Abstract
Purpose
Arsenite and arsenate leaching from iron (hydr)oxides is one major parameter affecting the mobility of arsenic in the natural environment. In the process of arsenic transfer to groundwater, the retention capacity of arsenic by different iron (hydr)oxides needs to be investigated. The aim of this study is to determine the retention capacity of arsenite or arsenate from the ferrihydrite, lepidocrocite, or magnetite-coated sand column in the leaching process as well as the influence factors on leaching.
Materials and methods
The leaching of arsenite and arsenate from columns loaded with ferrihydrite, magnetite, or lepidocrocite-coated quartz sand was examined, and the influence factors such as pH, phosphate, and humic acid (HA) contents on leaching and retention were also investigated.
Results and discussion
The retention performance of As(III) and As(V) depended on the type of iron (hydr)oxides: ferrihydrite > magnetite > lepidocrocite. The retention capacities of As(III) and As(V) by amorphous ferrihydrite versus magnetite and lepidocrocite are 3.25, 5.63 (As(III)) and 1.75, 3.65 (As(V)) times higher. The retention capacity of arsenic is largely affected by the pH of leaching solutions. The retention of As(III) by ferrihydrite is efficient in near-neutral or slightly acidic environments. The addition of phosphate or HA significantly affected the leaching and retention. The addition of phosphate severely inhibited the leaching and retention of As(III) and As(V) by ferrihydrite, and the inhibitory effect was more obvious along with the increase of phosphate concentration. The retention of As(III) and As(V) by ferrihydrite was significantly enhanced by the addition of low-dose HA but was inhibited by the addition of excessive HA.
Conclusions
Retention performance of As(III) and As(V) from a ferrihydrite-coated sand column is greater than a magnetite- or a lepidocrocite-coated sand column, and the influence factors such as pH, phosphate, and HA affect the leaching and retention of As(III) and As(V). The results theoretically underlie the application of iron (hydr)oxide in arsenic pollution control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Arsenic (As), a known poison, is widely distributed in the environment because of its natural existence and anthropogenic use in both agriculture and industry to control a variety of insect and fungal pests (Ruokolainen et al. 2000; Leist et al. 2003). Arsenic is well-known for its high toxicity and strong carcinogenicity (United States Environmental Protection Agency 1997). At the end of the twentieth century, the revision of the maximum contaminant level (MCL) for arsenic in drinking water was reduced from 50 to 10 μg/l by the Environmental Protection Agency (USEPA) and World Health Organization (WHO) (Ghosh et al. 2006a). Arsenic pollution has been a long concern, and such incidents occur frequently in recent years, especially in waters and soils (Jain and Ali 2000; Smedley and Kinniburgh 2002; Swartz et al. 2004; Jaime et al. 2007; Amstaetter et al. 2010; Jia et al. 2012; Tuna et al. 2013). In China, arsenic resources are widely associated with ore in nonferrous polymetallic deposits. These arsenic-bearing solid residuals (ABSR) from waste tailings are discarded in the periphery of mining areas. After a series of weathering conditions such as accumulation, oxidization, leaching, and dissolution, the arsenic-containing waste rocks and tailings will release arsenic and thus pollute and harm the surrounding groundwater, farmlands, and eco-environment to different degrees.
The common valence states of As found in natural waters, As(V) and As(III), exist as arsenate (As(V), as H x AsO4 x − 3) and arsenite (As(III), typically as H3AsO3) (Kocar et al. 2006). Both the redox states and chemical forms of arsenic are important because they determine its toxicity and environmental mobility (Keon et al. 2001; Hamon et al. 2004). Reduction from As(V) to As(III) for example leads to enhanced mobility and toxicity of the reduced species (Delaun et al. 1991; Dixit and Hering 2003; Oremland and Stolz 2005; Kulp et al. 2008). Removal of As(III) and As(V) by adsorption onto solid media is currently the most widely chosen treatment option, and it is effective to remove arsenic from water and soils (Ghosh et al. 2006b; Amstaetter et al. 2010). The adsorption of arsenite or arsenate is significantly associated with the oxides (hydroxides) of iron and aluminum and the clay content in soils (Douglas 1984; Raven et al. 1998). Arsenite or arsenate can be chemically adsorbed by specific iron (hydr)oxides to mainly form inner-sphere complexes (Pierce and Moore 1982; Dzombak and Morel 1990; Goldberg and Johnston 2001; Zhang et al. 2007a).
