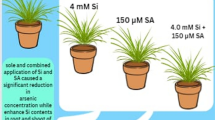
Abstract
Soil contamination with toxic heavy metals (such as arsenic (As)) is becoming a serious global problem due to rapid development of social economy, although the use of silicon (Si) and sodium hydrosulfide (NaHS) has been found effective in enhancing plant tolerance against biotic and abiotic stresses including the As toxicity. For this purpose, a pot experiment was conducted using the different levels of As toxicity in the soil, i.e., (0 mM (no As), 50, and 100 µM) which were also supplied with the different exogenous levels of Si, i.e., (0 (no Si), 1.5, and 3 mM) and also with the NaHS, i.e., (0 (no NaHS), 1, and 2 mM) on growth, photosynthetic pigments, gas exchange characteristics, oxidative stress biomarkers, antioxidant machinery (enzymatic and non-enzymatic antioxidants), and their gene expression, ion uptake, organic acid exudation, and As uptake of maize (Zea mays L.). Results from the present study showed that the increasing levels of As in the soil significantly (P < 0.05) decreased plant growth and biomass, photosynthetic pigments, gas exchange attributes, sugars, and nutritional contents from the roots and shoots of the plants. In contrast, increasing levels of As in the soil significantly (P < 0.05) increased oxidative stress indicators in terms of malondialdehyde, hydrogen peroxide, and electrolyte leakage and also increased organic acid exudation patter in the roots of Z. mays, although the activities of enzymatic antioxidants and the response of their gene expressions in the roots and shoots of the plants and non-enzymatic such as phenolic, flavonoid, ascorbic acid, and anthocyanin contents were initially increased with the exposure of 50 µM As, but decreased by the increasing the As concentration 100 µM in the soil. The negative impact of As toxicity can overcome the application of Si and NaHS, which ultimately increased plant growth and biomass by capturing the reactive oxygen species and decreased oxidative stress in Z. mays by decreasing the As contents in the roots and shoots of the plants. Our results also showed that the Si was more sever and showed better results when we compared with NaHS under the same treatment of As in the soil. Research findings, therefore, suggest that the combined application of Si and NaHS can ameliorate As toxicity in Z. mays, resulting in improved plant growth and composition under metal stress, as depicted by balanced exudation of organic acids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, rapid increases in urbanization and industrialization have caused the excessive release of heavy metals in farmlands with damaging effects on ecosystems (Ahmad et al. 2022; Alatawi et al. 2022). Heavy metal accumulation in soils is of great concern in agricultural production due to its adverse effects on food safety and marketability, crop growth due to phytotoxicity, and the environmental health of soil organisms (Bilen et al. 2019, Hussain et al. 2022, Madhu and Sadagopan 2020). Arsenic (As) is a highly toxic and carcinogenic element (Mondal et al. 2021), and the most widespread sources of As in soil and water are natural sources, such as volcanic activities, weathering, and erosion of minerals and rocks, and geothermal waters (Alsafran et al. 2022; Irshad et al. 2021; Ma et al. 2023; Sun et al. 2023; Turan et al. 2019). As is released from weathering of As containing minerals, natural processes (volcanic eruption and geothermal waters), anthropogenic activities (e.g., effluents from mining and metallurgical industries), and As containing pesticides (Saleem et al. 2022a, Sun et al. n.d.). There is an abundance of evidence that As negatively interferes with several biochemical and physiological processes within a plant, causing reduced plant growth and yield (Akram et al. 2018; Bhat et al. 2022; Tanveer et al. 2022; Turan 2020). Inside the plant cell, heavy metals induce oxidative stress by enhanced production of reactive oxygen species (ROS), which may cause cell death via oxidative processes, such as protein oxidation, enzyme inhibition, DNA and RNA damage, and lipid peroxidation (Afzal et al. 2020; Rehman et al. 2019; Saleem et al. 2020c; Turan 2019). Antioxidants, such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX), come into play to scavenge ROS. For example, SOD facilitates the conversion of superoxide (O−1) radicals to hydrogen peroxide (H2O2), whereas POD decomposes H2O2 into water (H2O) and molecular O2 (Al Jabri et al. 2022; Hafeez et al. 2022; Imran et al. 2021; Mumtaz et al. 2021; Saleem et al. 2020d). The sites contaminated with As need immediate attention because of the associated severe health risks.
Silicon (Si) is ubiquitous in nature and constitutes about 0.03% of biosphere. It comprised ca. 10% of the plant’s dry biomass which is much higher than the concentrations of other nutrient elements (Anwaar et al. 2015; Javed et al. 2020). It has previously been reported in many studies that Si application helps in ameliorating metal stress in plants (Hasanuzzaman et al. 2019; Kaya et al. 2020a; Pirooz et al. 2021). From soil, uptake of Si depends on the type of growth medium, soil properties, and plant species where plants are classified as high-, medium-, and low-Si accumulators (Adrees et al. 2015; Alamri et al. 2020). A number of studies revealed that Si application increased plant growth and biomass (Fan et al. 2016), mineral uptake (Wu et al. 2016), and gaseous exchange attributes (Javed et al. 2020); reduced oxidative stress by scavenging reactive oxygen species (ROS) (Saleem et al. 2022b); and diminished accumulation of organic acids in different plant species (Ma et al. 2022a). Among different gaseous compounds, exogenous use of sodium hydrosulfide (NaHS) has also gained more importance. NaHS is the donor of H2S that is a colorless soluble gas that can be toxic. However, during the last decade, NaHS has been recognized as a vital molecular signal in plants (Ma et al. 2022b; Ozfidan-Konakci et al. 2020). For instance, NaHS maintains the membrane integrity of Triticum aestivum root tips under Co toxicity, reduces chlorophyll degradation, and prevents oxidative damage (Ozfidan-Konakci et al. 2020). NaHS regulates different developmental processes, such as lateral root and adventitious root formation and germination (Kaya et al. 2020b; Panda et al. 2011). Therefore, NO has a significant ability to mediate plant responses to environmental stresses. Zea mays (maize), Triticum aestivum (wheat), and Oryza sativa (rice) are the major staple crops; among them, Z. mays holds prime importance due to its different uses in the food and feed industry (Ali et al. 2019; Hassan et al. 2022). As Z. mays is an important food crop, it is known to be a social security for farmers and is an important feed and industrial source (Iqbal et al. 2016; Kaya et al. 2020d). It is also known as the “Queen of Cereals” due to its huge biomass and production, and it is cultivated over 197.2 million ha in temperate region of the world (Ranum et al. 2014). In some countries, Z. mays is consumed as a staple food in the form of corn flakes, corn syrup, and corn oil; and it is used for the production of starch, ethanol, and plastic and in antibiotic agents (Du et al. 2019; El-Tohory et al. 2023).
Previously, few studies on Z. mays were executed to investigate its morphology and physiology under metal stress (Abbas et al. 2023; El-Tohory et al. 2023; Hadi et al. 2010; Shafigh et al. 2016); but synergistic application of Si and NaHS on various morphophysiological characteristics, ionomics, gene expression, and organic acid exudation potential of Z. mays was rarely investigated under metal stressed regimes. Therefore, the present study was conducted to study (I) the effect of different levels of Si and NaHS on plant growth, biomass, and gaseous exchange parameters of Z. mays under As stress; (II) oxidative stress and the responses of different antioxidative enzymes (enzymatic and non-enzymatic), as well as the response of the specific gene expression; and (III) essential minerals uptake, organic acid exudation, and As accumulation in different organs of Z. mays under As stress. The results from the present study gave a new insight that the use of Si and NaHS in heavy metal studies may be beneficial and can improve plant yield under As-contaminated soil.
Materials and methods
Experimental setup and growth treatment
The present study was conducted in the botanical garden under greenhouse environment belonging to the Department of Botany, Government College University, Faisalabad 38000, Punjab, Pakistan (31° 24/N, 73° 04/E). Healthy and mature seeds of maize (Zea mays L.) were surface sterilized with (0.1%) bleaching powder for 10–20 min and washed gently with deionized water before starting an experiment. Ten healthy seeds selected in the plastic pots (15 cm height × 20 cm width) were used in this study, each containing 5 kg of uncontaminated soil. The soil used for this experiment washed with distilled water then air dried and sieved with 5-mm. The physio-chemical of the soil used in this experiment are as follows: pH-6.9, EC-0.9 dS cm−1, organic matter-17 g kg−1, EK-21 mg kg−1, TP-0.17 g kg−1, and TN-16 g kg−1. Before starting the pot experiment, the soil was artificially spiked with different concentrations of As, i.e., 0 (no As), 50, and 100 µM, using sodium arsenate (Na2HAsSO4)·7H2O (Sigma). All pots have undergone two cycles of water saturation and air-drying, and the seeds were sown. After seed germination (more than 2-cm emergence) (Saleem et al. 2019), the sand was exogenously supplied with various levels of, i.e., (0 (no Si), 1.5, and 3 mM) by using K2SiO3 salt. These are the same levels of Si we have used in our previous study under the varying levels of As in the soil (Saleem et al. 2022a). Various levels of sodium hydrogen sulfide (NaHS), i.e., (0 (no NaHS), 1, and 2 mM) were administered for 1 week following the imposition of As stress in the soil. The levels of NaHS were selected on the basis of our previous study on wheat (Triticum aestivum L.) (Mfarrej et al. 2021). The total duration of experimental treatments was 2 months under controlled conditions where they received natural light with day/night temperature of 35/40 °C and day/night humidity of 60/70%. Irrigation with As free water and other intercultural operations was performed, when needed. The experiment was designed by two-factor completely randomized (CRD) having four replicates. All plants were harvested on the 28th day after given As treatment to measure various growth and physiological parameters.
