Abstract
Purpose
River sediment pollution by heavy metals/metalloids has attracted widespread attention due to a serious threat to the ecosystem and human health. As an effective and economical alternative, the stabilization method was considered by previous studies for the remediation of sediments polluted by metals/metalloids. However, a comprehensive study is required for an extensive comparison on the effects of metal/metalloid immobilization based on the application of different materials as sediment amendments.
Materials and methods
In this study, the Maozhou River was selected as the study area, and the stabilization method was applied for the remediation of the river sediment polluted by metals and metalloids. Five materials (CaCO3, Ca(OH)2, zeolite, kaolin, FeCl2) were selected as amendments for the metal/metalloid stabilization in the collected sediment. A modified BCR procedure was employed for the speciation analysis of heavy metals and metalloid in the sediment before and after remediation. A TCLP (toxicity characteristic leaching procedure) investigation was performed to further evaluate the immobilization of heavy metals in acidic environment.
Results and discussion
The sediment of the Maozhou River was heavily polluted by heavy metals and metalloid. The speciation of As, Pb, Cr, and Mn mainly exists as residual fraction (F4), while that of Ni, Cu, and Zn was identified as exchangeable metal and carbonate-associated fraction (F1) and fraction associated with Fe-Mn oxides (F2). Moreover, the F2 fraction of Co was observed as the major speciation. Through the application of five materials (CaCO3, Ca(OH)2, zeolite, kaolin, FeCl2) as sediment amendments, the metal/metalloid speciation was transferred into F4. When five amendments were compared, the stabilization effect can be ordered as CaCO3 > zeolite > FeCl2 > kaolin > Ca(OH)2 based on the modified BCR results. TCLP results showed that using Ca(OH)2 and CaCO3 as amendments can significantly reduce the metal leachability in an acidic environment, while zeolite is effective for most of the heavy metals and metalloid.
Conclusions
The results showed that the sediment of the Maozhou River was seriously polluted by a variety of heavy metals and metalloids. This study provided extensive information on the speciation of metals or metalloid and the effect of various amendments on metals and metalloid stabilization, which can be of vital importance for further remediation of metal/metalloid-polluted sediment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With increasing waste discharges by anthropogenic activities, a large number of rivers have been severely contaminated worldwide (Li et al. 2013; Guan et al. 2016; Sekulić et al. 2018), which has become a major environmental concern. A considerable amount of rivers are polluted by heavy metals and metalloids discharged from various industrial processes such as metal smelting and battery manufacture (Feng et al. 2004; Chen et al. 2009). The river sediment has been widely accepted as a carrier and potential source of the contaminants in an aquatic environment (Yu et al. 2001). Heavy metals and metalloids accumulated in the sediments tend to re-enter the overlying water body when the physicochemical properties (e.g., pH, redox potential, ionic strength, and salinity) of the water change. The released metals and metalloids from the sediment might cause secondary pollution to the river ecosystem and deteriorate the quality of the water (Zoumis et al. 2001; Filgueiras et al. 2002; Juwarkar et al. 2010). Recently, the sediment pollution caused by heavy metals and metalloids has attracted widespread attention due to a serious threat to the ecosystem and human health (Zhang et al. 2011a; Chabukdhara and Nema 2012; Xue et al. 2018).
Remediation agents are always needed to avoid further release of heavy metals and metalloids via changing the form of heavy metals and metalloids in the contaminated sediments. The sediment remediation involves a variety of physical, chemical, and biological technologies, in which the stabilization/solidification (S/S) is considered as an effective and economical method (Yi et al. 2017). S/S technology has been widely applied in soil remediation and solid waste treatment, through which the toxic metals can be immobilized by changing their speciation via the addition of S/S treatment materials (Lee et al. 2009; Yoo et al. 2013). With different physical and chemical bindings, the newly formed metal speciation may further reduce the mobility and bioavailability of the toxic metals (Lee et al. 2009). S/S has expressed the effect of long-term metal and metalloid immobilization, making its wide application for the remediation of the heavy metal and metalloid-contaminated sediment. Moreover, as the speciation of metals and metalloids determines their toxicity, mobility, and bioavailability (Jain 2004), the speciation analysis is recognized as one of the most reliable criteria to evaluate the potential environmental effect of the contaminated river sediments (Sundaray et al. 2011; Huang et al. 2016).
The sequential chemical extraction method is usually applied for metal/metalloid speciation analysis, which can further evaluate the direct and potential toxicity based on the metal/metalloid mobility and bioavailability. In this way, this method is recognized as a suitable means for evaluating the stabilization effect of heavy metal and metalloid-contaminated sediments (Obbard 2006; Sundaray et al. 2011; Yoo et al. 2013; Yi et al. 2017). There are numerous sequential extraction procedures applied for the speciation analysis of heavy metals and metalloids in the sludge, soil, and sediment, while the BCR (European Community Bureau of Reference) is one of the most widely used methods (Huang et al. 2016; Zhao et al. 2017). The modified BCR has been successfully applied to divide metals and metalloids in sediments into different binding forms, including exchangeable metal and carbonate-associated fraction (F1), fraction associated with Fe-Mn oxides (F2), fraction bound to organic matter (F3), and residual fraction (F4). The stability of the metal/metalloid speciation above can be listed in the order as follows: F1 < F2 < F3 < F4. As the most stable fraction, F4 is insoluble and almost unreactive because the residual solids occlude heavy metals in their crystalline structures (Fuentes et al. 2008). Therefore, the amount and/or the ratio of F4 can be adopted as the most critical data to evaluate the metal/metalloid stabilization effect after sediment remediation.
