Abstract
Purpose
Sorption to sediments and biofilms is thought to be a crucial mechanism controlling the fate and transport of emerging contaminants. Biofilm growth changes the properties of the sediments, which may further influence the sorption mechanism of emerging contaminants to sediments. This study is focused on the effects of biofilms on the linear and nonlinear sorption of fluoroquinolone antibiotic ofloxacin (OFL) by sediments.
Materials and methods
The top 5 cm of sediments and the overlying water containing natural microorganisms were collected from a shallow lake in summer. They were cultivated in the laboratory for the development of biofilms on the surface of sediments. Batch sorption experiments of OFL by original sediments and biofilm-coated sediments were conducted, and infrared spectrometry was used to obtain the main functional groups involved in sorption. Extracellular polymeric substances (EPS) were extracted from biofilms to investigate their interaction with OFL through three-dimensional excitation-emission matrix fluorescence spectroscopy and UV-Vis spectroscopies.
Results and discussion
The results showed that linear partition and nonlinear adsorption were simultaneously involved in the sorption process. The linear partition coefficients of OFL in sediments decreased by 50% and 60%, whereas the nonlinear adsorption capacities increased by 1.7 and 2.0 times after the biofilms colonized the sediment surface for 30 and 45 days, respectively. The decreased linear partition coefficients of OFL were related to the barrier created by biofilm coatings affecting hydrophobic interaction, whereas the increased nonlinear adsorption capacities were due to the increase in cation exchange capacities and the formation of hydrogen bonds between fluorine atoms in OFL and –OH groups in sediment. In addition, OFL and protein-like substances contained in EPS from biofilms could form complexes that affect the sorption processes.
Conclusions
The present study reveals that biofilms can inhibit hydrophobic interaction but facilitate cation exchange and hydrogen binding between OFL and sediments. Our work yields new insights into the interaction of aquatic solid sorbents, which is significant to understanding the transport and fate of organic contaminants in natural waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fluoroquinolone antibiotics have been widely used in the prevention and treatment of human and veterinary infectious diseases (Hu et al. 2018b). In aquatic environments, these antibiotics are usually derived from hospital and domestic wastewater (Wang et al. 2017b), livestock excrement (Chen et al. 2016), and effluents from sewage treatment plants (Hu et al. 2018a). The occurrence of fluoroquinolone antibiotics in natural waters at concentrations of ng L−1 or μg L−1 has been widely reported (Gothwal and Thatikonda 2017; Kafaei et al. 2018; Prutthiwanasan and Suntornsuk 2018). The residues of these antibiotics in the aquatic environment can produce long-term antibiotic resistance and many harmful effects in organisms and humans, which have drawn attention worldwide (Dhawde et al. 2018; Shen et al. 2018; Danner et al. 2019; Jurado et al. 2019). Therefore, investigations into the fate of fluoroquinolone antibiotics in the aquatic environment are required.
Fluoroquinolone antibiotics are resistant to hydrolysis and volatilization because of the quinolone ring stability and its ultralow Henry’s law constants (Babic et al. 2013; Dorival-Garcia et al. 2013). After the antibiotics are discharged into aquatic environments, they will undergo sorption, photodegradation, and biodegradation. Sorption to sediments is an important process for controlling the antibiotics’ environmental behaviors (Zhang and Dong 2008; Riaz et al. 2018). Fluoroquinolone antibiotics can be sorbed by sediments and soils through hydrophobic interaction and cation exchange (Tolls 2001; Li et al. 2018). In addition, the chemical substances in sediments may react with the functional groups in fluoroquinolone antibiotics through hydrogen bonding and inner-sphere surface complexation or formation of an inner-sphere complex with iron and aluminum oxides via the carboxylic group (Li et al. 2017; Liao et al. 2018). Therefore, the sorption of fluoroquinolone antibiotics onto sediments is related to the physicochemical properties of sediments.