Sampling and data collection
After 4 weeks, remaining three seedlings were uprooted and washed gently with the help of distilled water to eliminate the aerial dust and deposition. Functional leaf in each treatment was picked at a rapid growth stage during 09:00–10:30 a.m. The sampled leaves were washed with distilled water, immediately placed in liquid nitrogen, and stored in a freezer at − 80 °C for further analysis. All the harvested plants were divided into two parts, i.e., roots and shoots, to study different physio-biochemical traits. Leaves from each treatment group were picked for chlorophyll, carotenoid, oxidative stress, and antioxidant analysis. Root and shoot lengths were measured straightway after the harvesting by using measuring scale and digital weighting balance to measure fresh biomass. Roots were uprooted and immersed in 20 mM Na2EDTA for 15–20 min to remove As adhered to the root surfaces. Then, roots were washed thrice with distilled water and finally once with deionized water and dried for further analysis. The different parts of the plant (roots and shoots) were oven-dehydrated at 65 °C for 72 h for As determination, and the total plant dry weight was also measured. Although this experiment was conducted in pots, for the collection of organic acids, two seedlings were transferred to the rhizoboxes which consist of plastic sheet, nylon net, and wet soil (UdDin et al. 2015). After 48 h, plants were taken from the rhizoboxes, and the roots were washed with redistilled water to collect the exudates from root surface. The samples were filtered through a 0.45-μm filter (Millex HA, Millipore) and collected in Eppendorf tubes (Greger and Landberg 2008). The collected samples were mixed with NaOH (0.01 M) in order to analyze the organic acids. However, the samples used for analysis of oxalic acid were not treated with NaOH (Javed et al. 2013).
Determination of photosynthetic pigments and gas exchange characteristics
Leaves were collected for the determination of chlorophyll and carotenoid contents. For chlorophylls, 0.1 g of fresh leaf sample was extracted with 8 mL of 95% acetone for 24 h at 4 °C in the dark. The absorbance was measured by a spectrophotometer (UV-2550; Shimadzu, Kyoto, Japan) at 646.6, 663.6, and 450 nm. Chlorophyll content was calculated by the standard method of Arnon (1949).
Net photosynthesis (Pn), leaf stomatal conductance (Gs), transpiration rate (Ts), and intercellular carbon dioxide concentration (Ci) were measured from four different plants in each treatment group. Measurements were conducted between 11:30 and 13:30 on days with a clear sky. Rates of leaf Pn, Gs, Ts, and Ci were measured with a LI-COR gas-exchange system (LI-6400; LI-COR Biosciences, Lincoln, NE, USA) with a red-blue LED light source on the leaf chamber. In the LI-COR cuvette, CO2 concentration was set as 380 mmol mol−1, and LED light intensity was set at 1000 mmol m−2 s−1, which was the average saturation intensity for photosynthesis in Z. mays (Austin 1990).
Determination of oxidative stress indicators
The degree of lipid peroxidation was evaluated as malondialdehyde (MDA) contents. Briefly, 0.1 g of frozen leaves were ground at 4 °C in a mortar with 25 mL of 50 mM phosphate buffer solution (pH 7.8) containing 1% polyethene pyrrole. The homogenate was centrifuged at 10,000 × g at 4 °C for 15 min. The mixtures were heated at 100 °C for 15–30 min and then quickly cooled in an ice bath. The absorbance of the supernatant was recorded by using a spectrophotometer (xMark™ Microplate Absorbance Spectrophotometer, Bio-Rad, USA) at wavelengths of 532, 600, and 450 nm. Lipid peroxidation was expressed as l mol g−1 by using the formula: 6.45 (A532-A600)-0.56 A450. Lipid peroxidation was measured by using a method previously published by Heath and Packer (1968).
To estimate H2O2 content of plant tissues (root and leaf), 3 mL of sample extract was mixed with 1 mL of 0.1% titanium sulfate in 20% (v/v) H2SO4 and centrifuged at 6000 × g for 15 min. The yellow color intensity was evaluated at 410 nm. The H2O2 level was computed by the extinction coefficient of 0.28 mmol−1 cm−1. The contents of H2O2 were measured by the method presented by Jana and Choudhuri (1981).
Stress-induced electrolyte leakage (EL) of the uppermost stretched leaves was determined by using the methodology of Dionisio-Sese and Tobita (1998). The leaves were cut into minor slices (5 mm length) and placed in test tubes having 8 mL distilled water. These tubes were incubated and transferred into a water bath for 2 h prior to measuring the initial electrical conductivity (EC1). The samples were autoclaved at 121 °C for 20 min and then cooled down to 25 °C before measuring the final electrical conductivity (EC2). Electrolyte leakage was calculated by the following formula:
Determination of antioxidant enzyme activities and their gene expression
To evaluate enzyme activities, fresh leaves (0.5 g) were homogenized in liquid nitrogen and 5 mL of 50 mmol sodium phosphate buffer (pH 7.0), including 0.5 mmol EDTA and 0.15 mol NaCl. The homogenate was centrifuged at 12,000 × g for 10 min at 4 °C, and the supernatant was used for measurement of superoxide dismutase (SOD) and peroxidase (POD) activities. SOD activity was assayed in 3 mL reaction mixture containing 50 mM sodium phosphate buffer (pH 7), 56 mM nitro blue tetrazolium, 1.17 mM riboflavin, 10 mM methionine, and 100 μL enzyme extract. Finally, the sample was measured by using a spectrophotometer (xMark™ Microplate Absorbance Spectrophotometer; Bio-Rad). Enzyme activity was measured by using a method by Chen and Pan (1996) and expressed as U g−1 FW.
POD activity in the leaves was estimated by using the method of Sakharov and Ardila (1999) by using guaiacol as the substrate. A reaction mixture (3 mL) containing 0.05 mL of enzyme extract, 2.75 mL of 50 mM phosphate buffer (pH 7.0), 0.1 mL of 1% H2O2, and 0.1 mL of 4% guaiacol solution was prepared. Increases in the absorbance at 470 nm because of guaiacol oxidation were recorded for 2 min. One unit of enzyme activity was defined as the amount of the enzyme.
Catalase (CAT) activity was analyzed according to Aebi (1984). The assay mixture (3.0 mL) was comprised of 100 μL enzyme extract, 100 μL H2O2 (300 mM), and 2.8 mL 50 mM phosphate buffer with 2 mM ETDA (pH 7.0). The CAT activity was measured from the decline in absorbance at 240 nm as a result of H2O2 loss (ε = 39.4 mM−1 cm−1).
Ascorbate peroxidase (APX) activity was measured according to Nakano and Asada (1981). The mixture containing 100 μL enzyme extract, 100 μL ascorbate (7.5 mM), 100 μL H2O2 (300 mM), and 2.7 mL 25 mM potassium phosphate buffer with 2 mM EDTA (pH 7.0) was used for measuring APX activity. The oxidation pattern of ascorbate was estimated from the variations in wavelength at 290 nm (ε = 2.8 mM−1 cm−1).
The expression profile of the defense genes (i.e., Fe-SOD, POD, CAT, and APX) was carried out through RT q-PCR in rice plants grown after being treated with selected strains in a greenhouse experiment. For this, the selected gene sequences were taken from NCB1, followed by designing primers through the PrimerQuest tool; the primers are listed in Supplementary Materials Table S2. The housekeeping gene elongation factor 1-alpha (ef1) was used in the present study. Briefly, RNA was extracted from fresh rapeseed plant leaves inoculated with selected strains, and ddH2O was used as the control grown under infested and non-infested A. besseyi in greenhouse conditions after 4 days’ post-inoculation (dpi) through the TRizole method. Gene-targeting primers were designed based on mRNA or expressed sequence tag (EST) for the corresponding genes as follows: Fe-SOD (F: 5′ ACGGTGTGACCACTGTGACT 3′, R: 5′ GCACCGTGTTGTTTACCATC3′), POD (F: 5′ATGTTTCGTGCGTCTCTGTC3′, R: 5′ TACGAGGGTCCGATCTTAGC 3′), CAT (F: 5′ TCGCCATGCTGAGAAGTATC 3′, R: 5′ TCTCCAGGCTCCTTGAAGTT 3′), APX (F:5′ ATGAGGTTTGACGGTGAGC 3′, R:5′ CAGCATGGGAGATGGTAGG 3′) as an internal control. The Vazyme HiScript II Q RT SuperMix Kit (Vazyme, Nanjing, China) was used for cDNA synthesis. RT-qPCR was performed to analyze the expression profile of selected genes in rapeseed plants through a ABI 7500 Fast Real-Time PCR Detection System (Thermo Fisher Scientific, San Jose, CA, USA). The PCR machine was programmed using the following steps: initial denaturation at 95 °C for 30 s, including 40 cycles of 95 °C for 5 s, and 34 s at 60 °C. Finally, relative quantification was performed according to the comparative C method of 2 − ∆∆ CT as described by Kong et al. (2021). The threshold cycle (Ct) value of actin was subtracted from that of the gene of interest to obtain the ΔCt value.
