Abstract
Sensing temperature is vitally important to adapt our body to environmental changes. Local warm detection is required to initiate regulation of cutaneous blood flow, which is part of the peripheral thermoregulatory mechanisms, and thus avoid damage to surrounding tissues. The mechanisms mediating cutaneous vasodilation during local heat stress are impaired with aging. However, the impact of aging on the ability of the skin to detect subtle thermal changes is unknown. Among heat-activated cation channels, transient receptor potential vanilloid 3 (TRPV3) is a thermo-sensor predominantly expressed on keratinocytes and involved in local vascular thermoregulatory mechanisms of the skin in young mice. In the present study, using a murine in vivo model of local heat exposure of the skin, we showed that heat-induced vasodilation was reduced in old mice associated with reduced expression of TRPV3 channels. We also found a decrease in expression and activity of TRPV3 channel, as well as reduced TRPV3-dependent adenosine tri-phosphate release in human primary keratinocytes from old donors. This study shows that aging alters the epidermal TRPV3 channels, which might delay the detection of changes in skin temperature, thereby limiting the mechanisms triggered for local vascular thermoregulation in the old skin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The skin is the largest organ of our body. This envelop is involved in various functions, including temperature detection required to initiate regulation of cutaneous blood flow [1, 2]. During environmental thermal stress, changes in skin temperature occur prior to changes in core temperature, and allow a rapid and appropriate response to heat (vasodilation) or cold (vasoconstriction) [3]. Adaptation of the skin blood flow thus plays an essential role in thermoregulation, and therefore prevents damages to nearby tissues. The physiological regulation of the skin blood flow relies not only on the activation of the autonomic and sensory nervous system through the release of vasoactive neuropeptides [4] but also on the release of vasoactive factors by the endothelial cells [5, 6]. The mechanisms underpinning the cutaneous vasodilation during local heat stress have been extensively studied [3, 6,7,8], and include an initial increase of skin blood flow (axonal reflex), followed by a plateau predominantly dependent of the nitric oxide (NO) pathway. The effects of aging are also well characterized with a reduction in the ability to raise skin blood flow during local heat stress, due to a decrease of sympathetic transduction and endothelial dysfunction [9,10,11,12,13,14,15], including decrease of endothelium NO synthase (eNOS) activity and NO bioavailability. Although sensing environmental temperature changes is vitally important for the body to properly adapt its vascular thermoregulatory responses to local heat stress, temperature detection process remains poorly studied. It is therefore crucial to further improve our understanding of the impact of age progression on the physiological mechanisms of thermoregulation and in particular on the trigger mechanisms (local detection of moderate skin temperature changes).
Historically, sensory nerve endings passing through the epidermis were thought to be the exclusive transducers for temperature detection and, consequently, thermoregulation [3, 16, 17]. Since the discovery of thermo-sensors of transient potential vanilloid (TRPV) channels family [18,19,20], substantial advances have been made on thermal sensors biology. Interestingly, keratinocytes, the major cell population of the epidermis, also expressed several thermo-sensors such as TRPV1, TRPV3, and TRPV4 [21]. Our knowledge on the relationship between the thermo-TRP channels and the heat-evoked cutaneous vasodilation response is just emerging and has yet mainly focused on TRPV1 [22, 23]. Being able to accurately detect subtle 1 °C temperature deviations from our thermoneutral skin temperature of around 33 °C, TRPV3 and TRPV4 channels localized on the epidermal keratinocytes [19, 24, 25] are likely to be first activated by local warming of the skin, ultimately triggering cutaneous vasodilation. However, it has recently been shown that TRPV4 channels did not contribute to the regulation of cutaneous vasodilation in human skin during local thermal hyperemia [26]. In contrast, using a murine model, we previously demonstrated that epidermal TRPV3 acts as a crucial cutaneous thermo-sensor for the local thermoregulatory control of skin blood flow. Indeed, the local heat-evoked vasodilation was impaired in Trpv3-KO mice associated with local heat loss disruption, but not in Trpv1-KO mice [27]. This phenomenon requires mainly peptidergic nerve endings through calcitonin gene-related peptide (CGRP) release and NO produced by endothelial cells. Moreover, TRPV3 can answer to a heat stimulus by releasing various mediators such as adenosine tri-phosphate (ATP), NO, prostaglandin E2 (PGE2), and nerve growth factor (NGF) [28,29,30,31]. Deprivation of cutaneous heat-induced vasodilation in Trpv3-KO mice was associated with a faster increase of internal temperature in passive whole-body heating, exhibiting an attenuated physiological ability to dissipate heat due to a reduced cutaneous vasodilation [27]. Trpv3-KO mice also present a profound deficit in sensing warm external temperature and have a preference for cooler temperature (25 °C) [32]. Such deficit in response to thermal change was also observed in elderly people [12, 33, 34]. We thus hypothesized that a decrease of TRPV3 expression or activity in keratinocytes could be involved in the default of warm detection and thus cutaneous vascular thermoregulation during aging.
In the present study, we firstly compared the vascular response of the skin in young and old control mice, as well as young Trpv3-KO mice upon a local heat exposure. To explore the impact of aging on epidermal TRPV3 channels, we analyzed the expression of TRPV3 channel in biopsies from young and old humans, as well as in mouse skin. We also measured TRPV3 activity from young and old human primary keratinocytes using calcium imaging. To identify the impact of aging on the soluble mediators released by epidermal keratinocytes following TRPV3 activation, we evaluated cytokines and other factors such as ATP, a cell communication mediator, following TRPV3 activation in 2D culture of young and old human primary keratinocytes. Our data showed a default of heat-induced vasodilation in old mice, also observed in Trpv3-KO mice. Moreover, our results demonstrated a strong decline of both expression and activity of TRPV3 in old human primary keratinocytes, which could contribute to the impaired initial thermal detection (>33 °C) and consequently thermoregulation in the elderly population.
Materials and methods
Animals
Young (2 months) and old (22 months) C57BL6 mice and young (2 months) Trpv3-KO mice (raised on a C57BL6 background) were used in all experiments. Mice were housed with ad libitum access to food and water, in a temperature-controlled room (about 23 °C) with a 12-h light/dark cycle. All experiments were conducted in accordance with European Union recommendations for animal experimentation and approved by scientific and ethics committees (agreement, #33946 Rhone-Alpes ethics). Special effort was made to minimize the number as well as the stress and suffering of mice used in this study. As they age, mice can lose weight and lower their internal temperature. These two parameters were monitored at least once a week for aged mice (>15 months old).