Natural sediment surface has an extremely complex morphology and can adsorb various nutrients, which predisposes it to microbial colonization (Kraemer et al. 2013). It has been shown that exposed sediments in an aquatic environment can be rapidly colonized by microorganisms (Fang et al. 2012). When microorganisms are associated with the sediment surface, they secrete a matrix of extracellular polymeric substances (EPS) to form biofilms (Chen et al. 2017). The growth of biofilms may be promoted by eutrophication of the river and lake (Li et al. 2019) and possibly suppressed by sediment resuspension and shear stress (Fang et al. 2017). Besides, the biofilm formation can change the nutrient level in rivers and the change will inversely affect the biofilm formation (Ghanbari et al. 2016). Thus, the growth of biofilm is variable. Many studies have demonstrated that biofilms’ growth on the sediment changes the microtopography, sediment properties, and interparticle forces, subsequently changing the erosion response of sediment to the flow as well as the bedform and sediment transport (Fang et al. 2014; Gerbersdorf and Wieprecht 2015; Thom et al. 2015; Parsons et al. 2016; Cheng et al. 2018). However, little attention has been paid to the effects of biofilms on the sorption characteristics of emerging organic contaminants onto sediments. It has been reported that sediment organic matter comprises two important heterogeneous sorption domains: a rubbery amorphous domain and a glassy condensed domain (Ran et al. 2007; Sun et al. 2010). The structure of sediment organic matter affects the sorption of organic contaminants (Ran et al. 2007). The growth of biofilms can increase the content of amorphous organic matter in sediments (Ding et al. 2015). In addition, biofilms have an affinity for emerging organic contaminants due to the presence of biofilm EPS, which can interact with organic contaminants via Coulomb forces, hydrogen bonds, van der Waals forces, and hydrophobic interactions (Dong et al. 2017; Zhang et al. 2018b). Therefore, biofilms adhering to sediments can influence the latter’s sorption for organic contaminants. Ding et al. (2015) removed biofilms from sediments and found that the biofilm coverage of sediments had no effect on the sorption capacity of endocrine-disrupting chemicals. However, the sorption results provided by removal of biofilms from sediments may differ from those gained when considering the extent of biofilm growth by sorption. If the sorption of organic contaminants by original sediments was different from that by biofilm-coated ones, the concentration of organic contaminants left in aqueous phase will be changed and affect their ecological risk. Generally, a lower amount of organic contaminants sorbed on aquatic solids could pose a higher ecological risk in the aqueous phase (Lou et al. 2011; Zhang et al. 2011). It is necessary to find out the effect of biofilms on the sorption characteristic of sediments. However, it remains unknown whether changes in the growth of biofilms on sediments have an effect on the sorption of fluoroquinolone antibiotics in sediments, and knowledge of how biofilm EPS reacts with fluoroquinolone antibiotics is also limited.
In this study, ofloxacin (OFL), one of the most commonly reported fluoroquinolone antibiotics in aquatic environments (Dong et al. 2016; Gothwal and Shashidhar 2017; He et al. 2019), was chosen as a model fluoroquinolone agent. The aims of the study were (a) to investigate the effect of biofilms on sorption of OFL onto sediments by associating the chemical properties of original sediments and biofilm-coated sediments with their sorption characteristics and (b) to reveal the interaction between EPS from biofilms and OFL by three-dimensional excitation-emission matrix fluorescence spectroscopy and UV-Vis spectroscopy.
2 Materials and methods
2.1 Cultivation and characterization of biofilm-coated sediments
The top 5 cm of sediments and the overlying water containing natural microorganisms were collected from Nanhu Lake in Changchun, China, using a grab sampler in June 2016. Information on the lake is given in Table S1 in the Electronic Supplementary Material (ESM). The collected sediments and water samples were transferred to glass bottles and immediately transported to the laboratory. The sediments were air-dried at room temperature (20 °C), ground, and sieved through a 0.3-mm sieve to a mean particle size of 21.3 μm (measured by laser particle size distribution analyzer; Bettersize 2000; Bettersize, China). The water samples were filtered with a 3-μm mixed cellulose ester filter to remove suspended particles. Biofilm cultivation on sediments was performed according to a modified version described by Fang et al. (2017). Briefly, 20 mg of sediment and 40 mL of filtered natural water were added to a 50-mL Erlenmeyer flask. Mineral salt solutions were then added to facilitate the growth of biofilms (Zhang et al. 2018b). The cultivation was conducted under static state at 25 °C in natural light (10 h a day) for 30 and 45 days. The samples were flushed with air to provide O2. Sediments colonized by biofilms were referred to as biofilm-coated sediments (BS) to differentiate them from the original sediments (OS). The BS were named BS-30 or BS-45 according to its culture time. After culturing, BS-30 and BS-45 were rinsed twice with Milli-Q water to remove the culture medium and biofilms that did not adhere to sediments. Then, BS-30 and BS-45 were collected by centrifugation at 1000×g for 5 min, which did not shear off biofilms from sediments.
The total organic carbon (TOC) content of OS, BS-30, and BS-45 was measured with a TOC analyzer (TOC-L, Shimadzu, Japan) equipped with a solid sample module (SSM-5000A). The cation exchange capacities (CEC) of each sediment sample were determined by EPA method 9081 (Hahladakis et al. 2014).
2.2 Extraction of extracellular polymeric substances from biofilms
Extracellular polymeric substances (EPS) were separated from the biofilms to study their interaction with OFL. First, biofilms were colonized on glass slides held in polypropylene racks for 30 and 45 days, as reported in our previous study (Zhang et al. 2018b). The water and other conditions for biofilm cultivation were the same as for the simultaneous cultivation of biofilm-coated sediments. After culturing, the biofilms were scraped from the glass slides and suspended in Milli-Q water. The suspensions were then centrifuged for 10 min at 2700×g to remove the supernatant. Then, the biofilms separated by centrifugation were diluted to a concentration of 4 mg mL−1 (based on dry weight and volume conversion). Finally, EPS were extracted by centrifuging the biofilms at 10,000×g for 20 min at 4 °C (Dong et al. 2017). The supernatants were filtered with a 0.45 μM cellulose acetate membrane filter, and the filtrates were freeze-dried. The freeze-dried EPS were prepared at a concentration of 0.1 mg mL−1 and designated according to their culture times as EPS-30 and EPS-45.
