Abstract
Nanotechnology has achieved great attention due to its impressive performance especially engineered nanoparticles (ENPs). Copper-based nanoparticles offer favorable development in the fabrication of agrochemicals including fertilizers and pesticides in the field of agriculture. However, their toxic impact on melon plants (Cucumis melo) still needs to be investigated. Therefore, the aim of the current work was performed to focus on the toxic impact of Cu oxide nanoparticles (CuONPs) in hydroponically grown Cucumis melo. Our results demonstrated that CuONPs with 75, 150, and 225 mg/L significantly (P<0.005) suppressed the growth rate and badly affect physiological and biochemical activities in melon seedlings. Also, results revealed remarkable phenotypical changes besides significantly reduced fresh biomass and decreased levels of total chlorophyll contents in a dose-dependent manner. Atomic absorption spectroscopy (ASS) analysis exhibited that C. melo treated with CuONPs accumulates NPs in the shoot. Moreover, exposure to higher CuONPs (75–225mg/L) significantly increased the reactive oxygen species (ROS) accumulation, malondialdehyde (MDA), and hydrogen peroxide (H2O2) level in the shoot and induced toxicity in melon root with an increase in electrolyte leakage. Furthermore, antioxidant enzyme peroxidase (POD) and superoxide dismutase (SOD) activity in the shoot significantly increased under exposure to higher CuONPs. Exposure to higher concentrations of CuONPs (225 mg/L) significantly deformed the stomatal aperture. Furthermore, reducing the number and abnormal size of palisade mesophyll and spongy mesophyll cells were investigated especially at high doses of CuONPs. Overall, our current work demonstrates that CuONPs of 10–40 nm size provide direct evidence for a toxic effect in C. melo seedlings. Our findings were expected to inspire the safe production of NPs and agrifood security. Thus, CuONPs prepared from toxic route and its bioaccumulation into our food chain through crop plants possess a serious threat to the ecological system.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
ENPs (engineered nanoparticles) are small particles with a size of 1 to 100 nanometers in diameter (Yang et al. 2020). The physicochemical properties of metal and metal-based NPs differ from those of their native bulk salt material. The distribution of ENPs in agriculture and related industries, such as the chemical, pharmaceutical, biomedical, optical, food, and textile industries, has gained mainstream attraction (Arya et al. 2019, Staroń et al. 2020). The extensive use of nanoparticles has been achieved in their inescapable and irrevocable release into the agricultural zone and maintaining global food security (Pang et al. 2021). ENPs destructively impact the environment and organisms, which lead to a considerable source of concern as they become more widely used now. Plants could be directly contaminated by ENPs as they interact with the environment, soil, and water. As a result, regular assessment of toxicity of ENP phytotoxicity is considered vital in environmental protection (Jacobs et al. 2016, Yang et al. 2020, Zhang et al. 2019). The uncontrolled exposure of ENPs in agricrop eventually causes a hazardous effect on the food chain and human health. There have been multiple reports demonstrating the phytotoxicity of nanoparticles in plants (Handy et al. 2008, Mosa et al. 2018, Wiesner et al. 2006). Mostly, long-term exposure of toxic metal nanomaterial is released into the agrifield, consequently causing toxicity in the rhizosphere and leading to adverse effects on the microbial community of soil (Adeel et al. 2021).
Additionally, certain types of nanoparticles are purposefully made for their widespread use in a variety of applications, including biomedical and biological, owing to their unique properties. CuONPs have garnered substantial interest in agriculture as fungicides and insecticides (Ashraf et al. 2021, Vivekanandhan et al. 2021). CuONPs have attracted more researchers due to their bactericidal and biological activities (Salah et al. 2021). CuONPs are being used extensively, and their high-demand applications are increasing the likelihood of their release into the atmosphere and bioaccumulation into the soil via agricrops, presenting a major threat to human health (Liu et al. 2018). Consequently, it is critical to study the harmful effects of CuONPs on many crops (Naz et al. 2020). CuONPs have been reported to induce toxicity by causing several physiological and molecular consequences; at different dose-dependent concentrations, they are considered to have adverse effects on different crops (Yang et al. 2020). CuONPs are highly toxic than bulk salt form due to the positive charge on the surface assisting interactions among cells and nanoparticles. Moreover, the dissolution of CuONPs and toxicity depend on the temperature and pH of the solution (Adeel et al. 2021). CuONPs have suppressed the vegetative growth of Brassica juncea L., maize (Zea mays), and cotton (Gossypium hirsutum) in a dose-dependent manner (Le Van et al. 2016, Sui et al. 2014). Several studies of CuONP toxicity have been reported, which has a negative consequence on germination and vegetative development in several crops such as zucchini (Cucurbita pepo) (Stampoulis et al. 2009), barley (Hordeum vulgare) (Shaw et al. 2014), carrot (Daucus carota) (Ebbs et al. 2016), soybean (Glycine max) (Nair &Chung 2014a), tomato (Solanum lycopersicum) (Singh et al. 2017), spinach (Spinacia oleracea) (Zafar et al. 2017), lettuce (Lactuca sativa) (Shams et al. 2018), and cucumber (Cucumis sativus) (Mosa et al. 2018). Among these studies, it is investigated that the unregulated application of CuONPs deteriorates the uptake of beneficial nutrients such as molybdenum (Mo), magnesium (Mg), boron (B), manganese (Mn), iron (Fe), and zinc (Zn) (Nair & Chung 2014a). In addition, it was found that CuONPs with a size of 50 nm had a phytotoxic effect on cucumber plants cultivated hydroponically, with a considerable rise in ROS antioxidant enzymes like POD, CAT, and superoxide dismutase (Arif et al. 2018). Further, exogenous application of CuONPs deformed the stomatal aperture in the same like salt stress and that agglomerate CuONPs might block stomata (Rajput et al. 2015). Subsequently, the accumulation of CuONPs disrupted the ultrastructure of leaves, specifically in the photosystem, by reducing the amount of thylakoids, plastoglobules, starch content, and stomatal aperture (Olchowik et al. 2017). The molecular mechanisms by which CuONPs generate phytotoxicity however are still unknown. Additional investigation is recommended to have a better understanding of the mechanisms.