Assessment of skin blood flow
For the skin blood flow experiments, mice were anesthetized with thiopental (i.p. injection at 75 mg·kg−1 of body weight). Anesthesia was monitored to ensure an appropriate anesthetic depth to avoid the confounding effects of movement artifacts on laser Doppler flowmetry. Anesthetized mice were placed in an incubator maintained at 30 °C in order to better control cutaneous temperature throughout the experiment (35.5 ± 0.1 °C) and avoid anesthesia-mediated hypothermia. Cutaneous blood flow and cutaneous temperature were continuously recorded by a data acquisition system (Biopac) and subsequently analyzed (Acknowledge Biopac). In addition, systolic arterial blood pressure was monitored using a tail cuff system (IITC INC Life Science Inc.) before and after the experiments. At the end of each experiment, animals were killed and whole (epidermis and dermis) skin samples were taken in the plantar hindpaw and hairless back.
Heat-evoked response in the plantar hindpaw by laser Doppler flowmeter
Cutaneous blood flow was measured in the plantar hindpaw area using a laser Doppler probe (457, Perimed, Sweden) in anesthetized mice. The heating model consisted of recording 1-min baseline blood flow to ensure that the hemodynamic vascular responses had stabilized following anesthesia, as previously described [27]. Briefly, the hindpaw was exposed to local heating at a rate of 1 °C/min for 10 min (from 33 to 40 °C) using Temp Unit (PF 5020, Perimed, Sweden). The response to local heating consisted of an increase in cutaneous blood flow (vasodilation). Results are expressed as (1) arbitrary flux units during the entire recording period or (2) arbitrary flux units (× 103 flux units) measured as area under the recorded flux versus time for the entire recording period for 10-min temperature exposure or (3) a measure of maximum % increase in blood flow from preheating baseline to the end of the heat exposure (maximum vasodilation observed at 40 °C).
Endothelium-independent and -dependent responses on the dorsal skin by laser Doppler flowmeter coupled to iontophoresis
Skin blood flow was measured on a hairless area of the back of mice anesthetized with thiopental (75 mg·kg−1 body weight) using a laser Doppler probe (481-1, Perimed Sweden) for transcutaneous iontophoresis (probe area ≈ 1.08 cm2). Hair was removed using depilatory lotion 2 days prior to iontophoresis. Cutaneous blood flow was recorded during 1-min baseline period prior to iontophoresis. The endothelium-independent response was assessed by using iontophoretic delivery of sodium nitroprusside (SNP) (2%) with a cathodal current application of 100 mA for 20 s, while the endothelium-dependent responses were assessed by using iontophoretic delivery of acetylcholine (ACh) (2%) with an anodal current application of 100 mA for 20 s. The iontophoresis technique was chosen to assess in vivo skin microvascular function to avoid any systemic effects. Blood flow data were expressed as a measure of maximum % increase in blood flow from baseline over the entire recording period (20 min) following iontophoretic delivery.
Assessment of thermal function of skin sensory nerve endings
Tail flick
The conscious mouse was maintained in a restrainer and a cloth was used to cover their head. The tail (about 1.5 cm from the tip) was placed under a radiant heat source produced by a halogen lamp of a device (2TC series 8 Model, ITT Inc. Life Science, CA, USA) previously calibrated for 30 s to deliver 25 W of heat. The heating rate was 1.3 °C/s, and the delay in tail removal was measured. A cutoff time of 10 s was imposed to prevent tissue damage. Five measurements of the tail withdrawal latency were taken and averaged for each mouse to determine the nociceptive thermal threshold for the animal.
Hot plate
The conscious mouse was placed on a Bioseb hot plate (Bioseb, USA, #BIO-CHP) calibrate at 55 °C. The delay in mouse behavioral change (jump or paw licking) was manually measured using a chronometer. A cutoff time of 10 s was imposed to prevent tissue damage. Three measurements of latency were taken and averaged for each mouse to determine the nociceptive thermal threshold for the animal.
In situ hybridization
Human paraffin embedded tissue microarray (TMA: #SK244A and #SKN1001) was purchased from US Biomax (Derwood, MD, USA). Details on skin sample are presented on Supplementary Table 1. In situ hybridization of TRPV3 was performed using RNAscope® Multiplex Fluorescent Reagent Kit v2 (bio-techne, France, 323100) according to the manufacturer’s instructions. TRPV3 probes were labeled with Opal 570 (1:1500) supplied with the kit. At the end of the amplification and signal development, tissue sections were washed three times with PBS and blocked with PBS containing 5% goat serum, 2% BSA, and 0.1% Tween20 for at least 1 h at RT. After washing steps, primary antibodies targeting KRT14 (Abcam, ab181595, 1:400) were incubated overnight at 4 °C. Sections were washed and incubated with secondary antibodies (Thermo Fisher Scientific, #A-21245, 1:1000) 45 min at RT and nuclear staining was performed using ProLongTM Glass Antifade Mountant with NucBlueTM (Thermo Fisher Scientific, #P36981). Negative controls were performed using probe diluent supplied with RNAscope kit. Images were visualized using High Content Screening Yokogawa CQ1 microscope (Yokogawa, Tokyo, Japan), digitalized using sCMOS camera (Olympus, Hamburg, Germany), and analyzed using QuPath software (version 0.2.3).
Cell culture
Human primary keratinocytes (HPK) were isolated in-house from skin biopsies as previously described [6], or purchased from Lonza (Basel, Switzerland, #00192627). Skin biopsies were obtained from the DermoBioTec tissue bank at Lyon (Tissue Transfer Agreement n°214854) with the informed consent of adult donors (non-pathological tissues from abdomen or breast), in accordance with the ethical guidelines (French Bioethics law of 2021). Donor specifications are indicated in Supplementary Table 2. HPK were cultured in KBM Gold medium (Lonza, #00192060) at 37 °C and 5% CO2. The culture medium was renewed three times a week and cells were maintained to no more than passage 4.
RNA extraction and real-time PCR
For tissue RNA extraction, plantar hindpaw samples were immersed in RNA-later for 24 h at 4 °C and further stored at −80 °C. Total RNA was isolated using RNeasy® Fibrous Tissue Mini Kit (Qiagen, France, #74704), according to the manufacturer’s instructions. For cell RNA extraction, 6 h after treatment, cells were washed three times with PBS and RNA were extracted using NucleoSpin® RNA Plus (Macherey-Nagel, 740984.250). Quantity and purity of RNA were evaluated using NanoDrop ™ 2000. cDNA synthesis was performed from at least 200 ng of RNA using PrimeScriptTM RT reagent kit (Takara Bio Europe, #RR037A) and analyzed in real-time qPCR using SYBR® Premix ExTaqII (Takara Bio Europe, #RR820A) on an AriaMx Realtime PCR system (Agilent Genomics, Santa Clara, CA, USA). Results were normalized to TBP and RPL13A (human) or Rpl13a and Eif4h (mouse) housekeeping genes expression levels, using the 2−ΔΔCt quantification method. RT-qPCR program and primers are listed in Supplementary Table 3.
