Abstract
Application of municipal solid and wood waste, as dominant sources of biomass, could be a promising alternative for producing energy from renewables via thermochemical gasification technology. In this paper, a study of thermogravimetric analysis (TGA) and excurrent gas composition produced by the municipal solid waste (MSW) and wood biomass gasification is presented. Thermogravimetric and heat flow curves for waste samples were performed at the temperature interval of 20–890 °C with a heating rate of 10 °C min−1 under a nitrogen atmosphere. According to thermal analysis data, differential scanning calorimetry (DSC) curves, the degradation stages of waste samples was determined, which correspond to the mono- or bimodal evolution of volatile compounds and the degradation of the resulting carbon residue. The gasification experiments were conducted in a high-pressure quartz reactor at temperatures of 850, 900, and 950 °C, using steam (0.3 g/min) and argon (2 dm3/min) as the gasifying agents. To ascertain the syngas composition, gas chromatography was employed in conjunction with a thermal conductivity detector. Both types of biomass showed remarkably similar syngas compositions. The highest concentration of hydrogen-rich gases was recorded at 950 °C for wood biomass, with 42.9 vol% and 25.2 vol% for hydrogen (H2) and carbon monoxide (CO), and for MSW, with an average 44.2 vol% and 18 vol% for H2 and CO. Higher temperatures improved the syngas composition by promoting endothermic gasification reactions, increasing hydrogen yield while decreasing tar and solid yields. This research helped to comprehend the evolution of the gasification process and the relationship between increased H2 and CO production as the gasification temperature increased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the size of the effects that municipal solid waste (MSW) has on the environment and the effects that follow, technological processes need to be improved so that one or more types of marketable products can be made from waste. Despite some difficulties and a sufficient number of solutions, processing municipal solid waste continues to be a significant challenge because of the rise in generated waste and its accumulation.
According to Kaza et al. (2018), the amount of MSW produced annually in 2016 was 2010 million tonnes, and by the year 2050, that number is predicted to be close to 3400 million tonnes. An enormous amount of global waste (approximately 33%) is usually collected at open dump sites, which remain the preferred waste disposal method in underdeveloped and developing countries. Uzbekistan is the only country in Central Asia with a population of over 33 million citizens. Subsequently, when compared with other countries in Central Asia such as Kazakhstan, Tajikistan, Turkmenistan, and the Kyrgyz Republic, the amount of MSW generated is significantly larger. There are serious problems with proper solid waste disposal not only in Uzbekistan but also around the world. According to the report published by the State Committee on Statistics of the Republic of Uzbekistan, the republic produces 35 million m3 of municipal solid waste per year. In addition, each year, 100 million tons of waste from industries and municipalities has been disposed of by dumping it in landfills, which is the method that is now in use. So far, the country’s landfills have amassed more than 2 billion tons of waste (Zaynutdinova 2017; Adilova 2017; Sharma et al. 2018). Sharma et al. (2018) reported that the bulk organic fraction of waste in underdeveloped and developing countries surpasses 50%, resulting in the production of numerous hazardous gases such as H2S, SO2, CO2, CH4, and NH3 and leachate at waste disposal sites, posing severe environmental and health issues. Subsequently, it is crucial to identify simple cost-effective techniques for solid waste reduction or elimination which would equally help to not only achieve public health and environmental standards but also contribute in effective management of some global issues like climate change and global warming.
The depletion of fossil fuels is another challenging issue that has resulted in a severe energy crisis and is a barrier to the sustainable advancement of human society. It has proven to be extremely difficult to build a clean and sustainable energy system that uses hydrogen instead of fossil fuels. Biomass is a potential source of hydrogen for clean and sustainable energy (García-Nieto et al. 2023; Sudalaimuthu and Sathyamurthy 2023). As alternative fuels, municipal solid and wood wastes, as one of the sources of biomass, have drawn a lot of interest. These fuels are not appropriate for power generation through direct combustion, which has low efficiency on a small scale due to their wide distribution and low density. As a result, several researchers have investigated gasification technology in an effort to create a small-scale, high-efficiency power production system. Since chemical energy is transformed directly into electricity, fuel cell power generation, which employs hydrogen as fuel, can achieve higher efficiency in small-scale applications (Yue et al. 2021; Niakolas et al. 2016). Therefore, it is imperative to develop a technique for producing hydrogen-rich gas from municipal solid and wood wastes. However, knowledge of the specific features of these biomasses is of necessity to develop and implement measures for the economically and environmentally efficient operation of boiler units/reactors (Tursunov et al. 2017, 2018, 2019, 2020a, 2020b).
In this regard, methods of thermal analysis are used to study the processes occurring during the combustion of MSW and wood and the influence of various factors on the efficiency of burning. The sophistication of the technical tools used in thermal analysis research greatly influences its success. First of all, it concerns such physicochemical methods as thermogravimetric analysis (TGA) and differential thermal analysis.
TGA is currently the most extensively used technique for pyrolysis and gasification analysis, as well as in the study of kinetic parameters (Loredo-Medrano et al. 2016; Tursunov et al. 2017, 2020b; Marrot et al. 2022). Following the studies undertaken by Tursunov et al. (2020b), El-Sayed and Mostafa (2020) and Seo et al. (2010), they stated that TGA offers quick quantitative approaches for the investigation of processes within non-isothermal or isothermal circumstances and facilitates the determination of efficient kinetic parameters for diverse decomposition reactions. In recent years, the TGA research of typical MSW components has been widely studied, and great progress has been achieved on thermal waste treatment technologies (Kantarelis et al. 2011; Zhou et al. 2015; Tursunov et al. 2017). Garcia et al. (1995) found that the weight loss from MSW pyrolysis was caused by two separate reactions: (i) the breakdown of the cellulosic fraction happened between 300 and 380 °C and (ii) the breakdown of the other fraction (paper, plastics, and polyethylene mixtures) happened between 200 and 500 °C. Chen et al. (2015) used TGA to investigate the pyrolysis and gasification characteristics of varieties of solid waste. The results obtained showed that the pyrolysis properties of eight different kinds of materials were similar when the pyrolysis temperature was slightly higher than 600 °C and the gasification temperature was higher than 700 °C. A different team of researchers under the direction of Ansah et al. (2016) conducted research on the calorimetric and thermogravimetric characteristics during the pyrolysis of paper, wood, polyethylene terephthalate plastic, and textiles in MSW, as well as co-pyrolysis of plastic components and biomass with and without torrefaction p processes. The results obtained indicated that if the biomass fraction is dominant, there is considerable interaction between PET plastic and biomass during co-pyrolysis. In addition to aforementioned references, a number of authors (Zhaosheng et al. 2009; Maoyun et al. 2010; Nzioka et al. 2019) have also applied TGA for the description of the decomposition of MSW components and woody biomass.
