Abstract
Purpose
San Luis Potosí is one of the largest metal producers; mining activity has been responsible for metal emissions for over 100 years, from several sources (deposits, tailings, effluents, and dusts) generating effects in human and ecosystem health. The objective of this study was to evaluate the effect of the concentrations of heavy metals in the soil health of four municipalities of San Luis Potosí contaminated with mine tailings, using enzyme activity as a biochemical endpoint.
Materials and methods
Four municipalities contaminated with residues of historical mining activity were analyzed (25 topsoil samples per type of site contaminated and reference). The parameters that were analyzed included pH; organic matter (OM); electrical conductivity (EC); percentage of clay, As, Cd, Cr, Cu, Hg, Pb, and Zn; and arylsulfatase (ARS), β-glucosidase (BG), urease (UR), and fluorescein diacetate hydrolysis (FDA) activities in soil. Differences among the parameters per municipality and type of site were evaluated using a factorial analysis of variance. The relationships were analyzed by Pearson’s correlation and a stepwise distance-based linear model permutation test (DistLM). Results were visualized using a distance-based redundancy analysis (dbRDA). A hazard quotient (HQ) for metals was calculated in order to estimate the effects on soil microbial processes.
Results and discussion
A concentration gradient (mg/kg) of Zn (4744.5–65,585.7), Pb (1321.0–31,932.2), As (ND-8736.7), and Cu (130.9–8475.4) was found in the contaminated sites. The HQ showed a very high hazard level for the elements detected in all contaminated sites (1.4–655.8). The pattern of enzymatic inhibition found was ARS (95.8 %), UR (90.6 %), FDA (86.9 %), and BG (76.0 %). Strong negative relationships were observed among enzymatic activities and heavy metals in the following inhibitory effect Cu > As > Zn > Pb. Metals and covariables explained from 84 to 86 % of variability in enzyme activity. EC, Cu, and As showed a strong inhibitory effect; and parameters such as OM, pH, and clay were found to have a slightly inducing effect.
Conclusions
In this study, the heavy metal concentrations were higher than the ones obtained in other reports for this region. The HQ reveals the presence of possible risks for the health of life in the region. The decrease of enzyme activities in soil could trigger adverse changes in the flow of matter and energy in ecosystems. This study provides a field baseline that could be part of a long-term monitoring program for these locations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The mining industry in México is one of the oldest and most important economic activities, and globally, the state of San Luis Potosí occupies the first place in arsenic (As), second in tin (Sn), third in copper (Cu) and zinc (Zn), fifth in lead (Pb), sixth in silver (Ag), and seventh in gold (Au) production (Servicio Geológico Mexicano SGM 2007). Intensive mining activities have generated metal emissions from several as both effluents and dusts, leaving approximately 10,000 to 50,000 abandoned sites in the whole country. Metal pollution is a permanent threat to ecosystems and human health, inhibiting plant growth and reducing soil functionality through the selection of less metabolically efficient metal-resistant species, as well as the direct inhibition of enzyme activity (Mergeay 2000; D’Ascoli et al. 2006; Gomes et al. 2010).
Soil quality has been defined based on its capacity to support the agricultural production; however, soil also have important effects on the environment as it influence the quality of atmosphere and water and ultimately the biodiversity and human health (Karlen et al. 2004). A healthy soil has the ability to function as a living system, within the limits of the ecosystem and land use, and it is able to sustain biological productivity; promote quality of air and water environments; and maintain plant, animal, and human health (Dick 1997). Several indicators have been established in order to evaluate the quality and health of soil; the ones that are commonly used are the physicochemical, biochemical, and biological properties. Indicators can vary among localities depending on the type, use, function (interaction between soil biota and biological, chemical, and physical processes that can contribute to soil fertility) and formation factors (soil-forming processes that take place among the climate, relief, mountain rocks, organisms, and time) of the soil (Doran and Parkin 1994). Balanced, safe, and productive soil function exists when its biological, biochemical, physical, and chemical properties all correlate each other and contribute to sustainable evolution.
Enzyme activities play a fundamental role because of their intracellular or extracellular chemical reaction pathway in the nutrient cycling (Dick 1997). Several enzymes have been taken as an indicator of the rate of a whole metabolic process, mainly of microbial origin, and are regarded as sensitive to diverse groups of pollutants including heavy metals (Karaca et al. 2010). Urease and exopeptidases are important enzymes involved in N mineralization (Allison and Vitousek 2005), and urease is strongly inhibited by heavy metals (Hemida et al. 1997; Effron et al. 2004; Wyszkowska et al. 2006; Yang et al. 2006; Mikanova 2006). Soil β-glucosidase activity plays a key role in the degradation of cellobiose-releasing glucose used as energy source by soil microorganisms (Eivazi and Tabatabai 1990); arylsulfatase releases sulfate from arylesters (Klose et al. 2011), its activity is normally related to the amount of organic carbon in soil, and it has shown a reduction in soil polluted with Cu and Cd (Geiger et al. 1998; Renella et al. 2003); and phosphatase activities (phosphomonoesterases and phosphodiesterase) are key enzymes in P cycling (Acosta-Martínez and Tabatabai 2011). Hydrolysis of fluorescein diacetate (FDA) has been used to determine the proportion of active fungi and bacteria (Schnürer and Rosswall 1982; Dick 1997; Gaspar et al. 2001) and as a global indicator of microbial hydrolytic activity. Soil enzyme activities are inhibited by heavy metals and metalloids (Schnürer and Rosswall 1982; Haigh and Rennie 1994; Renella et al. 2003; Lorenz et al. 2006; Wyszkowska et al. 2006; Li et al. 2009; D’Ascoli et al. 2006; Biró et al. 2012).
