Abstract
In the construction industry, environmental behavior of aggregates has been monitored thanks to leaching tests, especially for alternative aggregates obtained from waste (e.g., construction and demolition waste, MSWI). Few studies were carried on the leaching behavior of natural aggregates, which are often not regulated for their substance release in most EU member states (as France). Leachable content of some heavy metals, halides, and sulfates on natural aggregates was investigated using up-flow percolation test EN 16637–3 and compared to threshold values. Only three samples (NS2, NG1, and NG8) show one element which exceeded threshold values (As, Zn, As, respectively), among the 19 natural aggregates tested for leaching. In this study, three natural aggregates (NG1, NS1, NS2) have been chosen because of their measurable leaching values. Total content was obtained through acid digestion. Influence of grain size on leaching results was investigated. Predominant release mechanisms were determined using EN 16637–3 — Annex D, based on percolation results such as pH, electrical conductivity, and leached content, and were then discussed. Detailed results for releases of As, Ba, Ni, Zn, SO42−, and F− were investigated. EN 16637–3 — Annex D shows some limits, especially for trace elements. The pH was found to be one of the most important factors influencing leaching release of most elements, being more important than grain size. By comparing total content with released quantities, it has been shown that As and Mo in NS2 are easily leached, hence present in a very soluble chemical form. Determining release mechanisms accurately in this study seems only possible for elements present in significant amounts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The evaluation of the environmental performance of aggregates used in the construction industry has been carried out with increasing frequency in recent years, especially with the growing importance of recycled and artificial aggregates (derived from slag, residues from municipal solid waste incineration, etc.) in the market. Numerous studies have therefore focused on the leaching behavior of different materials to understand how potentially hazardous elements for the environment are released when the material comes into contact with water. Indeed, for the purpose of preventing environmental risks, it is more relevant to monitor the leached quantities than the total content (Van der Sloot and Kosson 2012), since only a fraction of the content can be solubilized, according to various factors. Thus, several physicochemical factors have been identified for their importance in the release mechanisms, such as temperature, pH, or grain size (Van der Sloot and Dijkstra 2004). Knowing the importance of each of these parameters can help finding adapted solutions to limit the release of substances (by means of stabilization or extraction for example) from a material and predict its long-term behavior (Liu et al. 2021; Kim et al. 2018; Verbinnen et al. 2017; Engelsen et al. 2017).
Most studies on the subject have been conducted on alternative materials (Schafer et al. 2019; Roque et al. 2016; Wiebusch et al. 1998; Dijkstra et al. 2008; Diotti et al. 2020; Del Rey et al. 2015; Maia et al. 2018), but few have focused on natural aggregates traditionally used in construction. Yet, the Construction Products Regulation (Regulation (EU) No 305/2011 2011) requires to declare the release of all construction materials in the next few years. Moreover, some studies demonstrated that sometimes natural aggregates can leach higher quantities of substances (for Zn, Ni, Co, and Cr) than alternative aggregates (Vollprecht et al. 2019; Tossavainen and Forssberg 1999).
Cuney et al. (1995) conducted leaching tests on different natural rocks: granite, andesite, rhyolite, etc. Of eight unaltered rocks, two waste management regulatory thresholds (associated with standard NFX 31–210) in place at the time the study was carried out were exceeded: one for arsenic in a granite, and the other for nickel in a shale. However, total content analyses showed abnormally high levels of these elements in these two rocks. Suzuki et al. (2020) studied the release of arsenic in excavated metamorphic rocks and proved that arsenic shows a similar leaching behavior in different metamorphic rocks.
Several test methods, including some standardized ones, have been developed to determine the quantities of leached elements (compliance tests) and to understand the leaching behavior of the material (i.e., characterization tests) (Kosson et al. 2002). The horizontal up-flow percolation test NF EN 16637–3 (AFNOR 2024) has been published early 2024 as a European standard and will apply to granular construction products. In addition, this draft standard provides a method for identifying the predominant mechanisms in element leaching which is used and discussed in this study. Standard NF EN 14405 (AFNOR 2017) is a similar up-flow percolation test applied to waste materials. In Europe, the Netherlands and Germany also have similar standards (DIN 2009; NEN 2004) with threshold values associated to them, which are used in this study for comparison purposes.