Chemical compounds
The TRPV3 agonists carvacrol (Sigma-Aldrich, France, #W224511) and 2-aminoethoxydiphenyl borate (Sigma-Aldrich, France, #100065-100MG), and TRPV3 antagonist isochlorogenic acid B (MedChemExpress, Sweden, #HY-N0057) were sequentially diluted in DMSO (stock solutions) and further diluted in KGM-gold just before use. All chemical treatments were performed when cells reached 70% confluence, with, depending on the conditions, 50 μM 2-APB, 300 μM carvacrol, 100 μM IAB, or an equivalent volume of DMSO diluted in medium.
Ca2+ measurement
Cells were cultivated in μ-Dish 35 mm High (Ibidi GmbH, #81156). After 45 min incubation at 37 °C with 2 μM of fura-2-acetoxymethyl ester (Fura2-AM) (Thermo Fisher Scientific, #F1221) in culture medium, cells were washed twice for 5 min with a Hank’s Balanced Salt Solution (HBSS 1×) with 1.26 mM Ca2+ and 0.49 mM Mg2+ (Gibco, UK, #14025-050), and placed at RT on a DMI6000 inverted wide-field microscope (Leica microsystem). Images were acquired with an Orca-Flash 4.0 Scientific CMOS camera (Hamamatsu) using a 40× oil-immersion objective. Using a Lambda DG-4+ filter (Sutter instruments), Fura-2 AM was excited at 340 and 380 nm and the fluorescent signal emitted measured at 510 nm. Images (1024 × 1024 pixels) were taken with a 3 s interval. Fluorescence ratios (F340 nm/F380 nm) were analyzed with MetaFluor 6.3 (Universal Imaging) after removing background fluorescence [35]. After 1 min of baseline, cells were treated with or without IAB (100 μM) for 10 min and then with Carvacrol (300 μM) for 5 min, or 2-APB (50 μM) 2 min and Carvacrol (300 μM) 5 min. Results are expressed as ratio 340/380 for each cell (~30/field) of each donor.
Luminex multiplex assay
Procartaplex 17 Plex (Life Technologies, France, #PPX-17) were designed in order to identify cytokines in cell supernatant 16 h after treatment: EGF, FGF-2, FGF-21, INFα, IFNβ, INFγ, IL-1α, IL-1β, IL-6, IL-8, IL-17A, IL-23, IL-29, NGF-β, TGF-α, VEGF-A, and VEGF-D. Measurements of cytokines were done according to the manufacturer’s instruction with MAGPIX®. Cell culture supernatants were collected 16 h after treatment.
Extracellular ATP assay
RealTime-Glo™ Extracellular ATP Assay (Promega, France, #GA5010) was used to measure ATP released after 10 min following TRPV3 activation. Doxorubicin (Sigma-Aldrich #D1515) 50 nM was used as positive control.
Statistics
Data are expressed as mean ± SD or mean ± SEM or median (IQR). According to the normality (Shapiro-Wilk test), statistical significance was calculated by Student’s t-test or Mann-Whitney, one-way analysis of variance (ANOVA1) or Kruskal-Wallis, two-way analysis of variance (ANOVA2), and Pearson correlation using Prism software (version 7.0, GraphPad Software, San Diego, CA, USA). Mean differences were considered statistically significant when p < 0.05. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
Cutaneous heat-induced vasodilation is reduced in a similar fashion in young Trpv3-KO and old mice
Using the murine in vivo model as previously [27], we measured cutaneous blood flow on hindpaw of young wild-type (WT; 2 months), old wild-type (22 months), and young Trpv3-KO (2 months) mice in response to a local heat exposure (from 33 to 40°C) (Fig. 1A–B). Young WT mice displayed a blood flow increase immediately after the onset of local heating. With exception of Trpv3-KO female mice (max temperature achieved: 36 °C), the cutaneous blood flow of old and Trpv3-KO mice did not increase significantly from baseline compared to young WT mice (34.2°C for both male and female) (Fig. 1A–B). As expected, the heat-induced vasodilation was significantly lower in old mice compared to young WT mice (Fig. 1C–D). In contrast, heat-induced vascular response was not different between young Trpv3-KO mice and old WT mice (Fig. 1C–D). The cutaneous vascular function (on back skin) assessed with acetylcholine and sodium nitroprusside, the systolic arterial blood pressure and skin temperature at baseline were not different between all groups, strengthening that neither aging nor TRPV3 inactivation impaired endothelium or vascular smooth muscle integrity (Supplementary Fig. 1A–D). Thus, the delay of local heat-induced vasodilation in old WT mice was not due to a vascular defect. Using sensory tests (tail flick and hot plate), we showed that the nociceptive responses were well-preserved in old WT and Trpv3-KO mice compared to young WT mice (Supplementary Fig. 1E–F). These data suggest that the default of local heat-induced vasodilation in old mice was due to a failure of the initial heat detection (likely via warm-sensitive TRPV3) and/or consequent triggering neurovascular mechanisms, rather than peripheral sensory nerve impairments.
Cutaneous heat-induced vasodilation is reduced in a similar fashion in young Trpv3-KO and old WT mice. Relative paw blood flow in response to local heating in anesthetized male (A) or female (B) of 2 months (n = 12 ♂ and n = 15♀), 22 months (n = 11 ♂ and n = 13♀), and 2 months Trpv3-KO (n = 12 ♂ and n = 11♀). Results are expressed as mean ± SEM. C AUC and D maximal skin blood flow from baseline of young wild-type, old wild-type, and young Trpv3-KO mice (median (IQR)). A Kruskal-Wallis was used to compare mean of each group. Two-way ANOVA (mixed-model) with Sidak’s multiple comparison was used to compare blood flow from the baseline
Altogether, these results highly suggested that impairment of heat-induced vasodilation in old WT mice is related to a decreased ability to sense heat (≥33 °C), likely due to an altered TRPV3 function.
TRPV3 expression is decreased with aging in both mouse and human epidermis
As Trpv3-KO and old WT mice displayed a similar deficit in response to local heat, we explored the epidermal expression of Trpv3 in young and old WT mice. Using RT-qPCR, we found a ~ 47% decrease of Trpv3 transcripts in old mice as compared to young animals in hindpaw skin, the major skin area involved in thermoregulation with the tail (Fig. 2A). In contrast, this decrease in Trpv3 expression was not observed in the back skin, which is not involved as a thermoregulatory area (Supplementary Fig. 2A). It is noteworthy to note that the expression of Trpv3 is significantly higher in hindpaw skin than in back skin regardless of both age and sex (Supplementary Fig. 2A). These results demonstrate a specific decrease of epidermal Trpv3 expression with aging in a thermoregulatory area, which could contribute to the impaired vasodilation on exposure to local heat in old mice.