Determination of non-enzymatic antioxidants, sugars, and proline contents
Plant ethanol extracts were prepared for the determination of non-enzymatic antioxidants and some key osmolytes. For this purpose, 50 mg of dry plant material was homogenized with 10 mL ethanol (80%) and filtered through Whatman No. 41 filter paper. The residue was re-extracted with ethanol, and the 2 extracts were pooled together to a final volume of 20 mL. The determination of flavonoids (Pękal and Pyrzynska 2014), phenolics (Bray and Thorpe 1954), ascorbic acid (Azuma et al. 1999), anthocyanin (Lewis et al. 1998), and total sugars (Dubois et al. 1956) and also free amino acids was performed from the extracts.
Fresh leaf material (0.1 g) was mixed thoroughly in 5 mL aqueous sulphosalicylic acid (3%). The mixture was centrifuged at 10,000 × g for 15 min, and an aliquot (1 mL) was poured into a test tube having 1 mL acidic ninhydrin and 1 mL glacial acetic acid. The reaction mixture was first heated at 100 °C for 10 min and then cooled in an ice bath. The reaction mixture was extracted with 4 mL toluene, and test tubes were vortexed for 20 s and cooled. Thereafter, the light absorbance at 520 nm was measured by using a UV–VIS spectrophotometer (Hitachi U-2910, Tokyo, Japan). The free proline content was determined on the basis of the standard curve at 520 nm absorbance and expressed as µmol (g FW)−1 (Bates et al. 1973).
Determination of nutrient content
For nutrient analysis, plant roots and shoots were washed twice in redistilled water, dipped in 20 mM EDTA for 3 s and then, again, washed with deionized water twice for the removal of adsorbed metal on the plant surface. The washed samples were then oven-dried for 24 h at 105 °C. The dried roots and shoots were digested by using a wet digestion method in HNO3: HClO4 (7:3 V/V) until clear samples were obtained. Each sample was filtered and diluted with redistilled water up to 50 mL. The root and shoot contents of Ca2+, Mg2+, Fe2+, and P were analyzed by using Atomic Absorption Spectrophotometer (AAS) model Agilent 240FS-AA.
Determination of root exudates analysis and As concentration
In order to determine the concentration of organic acids, freeze-dried exudates were mixed with ethanol (80%), and 20 μL of the solutions were injected into the C18 column (Brownlee Analytical C-183 µm; length 150 mm × 4.6 mm2, USA). Quantitative analysis of organic acids in root exudates was executed with high-performance liquid chromatography (HPLC), having a Flexer FX-10 UHPLC isocratic pump (PerkinElmer, MA, USA). The mobile phase used in HPLC was comprised of an acidic solution of aceto-nitrile containing aceto-nitrile:H2SO4:acetic acid in ratios of 15:4:1, respectively, and pH of 4.9. The samples were analyzed at a flow rate of 1.0 mL min−1 for a time period of 10 min. The inner temperature of the column was fixed at 45 °C, and quantification of organic acids was carried out at 214-nm wavelength with the help of a detector (UV–VIS Series 200, USA) as described by UdDin et al. (2015). Freeze-dried samples were dissolved in redistilled water, and the pH of the exudates was recorded with LL micro-pH glass electrode by using a pH meter (ISTEK Model 4005–08007 Seoul, South Korea). For the determination of total As concentration in shoots and roots, samples were oven-dried at 65 °C for 24 h and ashed in a mufe furnace at 550 °C for 20 h. After that, the ash was incubated with 31% (m/v) HNO3 and 17.5% (v/v) H2O2 at 70 °C for about 2 h and added distilled water. The As concentration in the digest was determined using an atomic absorption spectrophotometer (AAS).
Statistical analysis
The normality of data was analyzed using IBM SPSS software (Version 21.0. Armonk, NY, USA: IBM Corp) through a multivariate post hoc test, followed by a Duncan’s test in order to determine the interaction among significant values. Thus, the differences between treatments were determined by using ANOVA, and the least significant difference test (P < 0.05) was used for multiple comparisons between treatment means where significant Tukey’s HSD post hoc test was used to compare the multiple comparisons of means. The analysis showed that the data in this study were almost normally distributed. The graphical presentation was carried out using Origin-Pro 2019.
Results
Si- and NaHS-mediated improvement in growth and photosynthetic pigments in Z. mays under As stress
In the present study, various growth and photosynthetic parameters were also measured in Z. mays grown under the different levels of As toxicity in the soil, i.e., (0 mM (no As), 50, and 100 µM) which were also supplied with the different exogenous levels of Si and NaHS. The data regarding shoot length, root length, number of leaves, leaf area, shoot fresh weight, root fresh weight, shoot dry weight, and root dry weight is presented in Fig. 1, and the data regarding the chlorophyll-a, chlorophyll-b, total chlorophyll, carotenoid content, net photosynthesis, stomatal conductance, transpiration rate, and intercellular CO2 is presented in Fig. 2. According to the results, it was noticed that the increasing levels of As in the soil significantly (P < 0.05) decreased plant growth and biomass and photosynthetic pigments in Z. mays without the application of Si and NaHS (Figs. 1 and 2). According to the given results, increasing levels of As, i.e., 50 and 100 µM in the soil, significantly (P < 0.05) decreased shoot length, root length, number of leaves, leaf area, shoot fresh weight, root fresh weight, shoot dry weight, root dry weight, chlorophyll-a, chlorophyll-b, total chlorophyll, carotenoid content, net photosynthesis, stomatal conductance, and transpiration rate in Z. mays, compared to the plants grown without the treatment of As in the soil. The exogenous application of Si and NaHS was also applied to measured various growths (Fig. 1) and photosynthetic attributes (Fig. 2) in Z. mays grown under the elevating levels of As in the soil. The application of EDTA and CA non-significantly increased shoot length, root length, number of leaves, leaf area, shoot fresh weight, root fresh weight, shoot dry weight, root dry weight, chlorophyll-a, chlorophyll-b, total chlorophyll, carotenoid content, net photosynthesis, stomatal conductance, and transpiration rate at all levels of As in the soil, compared to the plants which were grown without the application of Si and NaHS. Our results also showed that the Si were more sever and showed better results when we compared with NaHS under the same treatment of As in the soil. We have also noticed that As toxicity did not significantly affect intercellular CO2, and also application of Si and NaHS did not significantly influence intercellular CO2 in Z. mays under all levels of As in the soil (Fig. 2H).
Effect of exogenous application of various levels of Si (0 (no Si), 1.5, and 3 mM) and NaHS (0 (no NaHS), 1, and 2 mM) on shoot length (A), root length (B), number of leaves (C), leaf area (D), shoot fresh weight (E), root fresh weight (F), shoot dry weight (G), and root dry weight (H) of Zea mays grown under various stress levels of As (0 (no As), 50, and 100 μM). Values in the figures indicate just one harvest. Mean ± SD (n = 4). Thus, the differences between treatments were determined by using ANOVA, and the least significant difference test (P < 0.05) was used for multiple comparisons between treatment means where significant Tukey’s HSD post hoc test was used to compare the multiple comparisons of means. Different lowercase letters on the error bars indicate significant differences between the treatments
Effect of exogenous application of various levels of Si (0 (no Si), 1.5, and 3 mM) and NaHS (0 (no NaHS), 1, and 2 mM) on chlorophyll − a content (A), chlorophyll − b content (B), total chlorophyll content (C), carotenoid content (D), net photosynthesis, (E) stomatal conductance (F), transpiration rate (G), and intercellular CO2 (H) of Zea mays grown under various stress levels of As (0 (no As), 50, and 100 μM). Values in the figures indicate just one harvest. Mean ± SD (n = 4). Thus, the differences between treatments were determined by using ANOVA, and the least significant difference test (P < 0.05) was used for multiple comparisons between treatment means where significant Tukey’s HSD post hoc test was used to compare the multiple comparisons of means. Different lowercase letters on the error bars indicate significant differences between the treatments
Si- and NaHS-mediated oxidative stress and antioxidant capacity and nutrient profile in Z. mays under As stress
Malondialdehyde (MDA) contents, hydrogen peroxide (H2O2) initiation, and electrolyte leakage (%) increased in the roots and leaves of Z. mays under the increasing As, i.e., (0 mM (no As), 50, and 100 µM) in the soil medium without Si and NaHS as compared to the plants grown in 0 µM of As. The data regarding oxidative stress indicators in the leaves of Z. mays are presented in Fig. 3. Application of Si and NaHS significantly decreased the contents of MDA, H2O2, and EL (%) in the roots and leaves grown with As level of 100 µM under Si and NaHS application as compared to those plants grown with 100 µM of As without the application of Si and NaHS.