Soil enzyme activity also responds to other main soil properties such as organic matter content (OM), pH value, microbial biomass and activity, and soil use (Nannipieri et al. 2012). Therefore, more reliable sources of information about the impact of metal pollution on the biochemical activity of soil, such as the analysis of the reference site which is not influenced by the metal input, as well as the comparison of the measured activities with the chemical properties of soil are needed (D’Ascoli et al. 2006). The aim of this study was to evaluate the effects of As and other heavy metals in the soils of four municipalities of San Luis Potosí contaminated by historic mine tailings, in soil enzyme activity as a biochemical stress endpoint.
2 Methods
2.1 Study site
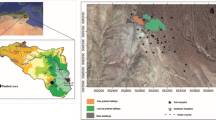
The arid region of the state of San Luis Potosí, México (Central Plateau), is characterized for having a bedrock of sedimentary origin made of limestone, lutite, and arsenite rock. Calcic Xerosols and Lithosols are the main types of soils distributed in the area. The predominant type of vegetation is the scrubland; the climate is dry, semi-desert steppe. The average altitude of the region is 2000 m above mean sea level, annual mean temperature is 17 °C, and there is an annual precipitation of 400 mm. Sampling was carried out during March–April on 2013 in four municipalities of the arid regions with environmental liabilities from mining activities in the state of San Luis Potosí, México (Fig. 1). The studied municipalities were (1) Cedral (CED), located in the plateau region of the northern part of the state (23° 49′ N latitude and 100° 43′ W longitude). The main mining area is located in the central part of the municipality, and it contains Hg and Sb deposits, with additional extraction of Zn, Cu, Pb, Ag, and Au taking place (skarn ore system). Even when mining activities in the municipality are currently active, there are numerous places with residues from as long as 243 years ago; (2) Charcas (CHA), located in the plateau region of the northern part of the state (23° 07′ N latitude and 101° 06′ W longitude). Numerous artisanal exploitations of Ag were found in this place, involving the use of Hg in the production processes (Gambusino method); this location has residues from as long as 442 years ago; (3) Villa de la Paz (VDP), located in the northern part of the state (23° 41′ N latitude and 100° 43′ W longitude). The region shows large accumulations of current and postcolonial tailings; Pb-Zn-Ag (Cu-Au) skarn ore system has been mined for since 1870. High concentrations of pollutants as well as effects on health and environment have been reported in the site (Yañez et al. 2003; Jasso-Pineda et al. 2007; Espinosa-Reyes et al. 2014); and (4) Cerro de San Pedro (CSP), located in the central part of the state (22° 12′ N latitude and 100° 47′ W longitude). The location has been exploited since the year 1545 and is still being exploited today, leaving many large current and colonial mine tailings exposed to open air.
In order to reach the objective of this study, two sampling stations were selected for every municipality and they were grouped according to the type of site: (1) contaminated, sites with residues of historical mining activity, and (2) reference, sites without mining activity located within a 6–10-km distance range from the contaminated soils and with similar biophysical conditions.
2.2 Soil sampling
Twenty-five pooled samples were taken per site; each pooled sample consisted of five subsamples of 10 cm3 of soil collected in a 1-m2 surface area (center and vertex) every 10 m following a random transect up to 250 m.
The samples were homogenized, divided, sieved (2 mm), and put in polyethylene bags, so that they could be transported in order to be analyzed in the laboratory. Fifty-gram aliquots of the samples were put inside sterile conical tubes for enzyme analysis and kept at 4 °C during transport to the laboratory, where they were then stored for 7 days at −20 °C.
2.3 Metal analysis
Element quantification was performed using X-ray fluorescence (XRF) with a portable analyzer (Thermo Scientific® Niton XL3t). This technique has the benefit of being non-destructive and gives a response in real time of the different elements present in a soil sample, which is why its use is widely recommended for environmental studies (Vogel-Mikuš et al. 2010). The measurement was done ex situ following the operating protocol of the instrument. Of the homogenized and previously oven-dried soil (40 °C), 100 mg was used for the quantification. Each sample was analyzed in triplicate using 60 s of analysis per beam/filter as default. Element concentrations were expressed in milligrams per kilogram of dry soil (mg/kg d.w.). Standard reference material analysis (Montana soil NIST-2711a) was conducted for quality control. Accuracy and precision of the XRF data were <20 % of difference (%diff) and <7 % of relative standard deviation (%RSD), respectively. The reported detection limits (mg/kg) for the analyzed elements were As (7.0), Cd (12.0), Cr (22.0), Cu (6.5), Hg (9.0), Pb (8.0), and Zn (10.0).
2.4 Physicochemical parameters
pH and electrical conductivity (EC) of soil were determined through the measurement method in water in a 1:2 (w/v) and 1:5 (w/v) soil:water relationship, respectively (Jones 2001). The measurements were achieved using a potentiometer (Hanna HI 2216). OM in the soil was obtained using the method of humid digestion (Combs and Nathan 2010; Metson et al. 1979); 1 g of each of the soil samples was mixed with 10 mL of Na2Cr2O7, 2H2O (0.5 M), and 20 mL of concentrated H2SO4. The samples were kept in constant agitation for 30 min. Of the deionized water, 70 mL was then added and they were then left unaltered for 24 h. Absorbance was measured using a spectrophotometer (Biomate 3 s Thermo Fisher Scientific) at 660 nm in 2 mL of the supernatant. OM in soil samples was expressed in percentage (%). The hydrometer method (Diario Oficial de la Federación DOF 2002) was used to analyze the clay content of soil samples.