TRPV3 expression is decreased with aging in both mouse and human skin. A Relative expression of Trpv3 normalized with mean Rpl13a and Eif4h in paw skin from young (5 months) and old (22–24 months) mice. In situ hybridization of TRPV3 (red dots) in a B 29-year-old man’s back humerus skin, C a 21-year-old woman’s breast skin, D a 78-year-old man’s back humerus skin, and E a 71-year-old women’s scalp skin, combined with KRT14 immunostaining (yellow) (scale bar 20 μm). F Percentage of TRPV3-positive cells and G number of TRPV3 transcripts per keratinocyte according to the age in nine women (circles) and seven men (squares) epidermis. H TRPV3 transcripts in basal cells (KRT14+) or differentiated cells (KRT14−) in young (19–52 years old) and old (66–78 years old) human skin. Results are expressed as median (IQR). A Mann-Whitney test was used to compare Trpv3 in young (n = 6 ♂ and n = 4 ♀) and old (n = 4 ♂ and n = 7 ♀) mouse hindpaws. Pearson correlation was performed to evaluate TRPV3 expression with aging (n = 16). A two-way ANOVA with Tukey’s multiple comparison test was used to compare TRPV3 transcripts in basal versus differentiated cells of young (n = 8) and old (n = 8) human epidermis
To further confirm the negative effect of aging on epidermal TRPV3, we looked at TRPV3 expression in human skin sections from both genders of several ages (Fig. 2B–E). As TRPV3 localization is still controversial in the literature [36,37,38], we used fluorescent RNA in situ hybridization of TRPV3 in combination with cytokeratin-14 (KRT14) immunostaining (Fig. 2B–E). We showed that the percentage of TRPV3-positive cells (Fig. 2F) and the number of TRPV3 transcripts per keratinocyte (Fig. 2G) gradually decrease with aging in men and women (see Supplementary Table 1 for more details). Accordingly, a strong negative correlation was determined between TRPV3-positive cells (r-0.7603) or estimated TRPV3 transcripts per cell (r-0.7799) and the age. In addition, the KRT14 immunostaining allowed to discriminate basal cells (KRT14+) and suprabasal cells (KRT14-) (Fig. 2H). Remarkably, when we compared the expression of TRPV3 in old human biopsies (mean age 71.25 years) with young ones (mean age 33.5 years), we only observed a decrease in basal cells, but not in differentiated keratinocytes. These results strongly suggest that aging negatively affects the expression of TRPV3 in the proliferating basal layer of human epidermis.
TRPV3 activity is reduced with aging in human primary keratinocytes
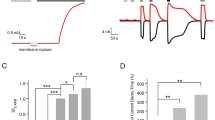
Since we already demonstrated that primary skin cells retain some features of aging when they are cultured in vitro [39,40,41], we took advantage of this 2D model to measure the TRPV3 channel intrinsic activity. To do so, we used a cytosolic Ca2+ ratiometric dye (Fura-2AM) to follow intracellular Ca2+ flux at the single cell level in human primary keratinocytes from young (mean age 32.6 years) and old (mean age 81.8 years) individuals (Fig. 3). We first confirmed the decrease of TRPV3 expression in “old” keratinocytes (Supplementary Fig. 3A). We next validated the specific activation of TRPV3 channel by its agonist carvacrol [42, 43], which effectively induced the intracellular Ca2+ entry in keratinocytes from young skin (Supplementary Fig. 3B–C). This effect was totally abolished by the co-treatment with the TRPV3 antagonist isochlorogenic acid B (IAB) [44], which confirm the specificity of TRPV3 activation (Supplementary Fig. 3B–C). In addition, TRPV3 can be sensitized and increases its response upon repetitive stimuli [45, 46]. Indeed, the sensitization with 2-APB followed by stimulation with carvacrol significantly increased the Ca2+ flux in keratinocytes compared to carvacrol only (Supplementary Fig. 3D). Therefore, we used this dual treatment (2-APB sensitization + carvacrol activation) to get the maximal stimulation of TRPV3. With this treatment, we observed an increase of intracellular Ca2+ flux in both young and old primary keratinocytes (Fig. 3). However, the maximal response of TRPV3 channel was ~31% lower in old keratinocytes (Fig. 3B). In addition, the TRPV3 antagonist IAB strongly reduced the intracellular Ca2+ flux in young keratinocytes but completely abolished the response in old ones (Fig. 3A–B). These results indicate that the intrinsic activity of the TRPV3 channels is less effective in old keratinocytes, likely due to a decrease of its expression with aging.
Aging reduces TRPV3 activity in human primary keratinocytes. A Changes in the intracellular Ca2+ concentration (mean curve), expressed as the fluorescence intensity ratio at 340 and 380 nm (F340/F380), were monitored in response to 50 μM 2-APB (2 min) and then 300 μM carvacrol (5 min) with (dotted line) or without (solid line) 100 μM IAB, in young (mean age 32.6 years) and old (mean age 81.8 years) human primary keratinocytes. B Measurement of the maximal Ca2+ response of keratinocytes from baseline (median (IQR)). A two-way ANOVA Tukey’s multiple comparison test was performed to determine the effect of aging and TRPV3 antagonist. (n = 4/group with at least 40 cells recorded in each biological replicate)
TRPV3-dependent ATP release is altered with aging in human primary keratinocytes
After exploring the expression and the activity of the TRPV3 channel, we aimed at characterizing the effect of aging on the release of TRPV3-dependent mediators. We first decided to focus on a well-known downstream pathway of TRPV3, the EGFR/TGF-α/NFκB pathway [36, 47, 48]. Using Luminex bead-based multiplex immunoassay, we found that TRPV3 stimulation (2-APB + carvacrol) induced an equivalent release of TGF-α in young and old keratinocytes (Fig. 4A). This TRPV3-dependent release of TGF-α was blocked by the TRPV3 antagonist IAB (Fig. 4A). The same tendency was observed by RT-qPCR for the following cytokines IL-1β, IL-6, IL-8, and NGF-β 6h (Fig. 4B–E), suggesting that the TRPV3/EGFR/TGF-α/NFκB pathway was not affected with aging in human primary keratinocytes.