Effect of exogenous application of various levels of Si (0 (no Si), 1.5, and 3 mM) and NaHS (0 (no NaHS), 1, and 2 mM) on MDA contents in the roots (A), MDA contents in the leaves (B), H2O2 contents in the roots (C), H2O2 contents in the leaves (D), EL percentage in the roots (E), and EL percentage in the leaves (F) of Zea mays grown under various stress levels of As (0 (no As), 50, and 100 μM). Values in the figures indicate just one harvest. Mean ± SD (n = 4). Thus, the differences between treatments were determined by using ANOVA, and the least significant difference test (P < 0.05) was used for multiple comparisons between treatment means where significant Tukey’s HSD post hoc test was used to compare the multiple comparisons of means. Different lowercase letters on the error bars indicate significant differences between the treatments
Various antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) in the roots and leaves of Z. mays and their specific gene expression such as Fe-SOD, POD, CAT, and APX and also the non-enzymatic compounds such as phenolic, flavonoid, ascorbic acid, and anthocyanin contents were also measured in the present study. The data regarding the activities of enzymatic antioxidants (SOD, POD, CAT, and APX) are presented in Fig. 4, and their specific gene expression such as Fe-SOD, POD, CAT, and APX are presented in Fig. 5, and also the results regarding the compounds of non-enzymatic antioxidants (phenolic, flavonoid, ascorbic acid, and anthocyanin) are presented in Fig. 6. The results showed that the activities of enzymatic antioxidants (SOD, POD, CAT, and APX) and their specific gene expression such as Fe-SOD, POD, CAT, and APX and the compounds of non-enzymatic antioxidants (phenolic, flavonoid, ascorbic acid, and anthocyanin) were increased up to a As level of 50 µM in the soil but decreased gradually by adding more concentration of As, i.e., 100 µM in the soil compared to the plants grown in 0 µM in the soil. Results also showed that the exogenous application of Si and NaHS non-significantly increased the activities of enzymatic antioxidants (SOD, POD, CAT, and APX) and their specific gene expression such as Fe-SOD, POD, CAT, and APX and also the compounds of non-enzymatic antioxidants (phenolic, flavonoid, ascorbic acid, and anthocyanin) at all levels of As (no As), 50, and 100 µM in the soil, compared to the plants which were not applied by the Si and NaHS.
Effect of exogenous application of various levels of Si (0 (no Si), 1.5, and 3 mM) and NaHS (0 (no NaHS), 1, and 2 mM) on SOD activity in the roots (A), SOD activity in the shoots (B), POD activity in the roots (C), POD activity in the shoots (D), CAT activity in the roots (E), CAT activity in the shoots (F), APX activity in the roots, (G), and APX activity in the shoots (H) of Zea mays grown under various stress levels of As (0 (no As), 50, and 100 μM). Values in the figures indicate just one harvest. Mean ± SD (n = 4). Thus, the differences between treatments were determined by using ANOVA, and the least significant difference test (P < 0.05) was used for multiple comparisons between treatment means where significant Tukey’s HSD post hoc test was used to compare the multiple comparisons of means. Different lowercase letters on the error bars indicate significant differences between the treatments
Effect of exogenous application of various levels of Si (0 (no Si), 1.5, and 3 mM) and NaHS (0 (no NaHS), 1, and 2 mM) on superoxide dismutase (Fe-SOD) (A), peroxidase (POD) (B), catalase (CAT) (C), and ascorbate peroxidase (APX) (D) of Zea mays grown under various stress levels of As (0 (no As), 50, and 100 μM). Values in the figures indicate just one harvest. Mean ± SD (n = 4). Thus, the differences between treatments were determined by using ANOVA, and the least significant difference test (P < 0.05) was used for multiple comparisons between treatment means where significant Tukey’s HSD post hoc test was used to compare the multiple comparisons of means. Different lowercase letters on the error bars indicate significant differences between the treatments
Effect of exogenous application of various levels of Si (0 (no Si), 1.5, and 3 mM) and NaHS (0 (no NaHS), 1, and 2 mM) on phenolic contents (A), flavonoid contents (B), ascorbic acid contents (C), anthocyanin contents (D), soluble sugar contents (E), reducing sugar contents (F), non-reducing sugar contents (G), and proline contents (H) of Zea mays grown under various stress levels of As (0 (no As), 50, and 100 μM). Values in the figures indicate just one harvest. Mean ± SD (n = 4). Thus, the differences between treatments were determined by using ANOVA, and the least significant difference test (P < 0.05) was used for multiple comparisons between treatment means where significant Tukey’s HSD post hoc test was used to compare the multiple comparisons of means. Different lowercase letters on the error bars indicate significant differences between the treatments
Soluble sugar, reducing sugar, non-reducing sugar, proline, and various nutrients, such as calcium (Ca2+), magnesium (Mg2+), iron (Fe2+), and phosphorus (P) contents from the roots and shoots of Z. mays, were also measured in the present study under the different levels of As (no As), 50, and 100 µM in the soil which were also supplied with the application of Si and NaHS. The data regarding the content of soluble sugar, reducing sugar, non-reducing sugar, and proline is presented in Fig. 6, and the data regarding the content of Ca2+, Mg2+, Fe2+, and P from the roots and shoots of the plants are presented in Fig. 7. Results from the present study is showing that the increasing levels of As in the soil significantly (P < 0.05) decreased the contents of nutrients (Ca2+, Mg2+, Fe2+, and P) in the roots and shoots of the plants and also decreased the sugar content (soluble sugar, reducing sugar, non-reducing sugar), compared to the plants which were grown in the soil which was not treated with As. However, the content of proline was increased by increasing the levels of As in the soil, compared to the plants which were not treated with As (Fig. 6H). The application of Si and NaHS was also applied to the plants exogenously and determined various sugar (Fig. 6) and phenolic and nutrient content (Fig. 7) from the roots and shoots of the plants. Results from the present study suggested that the application of Si and NaHS non-significantly increased sugar content (soluble sugar, reducing sugar, non-reducing sugar) and proline in the shoots and significantly increased nutrients (Ca2+, Mg2+, Fe2+, and P) in the roots and shoots of the plants, compared to the plants grown without the treatment of Si and NaHS, at all the levels of As in the soil.
Effect of exogenous application of various levels of Si (0 (no Si), 1.5, and 3 mM) and NaHS (0 (no NaHS), 1, and 2 mM) on calcium contents in the roots (A), calcium contents in the shoots (B), magnesium contents in the roots (C), magnesium contents in the shoots (D), iron contents in the roots (E), iron contents in the shoots (F), phosphorus contents in the roots (G), and phosphorus contents in the shoots (H) of Zea mays grown under various stress levels of As (0 (no As), 50, and 100 μM). Values in the figures indicate just one harvest. Mean ± SD (n = 4). Thus, the differences between treatments were determined by using ANOVA, and the least significant difference test (P < 0.05) was used for multiple comparisons between treatment means where significant Tukey’s HSD post hoc test was used to compare the multiple comparisons of means. Different lowercase letters on the error bars indicate significant differences between the treatments
Si- and NaHS-mediated organic acid exudation and As uptake in Z. mays under As stress
The contents of fumaric acid, formic acid, acetic acid, citric acid, malic acid, and oxalic acid in the roots and As concentration in the roots and shoots of Z. mays grown under toxic levels of As in the soil, with or without the application of Si and NaHS, are presented in Fig. 8. According to the given results, we have noticed that increasing the concentration of As in the soil (50 and 100 µM) induced a significant (P < 0.05) increased in the content of fumaric acid, formic acid, acetic acid, citric acid, malic acid, and oxalic acid in the roots and also As concentration in the roots and shoots of Z. mays, compared to those plants, which were grown in As level of 0 µM in the soil. Results also illustrated that the application of Si and NaHS decreased the contents of fumaric acid, formic acid, acetic acid, citric acid, malic acid, and oxalic acid in the roots while also decreased As concentration in the roots and shoots of Z. mays, compared with those plants, which were grown without the exogenous application with Si and NaHS.
Effect of exogenous application of various levels of Si (0 (no Si), 1.5, and 3 mM) and NaHS (0 (no NaHS), 1, and 2 mM) on fumaric acid contents (A), acetic acid contents (B), citric acid contents (C), formic acid contents (D), malic acid contents (E), oxalic acid contents (F), in the roots and As contents in the roots (G), and As contents in the shoots (H) of Zea mays grown under various stress levels of As (0 (no As), 50, and 100 μM). Values in the figures indicate just one harvest. Mean ± SD (n = 4). Thus, the differences between treatments were determined by using ANOVA, and the least significant difference test (P < 0.05) was used for multiple comparisons between treatment means where significant Tukey’s HSD post hoc test was used to compare the multiple comparisons of means. Different lowercase letters on the error bars indicate significant differences between the treatments
Discussion
As is an analog of phosphate (P) and competes forth same uptake carriers in the root plasmalemma of plants, and the As tolerance has been identified in a number of plant species (Bhat et al. 2022; Ulhassan et al. 2022a; Wen et al. 2020). Due to ever-increasing health concerns, As pollution demands effective and efficient removal system especially from fresh water ecosystem. Various physical and chemical methods such as adsorption, chemical precipitation, co-precipitation, electroplating, ion exchange, filtration, and reverse osmosis are being used for As decontamination (Mondal et al. 2021, Sun et al. n.d.). Changes in the As behaviors such as alteration in As species diversity, oxidation-reduction, precipitation, sorption, adsorption, dilution, volatilization, and formation of As-complex might be due to biological, physical, or chemical processes occurring in the environment (Faizan et al. 2021; Mushtaq et al. 2020). High concentrations of As significantly reduce plant productivity and photosynthetic pigmentations (Saleem et al. 2022a; Tanveer et al. 2022) as found in the present study that the increasing levels of As in the soil, i.e., (0 mM (no As), 50, and 100 µM) significantly (P < 0.05) decreased plant growth (Fig. 1) and photosynthesis (Fig. 2). As is a nonessential metalloid that is toxic and harmful with respect to agricultural production since it reduces biomass and photosynthesis in plants, in which it has also become a global burden and a source of great environmental concern (Sun et al. 2023; Tanveer et al. 2022). In plant cells, As(V) interferes with a metabolic pathway involving phosphate replacement during ATP (adenosine triphosphate) synthesis, causing the loss of energy (Lessl et al. 2015), and subsequently As(V) is reduced to As(III) in the cytoplasm, resulting in the stimulation of free radical formation and accumulation of reactive oxygen species (ROS) (Huq et al. 2019; Mushtaq et al. 2020).