Hydrogen production from biomass is a promising technology. This technology uses air, or oxygen-enriched air, and steam together as the agents of gasification to transform the biomass into a gaseous product. Hydrogen, carbon monoxide, and high-molecular-weight hydrocarbons are the main components of the excurrent gas that can coagulate at room temperature (Nyashina et al. 2023). Low-moisture biomass (less than 30%), such as MSW and wood, can be gasified to create gas in a partial oxidation environment at temperatures between 700 and 1200 °C. The main factor influencing the gasification process is fuel moisture. The use of fuel with a high moisture content is not restricted. However, fuels with moisture contents of 30% or more have issues with igniting and have a negative impact on the calorific value of syngas. In other words, the gasifier temperature will drop when feedstock has a high moisture content, resulting in an incomplete pyrolysis process during gasification (Dong et al. 2016; Tursunov et al. 2017, 2018, 2019, 2020a, 2020b). The syngas is used in a variety of processes, including the synthesis of ammonia and methanol in the chemical sector as well as the production of hydrogen. The energy sector also makes use of syngas (if obtained from biomass, it is regarded as renewable). Drying at 100 to 200 °C is the first stage of the gasification process. Between 200 and 500 °C, biomass is pyrolyzed, and its parts are broken down into char and gases like hydrogen (H2), carbon monoxide (CO), methane (CH4), and carbon dioxide (CO2). There is also a tiny amount of non-condensable gas like carbon dioxide (pyrolysis oil). This pyrolysis oil is broken down into explosive gases like hydrogen. Through a reforming procedure, it will ultimately transform into a non-condensable gas that is enriched in hydrogen (Uddin et al. 2013; Santamaria et al. 2021; Villafán-Vidales et al. 2022; Lopez et al. 2022). Over the past several decades, researchers have investigated theoretical and practical studies on biomass steam gasification. When steam is added to the biomass steam gasification process, Li et al. (2014), Lang et al. (2015), and Chianese et al. (2015) found that the H2 in H2O could be moved to make more H2 through a process called water–gas shift (WGS). However, throughout the gasification process, chemical reaction equilibrium limits the concentration of hydrogen (Li et al. 2011; Huang and Jin 2019). Additionally, during the biomass steam gasification process, significant volumes of CO and CO2, as well as negligible amounts of CH4 and tar, are produced simultaneously.
The impact of gasification temperature on the efficiency of the process and the generation of residues has been examined recently. Low temperatures led to low reaction rates and low H2 production, but higher temperatures promoted larger reaction rates in endothermic reactions such as the formation of water gas and methane reforming (Li et al. 2011; Adiya et al. 2017; Ambrosetti et al. 2022). These endothermic reactions have been shown to increase with temperature between 900 and 950 °C. Moreover, according to the literature, by increasing the gasification temperature, the carbon conversion efficiency and concentration of the resulting H2 increase, and the amount of tar in the syngas decreases (Guangul et al. 2012; Dascomb et al. 2013). When the feedstock and gasification process differ, the concentrations of CH4 and CO do not exhibit a consistent pattern for the same condition (Sikarwar et al. 2016; Ramachandriya et al. 2016; Mishra and Upadhyay 2021). To fully comprehend the advantages of manipulating the gasification temperature, it is necessary to conduct an experiment with a specific gasification method and feedstock.
To better understand the evolution of the gasification process, a novel experimental approach to municipal solid and wood waste gasification is proposed. This approach involves simultaneously studying the thermal decomposition process using TGA and the calorimetric characteristics of the wood waste and mixed MSW with the goal of determining the kinetic properties of the thermal conversion mechanism for wood and municipal solid wastes and the correlation between the production of hydrogen-rich gas and the gasification temperature, as well as the impact of the gasification temperature on the composition of the syngas. Additionally, the majority of the study on the gasification process that has been published in the literature relies on the ex situ method, in which measurements are made using char that has been produced in advance and cooled. Therefore, in order to more accurately represent the gasification process that takes place in commercial gasifiers, we adopted a novel strategy in which raw material samples rather than their char were exposed to gasification tests. This paper investigated the composition of the syngas from the gasification of Uzbekistan’s MSW and wood biomass at various temperatures for the aforementioned purposes. Also, this study tries to propose a method to improve the syngas quality by using a steam as a gasifying media for MSW and wood biomass gasification. This research makes part of the first large-scale fundamental study on thermochemical gasification of local MSW and wood biomass and their potential for energy generation in Uzbekistan. In addition, it is necessary to emphasize that this is the first such study that is carried out in Uzbekistan.
Materials and methods
MSW and wood biomass sampling
The method used for sampling the waste stream’s representatives of MSW and wood was based on international standards set out by the European PN-EN and American Society for Testing and Materials (ASTM) 5231. Approximately 100 kg of waste was collected for the sample and examined at the renewable energy research laboratory at TIIAME National Research University, Uzbekistan. The MSW samples were a mixture of 10 distinct components, including wood, kitchen waste, plastic bags, solid plastic, textiles, glass, rubber, and ferrous elements. Next, the waste was segregated according to the chosen categorization. The samples were crushed and sieved into particles that were roughly 2–3 mm in size prior to the experiments.
