Abstract
Background, aim, and scope
Feedstock recycling has received attention as an effective method to recycle waste plastics. However, estimating the reduction potential by life cycle assessment using coke oven and blast furnace in steel works has been a challenging task due to the complex structure of energy flow in steel works. Municipal waste plastics consist of several plastic resins. Previous studies have generally disregarded the composition of waste plastics, which varies significantly depending on the geographical area. If the reduction potentials by using each plastic resin in steel works can be quantified, the potential of municipal waste plastics (mixtures of plastic resins) can be estimated by summing up the potential of each resin multiplied by the composition of each resin in municipal waste plastics. Therefore, the goal of this study is to investigate the reduction potentials of CO2 emissions by using individual plastic resins (polyethylene (PE), polypropylene (PP), polystyrene (PS), polyethylene terephthalate (PET)) and those for municipal waste plastics in the coke oven and blast furnace.
Materials and methods
A model was developed to clarify the energy flow in steel works. In order to estimate the changes in energy and material balance in coke ovens when waste plastics are charged, the equations to calculate the coke product yield, gas product yield, and oil product yields of each plastic resin were derived from previous studies. The Rist model was adopted to quantify the changes in the inputs and outputs when plastics were fed into a blast furnace. Then, a matrix calculation method was used to calculate the change in energy balance before and after plastics are fed into a coke oven.
Results
It was confirmed that product yields of municipal waste plastics (mixtures of plastic resins) could be estimated by summing up the product yield of each plastic resin multiplied by the composition of each resin in municipal waste plastics. In both cases of coke oven and blast furnace feedstock recycling, the reduction potential of CO2 emissions varies significantly depending on the plastic resins. For example, in the case of coke oven chemical feedstock recycling, the reduction potential of PS and PP is larger than that of PE. On the other hand, in the case of blast furnace feedstock recycling, PE has the largest CO2 emissions reduction potential, whereas the CO2 emission reduction potential of PP is smaller than those of PE and PS. In both cases, PET has negative CO2 emission reduction potentials, i.e., there is an increase of CO2 emissions. In addition, the reduction potentials of CO2 emissions are slightly different in each city.
Discussions
The differences in the reduction potentials of CO2 emissions by coke oven chemical feedstock recycling of each plastic resin is attributable to the differences in calorific values and coke product yields of each plastic resin. On the other hand, the difference in the CO2 emission reduction potential for each plastic resin in blast furnace feedstock recycling is attributable to the difference in calorific values and the carbon and hydrogen content of each plastic resin, which leads to a difference in the coke substitution effect by each plastic resin. In both cases, the difference in those of municipal waste plastics is mostly attributable to the amount of impurities (e.g., ash, water) in the municipal waste plastics.
Conclusions
It was found that the reduction potential of CO2 emissions by coke oven and blast furnace feedstock recycling of municipal waste plastics (mixtures of plastic resins) could be estimated by summing up the potential of each resin multiplied by the composition of each resin in municipal waste plastics. It was also clarified that feedstock recycling of waste plastic in steel works is effective for avoiding the increase in CO2 emissions by incinerating waste plastics, such as those from household mixtures of different resins.
Recommendations and perspectives
With the results obtained in this study, reduction potentials of CO2 emissions can be calculated for any waste plastics because differences in composition are taken into account.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background, aim, and scope
Plastics are an indispensable material in our present economic society because they are light, tough, easily processed, and inexpensive. Large amounts of plastics are used for packing and container materials, which require these properties. The demand for plastics has remained high in developed countries, and the demand has been increasing in developing countries. In proportion, the amount of waste plastics has also increased, which has caused the most challenging waste issues that include a shortage of landfills and damage to eco-systems. In order to solve these problems, it is necessary to reduce waste plastics and promote recycling. In recent years, many countries such as Japan, Europe, the USA, India and China have been promoting recycling of waste plastics. As a result, the recycling rate has been growing year by year (Mutha et al. 2006; Subramanian 2000; Zhang et al. 2007; Patel et al. 2000; Bor et al. 2004; Braunegg et al. 2004). However, there is still a great difference between the recycling rates in each country. This is due to several differences in the enforcement of laws for the recycling of waste plastics, as well as the technologies and costs for recycling waste plastics in each country.
In Japan, recycling of waste plastics has been enhanced since 1997, when the “Containers and Packaging Recycling Law” was enforced. Currently, annual production of plastics in Japan is approximately 14.5 million tons, whereas the annual generation of municipal waste plastics was 5.2 million tons and that of industrial waste plastics was 4.86 million tons in 2005. As a result of many years of technological development, 62% of plastics (6.28 million tons) were recycled in 2005 by several methods. The recycling rate of waste plastics in Japan is relatively high compared to other countries; however, it is expected that the amount of waste plastics to be recycled will have to increase in the future because we have a landfill shortage problem and a responsibility to reduce CO2 emissions.
The substantial problem is that waste plastics are contained in Municipal Solid Waste (MSW), and each plastic resin is collected with other materials and may thus include many impurities. These impurities cause a barrier to effective recycling of waste plastics. Therefore, many technologies to separate waste plastics from MSW (Shent et al. 1999; Hu and Calo 2006) and to recycle them effectively have been developed. The recycling methods are grouped into three main categories:
-
1.
Mechanical recycling (Avila and Duarte 2003; Fortelny et al. 2004)
-
2.