Stress conditions can disturb the dynamic equilibrium of reactive oxygen species (ROS) production and elimination under normal growth in plants (Rehman et al. 2021; Yaseen et al. 2020), which promote ROS accumulation and membrane lipid peroxidation, and disrupt the structure and function of cell membrane system (Ahmad et al. 2018; Zafar-ul-Hye et al. 2020). High concentration of As in the soil induced oxidative damage by increasing the contents of MDA, initiation of H2O2, and increased percentage of EL which was observed in Glycine max (Ahmad et al. 2020), Oryza sativa (Faizan et al. 2021), and Spinacia oleracea (Saleem et al. 2022a). This ROS accumulation in plants is removed by a variety of antioxidant enzymes such as SOD, POD, CAT, and APX (Fig. 4) and non-enzymatic antioxidant (Fig. 6). However, the expression of specific genes, such as Fe-SOD, POD, CAT, and APX, under As-stressed environment plays a significant role in reducing As toxicity, which was reported in a number of studies under various plant species (Farooq et al. 2016; Mondal et al. 2021). Plants produce a variety of secondary metabolites such as proline, flavonoids, and phenolics that improve tolerance against metal toxicity (Kamran et al. 2020; Saleem et al. 2020a, b), although proline accumulation in plant tissue/organs in response to metal toxicity, might be associated with signal transduction and prevents membrane distortion, has been observed in many plant species (Rehman et al. 2020, Sakya and Prahasto 2020).
With increasing concentrations of As (50 and 100 µM) in the soil, the contents of Ca2+, Mg2+, Fe2+, and P in the roots and shoots of Z. mays were decreased significantly (P < 0.05) when compared to those plants grown without As addition (Fig. 7). The decrease in essential ion accumulation in different organs of Z. mays under varying As concentrations might also be due to the alteration in the physiological processes such as the failure of biosynthesis of chlorophyll and carotenoid contents (Irshad et al. 2021; Sun et al. 2023). Under metal toxicity, reduction in the contents of Fe2+ (Bashir et al. 2018), Mg2+ (Javed et al. 2017), P (Javed et al. 2017), and Ca2+ (Anwar et al. 2017) were recorded. Increasing As levels (50 and 100 µM) in the soil exerted a strong influence on exudation of organic acids from roots of C. annuum which probably is an adoptive mechanism for plant survival under Cd-stressed conditions (Fig. 8). The increasing contents of organic acids in the root exudates of Z. mays are likely to protect the plants against As stress and limit the uptake of metal from roots to aboveground plant parts due to metal-organic acid anion-complex formation (Javed et al. 2018; Saleem et al. 2022a). Outcomes of this study revealed that higher As levels (50 and 100 µM) resulted in a significant (P < 0.05) increase in the root and shoot As contents Z. mays (Fig. 8). Previously, increasing As contents in soil caused a significant (P < 0.05) increase in As contents of plant organs of Glycine max, Spinacia oleracea, and Solanum lycopersicum (Ahmad et al. 2020; Marmiroli et al. 2014; Shahid et al. 2019).
Si is found abundantly in the earth’s crust and is believed to be an important constituent in soil where it efficiently neutralizes the hazardous impacts of different stresses such as salinity, temperature, and various metal stresses on plants (Adrees et al. 2015; Kaya et al. 2020a). Although Si is not deliberated as an indispensable plant nutrient, it rather plays a major role in plant growth, especially under stressful conditions (Ahmad et al. 2019a; Hasanuzzaman et al. 2019). Si bears an ability to be promptly transported through specified transporters located in the cellular membranes of plant roots, and translocation from root cells to the aerial parts of plants is carried out through influx transporters identified in the xylem parenchyma cells (Heile et al. 2021; Kaya et al. 2020a). Numerous investigations have reported the ameliorating effects of Si against heavy metals in Triticum aestivum (Heile et al. 2021), Trachyspermum ammi (Javed et al. 2020), and Triticum turgidum (Rizwan et al. 2016). Under conditions of metal stress, the application of Si reduced the metal contents of plant organs and increased plant growth and composition, improved photosynthetic machinery, decreased in planta oxidative stress via increased antioxidative compounds, increased uptake of minerals, and influenced the exudation of organic acids from plant roots which were discussed in detail in reviews by Adrees et al. (2015) and Jia-Wen et al. (2013). Research findings depicted that the application of Si increased plant growth and biomass (Fig. 1) and also increased photosynthetic pigments (Fig. 2) in Z. mays grown under the As-contaminated soil. The application of Si bears a protective role and increases plant morphology and physiology under As, Cd, and Cr stress (Khan and Gupta 2018, Rizwan et al. 2012, Ulhassan et al. 2022b). This might result from the fact that Si application leads to a secretion of secondary metabolites which assist in ameliorating metal-stressed conditions and additionally due to the dilution effects of Si application which increase morpho-physiological traits by decreasing the contents of As in plant roots and shoots (Jia-Wen et al. 2013; Liang et al. 2007). The oxidative stress in plant cells and tissues can be reduced by the application of Si which increases the activities of antioxidants and capturing of stress-induced ROS (Ulhassan et al. 2022a). Our results illustrated that Si application decreased oxidative stress indicators (Fig. 3) and increased the activities of various antioxidant compounds such as SOD, POD, and CAT (Fig. 4) and their gene expression (Fig. 5) and also regulates the non-enzymatic compounds and sugar (Fig. 6) and nutrients (Fig. 7) in Z. mays. The application of Si induces the activities of antioxidative enzymes and, therefore, can be considered an indicator of enhanced ROS production and extenuation (Fig. 3). Moreover, our results showed that the application of Si decreased the uptake of As concentration in the roots and shoots of Z. mays under As-contaminated soil (Fig. 8). This might be due to the Si restricting apoplasmic transport of heavy metals and thus decreasing the concentration of free As ions in apoplasm (Siddiqui et al. 2022; Tripathi et al. 2012).
Sodium hydrogen sulfide (NaHS) is known as an excellent signaling molecule that regulates various physicochemical processes in plants (Mfarrej et al. 2021). Exogenously applied NaHS can increase plant growth (Fig. 1) by improving chlorophyll content (Fig. 2). Increases in plant growth after NaHS application could be related to increased antioxidant capacity and reduced ROS accumulation (Kaya et al. 2020c). However, inhibition of plant growth and reduction in chlorophyll content and photosynthetic parameters were greatly alleviated by exogenous application of NaHS (Figs. 1 and 2). NaHS has been reported to be the third gas transmitter after NO and CO in animals (Ali et al. 2014), but there is still little evidence demonstrating the role of H2S as a signal molecule in plants. Many studies have shown that sulfur-containing defense compounds, which include elemental sulfur (S0), H2S, glutathione, phytochelatins, various secondary metabolites, and sulfur-rich proteins, are crucial for the survival of plants under abiotic stresses. However, whether volatile NaHS belongs to the group of sulfur-containing defense compounds is still controversial. The involvement of NaHS in the alleviation of Pb (Ali et al. 2014), osmotic (Zhang et al. 2010) (Zhang et al. 2010), Al (Chen et al. 2013), and Cr (Ahmad et al. 2019b) stress has recently been reported.
Conclusion
On the basis of these findings, it can be concluded that the negative impact of As toxicity can be overcome by the external application of Si and NaHS. Our results depict that As toxicity induced severe metal toxicity in Z. mays by increasing the generation of ROS in the form of oxidative stress and also increasing the concentration of As in the roots and shoots of the plants. Furthermore, As toxicity also increased organic acid exudation and imbalance in the nutritional status of the plants, which ultimately decrease plant growth, yield, and photosynthetic efficiency. Hence, As toxicity was eliminated by the external application of Si and NaHS, which also decreased the As concentration in the plant tissues, degenerated ROS, and organic acid exudation, but increased the activities of antioxidants and essential nutrients in the plants. Therefore, long-term field studies should be executed to draw parallels among plants/crops root exudations, metal stress, nutrient fertigation regimes, nutrient mobility patterns, and plant growth in order to gain insights into the underlying mechanisms.
Data availability
Data and material are available for research purpose and for reference.