2.5 Enzyme activity assays
Urease (UR) activity was determined according to the Kandeler and Gerber (1988) method, modified by Kandeler et al. (2011). β-Glucosidase (BG) was determined using the method developed by Eivazi and Tabatabai (1988), modified by Deng and Popova (2011). Arylsulfatase (ARS) activity was determined according to the method reported by Tabatabai and Bremmer (1970), reported by Klose et al. (2011). Fluorescein diacetate hydrolysis (FDA) activity was determined according the method reported by Adam and Duncan (2001). Enzyme activities were determined 7 days after soil sampling. Concentrations of enzymes were adjusted to the moisture content of soil. All chemicals used were of analytical grade, and all measurements were carried out in triplicate and the mean was reported.
2.6 Hazard quotient
The estimation of the hazard quotient (HQ) of each of the analyzed metals was carried out by comparing the concentrations with the international guidelines, employing the following equation: HQ = C e /C r , where C e is the environmental concentration and C r is the reference concentration of Ecological Soil Screening Level (Eco-SSL) for protection against microbes and microbial processes in soil (Sutter 2007; Buchman 2008). The HQ is the ratio of potential exposure to the substance and the level at which no adverse effects are expected. If the HQ is greater than 1, then it is possible for adverse environmental health effects to appear.
2.7 Statistical analysis
Data are reported as the mean, standard error, and range. Samples with non-detectable values of arsenic and heavy metals were assigned half of the detection limit, when the number of data was at least half of the number for the variable. The data were transformed logarithmically in order to obtain a normal distribution. Differences among the concentrations of arsenic, heavy metals, enzymes, and covariables per municipality (CSP, VDP, CED, and CHA) and per type of site (contaminated or reference) were evaluated using a factorial analysis of variance followed by a multiple mean comparison test (least significant difference (LSD)). Relationships among arsenic, heavy metals, cofactors (physicochemical parameters), and enzymes were analyzed using Pearson’s correlation and a stepwise distance-based linear model permutation test (DistLM, selection criterion, AICc; 9999 permutation). Results were visualized using a distance-based redundancy analysis (dbRDA; Anderson et al. 2008). Statistical significance was determined using 5 and 1 %. Analysis was performed using Statistica version 12.0 (StatSoft®, Tulsa, Oklahoma, USA) and Primer 7+ Permanova add-on software package (v7.0.10 and v1.0.6; PRIMER-E Ltd., Ivybridge, UK) for univariate and multivariate analyses, respectively.
3 Results
3.1 Concentrations of As and heavy metals in soil samples
Heavy metal concentrations are shown in Table 1. No detectable levels of Cd, Cr, and Hg were found. In general, the levels of heavy metals and As were higher in the contaminated sites compared to the reference sites (from 40 to 1193 times). The observed concentration gradient in the sampled sites was Zn > Pb > Cu > As. Results of the ANOVA factorial test indicate the effect of the interaction of factors (municipality × site type) for all quantified elements, cofactors, and enzyme activities (Table 2). The post hoc analysis of the contaminated sites showed that CHA had the highest levels of Cu, Pb, and Zn. VDP had the highest concentrations of As; As was not detected in CHA or in CED. A positive correlation was found among the levels of Cu with Pb (r = 0.843, p < 0.01) and Zn (r = 0.871, p < 0.01) as well as among levels of As and Zn (r = 0.171, p < 0.05).
3.2 Hazard coefficients (HQ)
According to the limits set by Eco-SSL, HQ analysis performed for the microorganisms in the contaminated sites was as follows: in CSP, the results were Pb (51.0) > As (15.9) > Cu (13.0) > Zn (8.1). In VDP, they were As (87.37) > Zn (51.2) > Cu (13.2) > Pb (1.4). In CED, they were Zn (47.4) > Cu (17.8) > Pb (8.7). In CHA, they were Zn (655.8) > Cu (84.7) > Pb (35.4). Indexes show a very high hazard level for the elements detected in all contaminated sites. HQ levels for the other organisms (e.g., Eco-SSL for plants and mammals) including humans were 10 times higher than those obtained from microorganisms.
3.3 Physicochemical characteristics of soil
The results of the physicochemical characteristics of the studied sites are presented in Table 1. pH values varied within a range of 8.3 to 8.6 in the reference sites and from 4.1 to 7.9 in the contaminated sites. The pH of contaminated sites was lower compared to the reference sites by 18.6 % (LSD, MS = 0.005, p < 0.01). According to the classification established by the Mexican regulations (Diario Oficial de la Federación DOF 2002), pH values for the reference sites go from neutral to strongly alkaline and for the contaminated sites from neutral to moderately alkaline, except for CSP where soils were found to be strongly acidic (4.1). pH values showed a negative correlation with As, Pb, and Zn (Table 3).
EC (dS/m) levels found in reference sites ranged from 0.05 to 0.1 and from 0.7 to 1.6 in contaminated sites. EC of contaminated soils increased by 92.5 % compared to reference soils (LSD, MS = 0.018, p < 0.01). CSP was found to have the highest levels of electrical conductivity. According to the criteria established by Canadian Council of Ministers of the Environment (Canadian Council of Ministers of the Environment CCME 2014), the electrical conductivity in the reference sites of CSP and CHA indicated low and medium salinities, while in CED and VDP, there was low salinity. Contaminated sites of VDP and CED had soils ranging from medium to high salinity. In CSP, salinity ranged from very high to high levels, and in CHA, salinity went from low to medium. EC showed a positive association among Pb, Zn, Cu, and As (Table 3).