The main purpose of this work was to investigate a comprehensive study on melon (Cucumis melo) seedlings supplemented with CuONPs in a hydroponic system and examine the phytotoxicity and genotoxicity of CuONPs and investigate physiological factors, e.g., oxidative stress and impaired photosynthetic system, and subsequently the mechanism of uptake of CuONPs to areal parts of the plant, observation of stomatal apertures, and examine the anatomical changes in melon leaf cells. Thus, CuONPs prepared from toxic route and its bioaccumulation into our food chain through crop plants possess a serious threat to the ecological system. Future studies on the effect of CuONPs on other crops are needed to further elucidate their role in recovery from Cu deficiency.
Material and method
Experimental material
CuSO4·5H2O (0.1 M) (copper sulfate pentahydrate), C6H8O6 (0.2 M) (ascorbic acid), and NaOH (sodium hydroxide) and C6H10O5 (1.2 %) (starch) were used. All experiments were conducted using deionized water.
Preparation of CuONPs
Copper NPs were synthesized by a chemical reduction procedure that employed copper (II) sulfate pentahydrate which is a precursor salt and starch as a capping agent. CuONPs were synthesized by vigorously stirring 0.1 M copper (II) sulfate pentahydrate solution with 140 mL of starch (1.2 %) solution for 30 minutes. After that, 40 mL of 0.2 M ascorbic acid solution was added to the solution while continually stirring. Then, with quick stirring, 20 mL of 1 M NaOH (sodium hydroxide) solution was progressively added to the resulting solution and heated at 60 °C for 2 hours. After the reaction solution was finished, the resulting solution was taken from the heat and allowed to cool overnight before the supernatant was carefully collected. The precipitates were removed from the solution using a filtering process and rinsed three times with deionized water and ethanol to remove the excess starch that had attached to the nanoparticles. The resulting nanomaterials (assumed to be CuONPs) were dried in an oven at 80 °C for three hours before being kept in an airtight glass vial for future investigation (Khan et al. 2016).
Characterizations of CuONPs
The CuONPs were extensively characterized by SEM, XRD, TEM, and UV spectroscopy. The absorption spectrum of chemically synthesized CuONPs was evaluated via UV–Vis spectroscopy through UV-Vis Halo DB-20 spectrophotometer Australia within the range of 300–800 nm. XRD analysis was examined to determine the crystallographic structure of CuONPs using the Shimadzu Model Kyoto, Japan. The Debye-Scherrer equation (D = Kλ/β cos θ) was utilized to measure the particle size, where D represents crystal size perpendicular to the reflecting planes, while K is denoted constant (0.9). λ showed X-ray wavelength (1.5406 Å), while β justifies the angular full width at half-maximum in radians and θ is Bragg’s angle. The shape of particles and surface morphology of nanoparticles were investigated by SEM. The drop of CuONPs was placed on a carbon copper grid and visualized under the scanning electron microscope (SEM) using JEOL JSM-5910 SEM model. Further, the size of the CuONPs was investigated with high-resolution TEM microscopy using the JEM-100XX model. The diameter of the nanoparticles was used to establish the average size of the CuONPs.
Melon seedling growth and hydroponic system
The Bingxuecui (Jinan, China) melon cultivar seedlings were used to investigate the effect of CuONPs. The healthy melon seeds were sterilized with 70% alcohol for 60 seconds and 3% with NaClO for 10 min and thoroughly washed with double-distilled water (ddH2O). After it, seeds were germinated for 3 days in a thermostatically controlled incubator on ddH2O wetted filter paper in Petri plates at 28°C. Following, new emerging seedlings were shifted into the 32-hole tray with a substrate mixture of equal parts perlite, peat, and slag and kept in a greenhouse. The optimum temperature was kept at 26°C during the daytime and 18°C at nighttime, with a humidity of 60% and a 14-h photoperiod. Seedlings with two to three leaves completely developed were transplanted to the greenhouse using hydroponic containers with a half-Hoagland solution. A well-developed lateral root system was discovered after 7 days in the hydroponic pots. To keep the melon plants healthy, the boxes were replenished every three days with new Hoagland solutions.
CuONP treatment and analysis of morphological parameters
The synthesized CuONP powders in various concentrations (75, 150, and 225 mg/L) were fully dispersed in ddH2O and sonicated for 40 minutes. Further, the seedling was treated with fresh Hoagland solution containing different concentrations of CuONPs and put back in the greenhouse for 7 days. Each treatment had four replicate plants. Finally, seedlings were thoroughly washed to remove excess particles after 7 days of treatment and dried on tissue paper for 20 minutes. Each plant’s fresh biomass was measured using measuring balance after treatment.
Analysis of total chlorophyll contents, electrolyte leakage, and Cu analysis
The previously established method (Apodaca et al. 2017) was used to investigate the total chlorophyll contents, and the electrolyte leakage estimation was determined according to (Mosa et al. 2018). To check Cu uptake in treated seedling, oven-dried shoot tissues were balanced and crushed to make fine powder and then digested with nitric acid (HNO3) in a water bath at 90°C until brown fumes were finished. After the cooling process, ddH2O and 30% H2O2 were dropped gradually. Further, hydrochloric acid was dropped into the mixture and heated. The prepared samples were cooled at room temperature and filtered, and then, uptake of Cu contents was investigated using ASS (atomic absorption spectroscopy).