Feedstock recycling, e.g., monomerization, blast furnace feedstock recycling, coke oven chemical feedstock recycling, gasification, liquefaction, etc. (Garfort et al. 2004; Kim et al. 2002; Kaminsky et al. 2004; Kato et al. 2003; Okuwaki 2004; Asanuma and Ariyama 2004)
-
3.
Energy recovery, e.g., cement kilns, waste power generation, refuse-derived fuel (Yamakita et al. 2005; Xiao et al. 2007)
Among these recycling methods, mechanical recycling is only applicable to industrial plastics wastes and some particular waste plastics generated in households, e.g., polyethylene terephthalate (PET), because mechanical recycling requires clear separation of different types of resins with a low level of impurities. So, mechanical recycling has a limitation. In contrast, most waste plastics can be recycled by feedstock recycling and energy recovery if impurities such as metals are removed from them. So, feedstock recycling and energy recovery can be useful for recycling any type of waste plastics.
In Japan, the industry with the highest demand for coal is the steel works, with an annual consumption of approximately 60–70 million tons. So, the reduction of greenhouse gas, especially CO2 emissions, is currently a very important task for the steel industry due to the Kyoto Protocol. Since waste plastics are usually richer than coal in calorific value, the use of waste plastics instead of coal can reduce the consumption of fresh coal, leading to the reduction of CO2 emissions. In addition, the recycling of waste plastics uses the existing equipment in steel works, so it is not necessary to set up new facilities. Therefore, it is considered that steel works show great promise for the recycling of waste plastics. It is important to investigate the reduction potential of CO2 emissions by recycling waste plastics in steel works quantitatively. However, estimating the reduction potential has been a challenging task due to the complex structure of energy flow in such steel works.
2 Literature survey on estimation of reduction potential of CO2 when waste plastics are recycled in steel works
Studies on the estimation of the environmental effect of recycling waste plastics have been conducted since the 1990s, and they have primarily targeted mechanical recycling, energy recovery and recycling systems of waste plastics. In recent years, studies targeting feedstock recycling have been increasing, and the estimation of the environmental effect by feedstock recycling has attracted interest (Plastic Waste Management Institute 2005a; Molgaard 1995; Song et al. 1999; Arena et al. 2003; Ross and Evans 2003; Holmgren and Henning 2004; Finnveden et al. 2005; Noda et al. 2001; Perugini et al. 2005; Narita et al. 2001; Ziebik and Stanek 2001; Inaba et al. 2005).
There have been some attempts to estimate the reduction potential of environmental impact by feedstock recycling of waste plastics in steel works in which life cycle assessment (LCA) was applied (Narita et al. 2001; Ziebik and Stanek 2001; Inaba et al. 2005). Inaba et al. reviewed these previous studies in great detail and discussed the influence of setting system boundaries because there was no consistency in previous studies (Inaba et al. 2005). They also conducted comparative studies for feedstock recycling of waste plastics in coke ovens, blast furnaces, and energy recovery, based on the same functional unit. Their study clarified the following:
-
1.
The LCA results for feedstock recycling of waste plastics in steel works were quite dependent on setting system boundaries.
-
2.
Without careful investigation of the change in energy flows in related processes, the reduction potential of environmental impact by feedstock recycling of waste plastics in steel works would be incorrectly estimated.
-
3.
The reduction potential of environmental impact by feedstock recycling of waste plastics in blast furnaces was dependent on whether waste plastics would replace coke or pulverized coal.
-
4.
For comparison of different recycling technologies, collection of precise data about related processes from industries, studies based on representative data from statistics, and sensitivity analyses should be further conducted.
For estimating the reduction potential of CO2 emissions by recycling waste plastics in a coke oven or blast furnace, it is necessary to precisely analyze the changes in energy flow in steel works. Two issues should be investigated: one is the change of energy flow in the coke oven and blast furnace, and the other is the change of energy flow in the entire steel works induced by the change in inputs and outputs of the coke oven or blast furnace process.
In life cycle inventory (LCI) analyses, the process flow diagram method is often used. In the process flow diagram method, the functional unit is considered as the starting point, data is collected in the right amount per entity (substance, energy, service), and multiple processes are searched and added until no economic entity is unsatisfied. However, it has been reported that the existence of self-referring groups of processes, such as electricity, poses a problem in this approach (Heijungs 1994). Since the energy flow in steel works is complex and may comprise several loops, as shown in Fig. 1, the simple sequential method of calculating the energy balance does not work. In this case, a matrix calculation method proposed by Heijungs (1994) should be used. This method is quite effective when dealing with several processes, some of which are self-referring groups of processes, because the exact solution can be calculated for the system without using an iterative method or infinite progression (Suh and Huppes 2005). Suh and Huppes (2005) reviewed and compared methods for LCI compilation, which consisted of a process flow diagram, matrix calculation, economic input–output-based LCI, and hybrid analyses. The matrix calculation method was used for the development of a software tool, Chain Management by Life Cycle Assessment, CMLCA (Heijungs 2000). This method was also used in Inaba’s work (Inaba et al. 2005).
It should also be noted that previous studies paid little attention to the composition of waste plastics, which varies significantly depending on geographical area. The reduction potential of recycling waste plastics can also be dependent on the composition of waste plastics. Therefore, it is not adequate to compare the results obtained in previous studies if the compositions of waste plastics are different. So, it is necessary to develop a model for estimating the reduction potential of environmental impact in which the differences in the composition of waste plastics can be considered.