In France, acceptability guidelines for alternative materials used in road techniques have been made available since 2011 with threshold values associated with standard NF EN 12457 (AFNOR 2002), mainly used for environmental risks assessment in waste management. This standard corresponds to a batch leaching test. Therefore, these French acceptability guidelines may be revised and adapted to this new European standard to simplify the testing procedure to avoid double testing.
This study focuses on three natural granular samples selected for their high leaching values among 19 natural granular samples characterized by the up-flow percolation test. Therefore, these three samples are not representative of the geological diversity in France. Cumulative releases are compared to threshold values. The measured elements are heavy metals, halides, and sulfates, which have threshold values. Hypotheses regarding the nature of the predominant mechanisms in the leaching of each element are made with the help of various data such as pH, electrical conductivity, total content determined by acid digestion, and calculations provided by the standard NF EN 16637–3.
Materials
Three samples of natural aggregates were collected from two quarries: one natural gravel NG1 and two natural sands NS1 and NS2. These samples have been chosen from a large number of natural aggregates because of their relatively high leaching values compared to regulatory thresholds. Table 1 shows the samples’ characteristics. Samples NG1 and NS1 were collected from the same quarry. NG1 is an “as dug” gravel, while NS1 is a treated sand, implying a more advanced manufacturing process for the latter.
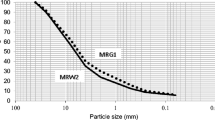
Figure 1 shows the grading curves of the three aggregates. No size reduction is applied. Absolute densities are obtained according to standard NF EN 1097–6 (AFNOR 2022) and are ranged between 2600 and 2630 kg m−3.
Methods
Percolation test
An up-flow percolation test is carried out on the samples according to standard NF EN 16637–3 (AFNOR 2024). The column (made of borosilicate glass) with a diameter of 10 cm and a height of 30 cm is equipped with layers of glass beads (1 mm in diameter) approximately 1 cm thick at the inlet and outlet of the column and with FEP tubes. The sample is then inserted into the column and compacted using a packing equipment. The mass of the sample is weighed and then converted to dry mass after performing a dry extract of the material (placed in an oven at 80 °C). The column is then saturated with distilled water, and an equilibrium is established for approximately 65 h. A flow rate of distilled water between 85 and 111 mL h−1 is imposed from bottom to top of the column using a diaphragm pump. Eluate fractions are collected at the outlet of the column depending on the ratio between the liquid volume and the dry mass of the sample (L/S in L kg−1): at 0.1, 0.2, 0.5, 1, 2, 5, and 10 L kg−1 (cumulative from the beginning of the test). The pH and electrical conductivity of each eluate are measured, and then, the eluates are vacuum-filtered with a 0.45-µm cellulose acetate filter and stored in borosilicate glass flasks before being analyzed (Fig. 2).
Standard NF EN 16637–3 – Annex D (AFNOR 2024) provides a method for determining the predominant release mechanisms. This method is based on calculations from the release results (concentrations, pH) to verify each hypothesis (Fig. 3). In this study, the method is applied as specified by the standard and then discussed.
Acid digestion
Acid digestion method is used to measure the total content of the three aggregates. In this study, total content is compared to released quantities obtained with percolation tests in order to observe how easily leached are substances. The samples are digested in aqua regia according to the technical specification CEN/TS 17196 — Method A (CEN/TC 351 2019). The test is carried out using a chemical reflux setup completed with an absorption vessel aimed at capturing volatile species (Fig. 4).