Aging reduces TRPV3-dependent release of ATP but not cytokines in human primary keratinocytes. A Relative TGF-α production following 16 h TRPV3 stimulation using agonists (50 μM 2-APB and 300 μM carvacrol) with or without the TRPV3 antagonist 100 μM IAB in young (n = 4, mean of age 34.3 years) and old (n = 5, mean of age 81.1 years) keratinocytes. Relative expression of IL-1β (B), IL-6 (C), IL-8 (D), and NGF-β (E) normalized to the mean of TBP and RPL13A 6 h following TRPV3 stimulation with or without TRPV3 blockade in young (n = 5, mean of age 32.6 years) and old (n = 5, mean of age 81.1 years) keratinocytes. A–E Results are expressed as a ratio treated/untreated. F Extracellular ATP release following TRPV3 stimulation with or without the TRPV3 antagonist IAB in young (n = 5, mean age 32.6 years) and old (n = 5, mean age 81.1 years) keratinocytes. Results are expressed as a ratio treated/untreated and then as percentage of variation from positive control (doxorubicin 50 mM) (n = 5). A two-way ANOVA (mixed-model) with Sidak’s multiple comparison was performed to determine effect of aging and TRPV3 antagonist groups. Results are expressed as mean ± SEM
ATP is a key messenger involved in cell communication and various biological processes [49,50,51,52], including in temperature transmission from keratinocytes to sensory nerve endings [28]. In order to evaluate the impact of aging on the ability of keratinocytes to release ATP in response to TRPV3 stimulation, we quantified extracellular ATP released by young and old keratinocytes using luminescence ATP assay. We found that ATP release after TRPV3 activation was strongly reduced in old compared to young keratinocytes (Fig. 4F). Moreover, IAB significantly decreased ATP release only in young cells. These results indicate that aging impairs TRPV3-induced ATP release in human primary keratinocytes.
Discussion
In this study, we provide the first evidence that aging alters epidermal TRPV3, which is necessary for temperature detection by the keratinocytes to trigger heat-evoked cutaneous vasodilation. The major findings are as follows: (i) aging is associated to a decrease of TRPV3 expression in mouse and human skin regardless of sex, (ii) the cutaneous vascular response upon local heat (from 33 to 40 °C) is almost abolished in old mice, as previously reported in young Trpv3-KO mice, and (iii) TRPV3 activity and ATP release in human primary keratinocytes are greatly diminished with aging.
We previously demonstrated that vasodilation in response to local heating was reduced in non-neuropathic old subjects (60–75 years old) compared to young subjects (25–30 years old) [34], suggesting that aging alters ability of cutaneous microvessels to adapt to local heat. In the present study, we showed that 22-month-old mice presented a lack of vasodilation upon local heat exposure on paw skin (from 33 to 40 °C), regardless of sex. This defect was similar to that observed in young male and female Trpv3-KO mice (2 months), as previously reported in males [27]. Although endothelial dysfunction has been described in very old mice (22–25 months) [10, 53, 54] and old individuals (~70 years old) [34, 55, 56], the old mice we used in the present study (22 months) displayed intact vasodilator function of the endothelium and smooth muscle in cutaneous microvessels, as assessed by the iontophoresis experiments using acetylcholine and sodium nitroprusside, respectively. As previously reported [27], we confirmed the intact vasodilator function of the endothelium and smooth muscle in cutaneous microvessels in Trpv3-KO mice. We also showed that old WT mice displayed intact sensory nerve integrity using tail flick and hot plate experiments. In a previous study, we already showed that motor and sensory nerve conduction velocities were not different between young adult C57BL6/J mice (6–7 months) and old mice (22–25 months) [53]. These data suggest that the impaired vasodilation on exposure to local heat in old mice is specifically due to a failure of the initial heat detection and/or consequent triggered neural mechanisms, rather than to downstream mechanisms required for heat-evoked vasodilation.
TRPV3 is an essential cutaneous sensor of warming, playing a primary role in the vascular response to heat exposure in young mice [27]. Accordingly, our findings demonstrated that Trpv3 expression was significantly reduced in the hindpaw skin of old mice compared to young mice. In addition, the higher expression of TRPV3 in hindpaw compared to the back skin, regardless of the age, underlines the importance of thermo-sensors in thermoregulatory areas [57,58,59]. We thus suggest that the reduced Trpv3 expression in the mouse epidermis alters the detection of moderate temperature in old mice and, in turn, triggers mechanisms necessary for the vascular response to local heat exposure. We also showed this decrease of TRPV3 expression in elderly subjects, further strengthening the critical role of TRPV3 to adapt to local heat in mammalian homeotherms. Here, we showed a strong negative correlation between TRPV3 expression and age for both women and men using in situ hybridization. We also observed that TRPV3 expression was quite identical in photo-exposed (groin, scalp, face) and photo-protected skin areas (back humeral, chest), suggesting that TRPV3 is homogeneously expressed in human skin regardless sun exposure. In contrast to TRPV3, TRPV1 channel expression has been shown to increase with aging especially in photo-exposed skin [60, 61], implying that TRPV1 expression is sensitive to sun exposure. The temperature activation of TRPV1 being > 42 °C, this may suggest that elderly are more able to detect painful/burning temperature, related to UV exposure, than moderate temperature of 33–39 °C (TRPV3 range) [62,63,64]. In addition to the decrease of TRPV3 expression in aged keratinocytes, we also point out for the first time an impaired intrinsic activity of the TRPV3 channel. Alongside the decreased expression of TRPV3, other alterations may occur with aging. For example, it has been shown that some post-translational modifications, such as hydroxylation of TRPV3 on N242, altered TRPV3 current in HEK cells [65, 66]. Indeed, hydroxylation at this site could destabilize TRPV3 multimer assembly or interactions with other protein partners that can modulate channel gating or addressing to the plasma membrane. However, changes in TRPV3 structure and post-translational modifications with aging remain unknown and would constitute a promising further study.
Activation of TRPV3 is associated with the release of various diffusible molecules that act on neighboring cells. Stimulation of the channel is well known to trigger a proinflammatory response [36], but the signaling cascade associated with local heat-mediated vasodilation is still not fully depicted. It has been already described that keratinocytes can convey heat [28] and touch [67] stimuli through release of ATP. In this study, we confirmed that TRPV3 stimulation induced an extracellular ATP release from young keratinocytes, but to a less extent from old keratinocytes. As expected, the level of proinflammatory cytokines was not changed in the same context. In addition to its function of an energetic molecule, ATP plays various biological roles including sensory transduction via its neuroactive property [67] or regulation of blood vessels via its vasoactive property [49, 68,69,70]. Since the discovery of synaptic-like contacts between keratinocytes and sensory neurons, it has been strongly suggested that keratinocytes could directly communicate with sensory nerve endings in the skin [71, 72]. Thus, the ATP release from keratinocytes after TRPV3 activation could act on purinergic receptors from sensory nerves [49] and might regulate vascular tone in response to heat. Thus, the loss of ATP release with aging may compromise communication between sensory nerve endings and keratinocytes in the elderly. In addition, skin blood vessel also expressed purinergic receptor [50]. We cannot thus exclude that the TRPV3-dependent release of ATP by keratinocytes will directly act on skin blood vessel to regulate vascular tone. Finally, keratinocytes can release other neuroactive and/or vasoactive substances than ATP, such as CGRP, NO, and PGI2 [29, 31, 73], which could also contribute to local heat-evoked vasodilation either by acting on sensory nerve endings or dermal blood vessels. However, how keratinocytes can inform and modulate the vascular tone needs further exploration.