Iron (hydr)oxides ubiquitous in soil and groundwater environment are commonly used as absorbents and antioxidants to remove heavy metals and organic matter from water or soils, owing to their large specific surface areas, high surface energy, high chemical activity, high oxidizability, wide availability, high contents, and low costs (Pierce and Moore 1982; Manning, et al. 1998; Raven, et al. 1998; Silva, et al. 2007; Demetriou and Pashalidis 2012). Therefore, characterizing the retention of arsenic by iron (hydr)oxides helps to understand the arsenic removal mechanism and thus the removal from the environment. However, there is little research about the different leaching migration characteristics between As(III) and As(V) from representative minerals (e.g., lepidocrocite, ferrihydrite, and magnetite) or about the effects of iron (hydr)oxides on arsenic leaching. Therefore, in this study, we selected these three representative iron (hydr)oxides and determined the retention capacity of arsenite or arsenate from ferrihydrite, lepidocrocite, and magnetite-coated sand columns in the leaching process as well as the influence factors on leaching. This study theoretically underlies the alleviation of the arsenite and arsenate pollution from groundwater environment and restoration from environmental pollution.
2 Materials and methods
2.1 Materials
All chemicals were analytical grade and purchased from Beijing Chemical Co. (Beijing, China). The As(III) and As(V) stock solutions were prepared with deionized water using NaAsO2 and Na2HAsO4, respectively. Arsenic working solutions were freshly prepared by diluting arsenic solutions with deionized water.
Experimental quartz sand (Sinopharm Chemical Reagent Co., Ltd.; purity > 98 %; average particle size 500 μm; evenness index 1.25) was used as a packing medium in simulation of soils. To remove the surface metallic oxides, the quartz sand was soaked in 0.01 mol/l NaOH and HCl for 24 h in due order, washed with deionized water and then dried at 105 °C.
2.2 Preparation of iron (hydr)oxides
The iron oxides were prepared according to the method by Schwertmann and Cornell (2000) with modification.
FeCl3 · 6H2O (54.06 g) was dissolved in 2 l of twice-distilled water, which was held in a 3-l closed container at 40 °C for 8 days. During this period, the bright gold solution became lighter yellow and compact yellow precipitates. The pH dropped from 1.7 to ~1.2 (lepidocrocite, γ-FeOOH). Then Fe(NO3)3 · 9H2O (40.00 g) was dissolved in 500 ml of distilled water and added with 330 ml of 1 mol/l KOH to bring the pH to 7.0–8.0. The last 20 ml of KOH was added dripped with constant checking of pH. Then, the mixture was stirred vigorously, centrifuged and then dialyzed rapidly until free from electrolytes (ferrihydrite, Fe5HO8 · 4H2O). FeSO4 · 7H2O (80.00 g) was soaked in 560 ml distilled water in a 1-l container. The container was placed in a water bath (90 °C) and a gas inlet for purge N2. Then, 240 ml of solution containing 6.46 g KNO3 and 44.9 g KOH was dripped over about 5 min. After addition of this solution, the resulting mixture was heated over 30–60 min, cooled overnight, and the black precipitates were washed (magnetite, Fe3O4). All the precipitates were dried in a vacuum drying box at 60 °C.
2.3 Column experiments
The quartz sand as-prepared (20.00 g) was slowly poured into a chromatographic column and tampered tightly to a filling height of 3.5 cm. This step was repeated five times and totally 100 g of quartz sand was filled in. Then, different amounts of an iron oxide sample were added into the column as per different dosage ratios.
About 1.00 g of an iron oxide (content 1 %) was added to the column. The pH 7 As(III) or As(V) stock solution was adjusted with 0.1 mol/l NaCl, 10 mg/l NaOH, and 10 mg/l HCl and then pumped from top to down into the packed column. During the experiment, the surface level of the packed column was 2 cm above the top of the quartz sand. The leaching speed of the leaching solutions was regulated to 1.0 ml/min by adjusting the peristaltic pump. The automatic collector collected at an interval of 10 min.
Different amounts of NaCl were added to control the ionic strength at 0.1 or 0.01 mol/l; 10 mg/l NaOH and 10 mg/l HCl were added to control the pH at 3, 5, 7, 9, or 11; different amounts of NaH2PO4 were added to control the molar ratio of As to PO4 3− (mol/l:mol/l) at 1:0.1 or 1:10; different amounts of HA were added to control the concentration ratio of As to HA (mg/l:mg/l) at 1:0.1 or 1:1 in the As(III) or As(V) stock solution. The following steps are the same as the second paragraph in Section 2.3.
2.4 Analysis methods
The total arsenic concentration in a leaching solution was measured by an iCAP 6300 inductance-coupling plasma (ICP) emission spectrometer (Thermo Fisher Scientific; USA), following the procedure as described in previous studies (Gil et al. 2007; Lopes et al. 2009). Different forms of arsenic concentration in the solution after leaching were measured by a liquid chromatography-atomic fluorescence spectrometer (LC-AFS) model 9700 (Beijing Haiguang Instrument Co., Ltd.). Aqueous pH was measured by a PHS-3C pH meter and an E-201-C pH composite electrode (Shanghai INESA Scientific Instrument Co., Ltd).