Antioxidant analysis of melon seedlings
Antioxidant enzymatic activity including peroxidase (POD) and superoxide dismutase (SOD) along with lipid peroxidation MDA assay (malondialdehyde contents) was examined in melon plants according to Wang et al. (2015) method. H2O2 of leaves was measured according to (Hayat et al. 2020).
Stomatal morphology under the SEM
The physical shape of stomata was studied in 7-day-old leaves of control and affected CuONPs using a scanning electron microscope (EM). The treated and control leaves were cut from the center without midrib with a sharp blade and prefixed with 2.5% glutaraldehyde at 24 h for observing the shape of the stomatal aperture. Consequently, the prepared specimens were thoroughly washed multiple times with phosphate buffer for 1 min. Finally, the samples were dehydrated in a series of ethanol grades starting from 30 to 90% for 15 min in each resultant solution. The final samples were washed thrice in 100% ethanol for 30 min. The Quorum K850 critical point dryer was used to dry the dehydrated sample. A scanning electron microscope (NOVA Nano SEM 230) was used to analyze samples at Shanghai Jiao Tong Analysis Centre Minhang Shanghai China.
Leaf anatomical changes under the transmission electron microscope
To observe the anatomical structure of the upper epidermis, lower epidermis, mesophyll cells, and palisade cells of control and treated CuONPs, the inoculated and uncalculated leaf samples were collected from seedlings and cut into 3 mm excluding midrib small segments and fixed in 2.5% glutaraldehyde in phosphate buffer (0.1 M at pH 7.0) for 24 h. The specimen was then dehydrated using a graded ethanol series of 30%, 50%, 70%, and 90%, clarified in xylene, embedded in paraffin wax, and cut into 8–10 m thick pieces. The prepared transverse leaf section was stained with toluidine blue solution and then observed under the electron microscope (Bx41, Olympus Optical Co. Ltd., Tokyo, Japan).
Statistical analysis
All data were evaluated by using a one-way statistical analysis of variance (ANOVA). Different letters represent significant differences between hormonal treatments at 0.005 probability level (t-test). Data are mean ± SE of three biological replicates.
Results and discussion
Characterizations of CuONPs
To understand the chemical and physical characteristics of the synthesized materials, we categorized the composite materials using important analytical methods. UV-Vis absorbance spectroscopy has shown to be a very effective tool for analyzing metal nanoparticles because the peak locations and shapes are highly sensitive to particle size (Xiong et al. 2011). The visible absorption bands for CuONPs have been performed to be in the range of 500–600 nm which depends on the optical properties of individual nanoparticles, including capping agents, size, and shape (El-Saadony et al. 2020). To demonstrate the influence of the capping agent ascorbic acid concentration on the UV-Vis spectroscopy of chemically produced Cu nanoparticles, the initial modest absorption small peaks with multiple intervals curves are located at around 385 nm, suggesting the ascorbic acid oxidation product. The second broadband surface plasmon resonance of copper nanoparticles has been found at around 570 nm with increasing capping agent intervals (Fig. 1a) (Lee et al. 2020). To check the plasmon resonance of prepared NPs, time is an important feature that enhances NP biotransformation and stability. In our current study, the first characteristic absorption peak was observed after 15 min of the reaction. The intensity of absorbance peaks was increased as the reaction progressed. This was caused by the development of copper nanoparticles. The reaction was completed after 15 min to reach the final time 150 min.
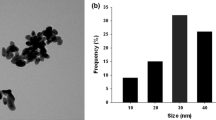
The crystallographic structure and chemical composition of the prepared CuONPs were confirmed by powder XRD. The data showed a sharp peak that the particles were in high nanocrystalline. The diffraction pattern of the synthesized CuONPs reveals six main peaks (Fig. 1b). The 2θ angle corresponding to 110, −111, 002, 111, 202, and 113 peaks, respectively, showed crystalline nature and high purity of the particles. The findings of our XRD analysis strongly resembled the previously reported XRD pattern (Rajendran et al. 2018). The XRD pattern illustrates the crystal nature of the particles which confirmed the preparation of the CuONPs (Sathiyavimal et al. 2018). The surface morphological and structural characterization studies were done by SEM and TEM analysis. CuONPs have a cubic form and are well distributed without aggregation, with diameters ranging from 40 to 80 nm, which are quite similar to the XRD predicted size (Fig.2a). The current findings were further confirmed by previously published work (Khan et al. 2016, Pugazhendhi et al. 2018). Moreover, the typical TEM images of the prepared CuONPs are in cube shape shown in Fig. 2b which is in close agreement with previously published results (Jana et al. 2000, Woo et al. 2012).
Biomass analysis of C. melo under CuONP treatment
Metal nanoparticle formulation has been investigated to show adverse effects on plant vegetative growth and development which can cause death in severe cases at various concentrations (Lee et al. 2010). In the outcomes of the present work, melon plants exposed to different concentrations of CuONPs exhibited clear phenotypic changes at the end of the treatment period (7 days), with yellow spots observed on treated plants. Following treatment, the total fresh biomass of C. melo seedlings was determined. Results showed a significant reduction of C. melo shoot treated with 150 and 225 mg/L CuONPs after 7 days compared with the biomass of the same plants at 0 mg/L CuONP concentration (Fig. 3). However, the seedlings treated with 75 mg/L CuONPs had no remarkable effect on the reduction of biomass as well. Such findings suggest that CuONPs exhibit a phytotoxic effect in a dose-dependent concentration. Furthermore, as predicted, the nontreated control plants (0 mg/L CuONPs) exhibited an increased but not noticeable rise in biomass after 7 days. Indeed, the toxic effect of dose-dependent concentration of CuONPs has been investigated in numerous crops (Mosa et al. 2018, Wang et al. 2012). The aim of this study was to investigate the phenotypic changes and anatomical changes in musk melon plants exposed to CuONPs. This article focuses on the acute toxicity based on various concentrations in terms of biomass reductions, which was similar to previous findings in rice, Zea mays, and cucumber plants grown hydroponically and in soil treated with CuONPs of 50 nm size (Kim et al. 2013, Mosa et al. 2018, Yang et al. 2020).