In conclusion, our study confirms that the initial detection of moderate temperature, through the warm-sensitive epidermal sensor TRPV3, is crucial for the skin to adapt to thermal stress such as heat. This offers a new function of the keratinocyte as a fundamental thermo-sensor and transducer of its thermal environment. With aging, decreased expression and activity of TRPV3 could reduce or delay the detection of changes in skin temperature, thereby limiting the mechanisms triggered for thermoregulation, including cutaneous vasodilation. Thus, TRPV3 may be a reliable target to counteract the thermoregulatory defect in the elderly. Aside TRPV3, decline of cold TRP channels with aging has been reported to influence temperature detection and may compromise vascular response to environmental cold in old individuals [74]. TRP channels are thus key actors in regulating the cutaneous vascular response to environmental temperature during aging, and need to be further considered.
References
Kolarsick PAJ, Kolarsick MA, Goodwin C. Anatomy and physiology of the skin. J Dermatol Nurses Assoc. 2011;3:203. https://doi.org/10.1097/JDN.0b013e3182274a98.
Yousef H, Alhajj M, Sharma S. Anatomy, Skin (integument), epidermis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
Johnson JM, Minson CT, Kellogg DL. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol. 2014;4:33–89. https://doi.org/10.1002/cphy.c130015.
Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–79. https://doi.org/10.1152/physrev.00026.2005.
Mitchell JA, Ali F, Bailey L, Moreno L, Harrington LS. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol. 2008;93:141–7. https://doi.org/10.1113/expphysiol.2007.038588.
Cracowski J-L, Roustit M. Human skin microcirculation. In: Comprehensive Physiology. John Wiley & Sons, Ltd; 2020. p. 1105–54.
Wong BJ, Hollowed CG. Current concepts of active vasodilation in human skin. Temperature (Austin). 2016;4:41–59. https://doi.org/10.1080/23328940.2016.1200203.
Smith CJ, Johnson JM. Responses to hyperthermia. Optimizing heat dissipation by convection and evaporation: neural control of skin blood flow and sweating in humans. Auton Neurosci. 2016;196:25–36. https://doi.org/10.1016/j.autneu.2016.01.002.
Bentov I, Reed MJ. The effect of aging on the cutaneous microvasculature. Microvasc Res. 2015;100:25–31. https://doi.org/10.1016/j.mvr.2015.04.004.
El Assar M, Angulo J, Vallejo S, Peiró C, Sánchez-Ferrer CF, Rodríguez-Mañas L. Mechanisms involved in the aging-induced vascular dysfunction. Front Physiol. 2012;3:132. https://doi.org/10.3389/fphys.2012.00132.
Cau SBA, Carneiro FS, Tostes RC. Differential modulation of nitric oxide synthases in aging: therapeutic opportunities. Front Physiol. 2012;3:218. https://doi.org/10.3389/fphys.2012.00218.
Holowatz LA, Thompson-Torgerson C, Kenney WL. Aging and the control of human skin blood flow. Front Biosci. 2010;15:718–39.
Balmain BN, Sabapathy S, Louis M, Morris NR. Aging and thermoregulatory control: the clinical implications of exercising under heat stress in older individuals. Biomed Res Int. 2018;2018 https://doi.org/10.1155/2018/8306154.
Greaney JL, Stanhewicz AE, Wolf ST, Kenney WL. Thermoregulatory reflex control of cutaneous vasodilation in healthy aging. Temperature (Austin). 8:176–87. https://doi.org/10.1080/23328940.2020.1832950.
Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 1985;2002(93):1644–9. https://doi.org/10.1152/japplphysiol.00229.2002.
Glatte P, Buchmann SJ, Hijazi MM, Illigens BM-W, Siepmann T. Architecture of the cutaneous autonomic nervous system. Front Neurol. 2019;0 https://doi.org/10.3389/fneur.2019.00970.
Stucky CL, Lewin GR. Isolectin B4-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–505. https://doi.org/10.1523/JNEUROSCI.19-15-06497.1999.
Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. https://doi.org/10.1146/annurev.biochem.75.103004.142819.
Kashio M. Thermosensation involving thermo-TRPs. Mol Cell Endocrinol. 2021;520:111089. https://doi.org/10.1016/j.mce.2020.111089.
Voets T. TRP channels and thermosensation. In: Nilius B, Flockerzi V, editors. Mammalian transient receptor potential (TRP) cation channels: volume II, Handbook of Experimental Pharmacology. Cham: Springer International Publishing; 2014. p. 729–41.
Caterina MJ, Pang Z. TRP channels in skin biology and pathophysiology. Pharmaceuticals (Basel). 2016;9 https://doi.org/10.3390/ph9040077.
Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong J, He H, Zhao Z, Cao T, Yan Z, et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010;12:130–41. https://doi.org/10.1016/j.cmet.2010.05.015.
Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, Lesnikov VA, Gavva NR, Romanovsky AA. Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci. 2011;31:1721–33. https://doi.org/10.1523/JNEUROSCI.4671-10.2011.
Peier AM. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–9. https://doi.org/10.1126/science.1073140.
Chung M-K, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem. 2003;278:32037–46. https://doi.org/10.1074/jbc.M303251200.
Fujii N, Kenny GP, McGarr GW, Amano T, Honda Y, Kondo N, Nishiyasu T. TRPV4 channel blockade does not modulate skin vasodilation and sweating during hyperthermia or cutaneous postocclusive reactive and thermal hyperemia. Am J Physiol Regul Integr Comp Physiol. 2021;320:R563–73. https://doi.org/10.1152/ajpregu.00123.2020.
Fromy B, Josset-Lamaugarny A, Aimond G, Pagnon-Minot A, Marics I, Tattersall GJ, Moqrich A, Sigaudo-Roussel D. Disruption of TRPV3 impairs heat-evoked vasodilation and thermoregulation: a critical role of CGRP. J Invest Dermatol. 2018;138:688–96. https://doi.org/10.1016/j.jid.2017.10.006.
Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, Patapoutian A, Fukumi-Tominaga T, Mizumura K, Tominaga M. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009;458:1093–102. https://doi.org/10.1007/s00424-009-0703-x.
Huang SM, Lee H, Chung M-K, Park U, Yu YY, Bradshaw HB, Coulombe PA, Walker JM, Caterina MJ. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci. 2008;28:13727–37. https://doi.org/10.1523/JNEUROSCI.5741-07.2008.
Seo SH, Kim S, Kim S-E, Chung S, Lee SE. Enhanced thermal sensitivity of TRPV3 in keratinocytes underlies heat-induced pruritogen release and pruritus in atopic dermatitis. J Invest Dermatol. 2020;140:2199–2209.e6. https://doi.org/10.1016/j.jid.2020.02.028.
Miyamoto T, Petrus MJ, Dubin AE, Patapoutian A. TRPV3 regulates NOS-independent nitric oxide synthesis in the skin. Nat Commun. 2011;2:369. https://doi.org/10.1038/ncomms1371.