The LC-AFS conditions were (Yun et al. 2010; Xiao et al. 2014): pH 5.92 phosphate buffer solution (PBS; Na2HPO4 and KH2PO4) as mobile phase; injection volume 100 μl; pumping flow 1.0 ml/min. To 5 % HCl as a load flow, 20 g/l alkaline KBH4 as a reductant was added and mixed to form a reductant H2; the separated substance reacted with H2 to form gaseous AsH3. The AFS conditions were as follows: flow rate of carrier gas and shielding gas were 300 and 900 ml/min, respectively; main current = 80 mA; auxiliary current = 40 mA; negative high voltage = 300 V; atomization temperature = 200 °C; height of atomization device = 10 mm.
All experimental data were compared by analysis of variance (ANOVA) and processed using the statistical software SPSS 19.0 of treatments and control samples.
2.5 Iron (hydr)oxides characterization
To determine the arsenic species adsorbed on the surface of the adsorbent after reaction with As(III) or As(V), we selected some samples and freeze-dried them for further analysis using scanning electron microscopy (SEM) and X-ray diffraction (XRD).
An S-4800 scanning electron microscope (SEM, Hitachi Ltd., Japan) and an XD-3 X-ray diffraction (XRD) analyzer (Beijing Purkinje General Instrument Co., Ltd.) were used to check lepidocrocite, ferrihydrite, and magnetite before and after leaching. A 2.6 × 20 cm glass column and an automatic sampling instrument were used for leaching experiments (Shanghai Huxi Analysis Instrument Factory Co., Ltd). SEM conditions were voltage 7–15 kV; working distance 8–12 mm. Samples were scanned from 20° to 80° (2θ) with a 1° (2θ) step-size and a 1-min count time. Results were interpreted with the support of the JADE 6.5 software package.
3 Results and discussion
3.1 As(III) and As(V) leaching from iron (hydr)oxide-coated sand column
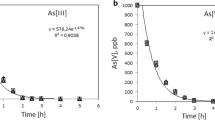
As(III) (a) and As(V) (b) leaching from columns loaded with ferrihydrite-, magnetite-, or lepidocrocite-coated quartz sand column are plotted and showed in Fig. 1. Results indicate that the concentrations of As(III) and As(V) in the eluents are changing before 800 min of leaching time. The retention performances of As(III) and As(V) depend on the type of iron (hydr) oxides: ferrihydrite > magnetite > lepidocrocite, and this order is consistent with a previous report (Raven et al. 1998), suggesting that the strongest retention performance of As(III) and As(V) is found in the ferrihydrite-coated quartz sand column. Throughout the experiment, the As(III) and As(V) concentrations in the eluents are within 0–3.5 mg/l (As(III)) and 0–6.0 mg/l (As(V)). Obviously, retention capacity of As(III) by ferrihydrite is stronger than the As(V). On the adsorption curve of magnetite, the arsenic concentrations within 0–200 min change rapidly first and then slowly and increase from 0.135 mg/l at the beginning to 8.06 mg/l at the end. The adsorption ability of lepidocrocite is relatively weaker than versus ferrihydrite and magnetite. After 400 and 700 min, the As(III) and As(V) concentrations in the elution solutions are balanced, respectively. In the As(III) leached by the lepidocrocite-coated sand column, the initial level (3.51 mg/l) and balanced level (8.93 mg/l) are larger versus the other two iron oxides. The retention abilities of As(III) and As(V) by ferrihydrite versus the magnetite and lepidocrocite are 3.25, 5.63(As(III)) and 1.75, 3.65(As(V)) times higher.
Ferrihydrite is an amorphous iron oxide possessing an extremely large specific surface area (SSA) and high reaction activity. The core of its structure is dominated by octahedrons, and its surface is occupied by abundant tetrahedral units. The surface unsaturation together with weak crystallinity and large SSA endows ferrihydrite with stronger retention ability compared with the other two iron oxides. Thus, ferrihydrite was selected and used to investigate the leaching and retention ability of As(III) and As(V).
3.2 Effects of ionic strength on As(III) and As(V) leaching
Ionic strength can affect the macroscopic adsorptive performances, which provide valuable information on the formation of surface complexes (Kim et al. 1988). In order to make identification effects of ionic strength on As(III) and As(V) leaching, we test the leaching and retention dynamics of As(III) and As(V) by a 1 % ferrihydrite-coated sand column (Fig. 2 a, b) with 0.1 or 0.01 mol/l NaCl as the supporting electrolyte. Results showed that the As(III) and As(V) concentrations in the two groups of solutions all increase with reaction time. The As(III) concentrations in the solutions increased slowly from 0 to 0.5 mg/l within 0–100 min then increased rapidly within 100–800 min but are gradually balanced after 800 min. Similarly, the As(V) concentrations in the eluents continue to increase before 800 min leaching time and increase from 0.01 mg/l at the beginning to 5.99 mg/l at the end. In different ionic strength solutions, the adsorption capacity of As(III) by ferrihydrite is stronger than the As(V). AVONA via least significant difference (LSD) was conducted on SPASS 19.0. The significance level is p > 0.05, so the adsorptions of arsenic by ferrihydrite are not significantly different among different ionic strengths in the leaching solutions. In other words, ionic strength is not a main influence factor on the leaching of As(III) or As(V) by the ferrihydrite-coated sand column.