Municipal waste plastics consist of several plastic resins, for example, polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), etc. If the reduction potentials of the environmental impact by using each plastic resin in steel works can be quantified, those of municipal waste plastics (mixtures of plastic resins) may be estimated by summing up the potential of each resin multiplied by the composition of each resin in municipal waste plastics. Therefore, in this work, the reduction potentials of CO2 emissions, by using the individual plastic resins (PE, PP, PS, PET), and those for municipal waste plastics were investigated by LCA. Because most PVC is removed from municipal waste plastics by the pre-treatment process in feedstock recycling in steel works, the reduction potential by using PVC is out of the scope of this paper.
3 Goal and scope definition
The goal of this study is to establish a model to clarify the energy balance in steel works and to estimate the reduction potential of CO2 emissions for the recycling of plastics in a coke oven and blast furnace in steel works. According to ISO14040, life cycle inventory analysis was conducted (ISO 2006). The functional unit was set as the treatment of 1 ton of plastic resin (PE, PP, PS, PET) or municipal plastic wastes. CO2 emissions were considered as the environmental impact. The system boundary included the processes in the steel works and a joint thermal power plant to produce electricity. The pre-treatment process to remove impurities from the waste plastics was also included. In addition, the processes of ferroalloy production, electric furnaces, forging, and casting were not considered because these processes were not affected by the use of waste plastics.
The CO2 emissions avoided due to the adoption of additional functions (i.e., generation of electricity and oil) were subtracted from the total CO2 emissions so that each case satisfied the same functional unit.
4 Materials and methods
In this chapter, methods for coke oven chemical feedstock recycling and blast furnace feedstock recycling are explained in Sections 4.1 and 4.2, respectively.
4.1 Coke oven chemical feedstock recycling
4.1.1 Outline of coke oven chemical feedstock recycling
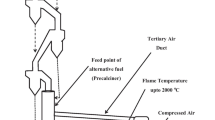
In coke ovens, the coals charged in the coke oven chambers are carbonized at a high temperature of about 1,373 K in a reducing atmosphere and are converted to products of coke, tar, light oil, and coke oven gas (COG), and so on. Ammonia liquor is used for flushing the ascension pipe exit at the top of the coke oven, and the high-temperature COG generated in the coke oven is cooled quickly to about 353 K or less. In addition, the COG is cooled in a primary gas cooler to about 308 K, the condensed liquid is separated into tar and ammonia liquor in a tar decanter, and the tar is recovered. It is thought that the carbonization conditions in a coke oven are suitable for waste plastic recycling because charged plastics decompose easily at high temperature in a reducing atmosphere.
Municipal waste plastics consist of bags, films, foamed plastics, and powders. Therefore, it is necessary to pre-treat the waste plastics and change their shape and size so that they are suitable for charging in the coke oven chambers. First, metals are removed from the waste plastics by magnetic sorting. Then the plastics are coarsely crushed and extraneous material is removed using a separator. After being finely crushed to about 10 mm, the volume is reduced using an agglomerator (screw kneader) at 393 K. The blocks are cut to a diameter of about 25 mm, air-cooled on a conveyor belt. After the waste plastics have been pre-treated, they are mixed with coal, charged in coke ovens, and carbonized. Waste plastics are carbonized at a high temperature and then decomposed into coke, tar, light oil, and gas. These materials are used as chemical raw materials. Coke is used to reduce the iron ore in a blast furnace, the tar and light oil are used as raw materials to make plastics, etc., and COG is used in power plants as an energy source.
Charging waste plastics in a coke oven may bring about some problems: one is the effect on coke quality, and another is the corrosion effect on coke ovens by chlorine in the waste plastics. Kato et al. (2002a) clarified that excess charging of waste plastics may make the coke brittle ; however, when waste plastics equivalent to 1 mass% of coal are added to coal, the strength of the coke is the same as when no waste plastics are added (Kato et al. 2002b). It was also clarified that the Cl of waste plastics has no harmful influence on coke ovens because most of the Cl in waste plastics reacts with ammmoniacal liquor at the top of the coke oven and only 1% of Cl is transferred into COG (Kato et al. 2002a). Actually, waste plastics equivalent to about 1 mass% of coal are added to the coke oven in Japanese steel industries.
4.1.2 Method to estimate the changes of energy and material balance by coke oven chemical feedstock recycling
As mentioned above, the following two changes must be considered to estimate the reduction potential of CO2 emissions when waste plastics are charged in a coke oven: (1) additional energy requirements for the pre-treating process of waste plastics, (2) changes of the energy and material balance in the coke oven. Methods to estimate these changes are shown as follows.
Pre-treating process
In this paper, it was assumed that PVC, water, ash and metals contained in waste plastics were removed in the pre-treating process. The annual average data about the energy consumption in the pre-treating processes for coke oven chemical feedstock recycling were obtained from the questionnaire survey collected from steel works. The energy consumption in the pre-treating process might vary depending on the composition of the waste plastics. However, since detailed data were not available, the average data were used regardless of the composition of waste plastics.