Three grams of aggregate sample is ground using a vibratory mill and then introduced into the reactor, to which 28 mL of aqua regia is added. The mixture is refluxed for 2 h. The digestate is then filtered using a 0.45-µm cellulose acetate filter and diluted in a 100-mL volumetric flask. The resulting solution is then analyzed by ICP-OES.
Analysis of eluates
The analyses presented here aim to quantify the compounds commonly measured during leaching tests. There are two of them: inductively coupled plasma spectroscopy (ICP) and ion exchange chromatography (IC).
ICP is used here to measure the quantities of trace metals and metalloids: Sb, As, Ba, Cd, Cr, Cu, Hg, Mo, Pb, Ni, Se, and Zn. The device used to perform these measurements is a Thermo Scientific iCAP Pro X ICP-OES, which operates under argon plasma. Before being analyzed, the eluate is acidified with 1% volume of nitric acid. Distilled water blank is also acidified in the same way. This blank is used to subtract any potential traces of elements already present in distilled water and/or nitric acid during measurements on the eluates.
IC is used here to measure the quantities of halides (F−, Cl−) and sulfates (SO42−). The device used to perform these measurements is a Thermo Dionex ICS900. An AS22 column associated with an AG22 pre-column is used. The measurements are done in a Na2CO3–NaHCO3 eluent. Eluates are introduced into the injection valve using a syringe and a 0.45-µm nylon syringe filter.
Based on the calibration range of both devices, limits of quantification (LoQ) have been determined. They are introduced in Table 2.
Results
Leaching percolation test
Choice of aggregates NS1, NS2, and NG1
A total of 19 natural aggregates have been characterized with the percolation test (Table 3).
The results are compared with the threshold values of different existing regulations. In France, there is no regulation on the release of pollutants from natural materials. The threshold values, associated with NF EN 14405 standard, of the Acceptability Guide for Alternative Road Materials (Cerema 2011) are therefore used. The Dutch (NEN 2004) and German (DIN 2009) regulatory thresholds for up-flow percolation tests are also used (see Table 4 for exact threshold values).
Most of the samples do not exceed any threshold (grey and white cells). Only three samples among all materials show an exceeding of at least one threshold for one element (red cell): NS2 and NG8 exceed As threshold values, and NG1 exceeds Ni, Zn, and SO42− threshold values. Some elements are present in quantities too low to be quantified by the analysis device (their cell is grayed out).
The three materials studied here (NG1, NS1, and NS2) were selected for their particularly visible leaching releases. NG8 was not selected as the threshold values for As were also exceeded by NS2. Moreover, NS1 has been selected to study the effect of grain size and manufacturing process by comparing with NG1 results.
Evolution of pH and electrical conductivity
The pH and electrical conductivity curves are presented in Fig. 5. The pH values of the eluates from NS1 (between 4 and 6) and NG1 (between 2 and 4) samples are acidic. A more acidic pH is observed for the NG1 gravel than for the NS1 sand (about 2 pH unit difference). The pH of the eluates from NS2 sand is slightly basic (between 8 and 10). In all three cases, an increase in pH of 1 to 2 units is observed during the test.
The electrical conductivity of the eluates from all studied samples decreases over time, which is consistent with the behavior observed during this type of test (Dijkstra et al. 2008). Most soluble elements are removed at the beginning of the test by wash-out phenomena. NS1 and NG1 samples have higher electrical conductivity at the beginning of the test (3000 µS cm−1 and 5600 µS cm−1 respectively) than the NS2 sample (700 µS cm−1). The electrical conductivity of the NG1 gravel remains higher than that of the NS1 sand during the test. Therefore, higher releases can be expected for the gravel than for the sand which is counterintuitive as many studies proved that a smaller grain size usually leads to higher leaching releases (Van der Sloot and Dijkstra 2004; Zhang et al. 2018).
These differences in pH and electrical conductivity between NS1 and NG1 are associated with the manufacturing process: While NG1 gravel is “as dug”, hence presents raw characteristics, NS1 sand has undergone multiple steps (screening, crushing, and washing).