The principle of choosing amendment is to increase the content and/or proportion of F4 fraction of heavy metals and metalloids after the application of the amendment. In this study, we selected five cost-effective materials with different properties, including calcium carbonate (CaCO3), calcium hydroxide (Ca(OH)2), artificial zeolite (Na2O·Al2O3·xSiO2·yH2O), kaolin (Al2Si2O5(OH)4), and iron chloride tetrahydrate (FeCl2·4H2O). Although some of the above amendments have been applied for the in situ immobilization of metal-contaminated sediment, none of the studies has made a comprehensive comparison on the effects of metal immobilization due to the varieties in experimental conditions. To provide technical guidance based on the effects of metal/metalloid immobilization under the same experimental condition, a systematic research was conducted in this study on metal immobilization through the application of the five materials. This work will determine the concentration level and the speciation of toxic metals/metalloids in the surface sediment of Maozhou River, as the most heavily polluted river in Shenzhen due to a variety of industrial activities but lack of published data on this issue. A series of experiments will be conducted extensively on the speciation of eight heavy metals and metalloid together with the effect of a variety of amendments on the metal/metalloid stabilization. Therefore, in this study, the speciation of eight metals and metalloid (Cr, Mn, Co, Ni, Cu, Zn, Pb, and As) will be explicated comprehensively before and after the addition of the amendments, while the leaching experiment modified from U.S. EPA SW-846 method 1311: toxicity characteristic leaching procedure (TCLP) will be conducted to further evaluate the effect of metal/metalloid stabilization. The results of this study will provide informative data for the heavy metal/metalloid pollution of the sediment and further propose an efficient methodology for the remediation of heavy metal/metalloid-contaminated sediments.
2 Materials and methods
2.1 Study area
Maozhou River, with a length of 31.3 km, is the largest river in Shenzhen and flows through the Guangming new area and Baoan district until finally into the Pearl River Estuary. Many industries (i.e., electroplating factory) distribute very densely around the watershed, which may cause serious pollution to the Maozhou River. The sediment of the river was dark, and the wastewater was kept being discharged from the drainage pipes along the river. Moreover, the city of Shenzhen has a total land area of 1949 km2 and a subtropical oceanic monsoon climate with an annual temperature of around 10–25 °C and annual precipitation range of 1600–2000 mm (Zhou et al. 2010).
2.2 Sample preparation and characterization
The subsurface sediment samples (0–20 cm depth) were randomly collected from the downstream of the Maozhou River (113.829° E, 22.791° N) in October 2018, and then homogenized and saved in the plastic bucket. The collected samples were freeze-dried, crushed, and then sieved through a 100-mesh nylon sieve, with particle size (d50) as 13.7 μm measured by a granulometer (Malvern, MASTERSIZER 3000) (Table 1 and Fig. S1 of the Electronic Supplementary Material—ESM). The pH value, moisture content (%), and the element compositions (%) were tested according to the standard method. For the measurement of sediment pH, 0.5 g of the air-dried sample was taken in ultrapure water (25 mL) and agitated for 24 h. Then the solution was left with occasionally shaking for 1 h before measuring the pH (Jain 2004). After drying the samples at 105 °C to a constant weight, the moisture content of the sediment was calculated accordingly. The elemental composition (C, H, N, and S) of the sediment was detected by a vario MICRO cube elemental analyzer (Elementar Analysen systeme GmbH, Germany).
The metal and metalloid contents of the sediment were determined by the microwave digestion using the acid mixture (HCl + HNO3 + HF = 3 + 9 + 4 mL). The samples were then transferred to Teflon bombs and digested in a high-performance microwave digestion system (ETHOS UP, Milestone, Italy). The concentrations of heavy metals and metalloid (Cr, Mn, Co, Ni, Cu, Zn, Pb, and As) were analyzed by an inductively coupled plasma-mass spectrometry (ICP-MS, 7700, Agilent Technologies, USA). Reagent blanks were implemented for each batch of samples.
All chemicals and reagents for experiments were of reagent grade, except hydroxylammonium chloride (guaranteed reagent (GR), 99%), hydrochloric acid (GR), and ammonium acetate (GR, 99%). All solutions were prepared in ultrapure water (18.2 MΩ·cm, Milli-Q). Ultrapure HCl, HNO3, HF, H2O2, and acetic acid were used as the sequential extraction reagents. A variety of materials were adopted in this study as the amendments for heavy metal and metalloid immobilization in the collected sediment, including calcium carbonate (CaCO3), calcium hydroxide (Ca(OH)2), artificial zeolite (Na2O·Al2O3·xSiO2·yH2O), kaolin (Al2Si2O5(OH)4), and iron chloride tetrahydrate (FeCl2·4H2O). Each type of amendment was mixed separately with the sediment at the ratio of 1:10 (w/w), and then, the mixture was added with ultrapure water and kept in a cool and dark environment for incubation of 1 week (Yi et al. 2017). Each series was conducted together with one control without any amendments, and all the experiments were carried out in triplicates.