Changes of energy and material balance in a coke oven
In order to estimate the changes of energy and material balance in coke ovens when waste plastics are charged into them, it is necessary to know the product yield of the waste plastics after carbonizing in the coke oven, that is, how much coke, oil, and gases are derived from waste plastics in coke ovens. Kato et al. conducted carbonization tests for municipal waste plastics from containers and packages using a commercial coke oven. The results showed that municipal waste plastics were converted into approximately 20% coke, 40% tar and light oil and 40% gas (Kato et al. 2002b).
However, these product yields of waste plastics must change depending on the composition of the waste plastics, so it is necessary to know the product yields of each plastic resin in a coke oven. The product yields of PE, PS, PET, PVC were obtained in previous studies (Kato et al. 2002b). The product yields of other plastic resin (PP) were not available in previous studies, so they were calculated as follows:
Studies on the model to estimate the coke product yield of coal in a coke oven (Nishioka et al. 1984) and the mechanism of coal carbonization (Nishioka and Yoshida 1984) showed that the coke product yield depends on the volatile matter content of coal. In the steel works in Japan, the volatile matter content is used as a parameter of the equation to calculate the coke product yield of coal (The Iron and Steel Institute of Japan 2006). It was also clarified that the gas product yield (Sakamoto 1986) and the oil product yield (Nisioka 1990) depend on the carbon content of coal, and the thermal decomposition mechanism of plastics is the same as that of coal (Koo et al. 1991).
Therefore, in this study, it was assumed that the product yields of plastics depend on the volatile matter content and carbon content, and the equations to calculate the coke product yield, gas product yield, and oil product yields of plastic resins were derived from least-squares approximations using the product yields of PE, PS, PET and PVC. The results are shown in Figs. 2, 3 and 4.
As is shown in Fig. 2, the coke product yield can be fairly linearly dependent on volatile matter. On the other hand, there are some deviations from the straight line obtained by the least-squares approximations in Figs. 3 and 4. So, it should be noted that further investigation and data collections are recommended to obtain the precise gas product and oil product yields for each resin. In this paper, we also conducted the validation of using these results (see the results in Section 5.1.1).
Next, the method to calculate the calorific value of gas derived from plastics is explained. In this study, the calorific value of the gas was calculated based on the energy balance in a coke oven. The inputs and outputs in the coke oven are shown in Fig. 5, and they were obtained from the literature (METI 2001). The energy inputs and outputs are 45,500 and 41,300 MJ, respectively, so the energy loss was calculated as 9.2%. When plastics (for example, PE) are charged, the energy output (41,800 MJ) can be calculated based on the energy loss (9.2%) and the energy input (46,100 MJ). The calorific value of gases (9,090 MJ) can be calculated by subtracting the calorific value of coke (30,100 MJ) and oil (2,650 MJ) from the total output energy (41,800 MJ). The calorific values of each plastic resin were calculated in the same way.
4.2 Blast furnace feedstock recycling
4.2.1 Overview of blast furnace feedstock recycling
In the blast furnace process of a steel works, iron ore, coke and auxiliary materials are used as feedstock, and iron ore is melted to produce pig iron. The coke acts as a reductant by removing oxygen from the iron ore (iron oxide). As plastics are made from PETroleum and natural gas, the main constituents are carbon and hydrogen. Therefore, it is possible to use waste plastics as a replacement for the coke reductant in the blast furnace process.
Plastic waste collected from households is cleaned of non-combustible matter and other impurities such as metals, and is then pulverized and packed to reduce its volume. The waste plastic is then fed into the blast furnace through a blast furnace tuyère and is gasified in a raceway. Some of the gases are used as a reducing agent to remove the oxygen from iron oxide. The remaining gases are used as a fuel in the blast furnace stove, or for generating electricity in a power plant. It is thus an important and a challenging task to quantify the change of energy and material balances when waste plastics are fed into a blast furnace, in order to properly estimate the CO2 emission reduction potential by blast furnace feedstock recycling.
4.2.2 Method to estimate the changes of energy and material balance by blast furnace feedstock recycling
Charging waste plastics into a blast furnace brings about two changes of energy and material balance in the blast furnace; one is the decrease of the amount of coke, because waste plastics substitute coke as a reductant, and the other is the change of the calorific value of gases (blast furnace gas (BFG)) because some of the gases from the waste plastics are used as reductants and changed into H2O or CO2.
To estimate the changes of inventory data for the blast furnace when waste plastics are used, it is necessary to determine the substitution rate of coke with waste plastics, i.e., how much coke is substituted by waste plastics.
A previous study on blast furnace feedstock recycling showed that the substitution rate was 1.1 (1.1 ton of coke is substituted by 1 ton of waste plastics; Research association for feedstock recycling of plastics 2005). However, the substitution rate will change depending on the composition of the waste plastics and the operating conditions of the blast furnace. Therefore, in this study, the Rist model (Rist and Meysson 1967) was applied to calculate the inputs and outputs of a blast furnace, and the substitution rate when waste plastics are fed into a blast furnace. According to a previous study (Asanuma and Ariyama 2004), it was assumed that the amount of waste plastics fed into the blast furnace was restricted to 5 mass% of the pig iron production.
PE, PP, PS, PET, and municipal waste plastics of seven cities were considered as plastic resins and waste plastics to be fed into the blast furnace. It was assumed that PVC, water, ash, and metals contained in the waste plastics were removed by a pre-treatment process (see “Pre-treating process” in Section 4.1.2). The inventory data for the pre-treatment process for blast furnace feedstock recycling were obtained from the literature (Vinyl Environmental Council 2001). The energy consumption in the pre-treating process was assumed to be constant regardless of the composition of the waste plastics. It is of interest to conduct sensitivity analyses in the further study, taking into account the change in energy consumption depending on the composition of waste plastics.