OM values (%) ranging from 3.7 to 4.6 for the reference sites and from 0.7 to 2.9 for the contaminated sites were obtained. OM in contaminated soils showed a 50.5 % decrease when compared to reference soils (LSD, MS = 0.020, p < 0.01). CED had the lowest levels of OM followed by CSP and VLP. CSP, CED, and VLP had an OM concentration ranging from medium to high in accordance with the Mexican regulations (Diario Oficial de la Federación DOF 2002). OM showed a negative correlation with Pb, Cu, and Zn (Table 3).
Presence of clays (%) ranged from 16.7 to 25.6 in the reference sites and from 6.5 to 13.3 in the contaminated sites. There was a 52.9 % decrease in the percentage of clays in the contaminated sites regarding the reference sites (LSD, MS = 0.031, p < 0.01). CED was found to have the lowest clay levels followed by VLP and CSP. Clay was also negatively correlated with Pb, Cu, Zn, and As (Table 3).
3.4 Soil enzyme activity
Results regarding enzymatic activities in the sampling sites are shown in Fig. 2. Contaminated sites were found to have less enzymatic activities than the reference sites. ARS activity (nmol p-nitrophenol/g h) ranged from 130.5 to 411.5 for the reference sites and from 8.2 to 13.5 for the contaminated sites. The inhibiting pattern for each of the sites was as follows: CHA (96.8 %), CSP (96.3 %), VDP (95.0 %), and CED (92.7 %). BG activity (nmol p-nitrophenol/g h) ranged from 28.2 to 55.7 and from 6.0 to 20.2 for reference and contaminated sites, respectively. Inhibition of BG per reference site was as follows: CHA (83.6 %), VPD (83.1 %), CED (78.2 %), and CSP (63.7 %). FDA activity (nmol fluorescein/g h) ranged from 1.9 to 2.7 for the reference site and from 0.18 to 0.54 for the polluted site. FDA inhibition regarding reference sites was as follows: CED (92.5 %), CSP (89.0 %), CHA (83.1 %), and VDP (73.5 %). URE showed values (nmol NH4-N/g 2 h) ranging from 62.9 to 346.9 for reference sites and from 4.5 to 40.7 for contaminated sites. The inhibiting pattern for URE was as follows: VDP (97.9 %), CSP (92.8 %), CHA (88.2 %), and CED (86.6 %). All enzyme activities showed correlation with each other (UR between FDA r = 0.70, p < 0.01; BG between ARS r = 0.928, p < 0.01; ARS between FDA r = 0.857, p < 0.01). The DistLM results (Table 4) show the multivariate relations among the elements, covariables, and enzymatic activities. All enzymes show a strong relationship with metals and covariates (from 84 to 0.87 %). EC, Cu, and As showed a strong inhibitory effect, and parameters such as OM, pH, and clay were found to have a slightly inducing effect. Figure 3 shows the dbRDA analysis, and it explains 84.1 of the total variation, where axis 1 determines the grouping of samples mainly based on the type of site (77.7 % of the total variation). The variation between samples is much longer in contaminated sites compared to reference sites, suggesting that contamination disturbance determines diverging responses. Axis 1 shows a negative association (p < 0.05) among Zn (r = −0.44), Cu (r = −0.41), EC (r = −0.39), As (r = −0.38), and Pb (r = −0.29) as well as a positive one between clay (r = −0.35), OM (r = −0.33), and pH (r = −0.11).
4 Discussion
4.1 Metals in soil samples and hazard coefficients (HQ)
Sites with mining activity were found to have significantly higher levels of metals than the reference sites. The main sources of contamination in these sites include tailings, dams, and slag deposits, from which pollutants mobilize, by being transported through wind and runoff (Monroy et al. 2002). The presence of this distribution pattern of pollution corresponds to what was observed in different studies done in similar environments (Razo et al. 2004a, b; Jasso-Pineda et al. 2007; Espinosa-Reyes et al. 2014). Even though previous studies have not been performed in all municipalities, the levels of metals found in this study were higher than the ones reported previously in studies conducted in VDP and CHA (Monroy et al. 2002; Razo et al. 2004a, b; Espinosa-Reyes et al. 2014). Pollutant concentrations exceed environmental protection criteria established by the international guidelines Squirt-NOOA (Eco-SSLs for soil microbiological effects). HQ results show a high-risk potential for bacterial life in the soil of the region. It is worth mentioning that hazard coefficients for protecting health of humans and biota (Avian, inverts, mammals, and plants) are even higher (ranging from 10 to 1000). In vitro bioaccessibility was determined in soil samples of VDP and CSP, obtaining values of >80 % for As and Pb (Razo et al. 2004b; Jasso-Pineda et al. 2007; Vazquez-Vazquez 2012). The combination and integration of these indexes, together with high bioaccessibility, suggest that immediate evaluation of the effects on human and ecological receptors ought to be performed, as well as the risk assessment associated with the exposure to polluted soils.
4.2 Effect on physicochemical parameters
When metals enter soil in great amounts, pH can be altered, causing it to acidify (Effron et al. 2004); this could be the explanation of the high correlation among heavy metal concentrations and pH values. pH values, temperatures, and oxidation of sulfide minerals, which are present in these areas, can favor the generation of acid mine drainage (Sherene 2010); CSP was the only place that showed evidence of the generation of acid mine drainage. On the other hand, in arid regions, sodium can accumulate in the form of sodium carbonate and its hydrolysis can produce strong alkali (NaOH), which is why the pH value can vary within a range of 8.5 to 10 in these soils, generating a buffer effect of pH on the ground.