Total chlorophyll contents and electrolyte leakage analysis
The reduction of chlorophyll content level in plants is the first indicator of phytotoxicity under abiotic stress conditions (Demiral & Türkan 2005). When the melon seedlings were treated with CuONPs for 7 days, obvious growth retardation was noticed in treated plants. The exposure of higher concentrations of CuONPs at the rate of 150–225 mg/L to treated plants showed a significant chlorophyll content reduction which might damage the stomatal aperture and photosynthetic system (Fig. 4a). Following our results, the total chlorophyll and carotenoid levels of B. juncea plants cultivated in the existence of CuONPs were significantly reduced. Under CuONP stress, the Cu-tolerant plant E. splendens showed a strong reduction in total chlorophyll content (Shi et al. 2014). Moreover, the substantial decline in total chlorophyll content could be due to lipid peroxidation changes in leaf thickness and a reduction in the availability of mineral elements under higher oxidative stress (Lequeux et al. 2010). The CuONPs exposed to higher concentration significantly reduced the total chlorophyll Chl-a and Chl-b content in many cultivated crops (Mosa et al. 2018, Nair et al. 2014, Singh et al. 2017, Yang et al. 2020). Most NPs adsorbed on root hair surfaces prevent the uptake of beneficial micro and macronutrients needed for plant growth and development, resulting in decreased chlorophyll biosynthesis levels (Mosa et al. 2018). The considerable decrease in total chlorophyll concentration after CuONP exposure might be attributed to membrane lipid peroxidation (Ma et al. 2013).
Exposure of CuONPs on total chlorophyll contents and electrolyte leakage in melon leaves. A The total chlorophyll fluorescence values of treated and control plants. The 0 mg/L (control) plants have higher total chlorophyll content, and when the concentration of CuONPs (75–225 mg/L) increases, the chlorophyll content decreases. B Electrolyte leakage analysis of CuONPs on C. melo plants. Error bars show standard errors of mean values of three replicate values. Statistically, the difference was calculated at ∗P ≤ 0.05 and ∗∗P ≤ 0.01
The plasma membrane permeability was investigated by measuring electrolyte leakage in control and CuONP-treated tissues. It was observed that exposure to 500 mg/L concentration of cerium oxide (CeO2) nanoparticles enhanced electrolyte leakage in rice seedlings (Hernandez-Viezcas et al. 2013). The plasma membrane integrity of C. melo seedlings treated with a target concentration of CuONPs was measured by electrolyte leakage analysis. The findings demonstrated higher electrolyte leakage in CuONP-treated plants compared to control plants (Fig. 4b). In our present work, C. melo was sensitive to CuONPs, and electrolyte leakage was significantly increased due to high reactivity. The exposure of CuONP toxicity altered the membrane permeability causing damage to the cell membranes and increasing the chances of NPs entering the cells. When CuONPs were treated to C. melo plants, their permeability increased, allowing them to accumulate in the roots and then be translocated to the areal parts through xylem vessels. Hence, CuONPs induced damage to the root plasma membrane integrity of C. melo as indicated by the significant increase in electrolyte leakage in 150 and 225 mg/L CuONP treatment. Additionally, a substantial decrease in electrolyte leakage was examined in agar root culture medium in lettuce plants with cerium oxide nanoparticles (CeO2 NPs) (Cui et al. 2014).
Cu level in shoot after treatment
Plants have played a vital role in the bioavailability of nanoparticles in the food chain (Human health) through the environment (Wang et al. 2012). To assess the bioaccumulation Cu contents, the concentrations of Cu in shoots of C. melo plants were determined by atomic absorbance spectroscopy after 7 days of CuONP treatment (Fig. 5). The results revealed an increased accumulation of Cu in these plants in proportion to the CuONP concentrations (150 and 225 mg/L). In this work, it was observed that exposing melon plants to different concentrations of CuONPs resulted in a significant increment in Cu content in areal branches (Wang et al. 2012). Based on previous literature, it was found that when B. juncea was treated with CuONPs that excess Cu was accumulated in plants’ shoots and roots (Nair & Chung 2015). When plants are supplemented with a higher concentration of CuONPs, the uptake of Cu from root to areal parts of plants translocates through xylem vessels (Feigl et al. 2013). Therefore, it was noticed that higher Cu contents in shoots of melon leave exposed to the higher concentrations of CuONPs might be due to direct contact with roots (Shi et al. 2014). In the hydroponic experiment, the phytotoxicity observed in seedlings was associated with exposure to CuONPs rather than soluble Cu ions in a nutrient solution, since a low concentration of Cu is required for plant development (Feigl et al. 2013). Plants can accumulate CuONPs, which can therefore damage plant growth, net photosynthetic rate, antioxidant enzyme activities, and nutritional element content, and even lead to DNA damage (Hong et al. 2015, Rizwan et al. 2017). Cu may transport electrons to molecular oxygen to generate ROS (O2-) and H2O2 to form OH group causing oxidative damage to cellular and subcellular components such nucleic acids, proteins, and lipids (Xiong et al. 2017).