Proximate and ultimate tests
Ultimate analysis determined the CHS in MSW and wood biomass samples. We used the CHS-580 analyzer by Eltra for carbon, hydrogen, and sulfur content detection.
The American ASTM-E871 and the European PN-EN 14774–3:2010 were used to figure out how much water, volatile matter, fixed carbon, and ash were in MSW and wood biomass. The Eltra TGA Thermostat analyzer was used to examine the above-mentioned parameters. The calorific value or high heating value (HHV) was calculated using a Leco AC calorimeter. Table 1 displays the findings from the proximate and ultimate analyses.
Thermogravimetric test
Thermogravimetric analysis was performed using Simultaneous Thermal Analysis (STA) 6000, PerkinElmer. The STA 6000 integrates two different analytical methodologies: TGA and DSC. The STA 6000 concurrently captures DSC heat flow data and TGA weight loss data. DSC heat flow is measured in milliwatts and joules per gram. The STA 6000 measures a sample’s temperature (relative to a reference) and weight as a function of the temperature and/or time. All TGA experiments were carried out in a nitrogen atmosphere at temperatures ranging from 20 to 890 °C and at a heating rate of 10 °C min−1. The results were displayed as a function of temperature, and the weight loss that occurred during measurement was continuously recorded.
Gasification procedure (thermovolumetry analysis)
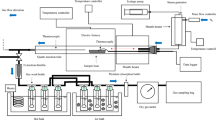
The experiments were conducted using specialized laboratory equipment that allows examination of the gasification process with high-pressure steam, not under atmospheric pressure. The applied equipment was extensively described in the works of Porada et al. (2016) and Śpiewak et al. (2021), and its diagram is shown in Fig. 1. The apparatus consists of three fundamental systems: a high-pressure reactor with a heating system, a system for feeding the reactor with gasifying agent (steam) and fuel, and a system for analyzing and collecting the produced gas. There is a 20-mm-diameter quartz retort with a grate inside the reactor. After stabilizing the parameters of the isothermal measurements, such as temperature of 850, 900, and 950 °C; pressure of 10 bar; steam flow of 0.3 g/min; and argon flow of 2 dm3/min, 1 g of the fuel sample was introduced onto the grate, and the resulting gas was analyzed. A piston feeder that has been properly built is used for this. To move the piston, the inlet valve on the pipe supplying the gas into the feeder chamber must be opened. Using an electric oven, the retort is heated while holding a sample. The quartz reactor’s pressure casing is made of a heat-resistant steel blanket, the ends of which are sealed off with lids fitted with pipes for providing gasifying agent and pipes for removing the produced gas. The insulation for the oven is made of mineral wool that is installed inside the casing. A thermocouple type K sensor that also helped to give commands to the controller-programmer to keep the sample at the required temperature measures the temperature of the biomass sample. The tar and water condensate are separated in the condenser, where they are then completely dried and removed from the filter. The resultant gas then runs through the filter. Following decompression, an analyzer based on infrared radiation adsorption continuously determines the amounts of carbon dioxide, carbon monoxide, and methane in the resultant gas. In addition, gas samples were taken for examination using a gas chromatograph that had a thermal conductivity detector (TCD) attached to it in order to determine the amount of hydrogen present in the samples. Each measurement was carried out at least twice to ensure repeatability of the results, while the manuscript presents the results obtained based on one repeatable measurement. The statistical range of the derived results varied based on the measurement, whereas the errors had no impact on the observed general dependencies.
Formation rate and carbon conversion degree estimation
The production rates for each of these byproducts were calculated by measuring how much carbon dioxide, hydrogen, methane, and carbon monoxide were in the gas that was made when the tested samples were gasified. The information acquired allowed for the estimation of the tested products’ yields and the degree of carbon conversion.
The equation below can be used to represent the formation rate of a specific product:
where t is time (min); \(\dot{V}\) volumetric flow of the resulting gas (cm3/min); and Ci(t) concentration of the given product at time t (vol.%).
The yields of a given product were calculated using the following equation:
where Ci(j + 1) is the concentration of a given product over time t(j + 1) (vol.%) and Ci(j) concentration of a given product over time t(j) (vol.%).
Finally, the carbon conversion degree can be estimated on the basis of the following formula:
where \({V}_{\mathrm{CO}},{V}_{{\mathrm{CO}}_{2}},{V}_{{\mathrm{CH}}_{4}}\) are volume of formed gas component (dm3/g), Mc molar mass of carbon (g/mol), m sample mass (g), Cdaf dry ash free carbon content (-), and Vmol volume of 1 mol of gas at temperature of 273 K and pressure of 101,325 Pa (dm3/mol).
Results and discussion
Thermogravimetric tests
Figure 2 shows the thermogravimetric and heat flow curves for a municipal solid waste sample heated to 890 °C with a heating rate of 10 °C min−1 under a nitrogen atmosphere using a thermogravimetric analyzer. The MSW is made up of many complex elements that are typically grouped into five categories: food residue, paper, wood and bamboo, rubber and plastic, and textiles (Zhou et al. 2014). The pyrolysis process is different for different types of municipal solid waste (MSW) (Zhou et al. 2015), and the curve shown in Fig. 3 is for MSW that has a lot of low molecular compounds (Nzioka 2017). As can be seen in Fig. 2, three zones can be distinguished, namely, (1) dehydration, (2) fast decomposition of high molecular substances such as cellulose and lignin, and (3) slow degradation of lower molecular compounds with the formation of char. Due to the release of surface tension–constrained external or superficial water as well as the elimination of physically absorbed water in the waste material, dehydration zones, which peak with a maximum at 49 °C, demonstrate an initial mass loss (of about 3.4%) between ambient temperature and 160 °C. Then, two zones of MWS waste devolatilization, associated with the decomposition of appropriate waste components, were observed.
