forms in the sediment. J Agro Environ Sci 29:2406–2412 (in Chinese)
Mudryk ZJ, Perlinski P, Antonowicz J, Robak D (2015) Number of bacteria decomposing organic phosphorus compounds and phosphatase activity in the sand of two marine beaches differing in the level of anthropopressure. Mar Pollut Bull 101:566–574
Müller S, Mitrovic SM, Baldwin DS (2016) Oxygen and dissolved organic carbon control release of N, P and Fe from the sediments of a shallow, polymictic lake. J Soils Sediments 16:1109–1120
Ni ZK, Wang SR, Zhang L, Wu ZQ (2015) Role of hydrological conditions on organic phosphorus forms and their availability in sediments from Poyang Lake, China. Environ Sci Pollut Res 22:10116–10129
Paerl HW, Xu H, Mccarthy MJ, Zhu G, Qin BQ, Li YP, Gardner WS (2011) Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): the need for a dual nutrient (N & P) management strategy. Water Res 45:1973–1983
Preston MD, Basiliko N (2016) Carbon mineralization in peatlands: does the soil microbial community composition matter? Geomicrobiol J 33:151–162
Qi YC, Peng Q, Dong YS, Xiao SS, Sun LJ, Liu XC, He YT, Jia JQ, Cao CC (2014) Reponses of soil total organic carbon and dissolved organic carbon to simulated nitrogen deposition in temperate typical steppe in Inner Mongolia, China. Environ Sci 35:3073–3082 (in Chinese)
Qian YC, Shi JY, Chen YX, Lou LP, Cui XY, Cao RK, Li PF, Tang J (2010) Characterization of phosphate solubilizing bacteria in sediments from a shallow eutrophic lake and a wetland: isolation, molecular identification and phosphorus release ability determination. Molecules 15:8518–8533
Rahutomo S, Kovar JL, Thompson ML (2018) Varying redox potential affects P release from stream bank sediments. Plos One 13:e0209208
Roden EE, Wetzel RG (2002) Kinetics of microbial Fe(III) oxide reduction in freshwater wetland sediments. Limnol Oceanogr 47:198–211
Rodrı́Guez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Rydin E, Malmaeus JM, Karlsson OM, Jonsson P (2011) Phosphorus release from coastal Baltic Sea sediments as estimated from sediment profiles. Estuar Coast Shelf Sci 92:111–117
Schiebel HN, Peri F, Chen RF (2020) Dissolved organic matter export from surface sediments of a New England salt marsh. Wetlands 40:693–705
Schindler DW, Hecky RE (2009) Eutrophication: more nitrogen data needed. Science 324:721–722
Shan BQ, Li J, Zhang WQ, Di ZZ, Jin X (2016) Characteristics of phosphorus components in the sediments of main rivers into the Bohai Sea. Ecol Eng 97:426–433
Shilla DA, Asaeda T, Kalibbala M (2009) Phosphorus speciation in Myall lake sediment, NSW, Australia. Wetl Ecol Manag 17:85–91
Song XJ, Li DP, Xu CT (2020) Using aeration to enhance phosphorus adsorption and immobilization by the sediment and LMB. Water Air Soil Poll 232:93–106
Su YP, Ma S, Dong SL (2005) Variation of alkaline phosphatase activity in sediments of shrimp culture ponds and its relationship with the contents of C, N and P. J Ocean U China 4:75–79
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Tiessen H, Stewart JWB, Cole CV (1984) Pathways of phosphorus transformations in soils of differing pedogenesis. Soil Sci Soc Am J 48:853–858
Torres IC, Turner BL, Reddy KR (2017) Phosphatase activities in sediments of subtropical lakes with different trophic states. Hydrobiologia 788:305–318
Wan J, Yuan XY, Han L, Ye HM, Yang XF (2020) Characteristics and distribution of organic phosphorus fractions in the surface sediments of the inflow rivers around Hongze Lake, China. Int J Env Res Pub He 17:648
Wang CY, Zhang Y, Li HL, Morrison RJ (2013) Sequential extraction procedures for the determination of phosphorus forms in sediment. Limnology 14:147–157
Wang SY, Li H, Xiao J, Zhou YY, Bi YH, Cao XY (2016) Enhancement of sediment phosphorus release during a tunnel construction across an urban lake (Lake Donghu, China). Environ Sci Pollut Res 23:17774–17783
Wang YY, Yin HB, Kong M, Zhang YM, Liu R (2020) Effects of lanthanum/aluminum modified attapulgite clay on organic phosphorus control in eutrophic lakes. China Environ Sci 40:3801–3809 (in Chinese)