4.3 Matrix calculation to estimate the energy and material flow in steel works
As already mentioned, when waste plastics are charged in the coke oven and blast furnace, the energy balance in steel works changes. The amount and qualities (e.g., heating values and carbon contents) of COG, BFG and hydrocarbon oil generated from plastic wastes differ from those generated from coal and coke. This brings about a change in the energy flow (e.g., a change in total coal consumption, production of hydrocarbon oil, and electricity) in the steel works.
The matrix calculation method was applied to estimate the energy and material balance in steel works. The data regarding the inputs and outputs for the main processes in steel works were obtained from the literature (METI 2001), some of which are shown in Figs. 6 and 7. By using the matrix calculation method with the commodity of a net output of the system of 1,000 ton of crude steel, the amount of production in each process was obtained.
If surplus gas was generated from the coal and waste plastics, the gas was assumed to be used for the generation of electricity in a joint thermal power plant with an efficiency of 38% (Narita et al. 2001). The CO2 emission intensity of electricity and oil production were assumed to be 0.45 kg CO2/kWh (Matsuno and Betz 2000). Other CO2 emission intensities were obtained from literature (Ministry of the Environment 2003) and LCA software (JEMAI 2001).
4.4 Characteristics of waste plastics and data concerning their use in steel works
As plastics to be fed into the coke oven and blast furnace, PE, PP, PS, PET and municipal waste plastics were investigated. The properties of each plastic resin are shown in Table 1. Table 2 shows the components of municipal waste plastics in seven cities, which were obtained in the literature (JCPRA 2007; METI 2004). Because the details of “others” in Table 2 were not available, we simply allocated the amount of “others” to other plastic resins (PE, PP, PS, PET and PVC) based on the ratio of each resin. So, the components of municipal waste plastics in seven cities were assumed as they are shown in Table 3. Further investigation is recommended to investigate the detailed composition of plastic resins in municipal waste for obtaining a more precise estimation of CO2 reduction potentials.
5 Results and discussions
The results for coke oven chemical feedstock recycling and blast furnace feedstock recycling are shown in Sections 5.1 and 5.2, respectively. Then, the discussions are carried out in Section 5.3.
5.1 Results for coke oven chemical feedstock recycling
5.1.1 Conversion rates of waste plastics in the coke oven
As already mentioned, the product yields of PE, PS, PET, PVC were obtained in previous studies (Kato et al. 2002b). Those of the other plastic resin (PP) were calculated by the equations shown in Figs. 2, 3, and 4. The coke product yield of PP was calculated by using the volatile matter content (Williams and Williams 1999), and the gas product yield and oil product yield were calculated by using the carbon content. The results are shown in Table 4, which shows that the product yield of each plastic resin is different.
For validation of the results, the product yields of the municipal waste plastics whose composition were reported in a previous study (PE, 21.4%; PS, 24.8%; PP, 13.7%; PVC, 5.2%; PET, 15.5%; others, 19.0%; Kato et al. 2002b) were calculated with the results of the product yields of plastic resins shown in Table 5. The results were 15% for coke, 38% for oil, and 43% for gases, which agreed well with the experimental results (20% for coke, 40% for oil, and 40% for gases) reported in the literature (Kato et al. 2002b). This result indicates that product yields of municipal waste plastics (mixtures of plastic resins) can be estimated by summing up the product yield of each plastic resin multiplied by the composition of each resin in municipal waste plastics. Therefore, with the results obtained in this study, the reduction potential of CO2 emissions by coke oven chemical feedstock recycling of waste plastics can be calculated, in which the differences in compositions can be taken into account.
The inventory data of the coke oven process when plastic resins are charged in the coke oven were calculated based on the inputs and outputs in the coke oven (see Fig. 6) and the product yields of each plastic resin (see Table 4). The calorific values of gases derived from each plastic resin are shown in the row “Coke oven gas” in Table 5.
5.1.2 Changes of input and output of steel works when using plastics in the coke oven
The total of inputs and outputs in steel works when the plastic resins are charged in the coke oven were calculated by the matrix calculation method. The results are shown in Table 6. As already mentioned, the functional unit was set as the treatment of 1 ton of plastic resins or waste plastics, not on the amount of steel product. So, there are small differences in the amount of steel product in each plastic resin due to the difference in coke product yield of each plastic resin.
5.1.3 Reduction potential of CO2 emissions when using 1 ton of plastic resins and waste plastics in the coke oven
To estimate the reduction potential of CO2 emissions, the differences of inputs and outputs in steel works before and after waste plastics were charged in the coke oven were calculated first. Then, the change of CO2 emissions when waste plastics were charged in the coke oven was calculated by multiplying the differences with the CO2 emission intensities of each input and output.
Table 7 shows the differences in inputs and outputs and CO2 emissions in steel works when 1 ton of plastic resin is charged in a coke oven. The results of PE are shown as an example. The decrease in CO2 emissions is attributable to the reduction in CO2 emission due to partial substitution of coal with PE (431 kg CO2), electricity generation (1,879 kg CO2) and the production of hydrocarbon oil from PE (88 kg CO2). In contrast, the CO2 emission is increased due to the consumption of energy in the pre-treating process (135 kg CO2) and combustion of COG generated from PE (2,218 kg CO2). Therefore, the total reduction in CO2 emissions is 45 kg CO2/t-PE.