2.3 Batch sorption experiments
Experiments to determine the batch sorption of OFL were performed for OS, BS-30, and BS-45. A stock solution of OFL (100 mg L−1) was dissolved in a background solution of 0.1 mol L−1 KCl and 100 mg L−1 NaN3. The stock solution was then diluted with the background solution to 10 different initial concentrations (0.1–1.0 mg L−1) in 1-L volumetric flasks. The initial concentrations of OFL used in this study were consistent with other recent laboratory studies of OFL sorption on sediments (Cao et al. 2017; Wang et al. 2017a). Although the concentrations used in the experiments are much higher than those observed in natural waters (Wang et al. 2017b; Hu et al. 2018b), they are necessary for conducting reliable laboratory investigations with quantitative analysis. A 20-mg (dry weight) sediment sample was mixed with 20-mL OFL solution in 40-mL Teflon-coated screw cap vials. The vials were stored in the dark and shaken for 24 h at 25 °C in an air bath shaker based on preliminary experiments. Simultaneously, blanks without OFL and control samples without sediment samples were equilibrated together with the other samples. Two replications were conducted for each treatment. After being equilibrated for 24 h, the vials were centrifuged at 1000×g for 5 min. The concentration of OFL in the supernatants was then quantified by high-performance liquid chromatography as described in previous literature (Pan et al. 2012).
2.4 Spectral analysis
Infrared spectrometry was used to obtain the main functional groups involved in the OFL sorption mechanism. Freeze-dried sediment samples were analyzed using an infrared spectrometer (IRAffinity-1S, Shimadzu, Japan) before and after OFL sorption experiments. The freeze-dried sediment samples and spectrally pure KBr were mixed at a ratio of 1:100 and homogenized in an agate grinder (Wang et al. 2014). The resulting mixture was pressed into pellets, which were then loaded on an infrared spectrometer, and spectra in the range of 4000–400 cm−1 were collected with 20 replications at a resolution of 2 cm−1.
To study the interaction between EPS and OFL, the properties of EPS, 0.5 mg L−1 OFL, and 0.5 mg L−1 OFL-mixed EPS were recorded with a fluorescence spectrophotometer (F-2700, Hitachi, Japan) and UV-Vis spectrophotometer (UV-1800, Shimadzu, Japan). The three-dimensional excitation-emission matrix (3D-EEM) fluorescence spectra were recorded at every 5 nm interval over an excitation range of 220–550 nm with an emission range of 220–550 nm and 5 nm intervals. The scan speed was set at 12,000 nm min−1. The excitation and emission slits were both set to 5-nm bandpass. The UV-Vis spectra were recorded over a range of 200–700 nm at every 0.5 nm interval, and the scan speed was set to medium speed. Milli-Q water was used as the blank for the entire spectral analysis. The entire procedure was performed in triplicate, and the mean values were used for discussion.
2.5 Data analysis
The sorption amounts of OFL on different sediment samples were calculated according to the difference of the OFL concentrations in the water phase before and after the sorption, which used Eq. (1):
where Qe is the amount of OFL sorbed onto the sediment sample, mg g−1; C0 is the initial concentration of OFL, mg L−1; Ce is the concentration of OFL at equilibrium, mg L−1; V is the volume of the sorption solution, L; and W is the dry weight of the sediment sample, g.
The linear model, Langmuir model, Freundlich model, and dual reactive domain model (DRDM) were used to fit the sorption process. These models can be expressed by Eqs. (2), (3), (4), and (5):
Linear model:
-
Langmuir model:
-
Freundlich model:
-
DRDM:
where KH is the linear partition coefficient, L g−1; Qmax is the Langmuir adsorption maximum capacity, mg g−1; KL is the Langmuir equilibrium constant, L g−1; KF is the Freundlich equilibrium coefficient, (mg g−1)/(mg L−1)n; n is the Freundlich nonlinear coefficient; and KP is the partition coefficient of the linear component of the DRDM, L g−1.
Because the number of parameters varied in the above models, the normal coefficient of determination (r2) could not be compared directly. The adjusted r2 (r2adj) was therefore calculated and compared (Peng et al. 2012).
where m is the number of data points used for fitting and b is the number of coefficients in the fitting equation.
The single-point distribution coefficients (KD) were calculated at selected equilibrium concentrations of OFL to compare the sorption capacity of each sediment sample. The equation was as follows:
where Ce′ is the selected equilibrium concentration of OFL, mg L−1; and Qe′ is the sorption amount calculated from the best fitting model at selected equilibrium concentration of OFL, mg g−1. Model fitting and analysis of variance were conducted using Origin (Version 9.0, OriginLab, US).
3 Results and discussion
3.1 Characterization of original sediment and biofilm-coated sediments
The TOC values of sediment samples increased after cultivation for 30 and 45 days (Table 1). In general, the metabolic activities of microorganisms in biofilms can increase the organic content of sediment (Shang et al. 2014). As such, the information from TOC data demonstrates the formation of biofilms on the sediment surface. The CEC values of sediment samples ranged from 42.40 to 64.83 mmol g−1 (Table 1), the same order of magnitude as that specified by Ashayeri et al. (2018).