Watts CJ (2000) The effect of organic matter on sedimentary phosphorus release in an Australian reservoir. Hydrobiologia 431:13–25
Worsfold PJ, Monbet P, Tappin AD, Fitzsimons MF, Stiles DA, Mckelvie ID (2008) Characterisation and quantification of organic phosphorus and organic nitrogen components in aquatic systems: a Review. Anal Chim Acta 624:37–58
Wu YH, Wen YJ, Zhou JX, Wu YY (2014) Phosphorus release from lake sediments: effects of pH, temperature and dissolved oxygen. KSCE J Civ Eng 18:323–329
Yuan HZ, Li Q, Kukkadapu RK, Liu EF, Yu JH, Fang H, Li H, Jaisi DP (2019) Identifying sources and cycling of phosphorus in the sediment of a shallow freshwater lake in China using phosphate oxygen isotopes. Sci Total Environ 676:823–833
Yuan HZ, Tai ZQ, Li Q, Liu EF (2020a) In-situ, high-resolution evidence from water-sediment interface for significant role of iron bound phosphorus in eutrophic lake. Sci Total Environ 706:136040
Yuan HZ, Tai ZQ, Li Q, Zhang FM (2020b) Characterization and source identification of organic phosphorus in sediments of a hypereutrophic lake. Environ Pollut 257:113500
Zhang YS, Werner W, Scherer HW, Sun X (1994) Effect of organic manure on organic phosphorus fractions in two paddy soils. Biol Fertil Soils 17:64–68
Zhou C, Song CL, Huang DZ, Liu YB, Cao XU, Zhou YY (2011) Isolation and characterization of organic phosphorus-mineralizing bacteria in sediment of a Chinese large shallow eutrophic lake (Lake Taihu). Geomicrobio J 28:660–666
Zhou YM, Zhang Q, Tang HX (2010) Sorption behavior of polycyclic aromatic hydrocarbons onto humic acid particulates. Acta Sci Circumst 30:1564–1571 (in Chinese)
Zhou YY, Song CL, Cao XY, Li JQ, Chen GY, Xia ZY, Jiang PH (2008) Phosphorus fractions and alkaline phosphatase activity in sediments of a large eutrophic Chinese lake (Lake Taihu). Hydrobiologia 599:119–125
Zhu J, He Y, Wang JH, Qiao ZC, Wang Y, Li ZH, Huang MS (2017a) Impact of aeration disturbances on endogenous phosphorus fractions and their algae growth potential from malodorous river sediment. Environ Sci Pollut Res 24:8062–8070
Zhu YR, Wu FC, Feng WY, Liu SS, Giesy JP (2016) Interaction of alkaline phosphatase with minerals and sediments: activities, kinetics and hydrolysis of organic phosphorus. Colloid Surface A 495:46–53
Zhu YR, Wu FC, He ZQ, Giesy JP, Feng WY, Mu YS, Feng CL, Zhao XL, Liao HQ, Tang Z (2015) Influence of natural organic matter on the bioavailability and preservation of organic phosphorus in lake sediments. Chem Geol 397:51–60
Zhu YR, Wu FC, He ZQ, Guo JY, Qu XX, Xie FZ, Giesy JP, Liao HQ, Guo F (2013) Characterization of organic phosphorus in lake sediments by sequential fractionation and enzymatic hydrolysis. Environ Sci Technol 47:7679–7687
Zhu YR, Wu FC, Liu Y, Wei Y, Liu SS, Feng WY, Giesy JP (2017b) Microbial biomass and community composition involved in cycling of organic phosphorus in sediments of lake Dianchi, Southwest China. Geomicrobiol J 34:249–260
Zou YC, Zhang LL, Wang LY, Zhang SJ, Yu XF (2018) Effects of aeration, vegetation, and iron input on total P removal in a lacustrine wetland receiving agricultural drainage. Water 10:61
Acknowledgements
We would like to thank Mr. Yang Haifeng for the construction of the fine bubble aeration at the sediment–water interface simulation experiment device.
Funding
The authors received financial support from the Foundation of National Special Item on Water Resource and Environment (No 2017ZX07603003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Peifang Wang
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, C., Chen, X., Xu, Y. et al. Effects of fine bubble aeration at the sediment‒water interface on distributions of organic phosphorus fractions and related microbial activity in a heavily urban river. J Soils Sediments 21, 3528–3539 (2021). https://doi.org/10.1007/s11368-021-03019-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-021-03019-5