The formation of outer sphere complexes was relative in that the adsorptive capacity decreases with the elevation of ionic strength. On the contrary, when ionic strength has little effect or favorable effect on the adsorption capacity, the formation of inner sphere complexes may be inferred (He et al. 2015). These As(III) and As(V) leaching results indicate that the inner sphere complexes might dominate in the leaching and retention of As(III) and As(V) onto the ferrihydrite accordingly (Manning et al. 1998).
3.3 Effects of eluent pH on As(III) and As(V) leaching
The leaching and retention kinetics of As(III) and As(V) over a wide pH range from 3.0 to 11.0 is illustrated in Fig. 3a, b. The As(III) and As(V) concentrations in the eluents gradually increase with the prolonging of reaction time and are balanced after 800 min leaching time. The retention ability of As(III) or As(V) by the ferrihydrite-coated quartz sand column varies with different pH values of solution. Quantitatively, the retained concentrations of As(III) increase from 3.57 at pH 3.0 to 6.96 mg/l at pH 5.0 and decrease from 6.42 at pH 9.0 to 5.16 mg/l at pH 11.0. When the solution pH value is 7.0, the retained concentration of As(III) is 6.10 mg/l after reaching equilibrium. The pH significantly impacts the adsorption capacity of As(III) by ferrihydrite. The adsorption ability changed as follows: pH 5.0 > pH 7.0 > pH 9.0 > pH 11.0 > pH 3.0, and the maximum adsorption quantity (at pH 5.0) is 1.95 times larger than the minimum one (at pH 3.0). Results show that both weak acid or weak alkali environments are favorable for the adsorption of As(III) by ferrihydrite. The leaching adsorption ability of As(III) by ferrihydrite is minimized at pH 3.0. The reason is that pH 3.0 is a strongly acidic environment relative to other pH levels, leading to the partial dissolution and severe reduction of the amount of iron mineral and resulting in the reduction of adsorption ability.
Similarly, the solution pH significantly impacts the leaching and retention of As(V) by the ferrihydrite-coated sand column. After leaching, solution concentration is stabilized, the concentration of As(V) in the leaching solution increases from 5.20 at pH 3.0 to 8.59 mg/l at pH 11.0. However, the concentration of As(III) leached by the ferrihydrite-coated sand column changes in the first trend and then increased trend with the pH increase.
As pH increases, the decreasing concentration of aqueous protons drives more protons from the surfaces of iron (hydr)oxides, thus making it more negatively charged (Ghosh et al. 2006b). The point of zero charge (PZC) of ferrihydrite is about pH 7.0 to 8.5 (Raven et al. 1998; Qi and Pichler 2014). Thus, the range of pH investigated brackets the PZC of the media and consequently brackets the largest change in surface charge per unit change in pH occurs. pKa values of arsenious acid (H3AsO3) and arsenic acid (H3AsO4) are as follows: pK 1 = 9.22, pK 2 = 12.13, and pK 3 = 13.4; pK 1 = 2.20, pK 2 = 6.97, and pK 3 = 11.53, respectively. Inflections or maxima in the retention envelopes of anions at pH values close to their pKa are a well-documented phenomenon (Raven et al. 1998). Arsenite retention ability is not reduced significantly until pH values were greater than 9.0. The higher fraction of aqueous arsenic in the adsorption experiments than in the desorption experiments might be due to the presence of an energy barrier, which must be overcome to mobilize absorbed arsenic and thus slow down the release (for desorption) compared to the uptake (for adsorption). This indicates the surface sites, on which arsenate is irreversibly bound in the operating conditions employed and is consistent with a shift from monodentate to bidentate surface bonding after retention.
To sum up, the wide pH range from 3.0 to 11.0 has a significant effect on the surface charge distribution of iron (hydr)oxides and ionization of As (III) and As (V), thus affecting the leaching and retention ability of As (III) and As (V) from iron (hydr)oxide-coated sand columns.
3.4 Effects of phosphate on As(III) and As(V) leaching
In this section, in order to investigate the effects of phosphate on As(III) and As(V) leaching, we test the leaching dynamics of As(III) and As(V) by the 1 % ferrihydrite-coated sand column (Fig. 4a, b) with a molar ratio of As to HPO4 2− as 1:0.1 or 1:10 in the stock solution. As shown in Fig. 4a, at the molar ratio of As to HPO4 2− of 1:0 or 1:0.1 in the stock solution, the retention concentration of As(III) by the ferrihydrite-coated sand column after balance dropped by 4.83 % from 6.01 to 5.72 mg/l. At the ratio of As to HPO4 2− of 1:10, the retention concentration of As(III) by ferrihydrite after equilibrium is only 3.99 mg/l, 66.39 % of the baseline (no addition of HPO4 2−). As illustrated in Fig. 4b, at the molar ratio of As to HPO4 2− of 1:0 or 1:0.1, the concentration of As(V) by the ferrihydrite-coated sand column in the eluent after equilibrium rises by 5.01 % from 5.98 to 6.28 mg/l. At the molar ratio of As to HPO4 2− of 1:10 in the stock solution, the adsorption concentration of As(V) by ferrihydrite after adsorption equilibrium is 1.59 mg/l, only 39.55 % of the baseline.