References
Abbas S, Javed MT, Shahid M, Tanwir K, Saleem MH, Niazi NK (2023) Deciphering the impact of Acinetobacter sp. SG-5 strain on two contrasting Zea mays L. cultivars for root exudations and distinct physio-biochemical attributes under cadmium stress. J Plant Growth Regul 1–18. https://doi.org/10.1007/s00344-023-10987-0
Adrees M, Ali S, Rizwan M, Zia-ur-Rehman M, Ibrahim M, Abbas F, Farid M, Qayyum MF, Irshad MK (2015) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Saf 119:186–197
Aebi H (1984): [13] Catalase in vitro, Methods in enzymology. Elsevier, pp 121–126
Afzal J, Saleem MH, Batool F, Elyamine AM, Rana MS, Shaheen A, El-Esawi MA, Tariq Javed M, Ali Q, Arslan Ashraf M, Hussain GS, Hu C (2020) Role of ferrous sulfate (FeSO4) in resistance to cadmium stress in two rice (Oryza sativa L.) genotypes. Biomolecules 10:1693
Ahmad P, Ahanger MA, Alyemeni MN, Wijaya L, Alam P (2018) Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 255:79–93
Ahmad P, Ahanger MA, Alam P, Alyemeni MN, Wijaya L, Ali S, Ashraf M (2019) Silicon (Si) supplementation alleviates NaCl toxicity in mung bean [Vigna radiata (L.) Wilczek] through the modifications of physio-biochemical attributes and key antioxidant enzymes. J Plant Growth Regul 38:70–82
Ahmad P, Alyemeni MN, Al-Huqail AA, Alqahtani MA, Wijaya L, Ashraf M, Kaya C, Bajguz A (2020) Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 9:825
Ahmad S, Mfarrej MFB, El-Esawi MA, Waseem M, Alatawi A, Nafees M, Saleem MH, Rizwan M, Yasmeen T, Anayat A, Ali S (2022) Chromium-resistant Staphylococcus aureus alleviates chromium toxicity by developing synergistic relationships with zinc oxide nanoparticles in wheat. Ecotoxicol Environ Saf 230:113142
Ahmad R, Ali S, Rizwan M, Dawood M, Farid M, Hussain A, Wijaya L, Alyemeni MN, Ahmad P (2019b) Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol Plant 168(2):289–300
Akram R, Turan V, Wahid A, Ijaz M, Shahid MA, Kaleem S, Hafeez A, Maqbool MM, Chaudhary HJ, Munis MFH (2018) Paddy land pollutants and their role in climate change. Environ Pollut Paddy Soils 113–124
Al Jabri H, Saleem MH, Rizwan M, Hussain I, Usman K, Alsafran M (2022) Zinc oxide nanoparticles and their biosynthesis: overview. Life 12:594
Alamri S, Hu Y, Mukherjee S, Aftab T, Fahad S, Raza A, Ahmad M, Siddiqui MH (2020) Silicon-induced postponement of leaf senescence is accompanied by modulation of antioxidative defense and ion homeostasis in mustard (Brassica juncea) seedlings exposed to salinity and drought stress. Plant Physiol Biochem 157:47–59
Alatawi A, Wang X, Maqbool A, Saleem MH, Usman K, Rizwan M, Yasmeen T, Arif MS, Noreen S, Hussain A, Ali S (2022) S-fertilizer (elemental sulfur) improves the phytoextraction of cadmium through Solanum nigrum L. Int J Environ Res Public Health 19:1655
Ali B, Mwamba TM, Gill RA, Yang C, Ali S, Daud MK, Wu Y, Zhou W (2014) Improvement of element uptake and antioxidative defense in Brassica napus under lead stress by application of hydrogen sulfide. Plant Growth Regul 74:261–273
Ali U, Shaaban M, Bashir S, Fu Q, Zhu J, Islam MS, Hu H (2019) Effect of rice straw, biochar and calcite on maize plant and Ni bio-availability in acidic Ni contaminated soil. J Environ Manage 259:109674
Alsafran M, Usman K, Ahmed B, Rizwan M, Saleem MH, Al Jabri H (2022) Understanding the phytoremediation mechanisms of potentially toxic elements: a proteomic overview of recent advances. Front Plant Sci 13. https://doi.org/10.3389/fpls.2022.881242
Anwaar SA, Ali S, Ali S, Ishaque W, Farid M, Farooq MA, Najeeb U, Abbas F, Sharif M (2015) Silicon (Si) alleviates cotton (Gossypium hirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environ Sci Pollut Res 22:3441–3450
Anwar S, Khan S, Ashraf MY, Noman A, Zafar S, Liu L, Ullah S, Fahad S (2017) Impact of chelator-induced phytoextraction of cadmium on yield and ionic uptake of maize. Int J Phytorem 19:505–513
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol 24:1
Austin RB (1990) Prospects for genetically increasing the photosynthetic capacity of crops. Plant Biology 10:395–409
Azuma K, Nakayama M, Koshioka M, Ippoushi K, Yamaguchi Y, Kohata K, Yamauchi Y, Ito H, Higashio H (1999) Phenolic antioxidants from the leaves of Corchorus olitorius L. J Agric Food Chem 47:3963–3966
Bashir A, Rizwan M, Ali S, urRehman MZ, Ishaque W, Riaz MA, Maqbool A (2018) Effect of foliar-applied iron complexed with lysine on growth and cadmium (Cd) uptake in rice under Cd stress. Environ Sci Pollut Res 25:20691–20699
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bhat JA, Faizan M, Bhat MA, Huang F, Yu D, Ahmad A, Bajguz A, Ahmad P (2022) Defense interplay of the zinc-oxide nanoparticles and melatonin in alleviating the arsenic stress in soybean (Glycine max L.). Chemosphere 288:132471
Bilen S, Bilen M, Turan V (2019) Relationships between cement dust emissions and soil properties. Polish J Environ Stud 28(5)
Bray H, Thorpe W (1954) Analysis of phenolic compounds of interest in metabolism. Methods Biochem Anal 27–52
Chen J, Wang W-H, Wu F-H, You C-Y, Liu T-W, Dong X-J, He J-X, Zheng H-L (2013) Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362:301–318
Chen C-N, Pan S-M (1996) Assay of superoxide dismutase activity by combining electrophoresis and densitometry. Bot Bullet Acad Sinica 37
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Du H, Gao W, Li J, Shen S, Wang F, Fu L, Zhang K (2019) Effects of digested biogas slurry application mixed with irrigation water on nitrate leaching during wheat-maize rotation in the North China Plain. Agric Water Manag 213:882–893
Dubois M, Gilles KA, Hamilton JK, Pt R, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
El-Tohory S, Zeng W, Huang J, Moussa MG, Dong L, Mohamed A, Khalifa O, Saleem MH, Zhran M, Salama MA, Wu J (2023) Effect of intercropping and biochar application on cadmium removal capacity by corchorus olitorius and zea mays. Environ Technol Innov 29:103033
Faizan M, Sehar S, Rajput VD, Faraz A, Afzal S, Minkina T, Sushkova S, Adil MF, Yu F, Alatar AA, Akhter F, Faisal M (2021) Modulation of cellular redox status and antioxidant defense system after synergistic application of zinc oxide nanoparticles and salicylic acid in rice (Oryza sativa) plant under arsenic stress. Plants 10:2254
Fan X, Wen X, Huang F, Cai Y, Cai K (2016) Effects of silicon on morphology, ultrastructure and exudates of rice root under heavy metal stress. Acta Physiol Plant 38:197
Farooq MA, Gill RA, Ali B, Wang J, Islam F, Ali S, Zhou W (2016) Subcellular distribution, modulation of antioxidant and stress-related genes response to arsenic in Brassica napus L. Ecotoxicology 25:350–366
Greger M, Landberg T (2008) Role of rhizosphere mechanisms in Cd uptake by various wheat cultivars. Plant Soil 312:195–205
Hadi F, Bano A, Fuller MP (2010) The improved phytoextraction of lead (Pb) and the growth of maize (Zea mays L.): the role of plant growth regulators (GA3 and IAA) and EDTA alone and in combinations. Chemosphere 80:457–462
Hafeez A, Tipu MI, Saleem MH, Al-Ashkar I, Saneoka H, Sabagh AE (2022) Foliar application of moringa leaf extract (MLE) enhanced antioxidant system, growth, and biomass related attributes in safflower plants. S Afr J Bot 150:1087–1095
Hasanuzzaman M, Alam MM, Nahar K, Mohsin SM, Bhuyan MB, Parvin K, Hawrylak-Nowak B, Fujita M (2019) Silicon-induced antioxidant defense and methylglyoxal detoxification works coordinately in alleviating nickel toxicity in Oryza sativa L. Ecotoxicology 28:261–276
Hassan A, Parveen A, Hussain S, Hussain I, Rasheed R (2022) Investigating the role of different maize (Zea mays L.) cultivars by studying morpho-physiological attributes in chromium-stressed environment. Environ Sci Pollut Res 29:72886–72897
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Heile AO, Zaman Qu, Aslam Z, Hussain A, Aslam M, Saleem MH, Abualreesh MH, Alatawi A, Ali S (2021) Alleviation of cadmium phytotoxicity using silicon fertilization in wheat by altering antioxidant metabolism and osmotic adjustment. Sustainability 13:11317
Huq ME, Fahad S, Shao Z, Sarven MS, Al-Huqail AA, Siddiqui MH, ur Rahman MH, Khan IA, Alam M, Saeed M (2019) High arsenic contamination and presence of other trace metals in drinking water of Kushtia district, Bangladesh. J Environ Manage 242:199–209