3.2 Effect of biofilms on ofloxacin sorption onto sediments
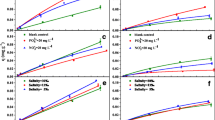
The sorption data were fitted with the linear, Langmuir, and Freundlich models as well as DRDM. The sorption parameters and values of r2adj are listed in Table S2 (ESM). The r2adj values suggested that the isotherms were well fitted by DRDM (r2adj > 0.943), and the DRDM isotherms are shown in Fig. 1. The DRDM could be further used to discuss changes in the linear partitioning (or dissolution) and nonlinear adsorption of OFL into or onto sediments after the growth of biofilms.
The relationship between partition coefficients and TOC values has been widely discussed in the literature. Previous authors usually reported a positive correlation between partition coefficients of organic contaminants and TOC of soils/clay minerals (Pan et al. 2006; Tan et al. 2018). However, the TOC values did not correlate significantly with the KP values (P > 0.05) in this study, which may have been a result of not having sufficient data points to see statistical significance or being limited by coexisting nonlinear adsorption (Xia et al. 2016). Although there was no significant correlation, the content of organic substances in the sediment samples increased, while their KP values decreased after the growth of biofilms (Fig. 2). The results showed that growth of biofilms decreased the KP values by 50% and 60%, but these values were still in accordance with the reported range from 0.39 to 16.54 g L−1 (Zhao et al. 2016). This implies that the sediments used in the previous studies may have been colonized by biofilms, although these studies’ authors did not identify the presence of biofilms on sediments. The decrease of KP after the growth of biofilms was related to the presence of EPS, which has been credited with both the interaction with, and the sequestering of, antibiotics (Martin et al. 2015; Zhang et al. 2018b; Wang et al. 2019). Thus, the growth of biofilms on the surface of sediments created a barrier, and OFL partitioned into the outer layer of biofilms. The partitioning process of organic contaminants into organic matter via hydrophobic interaction was related to the properties of organic matter (Chiou and Kile 1994; Pignatello et al. 2006; Yamamoto et al. 2009). Biofilm’s organic matter is less aromatic than that of sediment (Writer et al. 2011). Based on the similarity-intermiscibility theory, OFL containing aromatic ring structures will have a low affinity for low-aromaticity organic matter from biofilms. Therefore, the hydrophobic partitioning of OFL in sediments would be heavier than that in biofilms. In short, weak hydrophobic interaction between OFL and organic matter in the outer layer of biofilms leads to relatively low partition coefficients of OFL in biofilm-coated sediments.
Compared to the linear partition components, biofilms may have a different effect on the nonlinear Langmuir adsorption components. Because the KD values of the Langmuir adsorption components (KDL) in DRDM were related to the OFL equilibrium concentrations (Fig. S1–ESM), the mean KDL values were calculated at 100 selected OFL equilibrium concentrations ranging from 0 to 0.4 mg L−1. Then, the ratio of the mean KDL values of BS-30 or BS-45 to that of OS was calculated to quantitatively describe the changes in adsorption capacities. The calculated ratio values of BS-30 or BS-45 to OS were 1.7 ± 0.1 and 2.0 ± 0.7, respectively. The ratios were greater than one, indicating that the growth of biofilms enhanced nonlinear adsorption. Previous studies have shown that cation exchange contributed to the nonlinear adsorption process because the positively charged piperazinyl group of OFL exchanged H+ on the adsorbent surface (Zhang et al. 2018b). After the growth of biofilms, the increased CEC of sediments may promote cation exchange. Li et al. (2018) also reported that the higher CEC of sediments had a higher adsorption capacity for fluoroquinolone antibiotics. However, there was no significant correlation between CEC values and KDL (P > 0.05) in this study, suggesting the presence of other nonlinear adsorption mechanisms such as hydrogen bonding. It has been reported that hydroxyl hydrogen in sediments can provide binding sites for OFL sorption through the formation of hydrogen bonds (Wu et al. 2013). In the current study, the infrared spectra of OS and BS-30 were recorded to investigate the formation of hydrogen bonds (Fig. S2–ESM). After OFL sorption, the infrared absorption bands of –OH at 3400 cm−1 become lower due to red-shifts in O–H stretching frequencies (Fig. S2–ESM), which are caused by formation of hydrogen bonds as reported by Champagne et al. (2015). The most common are hydrogen bonds of the type X–H···Y, where X is O, N, or a halogen and Y is O, N, S, or a halide (Steiner 2002). Therefore, fluorine atoms with strong electronegativity in OFL could form hydrogen bonds with –OH groups in sediment samples. In an earlier study, the authors also reported the formation of hydrogen bonds between fluorine and hydrogen atoms (Uysal et al. 2013). However, other types of binding, except for hydrogen bonds, may occur with OFL and sediment, such as formation of an inner-sphere complex with iron and aluminum oxides in sediments via the carboxylic group in OFL (Li et al. 2017), which needs to be investigated in the future. In short, the growth of biofilms affects cation exchange and hydrogen bonds, thereby increasing the nonlinear adsorption capacities of sediments for OFL.