Effect of phosphate content on As(III) (a) and As(V) (b) elution from ferrihydrite-sand column; 10 g/kg of ferrihydrite added at sand column; initial concentration of As(III) or As(V) = 10 mg/l; flow rate = 1 ml/min; pH 7.0 in the eluent; the molar ratio of As to HPO4 2− at 1:0 was set up as the blank control
It is known that the sorption process of arsenate onto the iron (hydr)oxide is strongly disturbed by phosphate (Dixit and Hering 2003; Zhang et al. 2007b). Water contaminants are usually not as single ions in the water. In the previous chapter, the sorption of several iron-bearing mineral species was investigated at a constant ionic strength (added salt: NaCl) and a selected pH value, but without addition of any ion that is known to compete for the sorption sites. In this section, the influence of phosphate is investigated because of its competitive effect on the sorption of arsenate (Kolbe, et al. 2011). The addition of HPO4 2− severely inhibits the adsorption of As(III) and As(V) by ferrihydrite, and a higher HPO4 2− concentration is obvious more inhibitory. Since the elements of P and As are in the same main group of chemical periodic table, they have similar properties. HPO4 2− can compete with AsO2 − and AsO3 − for absorption, which reduces the amount of adsorption sites on surface of ferrihydrite as well as the adsorption ability of ferrihydrite. Ghosh’s study suggests a number of sites where arsenite or arsenate ions can be exchanged by phosphate, but the arsenite or arsenate adsorption to phosphate addition is indifferent at higher phosphate loadings, suggesting that not all arsenic or arsenate sites are exchangeable (Ghosh et al. 2006a).
3.5 Effects of HA on As(III) and As(V) leaching
The leaching dynamics of As(III) and As(V) by the 1 % ferrihydrite-coated sand column are showed in Fig. 5a, b with the As to HA concentration ratio 1:0.1 or 1:1. HA also shows a greater difference in the leaching stock solutions between the lowest and highest concentrations. When the HA concentration is 1 mg/l, the residual concentration of As(III) after balance decreases from 3.90 mg/l (without addition of HA, baseline) to 1.49 mg/l, about 61.8 % reduction. In the same conditions, the concentration of As(V) in the leaching solution after balance decreases from 5.99 mg/l (baseline) to 2.32 mg/l, about 61.3 % reduction (38.7 % of 5.99 mg/l). These results indicate that the addition of HA significantly improves the adsorption of As(III) and As(V) by ferrihydrite. However, with addition of 10 mg/l HA, the residual concentration of As(III) after balance increases from 3.90 mg/l (baseline) to 5.20 mg/l, about 1.33 times larger than baseline and 3.49 times larger than with addition of 1 mg/l HA. The residual concentration of As(V) after balance increases from 5.99 mg/l (baseline) to 8.41 mg/l, about 1.40 times larger than baseline and 3.63 times larger than with addition of 1 mg/l HA. These data indicate that addition of HA has a similar effect on As(III) and As(V) leaching from the ferrihydrite-coated sand column. In conclusion, the retention of As(III) and As(V) by ferrihydrite is significantly enhanced by the addition of low-dose HA, but is inhibited by the addition of excessive HA.
Effect of HA content on As(III) (a) and As(V) (b) elution from ferrihydrite-sand column; 10 g/kg of ferrihydrite added at sand column; initial concentration of As(III) or As(V) = 10 mg/l; flow rate = 1 ml/min; pH 7.0 in the eluent; the concentration ratio of As to HA at 1:0 was set up as the blank control
HA contains 50 % of NOM and is a unique anionic polyelectrolyte at all pH values (Warwick et al. 2005). It readily forms both aqueous and surface inner-surface complexes with cationic metals and metal oxides (Murphy et al. 1994). Arsenic uptake is suppressed by HA, and with the level of suppression increasing with HA concentration, the added HA hardly affects the adsorption of As (Warwick et al. 2005; Fakour and Lin 2014; Kong et al. 2014; Fakour and PanYF 2015). The suppression is attributed to the partial coverage of the adsorption sites, as confirmed by elemental analysis. HA may affect the retention ability of As(III) and As(V) from ferrihydrite through various mechanisms by (1) direct competition with the arsenic for surface sites (Parks, 1990), (2) absorption to the surface to create additional surface attraction and enhanced sorption at low HA concentration (Schwarzenbach et al. 1993), (3) acting as a soluble partitioning agent to bind the ions and keep them in solution (Stumm and Morgan 1996), (4) direct reaction with the sorbent surface to enhance dissolution of the surface and loss of sorption sites (Schwarzenbach et al. 1993), or (5) deposition of HA onto the solid surface to shield active sites (Ghosh et al. 2006b). The effects of HA on the retention of As(III) and As(V) from the ferrihydrite-coated sand column might be attributed to several mechanisms together, rather than a single mechanism.