Arid and dry locations generally tend to have soils with higher salinity levels because there is more evaporation taking place rather than precipitation and because they tend to have a pH that approximates or is close to 7. According to the Canadian Council of Ministers of the Environment (Canadian Council of Ministers of the Environment CCME 2014), electrical conductivity in residential soil or parks should not exceed 2 dS/m; however, contaminated soil from CSP doubled the established value (4.2 dS/m), and the contaminated site from CHA was at the maximum limit (2 dS/m). Higher salinity can increase the mobility of heavy metals through the following two possible means: positively charged ions associated to salts (Na and K) which can replace heavy metals in the absorption sites and negatively charged ions of chloride which can form stable soluble compounds with heavy metals (Cd, Zn, and Hg), with a tendency of generating acidic soils (Sherene 2010).
OM plays an important role in metal binding, complexation by organic ligands, control of metal solubility, and mobility. High amounts of heavy metals in polluted soil could change the mineralization rate of organic matter in soil and affect its accumulation and distribution (Zhang and Wang 2007). According to the classification established by Mexican regulations (Diario Oficial de la Federación DOF 2002), the amount of organic matter in soils from reference sites was considered high, and for contaminated sites, it was reduced to a medium level (50.5 % quantitatively).
Sites with higher concentrations of metals had fewer amounts of clay (predominantly sandy-loam soils). The negative association pattern in this study has also been observed in soils with fine textures contaminated mainly with lead (Sherene 2010). It is likely that the washing of clays which is an additional process to contamination can derive from the mechanical effect to which these soils have been exposed to.
4.3 Effect on enzyme activities
A strong negative relationship was also observed in the present study among enzymatic activities and heavy metals; the determination coefficients show the following inhibitory effect Cu > As > Zn > Pb. The effects of metals are modified when other variables are considered (Table 4).
Generally, enzyme activities are often enhanced by increasing pH in soil (except for acid phosphatase, which is predominant in acidic soils; Deng and Tabatabai 1997). According to the DistLM models, the inducing effect of pH was mainly found in BG and FDA (between 24 and 12 %, respectively). pH changes can affect the distribution of fungal and bacterial biomass in soil and can consequently modify patterns and soil enzyme activities, which can also heavily affect substrate-enzyme kinetics (Baath et al. 1992).
EC showed a great inhibitory effect in the enzymes evaluated in this study as well as a strong association with the toxic elements (Cu, As, Zn, and Pb). It has previously been reported that enzyme activities were reduced with increasing EC and that the degree of inhibition varied among the enzymes assayed and the nature and amounts of salts added. FDA, BG, and ARS were inhibited exponentially by increasing salinity and linearly by increasing sodicity (Zahran 1997). Carbonates can play an active role in metal immobilization such as Zn, Co, Ni, Hg, Ag, and Au (Adamo et al. 2006), but at alkaline pH values, the availability of the other elements (e.g., As, Cr, Mo, Se, and V) can increase.
ARS levels were inhibited in a 95.2 %, and the elements related to this inhibition were Cu, As, Pb, and Zn. Jadwiga et al. (2010) found a decrease of ARS activity of 46.8 % in the presence of 450 mg/kg of Cu. Speir et al. (1999) reported a 50 % inhibition of ARS derived from exposure to As. Borowik et al. (2014) found an inhibition of 85 % of ARS in soil polluted with Zn (2400 mg/kg). Hernández et al. (2006) found an inhibition of 64.3 % of ARS in soil polluted with Zn (18,900 mg/kg), Pb (4930 mg/kg), Cd (15.10 mg/kg), and Cu (11.9 mg/kg). This study also found that Cu was a strong inhibitor of BG. In this regard, Jadwiga et al. (2010) reported an inhibition of 37.5 % in the presence of 450 mg/kg of Cu, and D’Ascoli et al. (2006) found a negative association among concentrations of BG and Cu (229.34 ± 17.0 mg/kg on average). Kuperman and Carreiro (1997) reported inhibitions of 85 % of BG in soils contaminated with a mixture of heavy metals (As, Cd, Cu, Pb, and Zn in concentrations ranging from 121.0 to 3446.6 mg/kg of all evaluated metals).
FDA activity was significantly decreased by 84.5 %; metals involved in the inhibition were Zn, Pb, and As. Bhattacharyya et al. (2008) found that Zn (239 mg/kg) and Pb (333 mg/kg) caused a negative effect on FDA in contaminated soils experimentally. Kuperman and Carreiro (1997) reported an 81 % decrease in FDA activity in areas contaminated with metals derived from military operations. UR activity was inhibited by 77.2 % due to the presence of As and Pb. Yang et al. (2009) described an inhibition of the activity of UR by 75 % in agricultural soils contaminated with Cd (100 mg/kg) and Pb (500 mg/kg). Friedlová (2010) observed an inhibition of UR activity in alluvial soils containing high concentrations of Pb (5286 mg/kg), Zn (9288 mg/kg), and Cu (62.85 mg/kg). Wyszkowska et al. (2006) showed that UR activity was inhibited by 76.7 % in concentrations of 450 mg/kg of Cu when combined with Zn and Pb (50 mg/kg each). It is important to mention that in previously reported studies, inferior concentrations of heavy metals to the ones reported in this study were found or were determined in controlled conditions (laboratory experiments).