3.3 Interaction between extracellular polymeric substances and ofloxacin
As described in the previous section, EPS is likely to interact with OFL, which influences the sorption of the latter on biofilm-coated sediments. As such, the interaction between EPS and OFL was further investigated. The 3D-EEM fluorescence spectra showed that four fluorescence peaks were found in EPS-30 and EPS-45 (Fig. 3a, b). Peak T1 (Ex/Em = 225 nm/315–330 nm) and peak T2 (Ex/Em = 280 nm/330–345 nm) corresponded to protein-like fluorescence and could be further identified as aromatic protein and soluble microbial byproduct-like substances, respectively (Mayer et al. 1999; Dong et al. 2017). Peak A (Ex/Em = 255–275 nm/435–460 nm) and peak C (Ex/Em = 360–365 nm/440–460 nm) were associated with humic-like substances (Coble 1996; Kim et al. 2015). After the addition of OFL, the fluorescence intensities of peak T1 and T2 decreased (Fig. 3c, d). It is likely that this decrease was due to fluorescence quenching by OFL, suggesting that protein-like substances might react with OFL.
Fluorescence-quenching processes can be subdivided into static and dynamic processes that can be identified by UV-Vis spectroscopy. The dynamic quenching process can affect the excited state fluorophore but does not affect the UV-Vis spectra. In contrast, complexes formed during the static quenching process affect the UV-Vis spectra of the reagents (Dong et al. 2017). As shown in Fig. 4, the UV-Vis absorption spectrum of EPS (black solid line) in this study was obviously different from the spectrum of (EPS + OFL)-OFL (red dash line), which was calculated by subtracting the spectrum of OFL (blue dot line) from the spectrum of EPS + OFL (cyan dash dot line) to eliminate the influence of OFL in solution. This result implies the formation of a complex between EPS and OFL. In addition, the ratio of absorbance at 250–365 nm (E250/E365) of EPS-30 and EPS-45 decreased by 59.1% and 75.3% (Table S3–ESM), respectively, after the addition of OFL. It has been reported that E250/E365 negatively correlated with molecular size (Dong et al. 2018). The decreased values of E250/E365 in this study indicated an increase in the molecular size of EPS, further suggesting the formation of complexes between EPS and OFL. Therefore, the UV-Vis spectra confirmed that static quenching of the binding interaction between EPS and OFL is the basis upon which protein-like substances interact with OFL. This is corroborated by a previous study whose authors also reported that proteins in EPS play an important role in the sorption of fluoroquinolone antibiotics through the provision of binding sites (Zhang et al. 2018a). Thus, it appears that EPS–OFL complexes were formed in this study during the sorption process, which influenced the sorption of OFL on biofilm-coated sediments.
3.4 Implications
Biofilm growth on the sediment will change the properties of sediments, such as TOC, CEC, and functional group. Consequently, the sorption mechanisms of OFL onto biofilm-coated sediments will be different from that of original sediments, which shows the suppressed linear partition capacities and enhanced nonlinear Langmuir adsorption capacities. Generally, the sorption process dominated by Langmuir adsorption will transform into linear partition with the increase of sorbate concentrations when both linear partition and Langmuir adsorption are involved in sorption (Garcia-Zubiri et al. 2009; Cheng et al. 2017). Therefore, the enhanced effect of biofilms on sorption capacities of OFL onto sediments will transform into a suppressed effect with the increasing of OFL concentrations. It is clear that the less OFL is sorbed, the more there will be in water (without consideration of OFL degradation). The ecological risk of OFL to aquatic environment is mainly predicted by its concentration in water (He et al. 2019). Based on the present study, if the ecological risk of OFL was estimated from its concentration in water which was predicted by initial discharged concentration and the sorption capacity of original sediments, it will be incorrect due to the effect of biofilms on sorption capacities of OFL by sediments. Thus, when estimating the ecological risk of OFL in eutrophic waters, the growth of biofilms on sediments should be considered. However, other various factors including shear stress, nutrient level, microbial consortia, sediment composition, and antibiotic types may influence the results. Further experiments, with a focus on these influential factors, will be conducted in the future.
4 Conclusions
In this study, the growth of biofilms made OFL sorb onto the outer layer of biofilm-coated sediments. During the sorption process, both linear partitioning and nonlinear adsorption contributed to the sorption of OFL on original sediments and biofilm-coated sediments. The hydrophobic partitioning of OFL into sediments was suppressed, while nonlinear adsorption mechanisms, including cation exchange and hydrogen bonding, were enhanced after the growth of biofilms. Meanwhile, protein-like substances in EPS from biofilms interacted with OFL and affected the sorption of OFL to sediments. With the increase of OFL concentration, the combination of linear partitioning and nonlinear adsorption resulted in OFL sorption to biofilm-coated sediments being suppressed compared with the original sediments. The inhibited sorption of OFL onto biofilm-coated sediments increased the OFL concentration in the aqueous phase, which could further increase the ecological risk of OFL in waters. Therefore, freshwater biofilms play an important role in the sorption of OFL onto sediment surfaces.