Moqrich A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–72. https://doi.org/10.1126/science.1108609.
Holowatz LA, Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol. 1985;2010(109):1538–44. https://doi.org/10.1152/japplphysiol.00338.2010.
Fromy B, Sigaudo-Roussel D, Gaubert-Dahan M-L, Rousseau P, Abraham P, Benzoni D, Berrut G, Saumet JL. Aging-associated sensory neuropathy alters pressure-induced vasodilation in humans. J Invest Dermatol. 2010;130:849–55. https://doi.org/10.1038/jid.2009.279.
Raynard C, Ma X, Huna A, Tessier N, Massemin A, Zhu K, Flaman J-M, Moulin F, Goehrig D, Medard J-J, et al. NF-ΚB-dependent secretome of senescent cells can trigger neuroendocrine transdifferentiation of breast cancer cells. Aging Cell. 2022;21:e13632. https://doi.org/10.1111/acel.13632.
Szöllősi AG, Vasas N, Angyal Á, Kistamás K, Nánási PP, Mihály J, Béke G, Herczeg-Lisztes E, Szegedi A, Kawada N, et al. Activation of TRPV3 regulates inflammatory actions of human epidermal keratinocytes. J Invest Dermatol. 2018;138:365–74. https://doi.org/10.1016/j.jid.2017.07.852.
Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, Sinisi M, Birch R, Anand P. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. https://doi.org/10.1186/1471-2377-7-11.
Park CW, Kim HJ, Choi YW, Chung BY, Woo S-Y, Song D-K, Kim HO. TRPV3 channel in keratinocytes in scars with post-burn pruritus. Int J Mol Sci. 2017;18:2425. https://doi.org/10.3390/ijms18112425.
Muther C, Jobeili L, Garion M, Heraud S, Thepot A, Damour O, Lamartine J. An Expression screen for aged-dependent microRNAs identifies MiR-30a as a key regulator of aging features in human epidermis. Aging (Albany NY). 2017;9:2376–96. https://doi.org/10.18632/aging.101326.
Chevalier FP, Rorteau J, Ferraro S, Martin LS, Gonzalez-Torres A, Berthier A, El Kholti N, Lamartine J. MiR-30a-5p alters epidermal terminal differentiation during aging by regulating BNIP3L/NIX-dependent mitophagy. Cells. 2022;11:836. https://doi.org/10.3390/cells11050836.
Rorteau J, Chevalier FP, Bonnet S, Barthélemy T, Lopez-Gaydon A, Martin LS, Bechetoille N, Lamartine J. Maintenance of chronological aging features in culture of normal human dermal fibroblasts from old donors. Cells. 2022;11:858. https://doi.org/10.3390/cells11050858.
Broad LM, Mogg AJ, Eberle E, Tolley M, Li DL, Knopp KL. TRPV3 in drug development. Pharmaceuticals (Basel). 2016;9 https://doi.org/10.3390/ph9030055.
Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, Hatt H. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007;151:530–40. https://doi.org/10.1038/sj.bjp.0707245.
Qi H, Shi Y, Wu H, Niu C, Sun X, Wang K. Inhibition of temperature-sensitive TRPV3 channel by two natural isochlorogenic acid isomers for alleviation of dermatitis and chronic pruritus. Acta Pharm Sin B. 2022;12:723–34. https://doi.org/10.1016/j.apsb.2021.08.002.
Chung M-K, Lee H, Mizuno A, Suzuki M, Caterina M. 2-Aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci. 2004;24:5177–82. https://doi.org/10.1523/JNEUROSCI.0934-04.2004.
Liu B, Yao J, Zhu MX, Qin F. Hysteresis of gating underlines sensitization of TRPV3 channels. J Gen Physiol. 2011;138:509–20. https://doi.org/10.1085/jgp.201110689.
Cheng X, Jin J, Hu L, Shen D, Dong X, Samie MA, Knoff J, Eisinger B, Liu M, Huang SM, et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell. 2010;141:331–43. https://doi.org/10.1016/j.cell.2010.03.013.
Wang Y, Li H, Xue C, Chen H, Xue Y, Zhao F, Zhu MX, Cao Z. TRPV3 enhances skin keratinocyte proliferation through EGFR-dependent signaling pathways. Cell Biol Toxicol. 2020; https://doi.org/10.1007/s10565-020-09536-2.
Burnstock G, Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf). 2009;195:415–47. https://doi.org/10.1111/j.1748-1716.2009.01957.x.
Lohman AW, Billaud M, Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res. 2012;95:269–80. https://doi.org/10.1093/cvr/cvs187.
Raqeeb A, Sheng J, Ao N, Braun AP. Purinergic P2Y2 receptors mediate rapid Ca(2+) mobilization, membrane hyperpolarization and nitric oxide production in human vascular endothelial cells. Cell Calcium. 2011;49:240–8. https://doi.org/10.1016/j.ceca.2011.02.008.
Burnstock G. Purine and purinergic receptors. Brain Neurosci Adv. 2018;2:2398212818817494. https://doi.org/10.1177/2398212818817494.
Gaubert ML, Sigaudo-Roussel D, Tartas M, Berrut G, Saumet JL, Fromy B. Endothelium-derived hyperpolarizing factor as an in vivo back-up mechanism in the cutaneous microcirculation in old mice. J Physiol. 2007;585:617–26. https://doi.org/10.1113/jphysiol.2007.143750.
Matz RL, de Sotomayor MA, Schott C, Stoclet J-C, Andriantsitohaina R. Vascular bed heterogeneity in age-related endothelial dysfunction with respect to NO and eicosanoids. Br J Pharmacol. 2000;131:303–11. https://doi.org/10.1038/sj.bjp.0703568.
DeSouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, Stauffer BL. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. J Physiol. 2002;542:255–62. https://doi.org/10.1113/jphysiol.2002.019166.
Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol. 2003;284:H1662–7. https://doi.org/10.1152/ajpheart.00871.2002.
Škop V, Liu N, Guo J, Gavrilova O, Reitman ML. The contribution of the mouse tail to thermoregulation is modest. Am J Physiol Endocrinol Metab. 2020;319:E438–46. https://doi.org/10.1152/ajpendo.00133.2020.
Mota-Rojas D, Titto CG, de Mira Geraldo A, Martínez-Burnes J, Gómez J, Hernández-Ávalos I, Casas A, Domínguez A, José N, Bertoni A, et al. Efficacy and function of feathers, hair, and glabrous skin in the thermoregulation strategies of domestic animals. Animals (Basel). 2021;11:3472. https://doi.org/10.3390/ani11123472.
Hankenson FC, Marx JO, Gordon CJ, David JM. Effects of rodent thermoregulation on animal models in the research environment. Comp Med. 2018;68:425–38. https://doi.org/10.30802/AALAS-CM-18-000049.