2.3 Modified BCR procedure for sequential extraction
The modified version of the BCR sequential extraction procedure was selected for the speciation analysis of the heavy metals and metalloid in the collected sediments, including the fourth step—digestion of the residue after the third step using a microwave-assisted acid digestion procedure (Cuong and Obbard 2006). The detailed procedures are illustrated in Fig. 1. The control experiments were also conducted for the sediments without addition of any amendments.
2.4 Leaching experiments
The immobilization effect of metals and metalloid was further evaluated by their leachability via the TCLP. The extraction fluid #2 (0.1 mol L−1 acetic acid, pH 2.88 ± 0.05) was used at a solid/liquid ratio of 1:20 (m:V) and was rotated on a rotary extractor (GGC-X, China) at 30 ± 2 rpm for 18 h at room temperature. After leaching, the mixture was centrifugated at 4000 rpm for 20 min and the supernatant liquid was filtered through a 0.45-μm membrane filter. The pH of leachate was then measured and all the extracts were acidified with HNO3 before being analyzed by ICP-MS.
3 Results and discussion
3.1 Characterization of the river sediment
The physicochemical properties of the collected sediment were summarized in Table 1. The pH value of the collected sediment is 5.95–6.06, which is lower than the reported pH range (6.57–8.20) of the other river sediments (Olivares-Rieumont et al. 2005; De Jonge et al. 2012; Huang et al. 2016). The moisture content was measured as 60.19%, which is slightly higher than the value of the other river sediments (50.20–55.75%) (Yoo et al. 2013; Huang et al. 2016). In addition, it can be found that the content of P is obviously higher than the value of 9500 mg kg−1 reported by Mishra et al. (2008). Moreover, Table 1 also collates the contents of eight heavy metals and metalloid in the river sediment. When the data was compared with the corresponding value listed in the China’s Environmental Quality Standard for Soils (GB15618-1995) (Table S1 of the ESM), the contents of heavy metals and metalloid (Cr, Ni, Cu, Zn, Pb, and As) are up to grade II and grade III, except for Mn and Co which are not included in this standard. The results indicate that the sediment of the Maozhou River is seriously polluted by a variety of heavy metals and metalloid, which might pose a potential danger to the aquatic environment. The C, H, N, and S were also analyzed with very low compositions, indicating a small content of organic matters. The elemental compositions were further obtained by X-ray fluorescence (S8 Tiger, Bruker), and the results in oxide forms show major elements as aluminum (expressed as Al2O3 with 25.36%) and silicon (expressed as SiO2 with 51.73%) in the river sediment.
Table 2 further summarizes the content of heavy metals and metalloid in the sediments from different countries. Compared with the reported data for other sediments, the sediment of Maozhou River contains the largest content for most of the heavy metals, e.g., Cr (430.23 mg kg−1), Co (328.26 mg kg−1), Ni (286.66 mg kg−1), and Cu (1115.81 mg kg−1). Even for the metalloid, the content of As is only lower than the value reported for the sediment of Dongting Lake (Li et al. 2013), while the amount of Pb is higher than the majority of the collected data. The content of Zn in the sediment of Maozhou River (1186.86 mg kg−1) is slightly lower than the value reported for the sediment of Lianshui river (1299 mg kg−1) in the year of 2011 (Zhang et al. 2011a). However, it is dramatically higher than the corresponding data of other sediments (9.10–708.80 mg kg−1). Moreover, the content of Mn in the sediment of Maozhou River is in the middle level among the reported data worldwide including China, Turkey, and India. The significantly high metal content further confirms that the sediment of Maozhou River has been heavily polluted by the heavy metals and metalloid, which needs further remediation.