Analysis of uptake of Cu contents in shoot. Concentration of Cu element in shoots of melon plants grown for 5 days in hydroponic solution inoculated with 75–225 mg/L CuONPs and at 0 mg/L (control). The data was statistically calculated by t-test (*P < 0.05, **P < 0.01, ***P < 0.005). Data are mean ± SE of three biological replicates
Exposure of CuONPs on MDA and H2O2 level
For ROS-mediated oxidative damage to cell membrane integrity, we investigated MDA content in both control and CuONP-treated C. melo seedlings. As shown in Fig. 6a, C. melo plants treated with 150 and 225 mg/L CuONPs showed a significant increase in MDA contents as compared to the unexposed control plants. However, it was observed that lipid peroxidation levels were more prominent in higher doses of CuONP concentration. Collectively, previous results have revealed that higher exposure to CuONPs increases MDA levels in leaves (Azhar et al. 2020). According to numerous previous literature, CuONPs significantly increased MDA levels in rice, barley, and Arabidopsis seedlings (Nair & Chung 2014b, Shaw & Hossain 2013, Wang et al. 2014, Yang et al. 2020). The effect of various concentrations of CuONPs on H2O2 content was analyzed in shoots of melon plants. However, a significant enhancement in H2O2 levels was noticed in shoots as a result of exposure to 75–225 mg/L of CuONPs as compared to the control (P < 0.05) (Fig. 6b). Meanwhile, the exposure of TiO2NP to Hornwort (Hydrilla verticillata) illustrated an increment of H2O2 which was due to oxidative stress (Spengler et al. 2017). It is observed that exposure to higher concentration CuONPs enhances H2O2 activity in Cucumis sativus (Mosa et al., 2018).
Antioxidant activity
Plants have developed antioxidant enzymatic systems SOD, POD, and CAT to mitigate the oxidative damages produced by ROS accumulation (Ali et al. 2017, Bowler et al. 1992, Khan et al. 2019). Under CuONP stress, the antioxidant enzyme activities were differentially modulated. In our current findings, the peroxidase activity (POD) enzyme levels change significantly as a result of exposure to 75–225 mg/L of CuONP concentration as compared to the controls (Fig. 7a). However, dose-dependent concentration and a substantial upsurge in POD enzyme activity were noticed in shoots as a consequence of exposure to a higher concentration of CuONPs (Fig. 7a). Meanwhile, a significant increase in SOD enzyme activity was also detected in shoots of plants that were cultivated in the presence of 75–225 mg/L of CuONPs (Fig. 7b). Previous research has shown that when extra H2O2 is present, the POD enzymes’ activity increases, resulting in increased lignification of plant cells under Cu stress (Kováčik et al. 2010, Lin et al. 2005). CuONPs enhanced oxidative stress (ROS) in plants. Based on previous reports, upregulation in antioxidant enzyme activity indicates that the plant’s defense system against NPs has been activated (Shaw et al. 2014, Song et al. 2016). The SOD (superoxide dismutase) activity in plant cells regulates H2O2 and O2 concentrations and serves as the first line of defense against phytotoxicity produced by various stresses (Azhar et al. 2020).
Effect of CuONPs on guard cell
Stomata on the surface of melon leaves were analyzed to observe the effect of CuONPs (0 and 225 mg/L) on stomatal aperture and guard cells. The results revealed that CuONPs deformed the stomatal pores and altered stomatal aperture as compared to control seedlings (Fig. 8). The guard cells were shrinkage under CuONP stress. However, the plasmolysis of guard cells and damage to stomatal aperture were significant in treated leaves as compared to unexposed plants (Fig. 8C, D). These findings revealed that experience to higher concentrations of CuONPs could damage the stomatal aperture and shape of guard cells under CuONP stress.
Consequence of CuONPs on anatomical changes of leaf cells
The impact of CuONPs (0–225 mg/L) toxicity on the ultracellular structure was inspected in inoculated and uninoculated leaves of melon plants. The results significantly exhibited that under control conditions, leaves had well-developed palisade parenchyma cells, spongy parenchyma, mesophyll cells, vascular bundles, guard cells, and distinguished cell membrane and cell wall (Fig. 9A). The leaves exposed to CuONPs (225 mg/L) exhibited phytotoxic symptoms. The vascular bundles were deformed under CuONP stress. CuONPs reduced the palisade cells, spongy parenchyma, and mesophyll cells and increased the intercellular spaces. Moreover, significant abnormal size and reduced leaf thickness were significantly observed under 225 mg/L CuONP stress (Fig. 9D). However, these destructive cellular structures under CuONP toxicity were more noticeable in mesophyll cells treated as compared to control. CuONPs inhibited the photosynthesis system by modulating stomatal conductance and thylakoid membranes (Da Costa & Sharma 2016, Perreault et al. 2014). Based on the toxicological effect of CuONPs, the present investigation suggests the toxic effects of CuONPs on stomatal aperture (Fig. 8C, D). However, the destructive stomatal aperture and irregular shape of guard cells were significantly remarkable in exposed to 175 and 225 mg/L CuONPs as compared to control (Fig. 8). The more prominent ultrastructural changes and damages to guard cell aperture in exposed CuONP plant leaves might be due to the accumulation of reactive oxygen species (ROS) under CuONP stress (Choudhury & Panda 2005). Moreover, one report illustrated that higher concentrations of ZnONPs have been involved in the reduction of mesophyll cells (Salah et al. 2015). Similar alterations have been investigated in mesophyll cells of spring Hordeum vulgare shoot underexposure of CuONP stress (Rajput et al. 2018). Typically, leaf epidermal and mesophyll cells accumulate excessive amounts of heavy metals (Dobrikova et al. 2021).