According to research conducted by Buah et al. (2007) or Sorum et al. (2001), the dominant weight loss of the MSW cellulosic matter occurred between 250 and 400 °C; thermal degradation of polypropylene, polystyrene, high-density polyethylene, and low-density polyethylene occurred between 350 and 500 °C, whereas polyvinylchloride degraded in two major stages between (1) 200 and 380 °C (connected to the release of chlorine and occurred in the same temperature range as for the hemicellulose and cellulose fractions of paper) and (2) 380 and 550 °C (connected to degradation of the remaining hydrocarbons and occurred at similar temperatures as for the other plastics). Therefore, it may be concluded that the second zone, between 180 and 450 °C, was attributed to both devolatilizations of hemicelluloses and cellulose fractions from lignocellulosic and cellulosic-based materials. As a result, 19.7% of the initial sample weight has been lost. The third zone of weight loss, occurring between 450 and 890 °C and showing a peak with a maximum at 803 °C, may be due mainly to the degradation of plastic components (including PP devolatilization) with minor contributions from lignin degradation. In truth, these changes are usually observed in the temperature range up to 500 °C (Chen et al. 2015; Gunasee et al. 2016), but according to Nzioka et al. (2019), degradation of lower molecular compounds may occur until even 900 °C. As a consequence of the prolonged duration of Zone 3, the highest weight loss was recorded, amounting to about 23.7%.
Figure 3 shows the thermogravimetric and heat flow curves for a biomass waste sample heated to 890 °C with a heating rate of 10 °C min−1 under a nitrogen atmosphere using a thermogravimetric analyzer. Considering that cellulose, hemicellulose, lignin, and extractives are major parts of biomass and that the thermal degradation profiles of biomass samples are interpreted as the sum of the degradations of their major parts, Uzun et al. (2016) were able to identify some typical zones of biomass sample breakdown. In agreement with this fact, in Fig. 3, three main zones with corresponding peaks can be observed. The first zone, between the initial temperature and up to about 100 °C (peak at 47 °C), is responsible for the release of moisture retained in the biomass (and possibly gases adsorbed on the surface, such as CO2). During this zone, weight loss was low, on the order of 3.5%. As the temperature rises, the process of decomposition of hemicellulose, cellulose, and lignin begins. According to literature reports, hemicellulose decomposes between 220 and 320 °C; cellulose decomposes between 250 and 360 °C, whereas lignin undergoes gradual decomposition in a broad range of temperatures, between 80 and 550 °C (Yu et al. 2017). Nevertheless, these temperature ranges are contractual, and many reports indicate that decomposition of biomass components may occur within the broader limits and at higher temperatures; however, the superposition of the last three stages is commonly observed (Dorez et al. 2014). Thus, in Fig. 3, two more zones at different temperature ranges connected with the decomposition process may be distinguished. The second zone, between 220 and 490 °C, shows a maximum peak at 353 °C and corresponds to the pyrolysis process of hemicellulose and cellulose. In this zone, most of the weight loss occurs (above 17%). In turn, the third zone, during which 14.1% of mass loss was observed, commences at about 520 °C and lasts to the final temperature (with a peak at temperatures between 695 and 780 °C). This zone may be attributed to the decomposition process of lignin and cellulose, which occurs slowly and may exceed the temperature range of the pyrolytic reaction (Lapuerta et al. 2004; Zhai et al. 2016). Long et al. (2017) also reached a similar conclusion based on the peak at around 800 °C, regarded as representing the decomposition of lignin.
Changes in the formation rates of gas components
Figures 4 and 5 show how the gasification of MSW and wood biomass changes over time, as well as how the production rates of H2, CO, CO2, and CH4 change at 850, 900, and 950 °C. In any process, there are two distinct stages that can be identified: First, there is pyrolysis, which is the rapid release of volatiles that is seen as a peak at the start of the process. Due to the fact that analyzed samples belong to reactive materials, this stage was very intense, and formation rates of all gas components were the highest. Next comes gasification, which is a much slower, more prolonged stage of char conversion by reaction with steam. Syngas (H2 + CO) was created as a result. The syngas that persisted in the high-temperature zone for a sufficient amount of time may support further reactions, such as the water gas shift reaction (especially in wood samples that are known to contain alkali and alkaline earth metals in the mineral matter (Śpiewak et al. 2023). Thus, CO2 was produced; however, CH4 was only released during the pyrolysis stage. Following CO and CO2 (which had similar formation rates), hydrogen formation rates were highest during both stages of MSW and wood biomass gasification. Methane was released at a fast rate during pyrolysis, but because it was produced so quickly, CH4 was not an essential component of the resultant gas (syngas).
The major syngas component (hydrogen) formed at a faster rate when the temperature was raised, which also resulted in a shorter processing time. However, in each instance, the nature of particular curves remained the same; i.e., two fundamental processes took place (except CH4, which was released only during pyrolysis). The following subsections describe the specific impact of temperature on gasification reactions.
In general, high temperatures promote gasification by accelerating secondary hydrocarbon cracking and shifting processes toward hydrocarbon (CnH2n) conversion. This may be due to the contribution of reforming reactions that are favored and occur at high temperatures, thus becoming dominant at temperatures greater than 800 °C. The composition of the resulting gas is determined by the common reactions that lead to increased synthesis gas yields during the conversion of tar to synthesis gas.
The following stage involved the development of carbon conversion degree curves. Figure 6 shows the conversion degrees for the MSW and wood biomass at 850, 900, and 950 °C. The study of the given curves shows that temperature has a considerable influence on the degree of conversion. According to a comparative analysis of the samples, the higher reactivity for both of the analyzed biomass samples was at 900, especially at 950 °C. In comparison with wood biomass, the carbon conversion degree for MSW was slightly higher. These variations in conversion degree and reactivity could be attributed to the alkali index and the O/C ratio.