The reduction potential of other plastic resins (PP, PS, PET) were calculated in the same way. As shown in Fig. 8 (the positive values of the vertical scale indicate the reduction of CO2 is effective), CO2 emission reduction potentials vary significantly depending on the plastic resin. For example, PP and PS have a larger CO2 emission reduction potential, but PET even has a negative reduction potential. This is attributable to the fact that the coke product yield of PET is smaller, leading to a small substitution of coal by PET.
Based on the results of the CO2 emission reduction potential of each plastic resin, the reduction potentials of CO2 emission by coke oven chemical feedstock recycling of municipal waste plastics in seven cities were calculated. The results are shown in Fig. 9. In this study, it was assumed that the reduction potential was linearly related to the composition ratio of waste plastics (see Section 5.1). As shown in Fig. 9, the CO2 emission reduction potential is slightly different in each city. The difference is mostly attributable to the amount of impurities (ash, water) in the waste plastics (see the components of waste plastics in City 2, City 4, and City 6 in Table 4).
5.2 Results for blast furnace feedstock recycling
5.2.1 Changes of inventory data for the blast furnace when using plastic resins
Table 8 shows the inventory data for 50 kg of plastic resins, which are fed into the blast furnace. The amount of plastics fed into the blast furnace was set as 5 mass% of the pig iron production. Therefore, 1 ton of pig iron is produced when 50 kg of plastic resins is fed into the blast furnace. The amount of coke is decreased, due to the substitution of coke by the plastic resins, as shown in Table 8. The substitution effect is different depending on the type of plastic resins used. The substitution rate of plastic resins is shown to be directly correlated to the carbon content or calorific value, and the calorific value of gases (BFG) increases with all the plastic resins, because the calorific values of plastics are larger than that of coke (see the “Calorific value of BFG” in Table 8).
5.2.2 Changes of inputs and outputs of a steel works when using plastic resins in a blast furnace
The inputs and outputs in a steel works when six plastic resins were fed into a blast furnace were calculated by the matrix calculation method. The results are shown in Table 9. The amounts of coal required for producing the same amount of pig iron vary depending on the resins used, because the substitution rate of coke for each plastic resin is different, which results in the difference of total coal input.
5.2.3 CO2 emission reduction potential when using 1 ton of plastic resins and waste plastics in the blast furnace
Next, the differences of inputs and outputs in the steel work before and after the six plastic resins are fed into a blast furnace were calculated. The change of CO2 emissions from the blast furnace when using plastic resins as a coke was calculated by multiplying the differences with CO2 emission intensities of each input and output.
As an example, Table 10 shows the CO2 emissions when 1 ton of PE is used in a blast furnace. The decrease in CO2 emissions is attributable to the reduction of CO2 emissions, due to the partial substitution of coal by PE (4,213 kg CO2). On the other hand, the CO2 emission is increased due to the consumption of energy in the pre-treatment processing (146 kg CO2), the combustion of BFG from PE (3,140 kg CO2) and the increase of electricity generation (72 kg CO2). The total reduction in CO2 emissions is 964 kg CO2/t-PE.
The CO2 emission reduction potentials of other plastic resins (PP, PS, PET) were calculated in the same way, the results of which are shown in Fig. 10. A positive value on the vertical scale indicates that the reduction of CO2 is effective.
PE has the largest CO2 emission reduction potential when it is used in a blast furnace as a coke substitute. On the other hand, PET has negative reduction potentials, i.e., there is an increase of CO2 emissions. This is attributable to the relatively small calorific values and carbon and hydrogen content of PET, leading to a relatively small coke substitution effect by these plastic resins.
The CO2 emissions reduction potentials for municipal waste plastics fed into a blast furnace were then calculated using the results for each of the plastic resins. This calculation is based on the changes in inputs and outputs of a blast furnace obtained by the Rist model, which are linearly-related with the composition of the plastic resins. The positive value on the vertical scale indicates that the reduction of CO2 is effective. The CO2 emission reduction potential is slightly different for waste plastics from each city, ranging from 395–580 kg CO2/ton waste plastics, as shown in Fig. 11. The difference is mostly attributable to the amount of impurities (ash, water) in the waste plastics (e.g., the components of waste plastics in City 2, City 4, and City 6 (see Table 3)).
5.3 Discussions
Compared with coke oven chemical feedstock recycling, the blast furnace feedstock recycling of waste plastics has a larger CO2 emission reduction potential for plastics resins, except for PET and mixtures of different resins. However, the results calculated by the Rist model indicate the ideal potential for the reduction of CO2 for blast furnace feedstock recycling, so that it should be noted that the actual reduction effect when waste plastics are fed into a blast furnace changes depending on the operating conditions of the blast furnace.
CO2 emissions when PE, PP, PS and PET resins are incinerated can be calculated as 3.14, 3.14, 3.38, and 2.29 ton CO2/ton resin, respectively, from the carbon content of each plastic resin. So, if the municipal waste plastics in seven cities were simply incinerated (excluding PVC, ash, water), the CO2 emissions would be 1,950–2,600 kg CO2/ton. This increase in CO2 emissions by incineration of waste plastics can be avoided if the waste plastics are going into feedstock recycling in steel works. Therefore, it is clarified that feedstock recycling of waste plastic in steel works is effective for avoiding an increase in CO2 emissions by incinerating waste plastics, such as those from household mixtures of different resins.