References
Ashayeri NY, Keshavarzi B, Moore F, Kersten M, Yazdi M, Lahijanzadeh AR (2018) Presence of polycyclic aromatic hydrocarbons in sediments and surface water from Shadegan wetland–Iran: a focus on source apportionment, human and ecological risk assessment and sediment-water exchange. Ecotoxicol Environ Saf 148:1054–1066
Babic S, Perisa M, Skoric I (2013) Photolytic degradation of norfloxacin, enrofloxacin and ciprofloxacin in various aqueous media. Chemosphere 91(11):1635–1642
Cao WQ, Song J, Yang GP (2017) An adsorption and thermodynamic study of ofloxacin on marine sediments. Environ Chem 14(6):350–360
Champagne PA, Desroches J, Paquin JF (2015) Organic fluorine as a hydrogen-bond acceptor: recent examples and applications. Synthesis 47(03):306–322
Chen GL, Liu X, Tartakevosky D, Li M (2016) Risk assessment of three fluoroquinolone antibiotics in the groundwater recharge system. Ecotoxicol Environ Saf 133:18–24
Chen XD, Zhang CK, Paterson DM, Thompson CEL, Townend IH, Gong Z, Zhou Z, Feng Q (2017) Hindered erosion: the biological mediation of noncohesive sediment behavior. Water Resour Res 53(6):4787–4801
Cheng GH, Sun MY, Ge XL, Ou Y, Xu XH, Lin Q, Lou LP (2017) Adsorption-desorption characteristics of nonylphenol on two different origins of black carbon. Water Air Soil Pollut 228(8):311
Cheng W, Fang HW, Lai HJ, Huang L, Dey S (2018) Effects of biofilm on turbulence characteristics and the transport of fine sediment. J Soils Sediments 18(10):3055–3069
Chiou CT, Kile DE (1994) Effects of polar and nonpolar groups on the solubility of organic compounds in soil organic matter. Environ Sci Technol 28:1139–1144. https://doi.org/10.1021/es00055a026
Coble PG (1996) Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar Chem 51(4):325–346
Danner MC, Robertson A, Behrends V, Reiss J (2019) Antibiotic pollution in surface fresh waters: occurrence and effects. Sci Total Environ 664:793–804
Dhawde R, Macaden R, Saranath D, Nilgiriwala K, Ghadge A, Birdi T (2018) Antibiotic resistance characterization of environmental E. coli isolated from river Mula-Mutha, Pune District, India. Int J Environ Res Public Health 15(6):1247
Ding HX, Li Y, Hou J, Wang Q, Wu Y (2015) Sorption behavior and modeling of endocrine-disrupting chemicals on natural sediments: role of biofilm covered on surface. Environ Sci Pollut Res 22(2):1380–1388
Dong DM, Zhang LW, Liu S, Guo ZY, Hua XY (2016) Antibiotics in water and sediments from Liao River in Jilin Province, China: occurrence, distribution, and risk assessment. Environ Earth Sci 75(16):1202
Dong DM, Zhang LW, Guo ZY, Hua XY (2017) The role of extracellular polymeric substances on the sorption of pentachlorophenol onto natural biofilms in different incubation times: a fluorescence study. Chem Ecol 33(2):131–142
Dong DM, Li LF, Zhang LW, Hua XY, Guo ZY (2018) Effects of lead, cadmium, chromium, and arsenic on the sorption of lindane and norfloxacin by river biofilms, particles, and sediments. Environ Sci Pollut Res 25(5):4632–4642
Dorival-Garcia N, Zafra-Gomez A, Navalon A, Gonzalez J, Vílchez JL (2013) Removal of quinolone antibiotics from wastewaters by sorption and biological degradation in laboratory-scale membrane bioreactors. Sci Total Environ 442:317–328
Fang HW, Zhao HM, Shang QQ, Chen MH (2012) Effect of biofilm on the rheological properties of cohesive sediment. Hydrobiologia 694(1):171–181
Fang HW, Shang QQ, Chen MH, He GJ (2014) Changes in the critical erosion velocity for sediment colonized by biofilm. Sedimentology 61:648–659
Fang HW, Chen YS, Huang L, He GJ (2017) Biofilm growth on cohesive sediment deposits: laboratory experiment and model validation. Hydrobiologia 799(1):261–274
Garcia-Zubiri IX, González-Gaitano G, Isasi JR (2009) Sorption models in cyclodextrin polymers: Langmuir, Freundlich, and a dual-mode approach. J Colloid Interface Sci 337(1):11–18
Gerbersdorf SU, Wieprecht S (2015) Biostabilization of cohesive sediments: revisiting the role of abiotic conditions, physiology and diversity of microbes, polymeric secretion, and biofilm architecture. Geobiology 13:68–97
Ghanbari A, Dehghany J, Schwebs T, Müsken M, Häussler S, Meyer-Hermann M (2016) Inoculation density and nutrient level determine the formation of mushroom-shaped structures in Pseudomonas aeruginosa biofilms. Sci Rep 6:32097. https://doi.org/10.1038/srep32097
Gothwal R, Shashidhar (2017) Occurrence of high levels of fluoroquinolones in aquatic environment due to effluent discharges from bulk drug manufacturers. J Hazard Toxic Radioact Waste 21(3):05016003
Gothwal R, Thatikonda S (2017) Role of environmental pollution in prevalence of antibiotic resistant bacteria in aquatic environment of river: case of Musi river, South India. Water Environ J 31(4):456–462