The kinetic curves of the reactions that formed the studied gases, which were taken from the gasification experiments, made it possible to figure out how much product gas was made. Figures 7 and 8 show the values found for 1 g of MSW and wood biomass species in ash-free and dry states, as well as the total gas yields (vol. cm3), and their shares. An examination of the data presented points to the fact that, in most cases, the yields of syngas (hydrogen and carbon monoxide) increased with increasing temperature. However, in the case of MSW, which was gasified at 950 °C, carbon dioxide content was slightly higher than carbon monoxide, but it did not influence the quality of syngas, which was rich in hydrogen (908.2 vol/cm3).
Lahijani and Zainal (2011) found that as the gasification temperature went up, more hydrogen was formed in both MSW and wood biomass. This started endothermic methane steam reforming (Eqs. 4 and 5) and dry reforming (Eq. 6). The consumption of methane in these three reactions (Eqs. 4, 5, and 6) contributes to the reduction of the methane composition. Other studies (Liu et al. 2002; Parthasarathy and Narayanan 2014) also show that higher temperatures lead to more hydrogen in gas yields. This is because higher temperatures provide energy for endothermic reactions that produce hydrogen, which increases the amount of hydrogen in the gas yield. At high temperatures, the water–gas reaction has a greater contribution than the Boudouard reaction. In addition to high temperatures, water–gas shift reaction causes an increase in hydrogen concentration in the syngas and higher temperatures tend to favor steam reform of methane to CO and H2 (Albuloushi 2018; Ebrahimi et al. 2020; LeValley et al. 2022).
Also, wood biomass produces a lesser amount of H2, CO, CO2, and CH4 compared with MSW due to the lower volatile matter in wood biomass (32.09%) than in MSW (47.29%), which contributes to the lower reactivity in gasification applications (Bach et al. 2019).
The yields of tar and char decrease as the gasification temperature rises due to an increase in gas production (Ponzio et al. 2006; Feng et al. 2011; Cortazar et al. 2018). The larger release of gases during the initial devolatilization stage can be explained by the increased amount of gas produced at higher temperatures, as well as the secondary reactions that the tar and char undergo (tar cracking and reforming and char gasification) (Bronson et al. 2012; Ra et al. 2014; Bates et al. 2017). The composition of the gas is also influenced by temperature. Typically, when temperatures rise, H2 concentrations rise while CO and light hydrocarbons tend to decline. However, thermodynamic models have demonstrated that at exceedingly high reaction temperatures, the H2 yield decreases gradually (Mahishi and Goswami 2007; Ávila-Neto et al. 2009). Hence, it is critically important to control the gasification temperature to a certain level. Thereby, according to the overall analysis, it can be inferred that temperature has a significant impact on enhancing the synthesis gas ratio (H2 + CO) and concentration.
Conclusion
According to the study, TGA and heat flow curve tests showed the total weight loss for the decomposition of MSW (53.2%) and for wood (35%) between temperatures of 39 and 890 °C. The effect of gasification temperature on the resultant gases’ composition, particularly on syngas (H2 + CO), was conducted at 850, 900, and 950 °C in a high-pressure quartz reactor using steam and argon as gasifying agents. The composition of the syngas is influenced by a number of variables, including gasification temperature. At higher temperatures for wood gasification, such as 900 and 950 °C, gas concentrations of H2 and CO increased while CO2 and CH4 content decreased. As for the MSW gasification, gas concentrations of H2 and CO2 increased, while CH4 and CO decreased. If the composition of the gas would be the priority, 950 °C would be the ideal temperature to create the maximum concentration of syngas under the selected conditions.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Adilova M (2017) The problem of primary sorting of household waste. Env Saf Civ Ini 8:10–13
Adiya ZISG, Dupont V, Mahmud T (2017) Chemical equilibrium analysis of hydrogen production from shale gas using sorption enhanced chemical looping steam reforming. Fuel Process Technol 159:128–144. https://doi.org/10.1016/j.fuproc.2017.01.026
Albuloushi A (2018) Optimization of a water gas shift reaction. Undergraduate J Mathem Model: One + Two 8(2):5. https://doi.org/10.5038/2326-3652.8.2.4892
Ambrosetti M, Bonincontro D, Balzarotti R, Beretta A, Groppi G, Tronconi E (2022) H2 production by methane steam reforming over Rh/Al2O3 catalyst packed in Cu foams: a strategy for the kinetic investigation in concentrated conditions. Catal Today 387:107–118. https://doi.org/10.1016/j.cattod.2021.06.003
Ansah E, Wang L, Shahbazi A (2016) Thermogravimetric and calorimetric characteristics during co-pyrolysis of municipal solid waste components. Waste Manag 56:196–206. https://doi.org/10.1016/j.wasman.2016.06.015
Ávila-Neto CN, Dantas SC, Silva FA, Franco TV, Romanielo LL, Hori CE, Assis AJ (2009) Hydrogen production from methane reforming: thermodynamic assessment and autothermal reactor design. J Nat Gas Sci Eng 1(6):205–215. https://doi.org/10.1016/j.jngse.2009.12.003