Cumulative release
For each material, the total release (at a cumulative L/S ratio of 10 L kg−1) of each element studied is displayed in Table 4. The results are compared with the same threshold values than in Table 3.
It is observed that material NS1 does not exceed any threshold, material NS2 exceeds French (Cerema 2011) and German threshold values for arsenic by a factor of 10, and material NG1 exceeds Dutch threshold values for nickel, sulfates, and Dutch and German threshold values for zinc. For all elements, except for fluoride and chloride, the NG1 gravel shows greater releases than the NS1 sand from the same origin, which is counterintuitive: Indeed, a gravel with a lower specific surface area than a sand typically has less exchange with the leachate. This can nevertheless be explained by the difference in pH between these two materials: The NG1 gravel has an average pH during the test around 3, while the NS1 sand has an average pH of 5. It is accepted in the literature that an acidic pH leads to a greater release of most elements (Van der Sloot et al. 2009), except for halides like fluoride or chloride which are less sensitive to pH. It is also explained by the difference in the manufacturing process between the two samples: NG1 is “as dug,” while NS1 is treated, which means that part of the substances could have been already removed from NS1.
Leaching mechanisms
Standard NF EN 16637–3 — Annex D (AFNOR 2024) provides a method for determining the predominant release mechanisms. This method is based on calculations from the release results (concentrations and pH) to verify each hypothesis. The predominant release mechanisms for relevant elements (As, Ba, Ni, Zn, F−, and SO42−) in each material are presented in Table 5.
Except for the “overall low concentrations” category, determining the predominant mechanisms requires a preliminary choice from the operator: is the compound release apparently pH-dependent or not? According to the standard, this choice is made using concentration curves as a function of the pH of each eluate. If linearity is observed, the standard states that the release is apparently pH-dependent. As the standard does not provide any precise value on the slope or the coefficient of determination to determine linearity, this method lacks accuracy. Moreover, in this study, the pH ranges for each sample may be insufficiently large to observe pH dependence.
In this study, in order to follow closely the standard method, apart from some elements known to be pH-independent (e.g., Cl−), the apparent pH dependence was decided when the concentration curve of the compound as a function of pH showed a linear profile (see Fig. 6 as an example).
It is observed that the predominant mechanism can differ even for two aggregates of the same origin (NS1 and NG1). For NS1 sand, nickel is leached in a pH-dependent manner, with depletion of substance leaching. On the contrary, for NG1 gravel, nickel is leached in a pH-dependent manner, but with solubility control.
The concentration curves of the eluates as a function of the cumulative L/S ratio for the elements As, Ba, Ni, Zn, SO42−, and F− are presented in Fig. 7. Only results for samples with releases above the limit of quantification (LoQ) are displayed. In general, the measured elements, being present in the samples only in trace amounts, show a nearly systematic decrease in their leaching in the eluates during the test.
-
For the release of As, represented for sand NS2 only, an increase in the concentration of eluates is observed at the beginning of the test, reaching a peak between 0.2 and 0.5 L kg−1, followed by a decrease for the rest of the test. This is consistent with the behavior of arsenic leaching in metamorphic rocks, especially when a significant portion of arsenic is easily soluble in water (Suzuki and Katoh 2020). The arsenic released at the beginning of the test would mainly correspond to a non-specifically bound fraction of arsenic, and the second released part would correspond to a specifically bound fraction which requires more time to dissolve. According to the results from Table 4, the predominant mechanism for arsenic leaching in sample NS2 is the depletion of the substance: If the test were to continue for a longer time, concentrations of arsenic in eluates would soon reach low concentrations.
-
For the release of Ba, represented for sands NS1 and NS2, two different behaviors are observed: The release for the first three eluates (up to 0.5 L kg−1) is similar for both materials; then, the concentration of eluates from NS1 decreases more quickly than that of eluates from NS2 and reaches the LoQ from 5 L kg−1. It has been shown that the release of Ba was less dependent on pH (Zhang et al. 2018). This is contradictory to Table 4 for the release of Ba from the NS1 material, which has been determined to be pH dependent. Material NS2 therefore presents a larger fraction of easily soluble barium than material NS1.