Lee YM, Kim YK, Chung JH. Increased expression of TRPV1 channel in intrinsically aged and photoaged human skin in vivo. Exp Dermatol. 2009;18:431–6. https://doi.org/10.1111/j.1600-0625.2008.00806.x.
Lee YM, Kang SM, Chung JH. The role of TRPV1 channel in aged human skin. J Dermatol Sci. 2012;65:81–5. https://doi.org/10.1016/j.jdermsci.2011.11.003.
Kashio M, Tominaga M. TRP channels in thermosensation. Curr Opin Neurobiol. 2022;75:102591. https://doi.org/10.1016/j.conb.2022.102591.
Johnson AJ, Wilson AT, Laffitte Nodarse C, Montesino-Goicolea S, Valdes-Hernandez PA, Somerville J, Peraza JA, Fillingim RB, Bialosky J, Cruz-Almeida Y. Age differences in multimodal quantitative sensory testing and associations with brain volume. Innov. Aging. 2021;5:igab033. https://doi.org/10.1093/geroni/igab033.
Heft MW, Robinson ME. Age differences in suprathreshold sensory function. Age (Dordr). 2014;36:1–8. https://doi.org/10.1007/s11357-013-9536-9.
Karttunen S, Duffield M, Scrimgeour NR, Squires L, Lim WL, Dallas ML, Scragg JL, Chicher J, Dave KA, Whitelaw ML, et al. Oxygen-dependent hydroxylation by FIH regulates the TRPV3 ion channel. J Cell Sci. 2015;128:225–31. https://doi.org/10.1242/jcs.158451.
Zhang N, Fu Z, Linke S, Chicher J, Gorman JJ, Visk D, Haddad GG, Poellinger L, Peet DJ, Powell F, et al. The asparaginyl hydroxylase factor inhibiting HIF-1α is an essential regulator of metabolism. Cell Metab. 2010;11:364–78. https://doi.org/10.1016/j.cmet.2010.03.001.
Moehring F, Cowie AM, Menzel AD, Weyer AD, Grzybowski M, Arzua T, Geurts AM, Palygin O, Stucky CL. Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling. Elife. 2018;7:e31684. https://doi.org/10.7554/eLife.31684.
Verkhratsky A, Burnstock G. Biology of purinergic signalling: its ancient evolutionary roots, its omnipresence and its multiple functional significance. Bioessays. 2014;36:697–705. https://doi.org/10.1002/bies.201400024.
Fujii N, McGinn R, Halili L, Singh MS, Kondo N, Kenny GP. Cutaneous vascular and sweating responses to intradermal administration of ATP: a role for nitric oxide synthase and cyclooxygenase? J Physiol. 2015;593:2515–25. https://doi.org/10.1113/JP270147.
Fujii N, Halili L, Singh MS, Meade RD, Kenny GP. Intradermal administration of ATP augments methacholine-induced cutaneous vasodilation but not sweating in young males and females. Am J Physiol Regul Integr Comp Physiol. 2015;309:R912–9. https://doi.org/10.1152/ajpregu.00261.2015.
Talagas M, Lebonvallet N, Leschiera R, Marcorelles P, Misery L. What about physical contacts between epidermal keratinocytes and sensory neurons? Exp Dermatol. 2018;27:9–13. https://doi.org/10.1111/exd.13411.
Talagas M, Lebonvallet N, Leschiera R, Sinquin G, Elies P, Haftek M, Pennec J-P, Ressnikoff D, La Padula V, Le Garrec R, et al. Keratinocytes communicate with sensory neurons via synaptic-like contacts. Ann Neurol. 2020;88:1205–19. https://doi.org/10.1002/ana.25912.
Hou Q, Barr T, Gee L, Vickers J, Wymer J, Borsani E, Rodella L, Getsios S, Burdo T, Eisenberg E, et al. Keratinocyte expression of calcitonin gene-related peptide β: implications for neuropathic and inflammatory pain mechanisms. Pain. 2011;152:2036–51. https://doi.org/10.1016/j.pain.2011.04.033.
Thapa D, de Sousa Valente J, Barrett B, Smith MJ, Argunhan F, Lee SY, Nikitochkina S, Kodji X, Brain SD. Dysfunctional TRPM8 signalling in the vascular response to environmental cold in ageing. eLife. 2021;10:e70153. https://doi.org/10.7554/eLife.70153.
Acknowledgements
We acknowledge Jocelyne Vial and Geraldine Aimond for technical support and animal care facilities (AnexPeau facility, Lyon). We thank Theo Barthélemy and Emma Fraillon for their precious help for some experiments. We also thank Nicolas Lebonvallet, Ophelie Pierre, and Laurent Misery for their support and access to the calcium imaging platform of Laboratoire Interactions Epitheliums Neurones (LIEN/EA 4685/Brest). We thank Fabien Van Coppenolle (CarMeN/INSERM, U1060/Lyon) for his help in calcium imaging analysis and discussion. We thank PLATIM and especially Elodie Chatre for the microscopy. We thank Jerome Lamartine for critical reading of the manuscript.
Funding
This research project was supported by internal funds from CNRS and University Lyon 1. This work was supported by the French National Research Agency (KAST-ANR-23-CE14-0015).
Author information
Authors and Affiliations
Contributions
Conceptualization, L.S.M., F.P.C., and B.F.; methodology, L.S.M., A.J.-M., S.D., F.P.C., and B.F.; validation, L.S.M., T.E.J., F.P.C., and B.F.; formal analysis, L.S.M., A.J.-M., S.D., T.E.J., F.P.C., and B.F.; investigation, L.S.M., A.J.-M., S.D., F.P.C., and B.F.; resources, L.S.M., S.D., F.P.C., and B.F.; writing—original draft preparation, L.S.M., F.P.C., and B.F.; writing—review and editing, L.S.M., F.P.C., and B.F.; visualization, L.S.M., F.P.C., B.F.; S.D., T.E.J.; supervision, F.P.C. and B.F.; project administration, F.P.C. and B.F.; funding acquisition, F.P.C. and B.F.; all authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The animal study protocol was approved by the Animal Experimentation Committee of the University Claude Bernard Lyon I (protocol agreement #33946 approved on 25 November 2021).
Informed consent
Skin biopsies were obtained from the DermoBioTec tissue bank at Lyon (Tissue Transfer Agreement n_214854) with the informed consent of adult donors undergoing surgical discard (non-pathological tissues from breast, face, or abdomen), in accordance with the ethical guidelines (French Bioethics law of 2021).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 440 kb)
About this article
Cite this article
Martin, L.S., Josset-Lamaugarny, A., El Jammal, T. et al. Aging is associated with impaired triggering of TRPV3-mediated cutaneous vasodilation: a crucial process for local heat exposure. GeroScience 46, 3567–3580 (2024). https://doi.org/10.1007/s11357-023-00981-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00981-5