Bach QV, Gye HR, Song D, Lee CJ (2019) High quality product gas from biomass steam gasification combined with torrefaction and carbon dioxide capture processes. Int J Hydrog Energy 44:14387–14394. https://doi.org/10.1016/j.ijhydene.2018.11.237
Bates RB, Ghoniem AF, Jablonski WS, Carpenter DL, Altantzis Ch, Garg A, Barton JL, Chen R, Field RP (2017) Steam-air blown bubbling fluidized bed biomass gasification (BFBBG): multi-scale models and experimental validation. Part Technol Fluidization 63(5):1543–1565. https://doi.org/10.1002/aic.15666
Bronson B, Preto F, Mehrani P (2012) Effect of pretreatment on the physical properties of biomass and its relation to fluidized bed gasification. Environ Prog Sustain Energy 31(3):335–339. https://doi.org/10.1002/ep.11659
Buah WK, Cunliffe AM, Williams PT (2007) Characterization of products from the pyrolysis of municipal solid waste. Process Saf Environ Prot 85(5):450–457. https://doi.org/10.1205/psep07024
Chen S, Meng A, Long Y, Zhou H, Li Q, Zhang Y (2015) TGA pyrolysis and gasification of combustible municipal solid waste. J Energy Inst 88:332–343. https://doi.org/10.1016/j.joei.2014.07.007
Chianese S, Loipersböck J, Malits M, Rauch R, Hofbauer H, Molino A, Musmarra D (2015) Hydrogen from the high temperature water gas shift reaction with an industrial Fe/Cr catalyst using biomass gasification tar rich synthesis gas. Fuel Process Technol 132:39–48. https://doi.org/10.1016/j.fuproc.2014.12.034
Cortazar M, Alvarez J, Lopez G, Amutio M, Santamaria L, Bilbao J, Olazar M (2018) Role of temperature on gasification performance and tar composition in a fountain enhanced conical spouted bed reactor. Energy Convers Manag 171:1589–1597. https://doi.org/10.1016/j.enconman.2018.06.071
Dascomb J, Krothapalli A, Fakhrai R (2013) Thermal conversion efficiency of producing hydrogen enriched syngas from biomass steam gasification. Int J Hydrog Energy 38(27):11790–11798. https://doi.org/10.1016/j.ijhydene.2013.07.022
Dong J, Chi Y, Tang Yu, Ni M, Nzihou A et al (2016) Effect of operating parameters and moisture content on municipal solid waste pyrolysis and gasification. Energy Fuels 30(5):3994–4001. https://doi.org/10.1021/acs.energyfuels.6b00042
Dorez G, Ferry L, Sonnier R, Taguet A, Lopez-Cuesta JM (2014) Effect of cellulose, hemicellulose and lignin contents on pyrolysis and combustion of natural fibers. J Anal Appl Pyrolysis 107:323–331. https://doi.org/10.1016/j.jaap.2014.03.017
Ebrahimi P, Kumar A, Khraisheh M (2020) A review of recent advances in water-gas shift catalysis for hydrogen production. Emergent Mater 3:881–917. https://doi.org/10.1007/s42247-020-00116-y
El-Sayed SA, Mostafa ME (2020) Thermal pyrolysis and kinetic parameter determination of mango leaves using common and new proposed parallel kinetic models. RSC Adv 10:18160–18179. https://doi.org/10.1039/D0RA00493F
Feng Y, Xiao B, Goerner K, Cheng G, Wang J (2011) Influence of catalyst and temperature on gasification performance by externally heated gasifier. Smart Grid Renew Energy 2:177–183. https://doi.org/10.4236/sgre.2011.23021
Garcia A, Marcilla A, Font R (1995) Thermogravimetric kinetic study of the pyrolysis of municipal solid waste. Thermochim Acta 254:277–304. https://doi.org/10.1016/0040-6031(94)02002-6
García-Nieto PJ, García-Gonzalo E, Paredes-Sánchez BM et al (2023) Modelling hydrogen production from biomass pyrolysis for energy systems using machine learning techniques. Environ Sci Pollut Res 30:76977–76991. https://doi.org/10.1007/s11356-023-27805-5
Guangul FM, Sulaiman ShA, Raghavan VR (2012) Gasification and effect of gasifying temperature on syngas quality and tar generation: a short review. AIP Conf Proc 1440:491–498. https://doi.org/10.1063/1.4704254
Gunasee SD, Carrier M, Gorgens JF, Mohee R (2016) Pyrolysis and combustion of municipal solid wastes: evaluation of synergistic effects using TGA-MS. J Anal Appl Pyrol 121:50–61. https://doi.org/10.1016/j.jaap.2016.07.001
Huang A, Jin S (2019) Investigation of biomass (pine wood) gasification: experiments and Aspen Plus simulation. Energy Sci Eng 7:1178–1187. https://doi.org/10.1002/ese3.338
Kantarelis E, Yang W, Blasiak W, Forsgren C, Zabaniotou A (2011) Thermochemical treatment of E-waste from small household appliances using highly pre-heated nitrogen-thermogravimetric investigation and pyrolysis kinetics. Appl Energy 88:922–929. https://doi.org/10.1016/j.apenergy.2010.08.022
Kaza S, Yao L, Bhada-Tata P, Van Woerden F (2018) What a waste 2.0: a global snapshot of solid waste management to 2050. World Bank Publications, Washington, DC
Lahijani P, Zainal ZA (2011) Gasification of palm empty fruit bunch in a bubbling fluidized bed: a performance and agglomeration study. Biores Technol 102(2):2068–2076. https://doi.org/10.1016/j.biortech.2010.09.101
Lang Ch, Secordel X, Zimmermann Y, Kiennemann A, Courson C (2015) High-temperature water–gas shift catalysts for hydrogen enrichment of a gas produced by biomass steam gasification. C R Chim 18(3):315–323. https://doi.org/10.1016/j.crci.2014.12.004
Lapuerta M, Hernandez JJ, Rodriguez J (2004) Kinetics of devolatilisation of forestry wastes from thermogravimetric analysis. Biomass Bioenergy 27(4):385–391. https://doi.org/10.1016/j.biombioe.2003.11.010