-
For the release of Ni, represented for sand NS1 and gravel NG1, two slightly different behaviors are observed: Both materials present similar concentrations in the first eluate; then, the concentration of Ni for sand NS1 decreases rapidly, reaching the limit of quantification of the analysis apparatus at 2 L kg−1, whereas the concentration decreases less abruptly in gravel NG1. The results from Table 4 indicate that the predominant mechanism for Ni release is the wash out of the substance for material NS1 and controlled by solubility for material NG1. Nickel release is known to be maximized at acidic pH (Dijkstra et al. 2008; Van der Sloot and Dijkstra 2004). Vollprecht et al. (2019) showed that leached Ni can come from olivine.
-
For the release of Zn, represented for the three samples, two types of behaviors are observed: On the one hand, the concentrations of sand NS1 and gravel NG1 present a very similar decrease throughout the test. On the other hand, the concentration of Zn in sand NS2 presents a plateau at the beginning of the test (up to 0.5 L kg−1), followed by a decrease. At the end of the test, an increase in concentration is observed, possibly due to the contact of the leachate with surfaces that were not accessible at the beginning of the test. Zinc tends to be leached more easily at acidic pH, with a minimum release generally located around pH 9 (Dijkstra et al. 2008; Van der Sloot and Dijkstra 2004). Concentrations of the last eluate for all samples indicate that zinc would still be released for some time if the test were to continue further. This is coherent with the solubility-controlled mechanism determined in Table 4.
-
For the release of SO42−, represented for the three samples, two distinct behaviors are also observed: a similar decrease between sand NS1 and gravel NG1 and a more abrupt decrease in concentration for sand NS2, for which a low concentration plateau is observed from around 1 L kg−1. This plateau could correspond to a second fraction of the substance: While the first fraction is easily leached (probably from a sulfate salt), the second fraction would be solubility controlled which would explain the low and constant concentrations for the second part of the test.
-
For the release of F−, represented for the three samples, two distinct behaviors are also observed: a similar decrease between sand NS1 and gravel NG1 and a rapid decrease to depletion at around 1 L.kg−1 for sand NS2. It is noticed that gravel NG1 shows an increase in concentration between 0.2 and 0.5 L kg−1 and an apparent exhaustion of the substance at the end of the test at 10 L kg−1.
It is important to note that the first hypothesis regarding the apparent dependence on pH does not allow for a reliable result. It is therefore essential to also have knowledge of the general behavior of the compounds to be as close as possible to the correct answer. In addition, the low overall availability of the compounds monitored here almost always results in an apparent depletion of the compound. In this case, it is difficult to determine whether the leaching of a compound is pH-dependent or not. To go further, it would therefore be necessary to carry out static pH tests.
Acid digestion
The acid digestion method is used to measure total content of the samples. Leached quantities are part of the total content initially present in the material. The results of acid digestion of the natural aggregates are presented in Fig. 8. The results are expressed in mass of the targeted element in milligrams per kilogram of dry sample mass. Solid residues are visible at the end of the test for each sample. Among all the elements studied here, zinc is the one present in the largest quantity in all samples. Mercury is not detected in any of the materials. Compositions of similar orders of magnitude are observed for sand and gravel of the same origin (NS1 and NG1), except for zinc and copper. The NS2 sand has higher levels of arsenic and lead than the other two samples.
Discussion
The NS1 (sand) and NG1 (gravel) samples of the same quarry origin exhibit similar behaviors in the release of the various measured elements. However, differences are observed in the amounts leached (mg kg−1): NG1 gravel overall leaches more than NS1 sand despite similar total contents.