Hahladakis JN, Lekkas N, Smponias A, Gidarakos E (2014) Sequential application of chelating agents and innovative surfactants for the enhanced electroremediation of real sediments from toxic metals and PAHs. Chemosphere 105:44–52
He SN, Dong DM, Sun C, Zhang X, Zhang LW, Hua XY, Guo ZY (2019) Contaminants of emerging concern in a freeze-thaw river during the spring flood. Sci Total Environ 670:576–584
Hu J, Zhou J, Zhou S, Wu P, Tsang YF (2018a) Occurrence and fate of antibiotics in a wastewater treatment plant and their biological effects on receiving waters in Guizhou. Process Saf Environ Prot 113:483–490
Hu Y, Yan X, Shen Y, Di MX, Wang J (2018b) Antibiotics in surface water and sediments from Hanjiang River, Central China: occurrence, behavior and risk assessment. Ecotoxicol Environ Saf 157:150–158
Jurado A, Walther M, Díaz-Cruz S (2019) Occurrence, fate and environmental risk assessment of the organic microcontaminants included in the watch lists set by EU decisions 2015/495 and 2018/840 in the groundwater of Spain. Sci Total Environ 663:285–296
Kafaei R, Papari F, Seyedabadi M, Sahebi S, Tahmasebi R, Ahmadi M, Sorialh GA, Asgarii G, Ramavandi B (2018) Occurrence, distribution, and potential sources of antibiotics pollution in the water-sediment of the northern coastline of the Persian Gulf, Iran. Sci Total Environ 627:703–712
Kim LH, Jung Y, Yu HW, Chae KJ, Kim IS (2015) Physicochemical interactions between rhamnolipids and Pseudomonas aeruginosa biofilm layers. Environ Sci Technol 49(6):3718–3726
Li YD, Bi E, Chen HH (2017) Sorption behavior of ofloxacin to kaolinite: effects of pH, ionic strength, and cu (II). Water Air Soil Pollut 228(1):46
Li S, Huang Z, Wang Y, Liu YQ, Luo R, Shang JG (2018) Migration of two antibiotics during resuspension under simulated wind–wave disturbances in a water–sediment system. Chemosphere 192:234–243
Li SS, Peng CR, Cheng TS, Wang C, Guo LL, Li DH (2019) Nitrogen-cycling microbial community functional potential and enzyme activities in cultured biofilms with response to inorganic nitrogen availability. J Environ Sci 76:89–99
Liao QJH, Huang Z, Li S, Wang Y, Liu YQ, Luo R, Shang JG (2018) Effects of wind–wave disturbances on adsorption and desorption of tetracycline and sulfadimidine in water–sediment systems. Environ Sci Pollut Res 25(23):22561–22570
Lou LP, Wu BB, Wang LN, Luo L, Xu XH, Hou JA, Bei X, Hu BL, Chen YX (2011) Sorption and ecotoxicity of pentachlorophenol polluted sediment amended with rice-straw derived biochar. Bioresour Technol 102(5):4036–4041
Martin C, Low WL, Gupta A, Amin MCIM, Radecka I, Britland ST, Raj P, Kenward K (2015) Strategies for antimicrobial drug delivery to biofilm. Curr Pharm Des 21(1):43–66
Mayer LM, Schick LL, Loder TC III (1999) Dissolved protein fluorescence in two Maine estuaries. Mar Chem 64(3):171–179
Pan B, Xing BS, Liu WX, Tao S, Lin XM, Zhang YX, Yuan HS, Dai HC, Zhang XM, Xiao Y (2006) Two-compartment sorption of phenanthrene on eight soils with various organic carbon contents. J Environ Sci Health B 41(8):1333–1347
Pan B, Wang P, Wu M, Li J, Zhang D, Xiao D (2012) Sorption kinetics of ofloxacin in soils and mineral particles. Environ Pollut 171:185–190
Parsons DR, Schindler RJ, Hope JA, Malarkey J, Baas JH, Peakall J, Manning AJ, Ye L, Simmons S, Paterson DM, Aspden RJ, Bass SJ, Davies AG, Lichtman ID, Thorne PD (2016) The role of biophysical cohesion on subaqueous bed form size. Geophys Res Lett 43:1566–1573
Peng HB, Pan B, Wu M, Liu Y, Zhang D, Xing BS (2012) Adsorption of ofloxacin and norfloxacin on carbon nanotubes: hydrophobicity-and structure-controlled process. J Hazard Mater 233:89–96
Pignatello JJ, Lu YF, LeBoeuf EJ, Huang WL, Song JZ, Xing BS (2006) Nonlinear and competitive sorption of apolar compounds in black carbon-free natural organic materials. J Environ Qual 35:1049–1059
Prutthiwanasan B, Suntornsuk L (2018) Improved resolution of fluoroquinolones using cetyltrimethyl ammonium bromide–micellar electrokinetic chromatography and its application to residue analysis in surface water. J Chromatogr B 1092:306–312
Ran Y, Sun K, Yang Y, Xing BS, Zeng E (2007) Strong sorption of phenanthrene by condensed organic matter in soils and sediments. Environ Sci Technol 41:3952–3958
Riaz L, Mahmood T, Khalid A, Rashid A, Ahmed Siddique MB, Kamal A, Coyne MS (2018) Fluoroquinolones (FQs) in the environment: a review on their abundance, sorption and toxicity in soil. Chemosphere 191:704–720
Shang QQ, Fang HW, Zhao HM, He GJ, Cui ZH (2014) Biofilm effects on size gradation, drag coefficient and settling velocity of sediment particles. Int J Sediment Res 29(4):471–480