6 Conclusions
In this study, a model was developed to clarify the energy flow in steel works. A matrix calculation method was used to estimate the energy and material balance in the steel work, that is, the change in the total coal consumption, the production of hydrocarbon oil, and the surplus energy (electricity), as well as the CO2 emissions, when plastic resins and municipal waste plastics are fed into a coke oven and blast furnace.
In the case of coke oven chemical feedstock recycling, the results show that the reduction effects are caused primarily by the substitution of coal with plastics, and hydrocarbon oil production and electricity generation from surplus gases. The reduction potential of CO2 emissions is dependent on the plastic resins and composition of waste plastics because of the difference of coke product yields and calorific values. For example, the reduction potential of PET is smaller than that of other plastic resins because the coke product yield of PET means a small substitution of coal by PET.
In the case of blast furnace feedstock recycling, the Rist model was applied to calculate the changes in inputs and outputs of a blast furnace when plastics are used as feedstock. The results show that the CO2 emissions reduction potential is also dependent on the plastic resins used due to differences in the carbon and hydrogen contents and calorific values. PE has the largest CO2 emissions reduction potential when it is used in a blast furnace as a coke substitute. On the other hand, PET has negative reduction potentials, i.e., there is an increase of CO2 emissions. This is attributable to their relatively small calorific values and carbon and hydrogen contents, which leads to a relatively small coke substitution effect when using these plastic resins.
In both cases, the results of waste plastics mixed with several plastic resins show that the reduction potential is slightly different, depending on the ratio of impurities. Compared with coke oven chemical feedstock recycling, blast furnace feedstock recycling of waste plastics has a larger CO2 emissions reduction potential for various waste plastics and mixtures of different resins. However, it should be noted that the results calculated by the Rist model indicate the ideal potentials to reduce CO2 emissions by blast furnace feedstock recycling of waste plastics; therefore, the actual reduction effect when waste plastics are fed into a blast furnace will vary depending on the operating conditions of the blast furnace.
It is clarified that feedstock recycling of waste plastic in steel works is effective for avoiding the increase in CO2 emissions by incinerating waste plastics, such as those from household mixtures of different resins.
References
Arena U, Mastellone ML, Perugini F (2003) Life cycle assessment of a plastic packaging recycling system. Int J Life Cycle Assess 8(2):92–98
Asanuma M, Ariyama T (2004) Recycling of waste plastics in blast furnace. J I Energy 83:252–256
Avila AF, Duarte MV (2003) A mechanical analysis on recycled PET/HDPE composites. Polymer Degrad Stabil 80(2):373–382
Bor YJ, Chien Y, Hsu E (2004) The market-incentive recycling system for waste packaging containers in Taiwan. Environ Sci Pol 7(6):509–523
Braunegg G, Bona R, Schellauf F, Wallner E (2004) Solid Waste Management and Plastic Recycling in Austria and Europe. Polymer–Plast Technol Eng 43(6):1755–1767
Finnveden G, Johansson J, Lind P, Moberg G (2005) Life cycle assessment of energy from solid waste—part 1: general methodology and results. J Cleaner Prod 13(3):213–229
Fortelny I, Michalkova D, Krulis Z (2004) An efficient method of material recycling of municipal plastic waste. Polymer Degrad Stabil 85(3):975–979
Garfort AA, Ali S, Hernandez-Martınez J, Akah A (2004) Feedstock recycling of polymer wastes. Curr Opin Solid State Mater Sc 8(6):419–425
Heijungs R (1994) A generic method for the identification of options for cleaner products. Ecol Econ 10(1):69–81
Heijungs R (2000) Chain Management by Life Cycle Assessment (CMLCA). CML, Leiden University, The Netherlands, http://www.leidenuniv.nl/cml/ssp/cmlca.html, accessed 20.3.2008
Holmgren K, Henning D (2004) Comparison between material and energy recovery of municipal waste from an energy perspective: a study of two Swedish municipalities. Resour Conserv Recycl 43(1):51–73
Hu X, Calo JM (2006) Plastic particle separation via liquid-fluidized bed classification. Am Inst Chem Eng 52(4):1333–1342
Inaba R, Hashimoto S, Moriguchi Y (2005) Life cycle assessment of recycling in the steel industry for plastics containers and packaging. J Japan Soc Waste Manage 16(6):467–480 in Japanese
ISO 14040 (2006) Environmental management—life cycle assessment—principles and framework. International Organization for Standardization, Geneva
JCPRA (The Japan Containers and Packaging Recycling Association) (2007) Investigations on environmental impacts of recycling methods for plastics containers and packaging (in Japanese)
JEMAI (Japan Environmental Management Association for Industry) (2001) LCA-software ‘JEMAI-LCA’
Kaminsky W, Predel M, Sadiki A (2004) Feedstock recycling of polymers by pyrolysis in a fluidised bed. Polymer Degrad Stabil 85(3):1045–1050