Total content has no direct link to leached quantities and often a significant fraction of a substance cannot be leached in normal conditions. However, it is possible to elaborate on previous hypothesis and conclusions, by calculating percentages of leaching release (PR). The PR corresponds to the ratio between the quantity of an element released during leaching (Table 4) and the total content of that element in the sample measured by acid digestion (Fig. 8). A high PR indicates that the substance is easily leached in the conditions of the percolation test, and that it is found in soluble chemical forms in the sample. Figure 9 shows the PRs of natural aggregates.
Overall, higher PRs are observed for the NG1 aggregate than for the NS1 sand, despite similar total contents. It is commonly accepted that smaller particle size results in a larger specific surface area, leading to increased contact and exchange between the leachate and the material (Van der Sloot and Dijkstra 2004; Zhang et al. 2018). In this study, the different behavior between the sand and the coarse aggregate can be explained by a more advanced manufacturing process of the sand: NG1 is “as dug,” while NS1 is not. It could also be explained with the higher acidity of the NG1 (pH = 2.96). pH appears to be one of the most influential factors on the leaching of elements from these materials.
The PR in arsenic for NS2 reaches almost 30%. In this material, arsenic is therefore easily leachable; it is mainly found in a form and in mineralogical phases that are easily accessible to water and soluble (which is coherent with the depletion mechanism determined earlier for As in NS2, in Table 5). It is also interesting to note the high PR in molybdenum for this material: Indeed, it exceeds 20% while no release of this element is visible in the other samples (NS1 and NG1), although surprisingly, it is present only in small quantities in total content. Molybdenum is known to be most soluble at pH 9 (Van der Sloot and Dijkstra 2004) (which is the pH of NS2), which could explain the high release. Moreover, authors demonstrated that molybdenum can be present as adsorbed on iron hydroxides in natural rocks (Wang et al. 2018) and therefore is more leachable in this chemical form. Lead and zinc are very little released in NS2 although well present in the sample. For zinc, this is consistent with the solubility-controlled mechanism determined in Table 5.
Conclusion
Depending on the material studied, each element has a different leaching behavior and must be studied separately to analyze the predominant mechanisms in its release. However, it seems possible, at least in the case of arsenic, to observe a similar leaching trend in several different natural materials. Furthermore, in our study, pH and stage in the manufacturing process seem to exert a particular influence on the quantity of leached elements, while the influence of grain size seems limited. Some of the threshold values used for comparison in this study (French values) are only applicable to alternative materials, and natural quarry materials (as studied here) may need specific threshold values that are adapted.
Total content, obtained here by acid digestion, can be a useful tool to support hypotheses about the predominant leaching mechanisms in a material. By relating it to released quantities, it can be observed that certain elements are very easily leached in one material but not in others.
Annex D of the NF EN 16637–3 standard can guide hypotheses about the predominant mechanisms, but it remains limited, particularly in the case of elements released only in trace amounts, for which it is difficult to differentiate between substance depletion and pH dependence. Further research and additional testing, such as static pH tests or X-ray diffraction (XRD) tests would be necessary to confirm, refute or specify hypotheses established in this manner.
The results of these studies also make it interesting to conduct this type of testing on a larger number of materials from different sources to determine if it is possible to observe trends in element leaching behavior in certain types of materials.
Data availability
The data that support the findings of this study are available from the corresponding author, Martial Clavier, upon reasonable request.
References
AFNOR (2002) NF EN 12457 - Leaching — compliance test for leaching of granular waste materials and sludges
AFNOR (2017) NF EN 14405 - Characterization of waste — leaching behaviour test — up-flow percolation test (under specified conditions).
AFNOR (2022) NF EN 1097–6 - Tests for mechanical and physical properties of aggregates - part 6 : determination of particle density and water absorption. https://www.boutique.afnor.org/en-gb/standard/nf-en-10976/tests-for-mechanical-and-physical-properties-of-aggregates-part-6-determina/fa192181/321395.