LeValley TL, Richard AR, Fan M (2022) The progress in water gas shift and steam reforming hydrogen production technologies – a review. Int J Hydrog Energy 39(30):16983–17000. https://doi.org/10.1016/j.ijhydene.2014.08.041
Li B, Chen H, Yang H, Wang X, Zhang Sh, Dai Zh (2011) Modeling and simulation of calcium oxide enhanced H2 production from steam gasification of biomass. J Biobased Mater Bioenergy 5:378–384. https://doi.org/10.1166/jbmb.2011.1153
Li B, Wei L, Yang H, Wang X, Chen H (2014) The enhancing mechanism of calcium oxide on water gas shift reaction for hydrogen production. Energy 68:248–254. https://doi.org/10.1016/j.energy.2014.02.088
Liu ZhW, Jun KW, Roh HS, Park SE (2002) Hydrogen production for fuel cells through methane reforming at low temperatures. J Power Sources 111:283–287. https://doi.org/10.1016/S0378-7753(02)00317-8
Long Y, Meng A, Chen S, Zhou H, Zhang Y, Li Q (2017) Pyrolysis and combustion of typical wastes in a newly designed macro thermogravimetric analyzer: characteristics and simulation by model components. Energy Fuels 31(7):7582–7590. https://doi.org/10.1021/acs.energyfuels.7b00796
Lopez G, Santamaria L, Lemonidou A et al (2022) Hydrogen generation from biomass by pyrolysis. Nat Rev Methods Primers 2:20. https://doi.org/10.1038/s43586-022-00097-8
Loredo-Medrano JA, Bustos-Martínez D, Rivera De La Rosa J, Carrillo Pedraza ES, Flores-Escamilla GA, Ciuta S (2016) Particle pyrolysis modeling and thermal characterization of pecan nutshell. J Therm Anal Calorim 126:969–979. https://doi.org/10.1007/s10973-016-5541-4
Mahishi MR, Goswami DY (2007) Thermodynamic optimization of biomass gasifier for hydrogen production. Int J Hydrog Energy 32(16):3831–3840. https://doi.org/10.1016/j.ijhydene.2007.05.018
Maoyun H, Xiao B, Shiming L, Zhiquan H, Xianjun G, Siyi L, Fan Y (2010) Syngas production from pyrolysis of municipal solid waste (MSW) with dolomite as downstream catalysts. J Anal Appl Pyrol 87:181–187. https://doi.org/10.1016/j.jaap.2009.11.005
Marrot L, Candelier K, Valette J et al (2022) Valorization of hemp stalk waste through thermochemical conversion for energy and electrical applications. Waste Biomass Valor 13:2267–2285. https://doi.org/10.1007/s12649-021-01640-6
Mishra S, Upadhyay RK (2021) Review on biomass gasification: gasifiers, gasifying mediums, and operational parameters. Mater Sci Energy Technol 4:329–340. https://doi.org/10.1016/j.mset.2021.08.009
Niakolas DK, Daletou M, Neophytide SG et al (2016) Fuel cells are a commercially viable alternative for the production of “clean” energy. Ambio 45:32–37. https://doi.org/10.1007/s13280-015-0731-z
Nyashina G, Dorokhov V, Romanov D et al (2023) Gas composition during thermochemical conversion of dry solid fuels and waste-derived slurries. Environ Sci Pollut Res 30:24192–24211. https://doi.org/10.1007/s11356-022-23824-w
Nzioka AM (2017) Kinetic study of the thermal decomposition for mixed municipal solid waste using thermogravimetric analysis. Period Eng Nat Sci 5(3):355–363. https://doi.org/10.21533/pen.v5i3.123
Nzioka AM, Kim M, Hwang H, Kim Y (2019) Kinetic study of the thermal decomposition process of municipal solid waste using TGA. Waste Biomass Valori 10:1679–1691. https://doi.org/10.1007/s12649-017-0183-8
Parthasarathy P, Narayanan KSh (2014) Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield – a review. Renew Energy 66:570–579. https://doi.org/10.1016/j.renene.2013.12.025
Ponzio A, Kalisz S, Blasiak W (2006) Effect of operating conditions on tar and gas composition in high temperature air/steam gasification (HTAG) of plastic containing waste. Fuel Process Technol 87(3):223–233. https://doi.org/10.1016/j.fuproc.2005.08.002
Porada S, Rozwadowski A, Zubek K (2016) Studies of catalytic coal gasification with steam. Pol J Chem Technol 18:97–102. https://doi.org/10.1515/pjct-2016-0054
Ra HW, Seo MW, Yoon SJ et al (2014) Devolatilization characteristics of high volatile coal in a wire mesh reactor. Korean J Chem Eng 31:1570–1576. https://doi.org/10.1007/s11814-014-0061-z
Ramachandriya KD, Kundiyana DK, Sharma AM, Kumar A, Atiyeh HK, Huhnke RL, Wilkins MR (2016) Critical factors affecting the integration of biomass gasification and syngas fermentation technology. AIMS Bioeng 3(2):188–210. https://doi.org/10.3934/bioeng.2016.2.188
Santamaria L, Lopez G, Fernandez E, Cortazar M, Arregi A, Olazar M, Bilbao J (2021) Progress on catalyst development for the steam reforming of biomass and waste plastics pyrolysis volatiles: a review. Energy Fuels 35(21):17051–17084. https://doi.org/10.1021/acs.energyfuels.1c01666
Seo DK et al (2010) Study of the pyrolysis of biomass using thermogravimetric analysis (TGA) and concentration measurements of the evolved species. Anal Appl Pyrol 89(1):66–73. https://doi.org/10.1016/j.jaap.2010.05.008
Sharma B, Vaish B, Srivastava V, Singh S, Singh P, Singh RP (2018) An insight to atmospheric pollution-improper waste management and climate change nexus. In: Oves M, Khan MZ, Ismail IMI (eds) Modern age environmental problems and their remediation. Springer, Singapore, pp 23–47
Sikarwar VS, Zhao M, Clough P, Yao J, Zhong X, Memon MZ, Shah N, Anthony EJ, Fennell PS (2016) An overview of advances in biomass gasification. Energy Environ Sci 9:2939–2977. https://doi.org/10.1039/C6EE00935B