Effect of CuONPs on transverse leaf section. A 0 mg/L CuONPs. B 75 mg/L of CuONPs. C 150 mg/L of CuONPs. D 225 mg/L of CuONPs. Upon increasing CuONP exposure, leaves deformed cellular structures and had increased intracellular spaces. Scale bar: 100 μm. EP—epidermis, SP—spongy mesophyll cells, PP—palisade mesophyll cells, IS—intercellular spaces, VB—vascular bundles, GC—guard cells
Conclusion
Collectively, the current findings showed that CuONPs caused oxidative stress and reduced the vegetative growth of C. melo by affecting the plants on physiological, morphological, biochemical, phenotypical, and anatomical levels. Our results investigated that inoculated CuONPs of the size 4–40 nm were toxic to C. melo. CuONPs significantly decreased the total fresh biomass of C. melo-treated plants. Atomic absorbance spectroscopy study confirmed the accumulation of CuONPs in the areal part of plant tissues. Furthermore, CuONPs demonstrated a significant drop in total chlorophyll content, an increase in MDA and H2O2 levels, and a rise in electrolyte leakage, all of which resulted in damage to the melon root plasma membrane. Taken together, the exposure to CuONPs has increased antioxidant activity (SOD and POD) in C. melo. Finally, it is perceived that exposure to higher concentrations of CuONPs resulted in stomatal aperture deformation and altered the subcellular structure of treated melon leaves. Further investigation is required to examine the effect of CuONPs on the uptake of beneficial mineral contents.
Data availability
All data generated or analyzed during this study are included in this published article
Abbreviations
- CuONPs:
-
Cu oxide nanoparticles
- ENPs:
-
engineered nanoparticles
- ASS:
-
atomic absorption spectroscopy
- ROS:
-
reactive oxygen species
- MDA:
-
malondialdehyde
- H2O2 :
-
hydrogen peroxide
- POD:
-
peroxidase
- SOD:
-
superoxide dismutase
- EL:
-
electrolyte leakage
- NaOH:
-
sodium hydroxide
- SEM:
-
scanning electron microscope
- TEM:
-
transmission electron microscope
- XRD:
-
X-ray diffraction
References
Adeel M, Shakoor N, Shafiq M, Pavlicek A, Part F, Zafiu C, Raza A, Ahmad MA, Jilani G, White JC (2021) A critical review of the environmental impacts of manufactured nano-objects on earthworm species. Environ Pollut 290:118041
Ali I, Jan M, Wakeel A, Azizullah A, Liu B, Islam F, Ali A, Daud M, Liu Y, Gan Y (2017) Biochemical responses and ultrastructural changes in ethylene insensitive mutants of Arabidopsis thialiana subjected to bisphenol A exposure. Ecotoxicol Environ Saf 144:62–71
Apodaca SA, Tan W, Dominguez OE, Hernandez-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL (2017) Physiological and biochemical effects of nanoparticulate copper, bulk copper, copper chloride, and kinetin in kidney bean (Phaseolus vulgaris) plants. Sci Total Environ 599:2085–2094
Arif N, Yadav V, Singh S, Tripathi DK, Dubey NK, Chauhan DK, Giorgetti L (2018) Interaction of copper oxide nanoparticles with plants: uptake, accumulation, and toxicity, Nanomaterials in Plants, Algae, and Microorganisms. Elsevier, pp 297–310
Arya A, Mishra V, Chundawat TS (2019) Green synthesis of silver nanoparticles from green algae (Botryococcus braunii) and its catalytic behavior for the synthesis of benzimidazoles. Chemical Data Collections 20:100190
Ashraf H, Anjum T, Riaz S, Ahmad IS, Irudayaraj J, Javed S, Qaiser U, Naseem S (2021) Inhibition mechanism of green-synthesized copper oxide nanoparticles from Cassia fistula towards Fusarium oxysporum by boosting growth and defense response in tomatoes. Environmental Science: Nano 8(6):1729–1748
Azhar W, Khan AR, Muhammad N, Liu B, Song G, Hussain A, Yasin MU, Khan S, Munir R, Gan Y (2020) Ethylene mediates CuO NP-induced ultrastructural changes and oxidative stress in Arabidopsis thaliana leaves. Environmental Science: Nano 7:938–953
Bowler C, Mv M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Biol 43:83–116
Choudhury S, Panda SK (2005) Toxic effects, oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr.) Broth. under chromium and lead phytotoxicity. Water Air Soil Pollut 167:73–90
Cui D, Zhang P, Ma Y, He X, Li Y, Zhang J, Zhao Y, Zhang Z (2014) Effect of cerium oxide nanoparticles on asparagus lettuce cultured in an agar medium. Environmental Science: Nano 1:459–465
Da Costa M, Sharma P (2016) Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 54:110–119
Demiral T, Türkan I (2005) Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53:247–257
Dobrikova AG, Apostolova EL, Hanć A, Yotsova E, Borisova P, Sperdouli I, Adamakis I-DS, Moustakas M (2021) Cadmium toxicity in Salvia sclarea L.: an integrative response of element uptake, oxidative stress markers, leaf structure and photosynthesis. Ecotoxicol Environ Saf 209:111851
Ebbs SD, Bradfield SJ, Kumar P, White JC, Musante C, Ma X (2016) Accumulation of zinc, copper, or cerium in carrot (Daucus carota) exposed to metal oxide nanoparticles and metal ions. Environmental Science: Nano 3:114–126
El-Saadony MT, Abd El-Hack ME, Taha AE, Fouda MM, Ajarem JS, N. Maodaa S, Allam AA, Elshaer N (2020) Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials 10:587