3.2 Speciation of metals and metalloid in sediments with different amendments
3.2.1 Effect of different amendments on the speciation of metals and metalloid
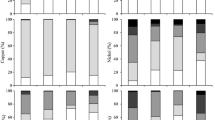
To evaluate the stabilization effect of the eight heavy metals and metalloid after the addition of amendments, the speciation analysis was conducted on the raw sediment and the sediments with different amendments by a modified BCR sequential extraction procedure. The speciation of the heavy metals and metalloid was compared and explicated to better understand the changes in metal and metalloid distribution due to the addition of amendments. Table 3 summarizes the fractions (in mg kg−1) of heavy metals and metalloid with different speciation in the raw and amended river sediments. Meanwhile, Table S2 of the ESM lists the value of water-soluble fraction of the eight heavy metals and metalloid in the raw and amended river sediments, which shows that the water-soluble fraction in most of the sediment is negligible except for Ni and Zn in the sediment with FeCl2 as amendment. Firstly, the recovery rate (R) in Table 3 is calculated within the range of 90.07–109.67%. The value is consistent with the results reported in other studies (Rosado et al. 2016; Yi et al. 2017), indicating the accuracy and credibility of the data in this study. Figure 2 further illustrates the percentages of different fractions from the speciation analysis for the heavy metals and metalloid, and therefore, the variation of the speciation results can be applied for a further evaluation on the metal and metalloid stabilization effect via using different amendments. As the most stable speciation of the heavy metals and metalloid, the value of the residual fraction may be regarded as one of the key criteria to evaluate the stabilization effect of the heavy metal and metalloid. Therefore, the value and percentage for each metal and metalloid will be described and compared after adding different amendments in the statement as follows:
Weight percentages of each fraction for the speciation of seven heavy metals and one metalloid (Cr, Mn, Co, Ni, Cu, Zn, Pb, and As). The metal and metalloid speciation includes water-soluble fraction (F0), exchangeable metal and carbonate-associated fractions (F1), fraction associated with Fe-Mn oxides (F2), fraction bound to organic matter (F3), and residual fraction (F4)
Chromium (Cr)
Table 3 shows that the average value of the residual fraction (F4) for Cr was increased from 185.79 mg kg−1 in the raw sediment to 197.06, 190.19, and 187.21 mg kg−1 in the sediment amended with CaCO3, zeolite, and FeCl2, respectively. When the F4 percentage of Cr was compared (Fig. 2), 44.57% of the Cr existed as the residual fraction in the raw sediment, while this percentage was increased to 48.57% and 45.13% after applying CaCO3 and zeolite as the amendment, respectively. Moreover, the F1 was increased only in the sediment adding with Ca(OH)2. The average value of F2 was increased when using Ca(OH)2, kaolin, and FeCl2 as amendments, while the F3 was increased in the sediment with zeolite, kaolin, and FeCl2.
Manganese (Mn)
The results in Table 3 show that the addition of CaCO3 and FeCl2 in the sediment can increase the residual fraction of Mn from its average initial value of 107.81 mg kg−1 to 115.66 and 113.76 mg kg−1, respectively. Compared to the percentage of F4 in the raw sediment (42.77%), the value was increased obviously to 47.26% and 48.93% after applying CaCO3 and FeCl2 for sediment remediation (Fig. 2). Moreover, in comparison with the metal speciation in the raw sediment, the average value of F1 was increased after adding Ca(OH)2 and kaolin while F3 grew up when using CaCO3, zeolite, kaolin, and FeCl2 as amendments.
Cobalt (Co)
The average value of the residual fraction for Co was found to increase slightly from 25.92 mg kg−1 to the range of 26.08–26.65 mg kg−1 when the raw sediment was amended with most of the materials in this study except Ca(OH)2 (Table 3). Compared to the original sediment without amendments, the residual fraction percentages of Co after applying CaCO3 and FeCl2 were increased from 8.16 to 8.45 and 8.63%, respectively (Fig. 2). The average value of F1 was increased slightly after adding the five agents, while the average value of F3 grew up after adding zeolite, kaolin, and FeCl2 as amendments.
Nickel (Ni)
It can be observed from Table 3 that the presence of CaCO3, zeolite, kaolin, or FeCl2 increased the residual fraction of Ni from the average initial value of 76.41 to 85.23, 91.48, 79.98, 81.73 mg kg−1, respectively. The F4 percentage of Ni was increased from 28.18 to 32.99, 32.26, 29.64% when using CaCO3, zeolite, and FeCl2 as the amendments, respectively (Fig. 2). Moreover, the average value of F1 was increased after applying Ca(OH)2 and kaolin in the river sediment. The F2 grew up when the CaCO3 was used as amendment, while F3 was increased in the sediment with zeolite and kaolin as amendments.
Copper (Cu)
Table 3 shows that the residual fraction of Cu was increased from the average initial value of 159.43 to 167.45 and 174.03 mg kg−1 after adding CaCO3 and zeolite as the amendment, respectively. Compared with the non-amended sediment (Fig. 2), the residual fraction percentage of Cu was increased from 14.99 to 16.44% due to the addition of CaCO3 in the river sediment. Adding kaolin in the river sediment has increased all fractions except F4. Adding Ca(OH)2 only increased F1, while the addition of FeCl2 was found to increase F1 and F3. Moreover, the use of zeolite as the amendment also resulted in an increase in the F3 of Cu in the river sediment.
Zinc (Zn)
When CaCO3 or zeolite was added for sediment remediation, the residual fraction of Zn grew from 232.20 mg kg−1 (the average value) in the raw sediment to 250.50 and 259.95 mg kg−1 (Table 3). It can be seen from Fig. 2 that the F4 percentage of Zn increased obviously from 20.35% (raw sediment) to 23.43% when the sediment is amended by CaCO3. Besides that, the F4 percentage of Zn was also detected to increase slightly (20.41%) when zeolite was applied for metal stabilization in the contaminated sediment. Moreover, the F1 and F3 were increased after adding kaolin, while both F2 and F3 were increased with CaCO3 and zeolite addition. Moreover, when the metals and metalloid were compared with each other, Zn showed the highest bioavailability, with the percentage of more than 76.57% as non-residual fractions in the original river sediment. Even after remediation by the five amendments, Zn was also observed with the highest percentage of the exchangeable and carbonate fractions.