Hussain I, Afzal S, Ashraf MA, Rasheed R, Saleem MH, Alatawi A, Ameen F, Fahad S (2022) Effect of metals or trace elements on wheat growth and its remediation in contaminated soil. J Plant Growth Regul 42(4(2023)):2258–2282
Imran M, Hussain S, Rana MS, Saleem MH, Rasul F, Ali KH, Potcho MP, Pan S, Duan M, Tang X (2021) Molybdenum improves 2-acetyl-1-pyrroline, grain quality traits and yield attributes in fragrant rice through efficient nitrogen assimilation under cadmium toxicity. Ecotoxicol Environ Saf 211:111911
Iqbal MA, Khalid M, Zahir ZA, Ahmad R (2016) Auxin producing plant growth promoting rhizobacteria improve growth, physiology and yield of maize under saline field conditions. Int J Agric Biol 18:37–45
Irshad S, Xie Z, Kamran M, Nawaz A, Faheem MS, Gulzar H, Saleem MH, Rizwan M, Malik Z, Parveen A, Ali S (2021) Biochar composite with microbes enhanced arsenic biosorption and phytoextraction by Typha latifolia in hybrid vertical subsurface flow constructed wetland. Environ Pollut 291:118269
Jana S, Choudhuri MA (1981) Glycolate metabolism of three submersed aquatic angiosperms: effect of heavy metals. Aquat Bot 11:67–77
Javed MT, Stoltz E, Lindberg S, Greger M (2013) Changes in pH and organic acids in mucilage of Eriophorum angustifolium roots after exposure to elevated concentrations of toxic elements. Environ Sci Pollut Res 20:1876–1880
Javed MT, Akram MS, Tanwir K, Chaudhary HJ, Ali Q, Stoltz E, Lindberg S (2017) Cadmium spiked soil modulates root organic acids exudation and ionic contents of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotoxicol Environ Saf 141:216–225
Javed MT, Akram MS, Habib N, Tanwir K, Ali Q, Niazi NK, Gul H, Iqbal N (2018) Deciphering the growth, organic acid exudations, and ionic homeostasis of Amaranthus viridis L. and Portulaca oleracea L. under lead chloride stress. Environ Sci Pollut Res 25:2958–2971
Javed MT, Saleem MH, Aslam S, Rehman M, Iqbal N, Begum R, Ali S, Alsahli AA, Alyemeni MN, Wijaya L (2020) Elucidating silicon-mediated distinct morpho-physio-biochemical attributes and organic acid exudation patterns of cadmium stressed Ajwain (Trachyspermum ammi L.). Plant Physiol Biochem 157:23–37
Jia-Wen W, Yu S, Yong-Xing Z, Yi-Chao W, Hai-Jun G (2013) Mechanisms of enhanced heavy metal tolerance in plants by silicon: a review. Pedosphere 23:815–825
Kamran M, Danish M, Saleem MH, Malik Z, Parveen A, Abbasi GH, Jamil M, Ali S, Afzal S, Riaz M (2020) Application of abscisic acid and 6-benzylaminopurine modulated morpho-physiological and antioxidative defense responses of tomato (Solanum lycopersicum L.) by minimizing cobalt uptake. Chemosphere 263:128169
Kaya C, Higgs D, Ashraf M, Alyemeni MN, Ahmad P (2020) Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol Plant 168:256–277
Kaya C, Şenbayram M, Akram NA, Ashraf M, Alyemeni MN, Ahmad P (2020) Sulfur-enriched leonardite and humic acid soil amendments enhance tolerance to drought and phosphorus deficiency stress in maize (Zea mays L.). Sci Rep 10:1–13
Kaya C, Ashraf M, Al-Huqail AA, Alqahtani MA, Ahmad P (2020a) Silicon is dependent on hydrogen sulphide to improve boron toxicity tolerance in pepper plants by regulating the AsA-GSH cycle and glyoxalase system. Chemosphere 257:127241
Kaya C, Sarıoğlu A, Ashraf M, Alyemeni MN, Ahmad P (2020) Gibberellic acid-induced generation of hydrogen sulfide alleviates boron toxicity in tomato (Solanum lycopersicum L.) plants. Plant Physiol Biochem 153:53–63
Khan E, Gupta M (2018) Arsenic–silicon priming of rice (Oryza sativa L.) seeds influence mineral nutrient uptake and biochemical responses through modulation of Lsi-1, Lsi-2, Lsi-6 and nutrient transporter genes. Sci Rep 8:1–16
Kong M, Liang J, Ali Q, Wen W, Wu H, Gao X, Gu Q (2021) 5-Methoxyindole, a chemical homolog of melatonin, adversely affects the phytopathogenic fungus Fusarium graminearum. Int J Mol Sci 22:10991
Lessl JT, Guan DX, Sessa E, Rathinasabapathi B, Ma LQ (2015) Transfer of arsenic and phosphorus from soils to the fronds and spores of arsenic hyperaccumulator Pteris vittata and three non-hyperaccumulators. Plant Soil 390:49–60
Lewis CE, Walker JR, Lancaster JE, Sutton KH (1998) Determination of anthocyanins, flavonoids and phenolic acids in potatoes. I: Coloured cultivars of Solanum tuberosum L. J Sci Food Agric 77:45–57
Liang Y, Sun W, Zhu Y-G, Christie P (2007) Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut 147:422–428
Ma J, Li Y, Chen F, Sun Y, Zhu Y, Wang L (2023) Bacillus mycoides PM35 in combination with titanium dioxide (TiO2)⎯ nanoparticles enhanced morpho-physio-biochemical attributes in Barley (Hordeum vulgare L.) under cadmium stress. Chemosphere 323:138224
Ma J, Ali S, Saleem MH, Mumtaz S, Yasin G, Ali B, Al-Ghamdi AA, Elshikh MS, Vodnar DC, Marc RA, Rehman A, Khan MN, Chen F, Ali S (2022a) Short-term responses of Spinach (Spinacia oleracea L.) to the individual and combinatorial effects of nitrogen, phosphorus and potassium and silicon in the soil contaminated by boron. Front Plant Sci 13. https://doi.org/10.3389/fpls.2022.983156
Ma J, Saleem MH, Yasin G, Mumtaz S, Qureshi FF, Ali B, Ercisli S, Alhag SK, Ahmed AE, Vodnar DC, Hussain I, Marc RA, Chen F (2022b) Individual and combinatorial effects of SNP and NaHS on morpho-physio-biochemical attributes and phytoextraction of chromium through Cr-stressed spinach (Spinacia oleracea L.). Front Plant Sci 13:973740. https://doi.org/10.3389/fpls.2022.973740
Madhu PM, Sadagopan RS (2020) Effect of heavy metals on growth and development of cultivated plants with reference to cadmium, chromium and lead–a review. J Stress Physiol Biochem 16:84–102
Marmiroli M, Pigoni V, Savo-Sardaro ML, Marmiroli N (2014) The effect of silicon on the uptake and translocation of arsenic in tomato (Solanum lycopersicum L.). Environ Exp Bot 99:9–17
Mfarrej M, Wang X, Hamzah Saleem M, Hussain I, Rasheed R, Arslan Ashraf M, Iqbal M, Sohaib Chattha M, Nasser Alyemeni M (2021) Hydrogen sulphide and nitric oxide mitigate the negative impacts of waterlogging stress on wheat (Triticum aestivum L.). Plant Biol 24(4):670–683
Mondal S, Pramanik K, Ghosh SK, Pal P, Mondal T, Soren T, Maiti TK (2021) Unraveling the role of plant growth-promoting rhizobacteria in the alleviation of arsenic phytotoxicity: a review. Microbiol Res 250:126809
Mumtaz S, Hameed M, Ahmad F, Ahmad MSA, Ahmad I, Ashraf M, Saleem MH (2021) Structural and functional determinants of physiological pliability in Kyllinga brevifolia Rottb. for Survival in Hyper-Saline Saltmarshes. Water Air Soil Pollut 232:424
Mushtaq T, Shah AA, Akram W, Yasin NA (2020) Synergistic ameliorative effect of iron oxide nanoparticles and Bacillus subtilis S4 against arsenic toxicity in Cucurbita moschata: polyamines, antioxidants, and physiochemical studies. Int J Phytorem 22:1408–1419
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Ozfidan-Konakci C, Yildiztugay E, Elbasan F, Kucukoduk M, Turkan I (2020) Hydrogen sulfide (H2S) and nitric oxide (NO) alleviate cobalt toxicity in wheat (Triticum aestivum L.) by modulating photosynthesis, chloroplastic redox and antioxidant capacity. J Hazard Mater 388:122061
Panda P, Nath S, Chanu TT, Sharma GD, Panda SK (2011) Cadmium stress-induced oxidative stress and role of nitric oxide in rice (Oryza sativa L.). Acta Physiol Plant 33:1737–1747
Pękal A, Pyrzynska K (2014) Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal Methods 7:1776–1782
Pirooz P, Amooaghaie R, Ahadi A, Sharififar F (2021) Silicon-induced nitric oxide burst modulates systemic defensive responses of Salvia officinalis under copper toxicity. Plant Physiol Biochem 162:752–761
Ranum P, Peña-Rosas JP, Garcia-Casal MN (2014) Global maize production, utilization, and consumption. Ann N Y Acad Sci 1312:105–112
Rehman M, Liu L, Bashir S, Saleem MH, Chen C, Peng D, Siddique KH (2019) Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper-contaminated soil. Plant Physiol Biochem 138:121–129
Rehman M, Fahad S, Saleem Mh, Hafeez M, Rahman Mh, Liu F, Deng G (2020) Red light optimized physiological traits and enhanced the growth of ramie (Boehmeria nivea L.). Photosynthetica 58(4):922–931
Rehman M, Saleem MH, Fahad S, Bashir S, Peng D, Deng G, Alamri S, Siddiqui MH, Khan SM, Shah RA (2021) Effects of rice straw biochar and nitrogen fertilizer on ramie (Boehmeria nivea L) morpho-physiological traits, copper uptake and post-harvest soil characteristics, grown in an aged-copper contaminated soil. J Plant Nutr 45(1):11–24