Sorum L, Gronli MG, Hustad JE (2001) Pyrolysis characteristics and kinetics of municipal solid wastes. Fuel 80(9):1217–1227. https://doi.org/10.1016/S0016-2361(00)00218-0
Śpiewak K, Czerski G, Porada S (2021) Effect of K, Na and Ca-based catalysts on the steam gasification reactions of coal. Part I: type and amount of one-component catalysts. Chem Eng Sci 229:116024. https://doi.org/10.1016/j.ces.2020.116024
Śpiewak K, Soprych P, Czerski G (2023) Influence of pressure and sunflower husks ash as catalyst on tire-char steam gasification. Energy Rep 9:1–15. https://doi.org/10.1016/j.egyr.2023.03.085
Sudalaimuthu P, Sathyamurthy R (2023) The clean energy aspect of plastic waste — hydrogen gas production, CO2 reforming, and plastic waste management coincide with catalytic pyrolysis — an extensive review. Environ Sci Pollut Res 30:66559–66584. https://doi.org/10.1007/s11356-023-26908-3
Tursunov O, Zubek K, Dobrowolski J, Czerski G, Grzywacz P (2017) Effect of Ni/Al2O3-SiO2 and Ni/Al2O3-SiO2 with K2O promoter catalysts on H2, CO and CH4 concentration by CO2 gasification of Rosa multiflora biomass. Oil Gas Sci Technol – Rev IFP Energies Nouvelles 72(6):37. https://doi.org/10.2516/ogst/2017037
Tursunov O, Dobrowolski J, Zubek K, Czerski G, Grzywacz P, Dubert F, Lapczynska-Kordon B, Klima K, Handke B (2018) Kinetic study of the pyrolysis and gasification of Rosa multiflora and Miscanthus giganteus biomasses via thermogravimetric analysis. Therm Sci 22:3057–3071. https://doi.org/10.2298/TSCI160524130T
Tursunov O, Isa KM, Abduganiev N, Mirzaev B, Kodirov D, Isakov A, Sergiienko SA (2019) A succinct review of catalyst dolomite analysis for biomass-MSW pyrolysis/gasification. Procedia Environ Sci Eng Manag 6(3):365–374
Tursunov O, Suleimenova B, Kuspangaliyeva B, Inglezakis VJ, Anthony EJ, Sarbassov Y (2020a) Characterization of tar generated from the mixture of municipal solid waste and coal pyrolysis at 800 °C. Energy Rep 6(1):147–152. https://doi.org/10.1016/j.egyr.2019.08.033
Tursunov O, Zubek K, Czerski G, Dobrowolski J (2020b) Studies of CO2 gasification of the Miscanthus giganteus biomass over Ni/Al2O3-SiO2 and Ni/Al2O3-SiO2 with K2O promoter as catalysts. J Therm Anal Calorim 139:3481–3492. https://doi.org/10.1007/s10973-019-08773-w
Uddin MN, Wan Daud WMA, Abbas HF (2013) Potential hydrogen and non-condensable gases production from biomass pyrolysis: Insights into the process variables. Renew Sust Energ Rew 27:204–224. https://doi.org/10.1016/j.rser.2013.06.031
Uzun BB, Varol EA, Pütü E (2016) Pyrolysis: a Sustainable way from biomass to biofuels and biochar. In: Viktor JB, Varol EA, Uzun BB, Liu J (eds) Biochar: a regional supply chain approach in view of climate change mitigation. Cambridge University Press, Cambridge (UK), pp 239–265
Villafán-Vidales HI, Lopez G, Santamaria L, Artetxe M, Olazar M (2022) An analysis of hydrogen production potential through the in-line oxidative steam reforming of different pyrolysis volatiles. J Anal Appl Pyrolysis 163:105482. https://doi.org/10.1016/j.jaap.2022.105482
Yu J, Paterson N, Blamey J, Millan M (2017) Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 191:140–149. https://doi.org/10.1016/j.fuel.2016.11.057
Yue M, Lambert H, Pahon E, Roche R, Jemei S, Hissel D (2021) Hydrogen energy systems: a critical review of technologies, applications, trends and challenges. Renew Sust Energ Rew 146:111180. https://doi.org/10.1016/j.rser.2021.111180
Zaynutdinova D (2017) The solid waste management system and possible ways to improve it. Env Saf Civ Ini 8:4–9
Zhai M, Guo L, Zhang Y, Dong P, Qi G, Huang Y (2016) Kinetic parameters of biomass pyrolysis by TGA. BioRes 11(4):8548–8557. https://doi.org/10.15376/biores.11.4.8548-8557
Zhaosheng Y, Xiaoqian M, Ao L (2009) Thermogravimetric analysis of rice and wheat straw catalytic combustion in air-and oxygen-enriched atmospheres. Energy Convers Manag 50:561–566. https://doi.org/10.1016/j.enconman.2008.10.022
Zhou H et al (2014) An overview of characteristics of municipal solid waste fuel in China: physical, chemical composition and heating value. Renew Sustain Energy Rev 36:107–122. https://doi.org/10.1016/j.rser.2014.04.024
Zhou H, Long Y, Meng A, Li Q, Zhang Y (2015) Thermogravimetric characteristics of typical municipal solid waste fractions during co-pyrolysis. Waste Manag 38:194–200. https://doi.org/10.1016/j.wasman.2014.09.027
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization was done by OT and KŚ. Methodology, validation, and writing–original draft preparation were carried out by OT, KŚ, and NA. Formal analysis was performed by OT, KŚ, IK, YY, and AK. Writing, review, and editing were done by OT, KŚ, and YY. Visualization was performed by YY, IK, and AK. Supervision was conducted by OT. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tursunov, O., Śpiewak, K., Abduganiev, N. et al. Thermogravimetric and thermovolumetric study of municipal solid waste (MSW) and wood biomass for hydrogen-rich gas production: a case study of Tashkent region. Environ Sci Pollut Res 30, 112631–112643 (2023). https://doi.org/10.1007/s11356-023-30368-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30368-0