Lead (Pb)
In Table 3, the presence of CaCO3 or kaolin has increased the residual fraction of Pb from 62.84 mg kg−1 (the average value) in the raw sediment obviously to 67.42 or 78.10 mg kg−1. When the CaCO3 or kaolin was added into the sediment, the F4 percentage of Pb was increased from 71.83 to 72.36, 78.26%, respectively (Fig. 2), which is in accordance with the findings of Wen et al. (2016). Moreover, adding CaCO3 or Ca(OH)2 could also increase the F1 and F2, while using kaolin was found to increase the F1 and F3. However, with the addition of FeCl2, the F3 was increased in the sediment.
Arsenic (As)
From the data shown in Table 3, all five amendments can increase the residual fraction of As from its average initial value of 24.46 mg kg−1 to the range of 24.49–27.02 mg kg−1. The F4 percentage of As was increased from 85.48 to 87.08, 87.94, 86.12% after adding CaCO3, zeolite, and FeCl2, respectively (Fig. 2). In addition, the F1 was increased when adding Ca(OH)2 or kaolin in the sediment. The amount of F2 grew up only in the sediment added with Ca(OH)2, while the F3 was increased in the sediment with zeolite, kaolin, or FeCl2 in comparison with the raw sediment.
3.2.2 Comparison on metal/metalloid speciation with different amendments
The addition of amendments like calcium hydroxide, calcium carbonate, and zeolite is to reduce the mobility and bioavailability of heavy metals (Castaldi et al. 2005). The amendments above can reduce the mobility and bioavailability of metal and metalloid in sediment by precipitation and/or sorption (Lee et al. 2009; Peng et al. 2009). The reason of using alkaline materials is that heavy metals can be well immobilized in the matrix with pH above neutral, which was recognized as one of the most critical mechanisms for heavy metal stabilization in soils and sediments (Kumpiene et al. 2008). Meanwhile, the increase in the environmental pH can further promote the adsorption capacity of the zeolite surface for heavy metal ions (Wen et al. 2016). Besides that, adding amendments can also increase the alkalinity of the sediment and subsequently cause a weakened competition of H+ with the other heavy metal ions for ligands (i.e., OH−, CO32−, SO42−, etc.) (Yi et al. 2017). Therefore, without competition from the H+ ions, the heavy metal ions will be easily combined with the ligands, resulting in more stable forms (Peng et al. 2009). In addition, the sorption of heavy metals will be enhanced due to the formation of metal carbonate precipitates, causing further decrease in metal availability (Aziz et al. 2008). Heavy metal such as Cu can be retained by the river sediment through cation exchange and specific adsorption, but precipitation may also be an important mechanism for their retention in the remediated sediment (Pagnanelli et al. 2004). Figure 3 compares the effect of metal and metalloid stabilization by the five amendments, which illustrates that the addition of CaCO3 has increased the residual fraction of the heavy metals and metalloid. In our experiments, the addition of CaCO3 could increase the pH value of the sediment up to 8, which further enhanced the heavy metal and metalloid stabilization (Houben et al. 2012). Increased pH and carbonate can lead to the formation of metal–carbonate precipitate complexes, which may further decrease the metal availability (Kumpiene et al. 2008). The limestone increased the residual fraction of Cu up to 8.02 mg kg−1, which is much higher than the value of 0.87 mg kg−1 reported previously (Yi et al. 2017). The addition of Ca(OH)2 as amendment was found to only increase the residual fraction of As (metalloid). This could be explained by the possible formation of As–Ca complexes like calcium hydrogen arsenate (CaHAsO4) and calcium arsenate (Ca3(AsO4)2) in the presence of calcium under moderate pH conditions (Porter et al. 2004). By adding zeolite as amendment, an obvious effect was observed on the stabilization of five heavy metals and one metalloid. Zeolites are usually recognized as a group of aluminosilicates with a relatively high cation exchange capacity and can also increase the alkalinity of the sediment (Garcıa-Sánchez et al. 1999). The use of zeolite in this study can increase the organic matter bounded fraction of Cu up to 98.92 mg kg−1, which is probably due to its more pronounced tendency for complexation with organic matters in the sediment (Balasoiu et al. 2001). Kaolin was found to increase the residual fraction of three heavy metals and one metalloid (Co, Ni, Pb, and As), which has been reported to be caused by the potential adsorption of the metals on kaolin (Zhang et al. 2011b). More negatively charged surface (kaolin) caused by the increase in pH value can further enhance the adsorption of the positively charged metal ions by the electrostatic force of attraction (Unuabonah et al. 2008). The addition of FeCl2 has increased the weight percentage of F3 and F4 fractions for six heavy metals and one metalloid except Zn, but the fraction of Zn was also found to largely move from F1 to F2. The reduction of Cr can be accelerated by the presence of divalent iron in the soil matrix (Kumpiene et al. 2008), which can reduce its toxicity. The mobility of As can be reduced by the Fe2+ due to the formation of insoluble secondary oxidation minerals, e.g., scorodite (FeAsO4·2H2O) (Sastre et al. 2004).