FDA was strongly associated (p < 0.01) with the rest of the enzymes in the following way: ARS (r = 0.85), BG (r = 0.83), and URE (r = 0.70). This can suggest that the decrease of enzymatic activity is mainly caused by the direct suppression of bacterial growth derived from the adverse conditions present in contaminated soils and subsequently by the direct interactions among enzymes and heavy metals (Kuperman and Carreiro 1997). In this regard, other studies carried out by our group (unpublished data) have shown strong inhibition (24 to 42 %) of microbial respiration of soil (%CO2) in the contaminated sites.
About the effects of metal aging in soil, Ciarkowska et al. (2016) reported an increase of soil enzymatic activity in soils with metal aging from 30 to 400 years. Our results did not show a temporary recovery pattern of the enzymatic activity. An important factor that influences the metal effects on soil enzymatic activities is the metal bioavailability that does not depend linearly on the metal total concentration. Assessment of the metal bioavailability in the studied soils deserves more attention in the future to understanding the potentials of soils to restore their ecological functionality on the long term.
5 Conclusions
In this study, heavy metal concentrations were higher than the ones obtained in other reports for contaminated sites in this region. The concentration and type of heavy metals, according to the HQ, reveal the presence of possible risks for the health of life in the region (biota and humans), which is why the location requires urgent intervention measures as well as constant monitoring and regulations of the levels of heavy metal in soil. Our results showed the relationship among different physicochemical parameters (pH, OM, EC, clay) and a mix of heavy metals on enzymatic activity in chronically contaminated soil under field conditions. The inhibition on enzymatic activity in this condition could be attributed to metal bioavailability. Furthermore, no decrease in toxic effects was observed through time in these sites; however, it could be inferred that the biological activity in these conditions could be due to the presence of certain groups of microorganisms that have acquired resistance characteristics with relevant importance. The damage done to the microbial component and the biological processes of the soil (e.g., mineralization and nutrient cycling) could trigger adverse changes in the flow of matter and energy in ecosystems. This study provides a baseline for the state of the enzymatic levels in soils from the mining region of San Luis Potosí; enzymatic determinations are quick, sensitive, and cheap methods that could become part of a long-term monitoring program for these locations.
References
Acosta-Martínez V, Tabatabai MA (2011) Sulfur cycle enzymes. In: Dick RP (ed) Methods of soil enzymology. Soil Science Society of America, Wisconsin, pp. 161–163
Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soil. Soil Biol Biochem 33:943–951
Adamo P, Zampella M, Gianfreda L, Renella G, Rutigliano FA, Terribile F (2006) Impact of the river overflowing on trace element contamination of volcanic soils in South Italy: part I trace element speciation in relation to soil properties. Environ Pollut 144:308–316
Allison SD, Vitousek PM (2005) Response of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37:937–944
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA + for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth, UK
Baath E, Frostergard A, Fritze H (1992) Soil bacterial biomass, activity, phospholipid fatty acid pattern, and pH tolerance in an area polluted with alkaline dust deposition. Appl Environ Microbiol 58:4026–4031
Bhattacharyya P, Tripathy S, Kim K, Kim SH (2008) Arsenic fraction and enzyme activities in arsenic contaminated soils by groundwater irrigation in West Bengal. Ecotox Environ Safe 71(1):149–156
Biró B, Domonkos M, Kiss E (2012) Catabolic FDA microbial activity as site-dependent monitoring tool in soil of an industrial town. Int Rev Sci Eng 3(1):41–46
Borowik A, Wyszkowska J, Kucharski J, Bacmaga M, Boros-Lajszner E, Tomkiel M (2014) Sensitivity of soil enzymes to excessive zinc concentrations. J Elem:637–648
Buchman MF (2008) NOAA Screening Quick Reference Tables (SQuiRTs) NOAA OR&R Report 08–1. Seattle WA Office of response and restoration division National Oceanic and Atmospheric Administration
Canadian Council of Ministers of the Environment CCME (2014) Soil Quality Guidelines for the Protection of Environmental and Human Health (SQG). Available from: http://www.ccme.ca/en/resources/canadian_environmental_quality_guidelines/index.html
Ciarkowska K, Gargiulo L, Mele G (2016) Natural restoration of soils on mine heaps with similar technogenic parent material: a case study of long-term soil evolution in Silesian-Krakow upland Poland. Geoderma 261:141–150
Combs SM, Nathan MV (2010) Soil organic matter. In: Brown JR (ed) Recommended chemical Soil Test Procedures of the North Central Region. Chapter 12. Missouri Agricultural Experiment station SB 1001, pp 53