Feigl G, Kumar D, Lehotai N, Tugyi N, Molnár Á, Ördög A, Szepesi Á, Gémes K, Laskay G, Erdei L (2013) Physiological and morphological responses of the root system of Indian mustard (Brassica juncea L. Czern.) and rapeseed (Brassica napus L.) to copper stress. Ecotoxicol Environ Saf 94:179–189
Handy RD, Von der Kammer F, Lead JR, Hassellöv M, Owen R, Crane M (2008) The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 17:287–314
Hayat K, Zhou Y, Menhas S, Bundschuh J, Hayat S, Ullah A, Wang J, Chen X, Zhang D, Zhou P (2020) Pennisetum giganteum: an emerging salt accumulating/tolerant non-conventional crop for sustainable saline agriculture and simultaneous phytoremediation. Environ Pollut 265:114876
Hernandez-Viezcas JA, Castillo-Michel H, Andrews JC, Cotte M, Rico C, Peralta-Videa JR, Ge Y, Priester JH, Holden PA, Gardea-Torresdey JL (2013) In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max). ACS Nano 7:1415–1423
Hong J, Rico CM, Zhao L, Adeleye AS, Keller AA, Peralta-Videa JR, Gardea-Torresdey JL (2015) Toxic effects of copper-based nanoparticles or compounds to lettuce (Lactuca sativa) and alfalfa (Medicago sativa). Environ Sci Process Impacts 17:177–185
Jacobs R, Meesters JA, Ter Braak CJ, van de Meent D, van der Voet H (2016) Combining exposure and effect modeling into an integrated probabilistic environmental risk assessment for nanoparticles. Environ Toxicol Chem 35:2958–2967
Jana NR, Wang ZL, Sau TK, Pal T (2000) Seed-mediated growth method to prepare cubic copper nanoparticles. CURRENT SCIENCE-BANGALORE 79:1367–1369
Khan A, Rashid A, Younas R, Chong R (2016) A chemical reduction approach to the synthesis of copper nanoparticles. International Nano Letters 6:21–26
Khan AR, Wakeel A, Muhammad N, Liu B, Wu M, Liu Y, Ali I, Zaidi SHR, Azhar W, Song G (2019) Involvement of ethylene signaling in zinc oxide nanoparticle-mediated biochemical changes in Arabidopsis thaliana leaves. Environmental Science: Nano 6:341–355
Kim S, Sin H, Lee S, Lee I (2013) Influence of metal oxide particles on soil enzyme activity and bioaccumulation of two plants. J Microbiol Biotechnol 23:1279–1286
Kováčik J, Grúz J, Klejdus B, Štork F, Marchiosi R, Ferrarese-Filho O (2010) Lignification and related parameters in copper-exposed Matricaria chamomilla roots: role of H2O2 and NO in this process. Plant Sci 179:383–389
Le Van N, Ma C, Shang J, Rui Y, Liu S, Xing B (2016) Effects of CuO nanoparticles on insecticidal activity and phytotoxicity in conventional and transgenic cotton. Chemosphere 144:661–670
Lee CW, Mahendra S, Zodrow K, Li D, Tsai YC, Braam J, Alvarez PJ (2010) Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environmental Toxicology and Chemistry: An International Journal 29:669–675
Lee Y-J, Kim K, Shin I-S, Shin KS (2020) Antioxidative metallic copper nanoparticles prepared by modified polyol method and their catalytic activities. J Nanopart Res 22:1–8
Lequeux H, Hermans C, Lutts S, Verbruggen N (2010) Response to copper excess in Arabidopsis thaliana: impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol Biochem 48:673–682
Lin C-C, Chen L-M, Liu Z-H (2005) Rapid effect of copper on lignin biosynthesis in soybean roots. Plant Sci 168:855–861
Liu J, Dhungana B, Cobb GP (2018) Environmental behavior, potential phytotoxicity, and accumulation of copper oxide nanoparticles and arsenic in rice plants. Environ Toxicol Chem 37:11–20
Ma H, Williams PL, Diamond SA (2013) Ecotoxicity of manufactured ZnO nanoparticles–a review. Environ Pollut 172:76–85
Mosa KA, El-Naggar M, Ramamoorthy K, Alawadhi H, Elnaggar A, Wartanian S, Ibrahim E, Hani H (2018) Copper nanoparticles induced genotoxicty, oxidative stress, and changes in superoxide dismutase (SOD) gene expression in cucumber (Cucumis sativus) plants. Front Plant Sci 9:872
Nair PMG, Chung IM (2014a) A mechanistic study on the toxic effect of copper oxide nanoparticles in soybean (Glycine max L.) root development and lignification of root cells. Biol Trace Elem Res 162:342–352
Nair PMG, Chung IM (2014b) Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignificaion, and molecular level changes. Environ Sci Pollut Res 21:12709–12722
Nair PMG, Chung IM (2015) Study on the correlation between copper oxide nanoparticles induced growth suppression and enhanced lignification in Indian mustard (Brassica juncea L.). Ecotoxicol Environ Saf 113:302–313
Nair PMG, Kim S-H, Chung IM (2014) Copper oxide nanoparticle toxicity in mung bean (Vigna radiata L.) seedlings: physiological and molecular level responses of in vitro grown plants. Acta Physiol Plant 36:2947–2958
Naz S, Gul A, Zia M (2020) Toxicity of copper oxide nanoparticles: a review study. IET nanobiotechnology 14(1):1–13
Olchowik J, Bzdyk RM, Studnicki M, Bederska-Błaszczyk M, Urban A, Aleksandrowicz-Trzcińska M (2017) The effect of silver and copper nanoparticles on the condition of english oak (Quercus robur L.) seedlings in a container nursery experiment. Forests 8:310
Pang L-J, Adeel M, Shakoor N, Guo K-R, Ma D-F, Ahmad MA, Lu G-Q, Zhao M-H, Li S-E, Rui Y-K (2021) Engineered nanomaterials suppress the soft rot disease (Rhizopus stolonifer) and slow down the loss of nutrient in sweet potato. Nanomaterials 11:2572