Weight percentages of each fraction for the speciation of the seven heavy metals and one metalloid before and after adding five amendments. The metal and metalloid speciation includes water-soluble fraction (F0), exchangeable metal and carbonate-associated fractions (F1), fraction associated with Fe-Mn oxides (F2), fraction bound to organic matter (F3), and residual fraction (F4)
3.3 Leaching behavior of metals and metalloid from the sediment
The TCLP leaching experiment was conducted to test the mobility of heavy metals and metalloid in the acidic environment and to further evaluate the effect of heavy metal and metalloid immobilization after remediation. Table 4 shows the amounts of leachable content of the seven heavy metals and one metalloid before (control experiment) and after the addition of five types of amendments. When the variation of seven heavy metals and one metalloid was concerned, it can be observed that the leachable Cr was decreased after adding amendments (except FeCl2) in comparison with the control experiment. The lowest leachable content of Cr was found to be 0.07 mg kg−1 when CaCO3 was used as the amendment, which is 30 times lower than that of the raw sediment. The leaching of Mn, Ni, Cu, and Zn cannot be prevented in the acidic environment even by adding kaolin or FeCl2. However, the above heavy metals reached minimum leachability when Ca(OH)2 was added for sediment remediation. For Co, its leachable content was decreased from 20.13 mg kg−1 in the raw sediment to 4.86 and 0.04 mg kg−1 when CaCO3 and Ca(OH)2 were used as amendments, respectively. The leachable contents of As and Pb were all decreased after adding any of the five amendments, especially that the content of Pb was lower than the detection limit in the leachate of sediment after adding Ca(OH)2, CaCO3, or zeolite.
Furthermore, the TCLP leaching results were further compared among the five amendments. Results show that the using Ca(OH)2 and CaCO3 as amendments can significantly reduce the leachability of all heavy metals and metalloid from the sediment, while zeolite for most of the heavy metals and metalloid except Co, kaolin for two heavy metals, and one metalloid (Cr, Pb, and As), FeCl2 for As, respectively. The substantial decline in the metal and metalloid leachability by the addition of Ca(OH)2 and CaCO3 may be mainly due to their effect on the significant increase in the pH value as alkaline amendments (Li et al. 2001; Yi et al. 2017). For example, the leachable content of Zn was found to be decreased by over 2000 times when Ca(OH)2 was added in the sediment, which might be due to the formation of the metal hydroxides. Similar results have been found for Cu (4.94 mg kg−1), which is consistent with the results of the sequential extraction due to the change of Cu phases as hydroxide for Ca(OH)2 (Li et al. 2001). Based on the results above, even using the same amendment, the evaluation of metal and metalloid stabilization effect is different when the TCLP experiment or the metal and metalloid speciation analysis is adopted.
4 Conclusions
This study showed that the Maozhou River sediment has been seriously polluted by a variety of heavy metals and metalloid. The speciation of metalloid and heavy metals like As, Pb, Cr, and Mn mainly existed as residual fraction, while that of Ni, Cu, and Zn was identified as exchangeable metal and carbonate-associated fractions (F1) and fraction associated with Fe and Mn oxides (F2). Moreover, the F2 fraction of Co was observed as the major speciation. Through the application of five materials (CaCO3, Ca(OH)2, zeolite, kaolin, FeCl2) as sediment amendments, the speciation of the heavy metals and metalloid was transferred into the residual fraction (F4), with the stabilization effect ordered as CaCO3 > zeolite > FeCl2 > kaolin > Ca(OH)2. The TCLP (toxicity characteristic leaching procedure) results showed that using Ca(OH)2 and CaCO3 as amendments can significantly reduce the metal leachability in acidic environment, while zeolite is effective for most of the heavy metals and metalloid. This study has provided informative data for the heavy metal and metalloid pollution of the river sediment, and a comprehensive evaluation on metal and metalloid remediation by using the five amendments.