D’Ascoli R, Rao MA, Adamo P, Renella G, Landi L, Rutigliano FA, Terribile F, Gianfreda L (2006) Impact of river overflowing trace element contamination of volcanic soils in South Italy: part II. Soil biological and biochemical properties in relation to trace element speciation. Environ Pollut 144:317–326
Deng S, Popova I (2011) Carbohydrate hydrolases. In: Dick RP (ed) Methods of soil enzymology. Soil Science Society of America, Wisconsin, pp. 185–198
Deng SP, Tabatabai MA (1997) Effect of tillage and residue management on enzyme activities in soils: III. Phosphatases and arylsulfatase. Biol Fertil Soils 24:141–146
Diario Oficial de la Federación DOF (2002) Norma Oficial Mexicana (NOM-021-SEMARNAT-2000) Que establece las especificaciones de fertilidad, salinidad y clasificación de suelos. Estudio, muestreo y análisis. [Official Mexican Standard, which fixed the fertility, salinity and soil classification. Study, sampling and analyses] Mexico. http://dof.gob.mx/nota_detalle.php?codigo=717582&fecha=31/12/2002. Retrieved October 20, 2015
Dick RP (1997) Soil enzyme activities as integrative indicators of soil health. In: Pankhurst CE, Doube BM, Gupta VVSR (eds) Biological indicators of soil health. CAB International, Wallingford, pp. 121–156
Doran JW, Parkin TB (1994) Defining and assessing soil quality. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA (eds) Defining soil quality for a sustainable environment. Soil Science Society of America, Madison, pp. 3–21
Effron D, de la Horra AM, Defrieri RL, Fontanive V, Palma PM (2004) Effect of cadmium, copper, and lead on different enzyme activities in a native forest soil. Comm Soil Sci Plant Anal 35:1309–1321
Eivazi F, Tabatabai MA (1988) Glucosidases and galactosidases in soils. Soil Biol Biochem 20:601–601. doi:10.1016/0038-0717(88)90141-1
Eivazi F, Tabatabai MA (1990) Factors affecting glucosidase and galactosidase activities in soils. Soil Biol Biochem 22:891–897
Espinosa-Reyes G, González-Mille DJ, Ilizaliturri-Hernández CA, Mejía-Saavedra J, Cilia-López G, Costilla-Salazar R, Díaz-Barriga F (2014) Effect of mining activities in biotic communities of villa de la Paz, San Luis Potosi, Mexico. Biomed Res Int. doi:10.1155/2014/165046
Friedlová M (2010) The influence of heavy metals on soil biological and chemical properties. Soil. Water Res 5(1):21–27
Gaspar ML, Cabello MN, Pollero R, Aon MA (2001) Fluorescein diacetate hydrolysis as a measure of fungal biomass in soil. Curr Microbio 42:339–344
Geiger G, Brandi H, Furner G, Schulin R (1998) The effect of copper on the activity of cellulose and β-glucosidase in the presence of montmorillonite or Al-montmorillonite. Soil Biol Biochem 30:1537–1544
Gomes NCM, Landu L, Smalla K, Nannipieri P, Brookes PC, Renella G (2010) Effects of Cd- and Zn-eriched sewage sludge on soil bacterial and fungal communities. Ecotoxicol Environ Safety 73:1255–1263
Haigh SD, Rennie AFK (1994) Rapid methods to assess the effects of chemicals on microbial activity in soil. Environ Toxic Water 9:347–354
Hemida SK, Omar SA, Abdel-Mallek AY (1997) Microbial populations and enzyme activity in soil treated with heavy metals. Water Air Soil Pollut 95:13–22
Hernández AJ, Beerril JM, Zárate O, Garbisu C (2006) Assessment of the efficiency of a metal phytoextraction process with biological indicators of soil health. Plant Soil 281:147–158
Jadwiga W, Miroslaw K, Jan K (2010) Activity of β-glucosidase, arylsulfatase and phosphatases in soil contaminated with cooper. J Elem 15(1):213–226
Jasso-Pineda Y, Espinosa-Reyes G, González-Mille D, Razo-Soto I, Carrizales L, Arturo Torres-Dosal A, Mejía-Saavedra J, Monroy M, Irina AI, Yarto M, Díaz-Barriga MF (2007) A n integrated health risk assessment approach to the study of mining sites contaminated with arsenic and lead. Interg Environ Assess Manag 3(3):344–350
Jones JJB (2001) Laboratory guide for conducting soil test and plant analysis, vol 28-31. CRC press, Washington DC, pp. 151–155
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Bio Fertil Soils 6:68–72. doi:10.1007/BF00257924
Kandeler E, Poll C, Frankenberger WT, Tabatabai MA (2011) Nitrogen cycle enzymes. In: Dick RP (ed) Methods of soil enzymology. Soil Science Society of America, Wisconsin, pp. 121–156
Karaca A, Cetin C, Turgay OC, Kizilkaya R (2010) Effects of heavy metals on soil enzyme activities. In: Sherameti I, Varma A (eds) Soil biology: soil heavy metals. Springer-Verlag Berlin Heidelberg, New York, pp. 239–245
Karlen DL, Andrews SS, Wienhold BJ (2004) Soil quality, fertility and health–historical context, status and perspectives. In: Schjønning P, Elmholt S, Christensen BT (eds) Managing soil quality: challenges in modern agriculture. CABI Publishing, Wallingford, pp. 17–33
Klose S, Bilen S, Tabatabai MA, Dick WA (2011) Sulfur cycle enzymes. In: Dick RP (ed) Methods of soil enzymology. Soil Science Society of America, Wisconsin, pp. 133–137
Kuperman RG, Carreiro MM (1997) Soil heavy metal concentrations, microbial biomass and enzyme activities in a contaminated grassland ecosystem. Soil Biol Biochem 29(2):179–190
Li YT, Rouland C, Benedetti M, Li FB, Pando A, Lavelle P, Dai J (2009) Microbial biomass, enzyme and mineralization activity in relation to soil organic C, N and P turnover influenced by acid metal stress. Soil Biol Biochem 41:969–977