Rizwan M, Meunier J-D, Miche H, Keller C (2012) Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J Hazard Mater 209:326–334
Rizwan M, Meunier J-D, Davidian J-C, Pokrovsky O, Bovet N, Keller C (2016) Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ Sci Pollut Res 23:1414–1427
Sakharov IY, Ardila GB (1999) Variations of peroxidase activity in cocoa (Theobroma cacao L.) beans during their ripening, fermentation and drying. Food Chem 65:51–54
Sakya A, Prahasto D (2020) The application of phosphorus and potassium to increase drought tolerance in Pereskia bleo (Kunt) DC with proline and antioxidant indicators, IOP Conference Series: Earth and Environmental Science. IOP Publishing, pp 012055
Saleem MH, Ali S, Seleiman MF, Rizwan M, Rehman M, Akram NA, Liu L, Alotaibi M, Al-Ashkar I, Mubushar M (2019) Assessing the correlations between different traits in copper-sensitive and copper-resistant varieties of jute (Corchorus capsularis L.). Plants 8:545
Saleem MH, Ali S, Hussain S, Kamran M, Chattha MS, Ahmad S, Aqeel M, Rizwan M, Aljarba NH, Alkahtani S (2020) Flax (Linum usitatissimum L.): a potential candidate for phytoremediation? Biological and Economical Points of View. Plants 9:496
Saleem MH, Ali S, Irshad S, Hussaan M, Rizwan M, Rana MS, Hashem A, Abd-Allah EF, Ahmad P (2020) Copper uptake and accumulation, ultra-structural alteration, and bast fibre yield and quality of fibrous jute (Corchorus capsularis L.) plants grown under two different soils of China. Plants 9:404
Saleem MH, Ali S, Rehman M, Rana MS, Rizwan M, Kamran M, Imran M, Riaz M, Soliman MH, Elkelish A (2020) Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 248:126032
Saleem MH, Fahad S, Khan SU, Ahmar S, Khan MHU, Rehman M, Maqbool Z, Liu L (2020) Morpho-physiological traits, gaseous exchange attributes, and phytoremediation potential of jute (Corchorus capsularis L.) grown in different concentrations of copper-contaminated soil. Ecotoxicol Environ Safety 189:109915
Saleem MH, Parveen A, Khan SU, Hussain I, Wang X, Alshaya H, El-Sheikh MA, Ali S (2022) Silicon fertigation regimes attenuates cadmium toxicity and phytoremediation potential in two maize (Zea mays L.) cultivars by minimizing its uptake and oxidative stress. Sustainability 14:1462
Saleem MH, Mfarrej MFB, Alatawi A, Mumtaz S, Imran M, Ashraf MA, Rizwan M, Usman K, Ahmad P, Ali S (2023) Silicon enhances morpho–physio–biochemical responses in arsenic stressed spinach (Spinacia oleracea L.) by minimizing its uptake. J Plant Growth Regul 42(3):2053–72
Shafigh M, Ghasemi-Fasaei R, Ronaghi A (2016) Influence of plant growth regulators and humic acid on the phytoremediation of lead by maize in a Pb-polluted calcareous soil. Arch Agron Soil Sci 62:1733–1740
Shahid M, Dumat C, Khalid S, Rabbani F, Farooq ABU, Amjad M, Abbas G, Niazi NK (2019) Foliar uptake of arsenic nanoparticles by spinach: an assessment of physiological and human health risk implications. Environ Sci Pollut Res 26:20121–20131
Siddiqui MH, Mukherjee S, Al-Munqedhi BMA, Kumar R, Kalaji HM (2022) Salicylic acid and silicon impart resilience to lanthanum toxicity in Brassica juncea L. seedlings. Plant Growth Regul 20:1–4
Sun Y, Ma L, Ma J, Li B, Zhu Y, Chen F (2022) Combined application of plant growth promoting bacteria and iron oxide nano-particles ameliorates the toxic effects of arsenic in Ajwain (Trachyspermum ammi L.). Front Plant Sci 13:5264. https://doi.org/10.3389/fpls.2022.1098755
Sun Y, Mfarrej MFB, Song X, Ma J, Min B, Chen F (2023) New insights in to the ameliorative effects of zinc and iron oxide nanoparticles to arsenic stressed spinach (Spinacia oleracea L.). Plant Physiol Biochem 107715. https://doi.org/10.1016/j.plaphy.2023.107715
Tanveer Y, Yasmin H, Nosheen A, Ali S, Ahmad A (2022) Ameliorative effects of plant growth promoting bacteria, zinc oxide nanoparticles and oxalic acid on Luffa acutangula grown on arsenic enriched soil. Environ Pollut 300:118889
Tripathi DK, Singh VP, Kumar D, Chauhan DK (2012) Rice seedlings under cadmium stress: effect of silicon on growth, cadmium uptake, oxidative stress, antioxidant capacity and root and leaf structures. Chem Ecol 28:281–291
Turan V (2019) Confident performance of chitosan and pistachio shell biochar on reducing Ni bioavailability in soil and plant plus improved the soil enzymatic activities, antioxidant defense system and nutritional quality of lettuce. Ecotoxicol Environ Saf 183:109594
Turan V (2020) Potential of pistachio shell biochar and dicalcium phosphate combination to reduce Pb speciation in spinach, improved soil enzymatic activities, plant nutritional quality, and antioxidant defense system. Chemosphere 245:125611
Turan V, Schröder P, Bilen S, Insam H, Juárez MF-D (2019) Co-inoculation effect of Rhizobium and Achillea millefolium L. oil extracts on growth of common bean (Phaseolus vulgaris L.) and soil microbial-chemical properties. Sci Rep 9:1–10
UdDin I, Bano A, Masood S (2015) Chromium toxicity tolerance of Solanum nigrum L. and Parthenium hysterophorus L. plants with reference to ion pattern, antioxidation activity and root exudation. Ecotoxicol Environ Saf 113:271–278
Ulhassan Z, Bhat JA, Zhou W, Senan AM, Alam P, Ahmad P (2022) Attenuation mechanisms of arsenic induced toxicity and its accumulation in plants by engineered nanoparticles: a review. Environ Pollut 302:119038
Ulhassan Z, Khan I, Hussain M, Khan AR, Hamid Y, Hussain S, Allakhverdiev SI, Zhou W (2022) Efficacy of metallic nanoparticles in attenuating the accumulation and toxicity of chromium in plants: current knowledge and future perspectives. Environ Pollut 315:120390
Wen E, Yang X, Chen H, Shaheen SM, Sarkar B, Xu S, Song H, Liang Y, Rinklebe J, Hou D (2020) Iron-modified biochar and water management regime-induced changes in plant growth, enzyme activities, and phytoavailability of arsenic, cadmium and lead in a paddy soil. J Hazard Mater 407:124344.
Wu J, Geilfus C-M, Pitann B, Mühling K-H (2016) Silicon-enhanced oxalate exudation contributes to alleviation of cadmium toxicity in wheat. Environ Exp Bot 131:10–18
Yaseen R, Aziz O, Saleem MH, Riaz M, Zafar-ul-Hye M, Rehman M, Ali S, Rizwan M, Nasser Alyemeni M, El-Serehy HA (2020) Ameliorating the drought stress for wheat growth through application of ACC-deaminase containing rhizobacteria along with biogas slurry. Sustainability 12:6022
Zafar-ul-Hye M, Naeem M, Danish S, Khan MJ, Fahad S, Datta R, Brtnicky M, Kintl A, Hussain GS, El-Esawi MA (2020) Effect of cadmium-tolerant rhizobacteria on growth attributes and chlorophyll contents of bitter gourd under cadmium toxicity. Plants 9:1386
Zhang H, Hu L-Y, Li P, Hu K-D, Jiang C-X, Luo J-P (2010) Hydrogen sulfide alleviated chromium toxicity in wheat. Biol Plant 54:743–747
Acknowledgements
This research was supported by the Higher Education Commission, Islamabad, Pakistan. The Teaching & Research Practice Field for School of Public Administration provided the pot experiment site.
Funding
This work was supported by the National Natural Science Foundation of China (no. 51974313, 41907405).
Author information
Authors and Affiliations
Contributions
Formal analysis: Sadia Javed, Sahar Mumtaz, Muhammad Ahsan Asghar, Aishah Alatawi, Romina Alina Marc. Funding acquisition: Shafaqat Ali, Sadia Javed, Sahar Mumtaz. Methodology: Sadia Javed, Mohsen Mohamed Elsharkawy, Romina Alina Marc, Ghulam Yasin, Sahar Mumtaz. Software: Manar Fawzi Bani Mfarrej, Sadia Javed, Shah Fahad, Aishah Alatawi, Sahar Mumtaz, Ghulam Yasin Mohsen Mohamed Elsharkawy, Romina Alina Marc, Javeed Lone. Supervision: Javeed Lone, Romina Alina Marc, Shafaqat Ali. Writing—original draft: Mohsen Mohamed Elsharkawy, Aishah Alatawi, Shah Fahad, Muhammad Ahsan Asghar, Rana M. Alshegaihi, Manar Fawzi Bani Mfarrej, Shafaqat Ali. Writing—review and editing: Shah Fahad, Aishah Alatawi, Rana M. Alshegaihi, Ghulam Yasin, Manar Fawzi Bani Mfarrej. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Written consent was sought from each author to publish the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alatawi, A., Mfarrej, M.F.B., Alshegaihi, R.M. et al. Application of silicon and sodium hydrosulfide alleviates arsenic toxicity by regulating the physio-biochemical and molecular mechanisms of Zea mays. Environ Sci Pollut Res 30, 76555–76574 (2023). https://doi.org/10.1007/s11356-023-27739-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27739-y