3.6 Retention mechanisms of As(III) and As(V) on iron (hydr)oxides
In the leaching process, the retention capacity of As(III) and As(V) from iron (hydr)oxide-coated sand columns depends on the adsorption capacity of As(III) and As(V) by different iron (hydr)oxides. Numerous studies show that iron (hydr)oxides can adsorb of As(III) and As(V) effectively. Moreover, the adsorption performances of As(III) and As(V) depend on the type of iron oxides: ferrihydrite > magnetite > lepidocrocite, and this order is consistent with retention performance in the leaching process.
In order to better understand the mobilization and repartition of arsenic during the dissolution and coprecipitation process, we identify morphological changes of ferrihydrite, lepidocrocite, magnetite, and speciation of secondary minerals by technology of SEM (Fig. 6) and XRD (Fig. 7).
Clearly, in the leaching reaction, all three iron-bearing minerals underwent obvious geometric and morphologic changes, such as agglomeration, rounding, and smoothing, indicating the presence of interparticle magnetic force, surface tension, and oxidoreduction on the surface of iron (hydr)oxides. Before leaching reaction, lepidocrocite was shaped as rod-like spindles each 1 μm long and 300 nm wide; the whole size was 2–7 μm, which was similar to a previous report. After As(III) or As(V) leached, lepidocrocite was nest-shaped under the interparticle surface tension, and the complexation via adsorption and coprecipitation with arsenic. The diameter of a unit increased from 2–7 μm to about tens of μm. The unreacted ferrihydrite was obviously gully on the surface and sponge-like inside. The surface of reacted ferrihydrite was smoother without clear gully, while the sponges inside were more filled-up. Because of small grain-size and weak crystallization, the ferrihydrite did not show obvious peaks on SEM. The magnetite was like sharp-crystal cubes, in diameter of 50–100 nm, indicating a typical micro-nanostructure. After As(III) or As(V) leached, the sharp-crystal cubes gradually smoothed to irregular spheres.
Before As(III) and As(V) leached, lepidocrocite and magnetite show clear characteristic peaks (Fig. 7), which are consistent with a previous report that ferrihydrite is featured by wide distribution, small grain-size, and weak crystallization. Ferrihydrite shows two unclear characteristic peaks, which are consistent with the prepared two-line ferrihydrite. With the prolonging of reaction time, the As(III) or As(V) contents in the three iron-bearing minerals all increase, and the mineralogy also changes, indicating that the iron ions in the iron-bearing minerals are gradually released to flocculate and coprecipitate with arsenic ions in the solutions to form new minerals. Moreover, Pedersen’s research suggested that ferrihydrite could be transformed into lepidocrocite and goethite at low Fe2+ concentrations and into goethite and magnetite at high Fe2+ concentrations (Pedersen, et al. 2006). Huang’s research indicated that magnetite and goethite could be formed at suitable pH value and initiated by adsorption of Fe2+ onto the ferrihydrite surface (Huang, et al. 2015). Iron (hydr)oxides have highly reactive surface areas than other iron oxides due to bidentate and bimolecular surface complexes between arsenic species and =FeOOH (Sakthivel et al., 2011; Maji et al., 2012; Rahman et al., 2013). Thus, under aqueous environment, the iron (hydr)oxides could effectively bind arsenic species by ligand exchanges on the iron (hydr)oxides containing adsorbent’s internal and external surfaces (Eqs. 1 and 2) and form the new minerals (Shams et al., 2014). In our study, the newly formed substances were identified via library searching to be Fe4O3(AsO4)2 (Angelelite, PDF 13–0121), FeAs2 (Loellingite, PDF 53–1197), FeFe3(As5O13) (Scheiderhoehnite, PDF 35–0462), and FeAsO4(H2O)2 (Scorodite, PDF 37–0468). These second minerals were obtained from flocculation and coprecipitation between Fe and As.
4 Conclusions
In this study, the retention performance of As(III) and As(V) from a sand column coated with ferrihydrite is greater than that coated with magnetite or lepidocrocite, and the influence factors such as pH, phosphate, and humic acid (HA) affect the leaching and retention of As(III) and As(V). For solutions with different pH levels, the retention of As(III) by ferrihydrite changes as follows: pH 5.0 > pH 7.0 > pH 9.0 > pH 11.0 > pH 3.0; the adsorption of As(V) by ferrihydrite decreases with the increase of pH in the leaching solution. Weak acid environments are favorable for the adsorption of As(III) by ferrihydrite. The addition of phosphate severely inhibits the leaching adsorption of As(III) and As(V) from the ferrihydrite-coated sand column, and the inhibitory effect is more obvious along with the increase of phosphate concentration. The adsorption of As(III) and As(V) by ferrihydrite is significantly enhanced by the addition of low-dose HA, but is inhibited by the addition of excessive HA. The results will provide the theoretical underline for the application of iron (hydr)oxide in arsenic pollution control.