References
Anthemidis A, Zachariadis G, Voutsa D, Kouras A, Samara C (2002) Variation of heavy metals and other toxic element concentrations in surface water of Macedonia. In Proceedings of 1st Environmental Conference of Macedonia, Thessaloniki, Greece, vol 104
Aziz HA, Adlan MN, Ariffin KS (2008) Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: post treatment by high quality limestone. Bioresour Technol 99(6):1578–1583
Balasoiu CF, Zagury GJ, Deschenes L (2001) Partitioning and speciation of chromium, copper, and arsenic in CCA-contaminated soils: influence of soil composition. Sci Total Environ 280(1–3):239–255
Castaldi P, Santona L, Melis P (2005) Heavy metal immobilization by chemical amendments in a polluted soil and influence on white lupin growth. Chemosphere 60(3):365–371
Chabukdhara M, Nema AK (2012) Assessment of heavy metal contamination in Hindon River sediments: a chemometric and geochemical approach. Chemosphere 87(8):945–953
Chen Q, Ke Y, Zhang L, Tyrer M, Hills CD, Xue G (2009) Application of accelerated carbonation with a combination of Na2CO3 and CO2 in cement-based solidification/stabilization of heavy metal-bearing sediment. J Hazard Mater 166(1):421–427
Chen C, Lu Y, Hong J, Ye M, Wang Y, Lu H (2010) Metal and metalloid contaminant availability in Yundang Lagoon sediments, Xiamen Bay, China, after 20 years continuous rehabilitation. J Hazard Mater 175(1–3):1048–1055
Cuong DT, Obbard JP (2006) Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Appl Geochem 21(8):1335–1346
De Jonge M, Teuchies J, Meire P, Blust R, Bervoets L (2012) The impact of increased oxygen conditions on metal-contaminated sediments part I: effects on redox status, sediment geochemistry and metal bioavailability. Water Res 46(7):2205–2214
Feng H, Han X, Zhang W, Yu L (2004) A preliminary study of heavy metal contamination in Yangtze River intertidal zone due to urbanization. Mar Pollut Bull 49(11–12):910–915
Filgueiras AV, Lavilla I, Bendicho C (2002) Chemical sequential extraction for metal partitioning in environmental solid samples. J Environ Monit 4(6):823–857
Fu J, Zhao C, Luo Y, Liu C, Kyzas GZ, Luo Y, Zhu H (2014) Heavy metals in surface sediments of the Jialu River, China: their relations to environmental factors. J Hazard Mater 270:102–109
Fuentes A, Lloréns M, Sáez J, Aguilar MI, Ortuño JF, Meseguer VF (2008) Comparative study of six different sludges by sequential speciation of heavy metals. Bioresour Technol 99(3):517–525
Garcıa-Sánchez A, Alastuey A, Querol X (1999) Heavy metal adsorption by different minerals: application to the remediation of polluted soils. Sci Total Environ 242(1–3):179–188
Gewurtz SB, Helm PA, Waltho J, Stern GA, Reiner EJ, Painter S, Marvin CH (2007) Spatial distributions and temporal trends in sediment contamination in Lake St. Clair. J Great Lakes Res 33(3):668–685
Guan Q, Wang L, Pan B, Guan W, Sun X, Cai A (2016) Distribution features and controls of heavy metals in surface sediments from the riverbed of the Ningxia-Inner Mongolian reaches, Yellow River, China. Chemosphere 144:29–42
Houben D, Pircar J, Sonnet P (2012) Heavy metal immobilization by cost-effective amendments in a contaminated soil: effects on metal leaching and phytoavailability. J Geochem Explor 123:87–94
Huang D, Xue W, Zeng G, Wan J, Chen G, Huang C, Zhang C, Cheng M, Xu P (2016) Immobilization of Cd in river sediments by sodium alginate modified nanoscale zero-valent iron: impact on enzyme activities and microbial community diversity. Water Res 106:15–25
Jain CK (2004) Metal fractionation study on bed sediments of River Yamuna, India. Water Res 38(3):569–578
Juwarkar AA, Singh SK, Mudhoo A (2010) A comprehensive overview of elements in bioremediation. Rev Environ Sci Biotechnol 9(3):215–288
Kishe MA, Machiwa JF (2003) Distribution of heavy metals in sediments of Mwanza Gulf of Lake Victoria, Tanzania. Environ Int 28(7):619–625
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments–a review. Waste Manag 28(1):215–225
Lee SH, Lee JS, Choi YJ, Kim JG (2009) In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments. Chemosphere 77(8):1069–1075
Li XD, Poon CS, Sun H, Lo IMC, Kirk DW (2001) Heavy metal speciation and leaching behaviors in cement based solidified/stabilized waste materials. J Hazard Mater 82(3):215–230
Li F, Huang J, Zeng G, Yuan X, Li X, Liang J, Wang X, Tang X, Bai B (2013) Spatial risk assessment and sources identification of heavy metals in surface sediments from the Dongting Lake, Middle China. J Geochem Explor 132:75–83
Mishra VK, Upadhyaya AR, Pandey SK, Tripathi BD (2008) Heavy metal pollution induced due to coal mining effluent on surrounding aquatic ecosystem and its management through naturally occurring aquatic macrophytes. Bioresour Technol 99(5):930–936
Obbard JP (2006) Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Appl Geochem 21(8):1335–1346
Olivares-Rieumont S, De la Rosa D, Lima L, Graham DW, Katia D, Borroto J, Martínez F, Sánchez J (2005) Assessment of heavy metal levels in Almendares River sediments—Havana City, Cuba. Water Res 39(16):3945–3953