Kato K, Nomura S, Fukuda K, Uematsu H, Takamatsu N, Kondo H (2002a) Effect of waste plastics recycling technology using coke ovens on energy consumption. J Iron Steel Inst Jap 15(4):756
Kato K, Nomura S, Uematsu H (2002b) Development of waste plastics recycling process using coke oven. ISIJ Int 42(Suppl):S10–S13
Kato K, Nomura S, Uematsu H (2003) Waste plastics recycling process using coke ovens. J Mater Cycle Waste Manage 5(2):98–101
Kim D, Shin S, Sohn S, Choi J, Ban B (2002) Waste plastics as supplemental fuel in the blast furnace process: improving combustion efficiencies. J Hazard Mater 94(3):213–222
Koo J, Kim S, Seo Y (1991) Characterization of aromatic hydrocarbon formation from pyrolysis of polyethylene polystyrene mixtures. Resour Conserv Recycl 5(4):365–382
Matsuno Y, Betz M (2000) Development of life cycle inventories for electricity grid mixes in Japan. Int J Life Cycle Assess 5(5):295–305
METI (Research and Statistics Department, Economic and Industrial Policy Bureau, Ministry of Economy, Trade and Industry) (2001) 2001 Yearbook of Iron and Steel Statistics (in Japanese)
METI (Research and Statistics Department, Economic and Industrial Policy Bureau, Ministry of Economy, Trade and Industry) (2004) Report on recyclability of containers and packaging, plastic bale (in Japanese)
Ministry of the Environment (2003) Report on a method to calculate amount of emission of greenhouse effect gas (in Japanese)
Molgaard C (1995) Environmental impacts by disposal of plastic from municipal solid waste. Resour Conserv Recycl 15(1):51–63
Mutha NH, Patel M, Premnath V (2006) Plastics materials flow analysis for India. Resour Conserv Recycl 47(3):222–244
Narita N, Sagisaka M, Inaba A (2001) Reduction effects of CO2 emission from steel products by reduction agent injection into blast furnace. J Japan Inst Metals 65(7):589–595 (in Japanese)
Nishioka K, Yoshida S (1984) Investigation of bonding pattern of coal particles and factors for determination of coke properties during carbonization. Tetsu to hagane 70(3):351–357
Nishioka K, Yoshida S, Hariki M (1984) Development of the carbonization simulation model with consideration of coking mechanism. Tetsu to hagane 70(3):358–365
Nisioka K (1990) Sun fossil: Coal (in Japanese). Agne Gijutsu Center, Tokyo
Noda R, Komatsu M, Sumi E, Kasakura T (2001) Evaluation of material recycling for plastics: environmental aspects. J Mater Cycles Waste Manage 3(2):118–125
Okuwaki A (2004) Feedstock recycling of plastics in Japan. Polymer Degrad Stabil 85(3):981–988
Patel M, von Thienen N, Jochem E, Worrell E (2000) Recycling of plastics in Germany. Resour Conserv Recycl 29(1–2):65–90
Perugini F, Mastellone ML, Arena U (2005) A life cycle assessment of mechanical and feedstock recycling options for management of plastic packaging wastes. Environ Prog 24(2):137–154
Plastic Waste Management Institute (2005a) An Introduction to Plastic Recycling in Japan 2004, 4 Life Cycle Assessment, Environmental and resource impact assessment of recycling methods by LCA. http://www.pwmi.or.jp/ei/ei_pk.htm, accessed 16.10.2008
Rist A, Meysson N (1967) A dual graphic representation of blast-furnace mass and heat balances. J Metals 9(4):50–56
Ross S, Evans D (2003) The environmental effect of reusing and recycling a plastic-based packaging system. J Cleaner Prod 11(5):561–571
Sakamoto K (1986) Development of carbonization in coke ovens: estimation of gas evolution patterns. Tetsu to Hagane 72(12):S840
Shent H, Pugh RJ, Forssberg E (1999) A review of plastics waste recycling and the flotation of plastics. Resour Conserv Recycl 25(2):85–109
Song H, Moon K, Hyun JC (1999) A life-cycle assessment (LCA) study on the various recycle routes of PET bottles. Korean Chem Eng 16(2):202–207
Subramanian PM (2000) Plastics recycling and waste management in the US. Resour Conserv Recycl 28(3–4):253–263
Suh S, Huppes G (2005) Methods for life cycle inventory of a product. J Cleaner Prod 13(7):687–697
The Iron and Steel Institute of Japan (2006) Iron and steel handbook
Vinyl Environmental Council (2001) Report on LCI for recycling and waste management of PVC products
Williams PT, Williams EA (1999) Interaction of plastics in mixed-plastics Pyrolysis. Energy Fuels 13(1):188–196
Xiao R, Jin B, Zhou H, Zhong Z, Zhang M (2007) Air gasification of polypropylene plastic waste in fluidized bed gasifier. Energ Convers Manage 48(3):778–786
Yamakita R, Miura K, Ishino Y, Ohiwa N (2005) An investigation on thermal recycling of recycled plastics resin. JSME Int J 48(1):83–91
Zhang G-H, Zhua J-F, Okuwaki A (2007) Prospect and current status of recycling waste plastics and technology for converting them into oil in China. Resour Conserv Recycl 50(3):231–239
Ziebik A, Stanek W (2001) Forecasting of the energy effects of injecting waste plastics into the blast furnace in comparison with other auxiliary fuels. Energy 26(12):1159–1173
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sekine, Y., Fukuda, K., Kato, K. et al. CO2 reduction potentials by utilizing waste plastics in steel works. Int J Life Cycle Assess 14, 122–136 (2009). https://doi.org/10.1007/s11367-008-0055-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-008-0055-3