References
Amstaetter K, Borch T, Larese-Casanova P et al (2010) Redox transformation of arsenic by Fe(II)-activated goethite (α-FeOOH). Environ Sci Technol 44(1):102–108
Delaun RD, Masscheleyn PH, Patrick WH Jr (1991) Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environ Sci Technol 25(8):1414–1419
Demetriou A, Pashalidis I (2012) Spectophotometric studies on the competitive adsorption of boric acid (B(III)) and chromate (Cr(IV)) onto iron (oxy)hydroxide (Fe(O)OH). Global Nest J 14(1):32–39
Dixit S, Hering JG (2003) Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals. Environ Sci Technol 37(18):4182–4189
Douglas MR (1984) Principles of adsorption and adsorption processes. Wiley, New York
Dzombak DA, Morel FMM (1990) Surface complexation modeling: hydrous ferric oxides. Wiley, New York
Fakour H, Lin TF (2014) Effect of humic acid on as redox transformation and kinetic adsorption onto iron oxide based adsorbent (IBA). Int J Env Res Pub He 11(10):10710–10736
Fakour H, PanYF LTF (2015) Effect of humic acid on arsenic adsorption and pore blockage on iron-based adsorbent. Water Air Soil Poll 226:14
Ghosh A, Mukiibi M, Saez AE et al (2006a) Leaching of arsenic from granular ferric hydroxide residuals under mature landfill conditions. Environ Sci Technol 40(19):6070–6075
Ghosh A, Saez AE, Ela W (2006b) Effect of pH, competitive anions and NOM on the leaching of arsenic from solid residuals. Sci Total Environ 363(1–3):46–59
Gil RA, Ferrua N, Salonia JA et al (2007) On-line arsenic co-precipitation on ethyl vinyl acetate turning-packed mini-column followed by hydride generation-ICP-OES determination. J Hazard Mater 143(1–2):431–436
Goldberg S, Johnston CT (2001) Mechanisms of arsenic adsorption on amorphous oxides evaluated using macroscopic measurements, vibrational spectroscopy, and surface complexation modeling. J Colloid Interf Sci 234(1):204–216
Hamon RE, Lombi E, Fortunati P et al (2004) Coupling speciation and isotope dilution techniques to study arsenic mobilization in the environment. Environ Sci Technol 38(6):1794–1798
He Z, Liu RP, Liu HJ et al (2015) Adsorption of Sb(III) and Sb(V) on freshly prepared ferric hydroxide (FeOxHy). Environ Eng Sci 32(2):95–102
Huang FG, Jia SY, Liu Y et al (2015) Reductive dissolution of ferrihydrite with the release of As(V) in the presence of dissolved S(−II). J Hazard Mater 286:291–297
Jaime WVM, Talbott JL, Scott J et al (2007) Arsenic speciation in arsenic-rich Brazilian soils from gold mining sites under anaerobic incubation. Environ Sci Pollut Res 14(6):388–396
Jain CK, Ali I (2000) Arsenic: occurrence, toxicity and speciation techniques. Water Res 34(17):4304–4312
Jia YF, Zhang DN, Pan RR et al (2012) A novel two-step coprecipitation process using Fe(III) and Al(III) for the removal and immobilization of arsenate from acidic aqueous solution. Water Res 46(2):500–508
Keon NE, Swartz CH, Brabander DJ et al (2001) Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environ Sci Technol 35(13):2778–2784
Kim FH, Charalambos P, James OL (1988) Modeling ionic strength effects on anion adsorption at hydrous oxide/solution interfaces. J Colloid Interf Sci 2(125):717–726
Kocar BD, Herbel MJ, Tufano KJ et al (2006) Contrasting effects of dissimilatory iron(III) and arsenic(V) reduction on arsenic retention and transport. Environ Sci Technol 40(21):6715–6721
Kolbe F, Weiss P, Morgenstern P et al (2011) Sorption of aqueous antimony and arsenic species onto akaganeite. J Colloid Interf Sci 357(2):460–465
Kong SQ, Wang YX, Zhan HB et al (2014) Competitive adsorption of humic acid and arsenate on nanoscale iron-manganese binary oxide-loaded zeolite in groundwater. J Geochem Explor 144:220–225
Kulp TR, Hoeft SE, Asao M et al (2008) Arsenic(III) fuels anoxygenic photosynthesis in hot spring biofilms from Mono Lake, California. Science 321(5891):967–970
Leist M, Casey RJ, Caridi D (2003) The fixation and leaching of cement stabilized arsenic. Waste Manage 23(4):353–359
Lopes WD, Santelli RE, Oliveira EP et al (2009) Application of multivariate techniques in the optimization of a procedure for the determination of bioavailable concentrations of Se and As in estuarine sediments by ICP OES using a concomitant metals analyzer as a hydride generator. Talanta 79(5):1276–1282
Maji SK, Kao YH, Wang CJ et al (2012) Fixed bed adsorption of As(III) on iron-oxide-coated natural rock (IOCNR) and application to real arsenic-bearing groundwater. Chem Eng J 203:285–293