Opfer SE, Farver JR, Miner JG, Krieger K (2011) Heavy metals in sediments and uptake by burrowing mayflies in western Lake Erie basin. J Great Lakes Res 37(1):1–8
Pagnanelli F, Moscardini E, Giuliano V, Toro L (2004) Sequential extraction of heavy metals in river sediments of an abandoned pyrite mining area: pollution detection and affinity series. Environ Pollut 132(2):189–201
Peng JF, Song YH, Yuan P, Cui XY, Qiu GL (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161(2–3):633–640
Porter SK, Scheckel KG, Impellitteri CA, Ryan JA (2004) Toxic metals in the environment: thermodynamic considerations for possible immobilization strategies for Pb, Cd, As, and Hg. Crit Rev Environ Sci Technol 34(6):495–604
Rath P, Panda UC, Bhatta D, Sahu KC (2009) Use of sequential leaching, mineralogy, morphology and multivariate statistical technique for quantifying metal pollution in highly polluted aquatic sediments—a case study: Brahmani and Nandira Rivers, India. J Hazard Mater 163(2–3):632–644
Rosado D, Usero J, Morillo J (2016) Assessment of heavy metals bioavailability and toxicity toward Vibrio fischeri in sediment of the Huelva estuary. Chemosphere 153:10–17
Sastre J, Hernández E, Rodrıguez R, Alcobé X, Vidal M, Rauret G (2004) Use of sorption and extraction tests to predict the dynamics of the interaction of trace elements in agricultural soils contaminated by a mine tailing accident. Sci Total Environ 329(1–3):261–281
Sekulić MT, Pap S, Stojanović Z, Bošković N, Radonić J, Knudsen TŠ (2018) Efficient removal of priority, hazardous priority and emerging pollutants with Prunus armeniaca functionalized biochar from aqueous wastes: experimental optimization and modeling. Sci Total Environ 613:736–750
Singh M, Ansari AA, Müller G, Singh IB (1997) Heavy metals in freshly deposited sediments of the Gomati River (a tributary of the Ganga River): effects of human activities. Environ Geol 29(3–4):246–252
Sundaray SK, Nayak BB, LinS BD (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—a case study: Mahanadi basin, India. J Hazard Mater 186(2–3):1837–1846
Unuabonah EI, Adebowale KO, Olu-Owolabi BI, Yang LZ, Kong L (2008) Adsorption of Pb (II) and Cd (II) from aqueous solutions onto sodium tetraborate-modified kaolinite clay: equilibrium and thermodynamic studies. Hydrometallurgy 93(1–2):1–9
Varol M (2011) Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J Hazard Mater 195:355–364
Wen J, Yi Y, Zeng G (2016) Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction. J Environ Manag 178:63–69
Xue W, Huang D, Zeng G, Wan J, Zhang C, Xu R, Cheng M, Deng R (2018) Nanoscale zero-valent iron coated with rhamnolipid as an effective stabilizer for immobilization of Cd and Pb in river sediments. J Hazard Mater 341:381–389
Yi Y, Wen J, Zeng G, Zhang T, Huang F, Qin H, Tian S (2017) A comparative study for the stabilisation of heavy metal contaminated sediment by limestone, MnO2 and natural zeolite. Environ Sci Pollut Res 24(1):795–804
Yoo JC, Lee CD, Yang JS, Baek K (2013) Extraction characteristics of heavy metals from marine sediments. Chem Eng J 228:688–699
Yu KC, Tsai LJ, Chen SH, Ho ST (2001) Chemical binding of heavy metals in anoxic river sediments. Water Res 35(17):4086–4094
Zhang C, Qiao Q, Piper JD, Huang B (2011a) Assessment of heavy metal pollution from a Fe-smelting plant in urban river sediments using environmental magnetic and geochemical methods. Environ Pollut 159(10):3057–3070
Zhang X, Lin S, Chen Z, Megharaj M, Naidu R (2011b) Kaolinite-supported nanoscale zero-valent iron for removal of Pb2+ from aqueous solution: reactivity, characterization and mechanism. Water Res 45(11):3481–3488
Zhao B, Xu X, Xu S, Chen X, Li H, Zeng F (2017) Surface characteristics and potential ecological risk evaluation of heavy metals in the bio-char produced by co-pyrolysis from municipal sewage sludge and hazelnut shell with zinc chloride. Bioresour Technol 243:375–383
Zhou H, Shi P, Wang JA, Yu D, Gao L (2010) Rapid urbanization and implications for river ecological services restoration: case study in Shenzhen, China. J Urban Plan Dev 137(2):121–132
Zoumis T, Schmidt A, Grigorova L, Calmano W (2001) Contaminants in sediments: remobilisation and demobilisation. Sci Total Environ 266(1–3):195–202
Funding
This work is supported financially by the Shenzhen Science and Technology Innovation Committee (JCYJ20160429191618506) and the National Natural Science Foundation of China (NSFC) (21707063). This work is also sponsored by Shenzhen Peacock Plan (KQTD2016022619584022), Guangdong Provincial Key Laboratory of Soil and Groundwater Pollution Control (No. 2017B030301012), and State Environmental Protection Key Laboratory of Integrated Surface Water-Groundwater Pollution Control.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hocheol Song
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 287 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Tang, Y., Zhong, G. et al. A comparison study on heavy metal/metalloid stabilization in Maozhou River sediment by five types of amendments. J Soils Sediments 19, 3922–3933 (2019). https://doi.org/10.1007/s11368-019-02310-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02310-w