AFNOR (2024) NF EN 16637–3 - Construction products: assessment of release of dangerous substances - part 3: horizontal up-flow percolation test
CEN/TC 351 (2019) CEN/TS 17196:2019 - Construction products: assessment of release of dangerous substances — digestion by aqua regia for subsequent analysis of inorganic substances
Cerema (2011) Acceptabilité de matériaux alternatifs en technique routière - Evaluation environnementale
Cuney M, Le Cloirec M, Ollivier J (1995) Application Des Tests de Lixiviation (NFX 31–210) à Des Produits Naturellement Exposés à l’action de l’eau, March
Del Rey I, Ayuso J, Galvín AP, Jiménez JR, López M, García-Garrido ML (2015) Analysis of chromium and sulphate origins in construction recycled materials based on leaching test results. Waste Manage 46(December):278–286. https://doi.org/10.1016/j.wasman.2015.07.051
Dijkstra JJ, Meeussen JCL, Van der Sloot HA, Comans RNJ (2008) A consistent geochemical modelling approach for the leaching and reactive transport of major and trace elements in MSWI bottom ash. Appl Geochem 23(6):1544–1562. https://doi.org/10.1016/j.apgeochem.2007.12.032
DIN (2009) DIN 19528 - Leaching of solid materials - percolation method for the joint examination of the leaching behaviour of inorganic and organic substances
Diotti A, Galvin AP, Piccinali A, Plizzari G, Sorlini S (2020) Chemical and leaching behavior of construction and demolition wastes and recycled aggregates. Sustainability 12(December):10326. https://doi.org/10.3390/su122410326
Engelsen CJ, van der Sloot HA, Petkovic G (2017) Long-term leaching from recycled concrete aggregates applied as sub-base material in road construction. Sci Total Environ 587–588(June):94–101. https://doi.org/10.1016/j.scitotenv.2017.02.052
Kim K, Yang W, Nam K, Choe JK, Cheong J, Choi Y (2018) Prediction of long-term heavy metal leaching from dredged marine sediment applied inland as a construction material. Environ Sci Pollut Res 25(27):27352–27361. https://doi.org/10.1007/s11356-018-2788-2
Kosson DS, van der Sloot HA, Sanchez F, Garrabrants AC (2002) An integrated framework for evaluating leaching in waste management and utilization of secondary materials. Environ Eng Sci 19(3):159–204. https://doi.org/10.1089/109287502760079188
Liu H, Zhang J, Li B, Zhou N, Li D, Zhang L, Xiao X (2021) Long term leaching behavior of arsenic from cemented paste backfill made of construction and demolition waste: experimental and numerical simulation studies. J Hazard Mater 416(August):125813. https://doi.org/10.1016/j.jhazmat.2021.125813
Maia, Margarida Braga, Jorge De Brito, Isabel M. Martins, Silvestre JD (2018) Toxicity of recycled concrete aggregates: review on leaching tests. Open Construct Build Technol J 12(1). https://doi.org/10.2174/1874836801812010187
NEN (2004) NEN 7373 - Leaching characteristics - determination of the leaching of inorganic components from granular materials with a column test - solid earthy and stony materials.
Regulation (EU) No 305/2011 (2011) of the European Parliament and of the Council of 9 March 2011 laying down harmonised conditions for the marketing of construction products and repealing Council Directive 89/106/EEC text with EEA relevance. OJ L. Vol. 088. http://data.europa.eu/eli/reg/2011/305/oj/eng.