Manning BA, Fendorf SE, Goldberg S (1998) Surface structures and stability of arsenic (III) on goethite: spectroscopic evidence for inner-sphere complexes. Environ Sci Technol 32(16):2383–2388
Murphy EM, Zachara JM, Smith SC et al (1994) Interaction of hydrophobic organic compounds with mineral-bound humic substances. Environ Sci Technol 28(7):1291–1299
Oremland RS, Stolz JF (2005) Arsenic, microbes and contaminated aquifers. Trends Microbiol 13(2):45–49
Parks GA (1990) Surface energy and adsorption at mineral/water interfaces: an introduction. Rev Mineral Geochem 23(1):133–175
Pedersen HD, Postma D, Jakobsen R (2006) Release of arsenic associated with the reduction and transformation of iron oxides. Geochim Cosmochim Acta 70(16):4116–4129
Pierce ML, Moore CB (1982) Adsorption of arsenite and arsenate on amorphous iron hydroxide. Water Res 16(7):1247–1253
Qi PF, Pichler T (2014) Closer look at As(III) and As(V) adsorption onto ferrihydrite under competitive conditions. Langmuir 30(37):11110–11116
Rahman IMM, Begum ZA, Sawai H et al (2013) Decontamination of spent iron-oxide coated sand from filters used in arsenic removal. Chemosphere 92(2):196–200
Raven KP, Jain A, Loeppert RH et al (1998) Arsenite and arsenate adsorption on ferrihydrite: kinetics, equilibrium, and adsorption envelopes. Environ Sci Technol 32(3):344–349
Ruokolainen M, Pantsar-Kallio M, Haapa A et al (2000) Leaching, runoff and speciation of arsenic in a laboratory mesocosm. Sci Total Environ 258(3):139–147
Sakthivel R, Jayasankar K, Das SK et al (2011) Effect of planetary ball milling on phase transformation of a silica-rich iron ore. Powder Technol 208(3):747–751
Schwarzenbach R, Gschwend P, Imboden D (1993) Environmental organic chemistry. Wiley, New York
Schwertmann U, Cornell RM (2000) Iron oxides in the laboratory-preparation and characterization , Second, completely revised and extended editionth edn. John Wiley and Sons Incorporated, New York
Shams AB, Jin Z, Lisha T et al (2014) Influence of calcination on magnetic honeycomb briquette cinders composite for the adsorptive removal of As(III) in fixed-bed column. Chem Eng J 257:1–9
Silva J, Mello JWV, Gasparon M et al (2007) Arsenate adsorption onto aluminium and iron (hydr)oxides as an alternative for water treatment. International Mine Water Association Symposium, Italy
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17(5):517–568
Stumm W, Morgan J (1996) Aquatic chemistry. JWiley, New York
Swartz CH, BluteNK BB et al (2004) Mobility of arsenic in a Bangladesh aquifer: inferences from geochemical profiles, leaching data, and mineralogical characterization. Geochim Cosmochim Acta 68(22):4539–4557
Tuna AOA, Ozdemir E, Simsek EB et al (2013) Removal of As(V) from aqueous solution by activated carbon-based hybrid adsorbents: impact of experimental conditions. Chem Eng J 223:116–128
United States Environmental Protection Agency (1997) Report on the expert panel on arsenic carcinogenicity: review and workshop. National Center for Environmental Assessment, USEPA, Washington DC
Warwick P, Inam E, Evans N (2005) Arsenic’s interaction with humic acid. Environ Chem 2(2):119–124
Xiao YB, Zhang M, Wen HW (2014) Simultaneous determination of arsanilic, nitarsone and roxarsone residues in foods of animal origin by ASE-LC-AFS. Spectrosc Spectral Anal 34(4):1100–1103
Yun HX, Zhang L, Li XW, Zhao YF et al (2010) Determination of inorganic arsenic in rice by liquid chromatography-atomic fluorescence spectrometry. J Hyg Res 39(3):316–320
Zhang GS, Qu JH, Liu HJ et al (2007a) Preparation and evaluation of a novel Fe-Mn binary oxide adsorbent for effective arsenite removal. Water Res 41(9):1921–1928
Zhang JS, Stanforth RS, Pehkonen SO (2007b) Effect of replacing a hydroxyl group with a methyl group on arsenic(V) species adsorption on goethite (alpha-FeOOH). J Colloid Interf Sci 306(1):16–21
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 41171254), and the Project of Agricultural Public Welfare Scientific Research (201303101–06).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jan Schwarzbauer
Rights and permissions
About this article
Cite this article
Wang, Y., Sun, L., Han, T. et al. Arsenite and arsenate leaching and retention on iron (hydr)oxide-coated sand column. J Soils Sediments 16, 486–496 (2016). https://doi.org/10.1007/s11368-015-1230-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1230-3