Shen B, Wen XH, Korshin GV (2018) Electrochemical oxidation of ciprofloxacin in two different processes: the electron transfer process on the anode surface and the indirect oxidation process in bulk solutions. Environ Sci Proc Impacts 20:943–955
Steiner T (2002) The hydrogen bond in the solid state. Angew Chem Int Ed 41(1):48–76
Sun K, Gao B, Zhang ZY, Zhang GX, Liu XT, Zhao Y, Xing BS (2010) Sorption of endocrine disrupting chemicals by condensed organic matter in soils and sediments. Chemosphere 80:709–715
Tan RJ, Liu RX, Li B, Liu XL, Li ZS (2018) Typical endocrine disrupting compounds in rivers of Northeast China: occurrence, partitioning, and risk assessment. Arch Environ Contam Toxicol 75:213–223
Thom M, Schmidt H, Gerbersdorf SU, Wieprecht S (2015) Seasonal biostabilization and erosion behavior of fluvial biofilms under different hydrodynamic and light conditions. Int J Sediment Res 30:273–284
Tolls J (2001) Sorption of veterinary pharmaceuticals in soils: a review. Environ Sci Technol 35(17):3397–3406
Uysal I, Severcan F, Evis Z (2013) Characterization by Fourier transform infrared spectroscopy of hydroxyapatite co-doped with zinc and fluoride. Ceram Int 39(7):7727–7733
Wang ZC, Gao MC, Wang S, Xin YJ, Ma D, She ZL, Wang Z, Chang QB, Ren Y (2014) Effect of hexavalent chromium on extracellular polymeric substances of granular sludge from an aerobic granular sequencing batch reactor. Chem Eng J 251:165–174
Wang P, Zhang D, Zhang H, Li H, Ghosh S, Pan B (2017a) Impact of concentration and species of sulfamethoxazole and ofloxacin on their adsorption kinetics on sediments. Chemosphere 175:123–129
Wang Z, Du Y, Yang C, Liu X, Zhang JQ, Li EH, Zhang Q, Wang XL (2017b) Occurrence and ecological hazard assessment of selected antibiotics in the surface waters in and around Lake Honghu, China. Sci Total Environ 609:1423–1432. https://doi.org/10.1016/j.scitotenv.2017.08.009
Wang CQ, Dong DM, Zhang LW, Song ZW, Hua XY, Guo ZY (2019) Response of freshwater biofilms to antibiotic florfenicol and ofloxacin stress: role of extracellular polymeric substances. Int J Environ Res Public Health 16(5):715
Writer JH, Ryan JN, Barber LB (2011) Role of biofilms in sorptive removal of steroidal hormones and 4-nonylphenol compounds from streams. Environ Sci Technol 45(17):7275–7283
Wu M, Pan B, Zhang D, Xiao D, Li H, Wang C, Ning P (2013) The sorption of organic contaminants on biochars derived from sediments with high organic carbon content. Chemosphere 90(2):782–788
Xia H, Gomez-Eyles JL, Ghosh U (2016) Effect of polycyclic aromatic hydrocarbon source materials and soil components on partitioning and dermal uptake. Environ Sci Technol 50(7):3444–3452
Yamamoto H, Nakamura Y, Moriguchi S, Nakamura Y, Honda Y, Tamura I, Hirata Y, Hayashi A, Sekizawa J (2009) Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: laboratory photolysis, biodegradation, and sorption experiments. Water Res 43(2):351–362
Zhang JQ, Dong YH (2008) Effect of low-molecular-weight organic acids on the adsorption of norfloxacin in typical variable charge soils of China. J Hazard Mater 151(2–3):833–839
Zhang GX, Zhang Q, Sun K, Liu XT, Zheng WJ, Zhao Y (2011) Sorption of simazine to corn straw biochars prepared at different pyrolytic temperatures. Environ Pollut 159(10):2594–2601
Zhang HQ, Jia YY, Khanal SK, Lu H, Fang HT, Zhao Q (2018a) Understanding the role of extracellular polymeric substances (EPS) on ciprofloxacin (CIP) adsorption in aerobic sludge, anaerobic sludge and sulfate-reducing bacteria (SRB) sludge systems. Environ Sci Technol 52(11):6476–6486
Zhang LW, Dong DM, Hua XY, Guo ZY (2018b) Inhibitory effects of extracellular polymeric substances on ofloxacin sorption by natural biofilms. Sci Total Environ 625:178–184
Zhao S, Liu X, Cheng D, Liu G, Liang B, Cui B, Bai J (2016) Temporal–spatial variation and partitioning prediction of antibiotics in surface water and sediments from the intertidal zones of the Yellow River Delta, China. Sci Total Environ 569:1350–1358
Funding
This study was supported by the National Natural Science Foundation of China (No. 21876060, 21577047, and 21307041) and the Science and Technology Program of Education Department of Jilin Province, China (No.JJKH20190124KJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Ian G. Droppo
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 244 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Dong, D., Hua, X. et al. Sorption of the fluoroquinolone antibiotic ofloxacin by aquatic sediments: influence of biofilm development at the sediment-water interface. J Soils Sediments 19, 4063–4072 (2019). https://doi.org/10.1007/s11368-019-02356-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02356-w