Lorenz N, Hintemann T, Kramarewa T, Katayama A, Yasuta T, Marschner P, Kandeler E (2006) Response of microbial activity and microbial community composition, in soils to long-term arsenic and cadmium exposure. Soil Biol Biochem 38:1430–1437
Mergeay M (2000) Bacteria adapted to industrial biotopes: the metal resistant Ralstonia. In: Hengge-Aronis R, Storz G (eds) Bacterial stress responses. ASM Press, Washington, DC, USA, pp. 403–414
Metson AJ, Blakemore LC, Rhoades DA (1979) Methods for the determination of soil organic carbon review and application to New Zealand soils. New Zeal J Sci 22:205–228
Mikanova O (2006) Effects of heavy metals on some soil biological parameters. J Geochem Explor 88:220–223
Monroy M, Díaz-Barriga MF, Razo I, Carrizales L (2002) Evaluación de la contaminación por arsénico y metales pesados (Pb, Cu, Zn) y análisis de riesgo en salud en Villa de la Paz-Matehuala, S.L.P. Instituto de Metalurgia, Universidad Autónoma de San Luis Potosí, México
Nannipieri P, Giagnoni L, Renella G, Puglisi E, Ceccanti B, Masciandaron G, Fornasier F, Moscatelli MC, Marinari S (2012) Soil enzymology: classical and molecular approaches. Biol Fertil Soils 48:743–762
Razo I, Carrizales L, Castro J, Díaz-Barriga MF, Monroy M (2004a) Arsenic and heavy metal pollution of soil, water and sediments in a semi-arid mining area in México. Water Air Soil Poll 152:129–152
Razo I, Téllez J, Monroy M, Carrizales L, Díaz-Barriga F, Castro J (2004b) As and Pb bioaccesibility in polluted soils from a mining site under semiarid climate in Mexico. In: Proceedings Tailings and Mine Waste. CRC Press, pp 173–182
Renella G, Ortigoza ALR, Landi L, Nannipieri P (2003) Additive effects of copper and zinc on cadmium toxicity on phosphatase activities and ATP content of soil as estimated by the ecological dose (ED50). Soil Biol Biochem 35:1203–1210
Schnürer J, Rosswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol 43:1256–1261
Servicio Geológico Mexicano SGM (2007) Anuario estadístico de la Minería Mexicana ampliada. Servicio Geológico Mexicano y Secretaría de Economía. http://www.economia.gob.mx/files/comunidad_negocios/industria_comercio/informacionSectorial/minero/anuario_mineria_mexicana_2012_ed2013.pdf. Retrieved October 10, 2015
Sherene T (2010) Mobility and transport of heavy metals in polluted soil environment. BFAIJ 2(2):112–121
Speir TW, Kettles HA, Parshotam A, Searle PL, Vlaar LNC (1999) Simple kinetic approach to determine the toxicity of as [V] to soil biological properties. Soil Biol Biochem 31:705–713
Sutter GW II (2007) Ecological risk assessment, 2 edn. CRC Press, Florida
Tabatabai MA, Bremner JM (1970) Arylsulfatase activity of soils. Soil Sci Soc Am Proc 34:225-229. doi:10.2136/sssaj1970.03615995003400020016x
Vazquez-Vazquez SE (2012) Caracterizacion de un depósito no controlado de residuos mineros y evaluacion de su impacto en suelo superficial. Tesis de Maestría. Posgrado Multidisciplinario de Posgrado en Ciencias Ambientales. pp 118
Vogel-Mikuš K, Kump P, Nečemer M, Pelicon P, Arčon I, Pongrac P, Povh B, Regvar M (2010) Quantitative analyses of trace elements in environmental samples: options and (Im) possibilities. In: Sherameti I, Varma A (eds) Soil biology: soil heavy metals. Springer-Verlag Berlin Heidelberg, New York, pp. 114–122
Wyszkowska J, Kucharski J, Lajszner W (2006) The effects of copper on soil biochemical properties and its interaction with other heavy metals. Pol J Environ Stud 15(6):927–934
Yáñez L, García-Nieto E, Rojas E, Carrizales L, Mejía J, Calderón J, Razo I, Díaz-Barriga MF (2003) DNA damage in blood cells from children exposed to arsenic and lead in a mining area. Environ Res 93:231–240
Yang Z, Liu S, Zheng D, Feng S (2006) Effects of cadmium, zinc, and lead on soil enzyme activities. J Environ Sci 18:1135–1141
Yang G, Pei Z, Liang M, Yue-Er Z, Wan-Yun S (2009) Assessment of effects of heavy metals combined pollution soil enzyme activities and microbial community structure: modified ecological dose–response model and PCR-RAPD. Environ Earth Sci 60:603–612
Zahran HH (1997) Diversity, adaptation and activity of the bacterial flora in saline environments. Biol Feril Soils 25:211–223
Zhang MK, Wang LP (2007) Impact of heavy metals pollution on soil organic matter accumulation. Ying Yong Sheng Tai Xue Bao 18(7):1479–1483
Acknowledgments
This work was supported by a grant from the Consejo Nacional de Ciencia y Tecnología (CB-178778) and Universidad Autónoma de San Luis Potosí (C12-FAI-03-67.67). Special thanks to Miss Laura Carmen Martínez Turrubiartes for English language editing of the manuscript and PhD. Edlin Guerra Castro for the multivariate analysis advice. None of the authors has any conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Chengrong Chen
Rights and permissions
About this article
Cite this article
Martínez-Toledo, Á., Montes-Rocha, A., González-Mille, D.J. et al. Evaluation of enzyme activities in long-term polluted soils with mine tailing deposits of San Luis Potosí, México. J Soils Sediments 17, 364–375 (2017). https://doi.org/10.1007/s11368-016-1529-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1529-8