Roque AJ, Martins IM, Freire AC, Neves JM, Antunes ML (2016) Assessment of environmental hazardous of construction and demolition recycled materials (C&DRM) from laboratory and field leaching tests application in road pavement layers. Procedia Eng 143:204–211. https://doi.org/10.1016/j.proeng.2016.06.026
Schafer ML, Clavier KA, Townsend TG, Kari R, Worobel RF (2019) Assessment of the total content and leaching behavior of blends of incinerator bottom ash and natural aggregates in view of their utilization as road base construction material. Waste Manage 98(October):92–101. https://doi.org/10.1016/j.wasman.2019.08.012
Suzuki S, Katoh M (2020) Estimation of potential arsenic leaching from its phases in excavated sedimentary and metamorphic rocks. Environ Geochem Health 42(2):407–418. https://doi.org/10.1007/s10653-019-00371-x
Tossavainen M, Forssberg E (1999) The potential leachability from natural road construction materials. Sci Total Environ 239(1):31–47. https://doi.org/10.1016/S0048-9697(99)00283-1
Van der Sloot HA, Dijkstra J (2004) Development of horizontally standardized leaching tests for construction materials: a material or release based approach? Identical leaching mechanisms for different materials. https://doi.org/10.13140/RG.2.2.11986.76486
Van der Sloot HA, Kosson DS (2012) Use of characterisation leaching tests and associated modelling tools in assessing the hazardous nature of wastes. Journal of Hazardous Materials, Selected papers presented at the 2nd International Conference CRETE 2010, October 2010 - Industrial and Hazardous Waste Management, 207–208 (March):36–43. https://doi.org/10.1016/j.jhazmat.2011.03.119
Van der Sloot HA, Meeussen JCL, Garrabrants AC, Kosson DS, Fuhrmann M (2009) Review of the physical and chemical aspects of leaching assessment. Energy Res Centre Netherlands
Verbinnen B, Van Caneghem Jo, Billen P, Vandecasteele C (2017) Long term leaching behavior of antimony from MSWI bottom ash: influence of mineral additives and of organic acids. Waste Biomass Valorization 8(7):2545–2552. https://doi.org/10.1007/s12649-016-9796-6
Vollprecht D, Berger M, Altenburger-Junker I, Neuhold S, Sedlazeck KP, Aldrian A, Dijkstra JJ, van Zomeren A, Raith JG (2019) Mineralogy and leachability of natural rocks–a comparison to electric arc furnace slags. Minerals 9(8):501. https://doi.org/10.3390/min9080501
Wang Z, Ma J, Li J, Wei G, Zeng Ti, Li L, Zhang Le, Deng W, Xie L, Liu Z (2018) Fe (hydro) oxide controls Mo isotope fractionation during the weathering of granite. Geochim Cosmochim Acta 226(April):1–17. https://doi.org/10.1016/j.gca.2018.01.032
Wiebusch, Bernd, Masaaki Ozaki, Haruki Watanabe, Carl Franz Seyfried (1998) Assessment of leaching tests on construction material made of incinerator ASH (sewage sludge): investigations in Japan and Germany. Water Science and Technology, Water Quality International ‘98, 38 (7):195–205. https://doi.org/10.1016/S0273-1223(98)00621-0
Zhang, Yibo, Jiannan Chen, Matthew Ginder-Vogel, Tuncer Edil (2018) Effect of pH and grain size on the leaching mechanism of elements from recycled concrete aggregate. In, 325–34. https://doi.org/10.1007/978-981-13-0128-5_37
Acknowledgements
This work was supported by Union Nationale des Producteurs de Granulats (UNPG) and Routes de France. Thanks to Isabelle Laurent, analytical chemist from the Chemistry Department of CY Cergy Paris Université for her help performing ICP-OES and IC.
Funding
This work was supported by Union Nationale des Producteurs de Granulats (UNPG) and Routes de France. The authors have no financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, experimental data collection, and analysis were performed by Martial Clavier. First draft of the manuscript was written by Martial Clavier. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The manuscript does not contain data which requires ethical approval.
Consent to participate
All the authors have consented to participate.
Consent for publication
All the authors have consented to publish.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Clavier, M., Ndiaye, K., Aggoun, S. et al. Investigation on the leaching behavior of natural aggregates using percolation test and total content. Environ Sci Pollut Res 31, 43339–43350 (2024). https://doi.org/10.1007/s11356-024-33972-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33972-w