Perreault F, Popovic R, Dewez D (2014) Different toxicity mechanisms between bare and polymer-coated copper oxide nanoparticles in Lemna gibba. Environ Pollut 185:219–227
Pugazhendhi A, Kumar SS, Manikandan M, Saravanan M (2018) Photocatalytic properties and antimicrobial efficacy of Fe doped CuO nanoparticles against the pathogenic bacteria and fungi. Microb Pathog 122:84–89
Rajendran A, Siva E, Dhanraj C, Senthilkumar S (2018) A green and facile approach for the synthesis copper oxide nanoparticles using Hibiscus rosa-sinensis flower extracts and It’s antibacterial activities. J Bioprocess Biotech 8:324
Rajput V, Chen Y, Ayup M (2015) Effects of high salinity on physiological and anatomical indices in the early stages of Populus euphratica growth. Russ J Plant Physiol 62:229–236
Rajput V, Minkina T, Fedorenko A, Sushkova S, Mandzhieva S, Lysenko V, Duplii N, Fedorenko G, Dvadnenko K, Ghazaryan K (2018) Toxicity of copper oxide nanoparticles on spring barley (Hordeum sativum distichum). Sci Total Environ 645:1103–1113
Rizwan M, Ali S, Qayyum MF, Ok YS, Adrees M, Ibrahim M, Zia-ur-Rehman M, Farid M, Abbas F (2017) Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: a critical review. J Hazard Mater 322:2–16
Salah SM, Yajing G, Dongdong C, Jie L, Aamir N, Qijuan H, Weimin H, Mingyu N, Jin H (2015) Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci Rep 5:1–14
Salah I, Parkin IP, Allan E (2021) Copper as an antimicrobial agent: recent advances. RSC Adv 11:18179–18186
Sathiyavimal S, Vasantharaj S, Bharathi D, Saravanan M, Manikandan E, Kumar SS, Pugazhendhi A (2018) Biogenesis of copper oxide nanoparticles (CuONPs) using Sida acuta and their incorporation over cotton fabrics to prevent the pathogenicity of Gram negative and Gram positive bacteria. J Photochem Photobiol B Biol 188:126–134
Shams M, Yildirim E, Guleray A, Ercisli S, Dursun A, Ekinci M, Raziye K (2018) Nitric oxide alleviates copper toxicity in germinating seed and seedling growth of Lactuca sativa L. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 46:167–172
Shaw AK, Hossain Z (2013) Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere 93:906–915
Shaw AK, Ghosh S, Kalaji HM, Bosa K, Brestic M, Zivcak M, Hossain Z (2014) Nano-CuO stress induced modulation of antioxidative defense and photosynthetic performance of Syrian barley (Hordeum vulgare L.). Environ Exp Bot 102:37–47
Shi J, Peng C, Yang Y, Yang J, Zhang H, Yuan X, Chen Y, Hu T (2014) Phytotoxicity and accumulation of copper oxide nanoparticles to the Cu-tolerant plant Elsholtzia splendens. Nanotoxicology 8:179–188
Singh A, Singh N, Hussain I, Singh H (2017) Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and Brassica oleracea var. botrytis. J Biotechnol 262:11–27
Song G, Hou W, Gao Y, Wang Y, Lin L, Zhang Z, Niu Q, Ma R, Mu L, Wang H (2016) Effects of CuO nanoparticles on Lemna minor. Bot Stud 57:1–8
Spengler A, Wanninger L, Pflugmacher S (2017) Oxidative stress mediated toxicity of TiO2 nanoparticles after a concentration and time dependent exposure of the aquatic macrophyte Hydrilla verticillata. Aquat Toxicol 190:32–39
Stampoulis D, Sinha SK, White JC (2009) Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol 43:9473–9479
Staroń A, Długosz O, Pulit-Prociak J, Banach M (2020) Analysis of the exposure of organisms to the action of nanomaterials. Materials 13:349
Sui HJ, Zhang JZ, Wang ZY (2014) Toxicity of copper oxide engineered nanoparticles to maize (Zea mays L.) at different aging times, Advanced Materials Research. Trans Tech Publ:972–975
Vivekanandhan P, Swathy K, Thomas A, Kweka EJ, Rahman A, Pittarate S, Krutmuang P (2021) Insecticidal efficacy of microbial-mediated synthesized copper nano-pesticide against insect pests and non-target organisms. Int J Environ Res Public Health 18:10536
Wang Z, Xie X, Zhao J, Liu X, Feng W, White JC, Xing B (2012) Xylem-and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ Sci Technol 46:4434–4441
Wang S-L, Zhang Y-X, Liu H-Z, Xin H (2014) Phytotoxicity of copper oxide nanoparticles to metabolic activity in the roots of rice. Huan jing ke xue= Huanjing kexue 35:1968–1973
Wang M, Wu C, Cheng Z, Meng H (2015) Growth and physiological changes in continuously cropped eggplant (Solanum melongena L.) upon relay intercropping with garlic (Allium sativum L.). Front Plant Sci 6:262
Wiesner MR, Lowry GV, Alvarez P, Dionysiou D, Biswas P (2006) Assessing the risks of manufactured nanomaterials. ACS Publications
Woo H, Kang H, Kim A, Jang S, Park JC, Park S, Kim B-S, Song H, Park KH (2012) Azide-alkyne huisgen [3+ 2] cycloaddition using CuO nanoparticles. Molecules 17:13235–13252
Xiong J, Wang Y, Xue Q, Wu X (2011) Synthesis of highly stable dispersions of nanosized copper particles using L-ascorbic acid. Green Chem 13:900–904
Xiong T, Dumat C, Dappe V, Vezin H, Schreck E, Shahid M, Pierart A, Sobanska S (2017) Copper oxide nanoparticle foliar uptake, phytotoxicity, and consequences for sustainable urban agriculture. Environ Sci Technol 51:5242–5251
Yang Z, Xiao Y, Jiao T, Zhang Y, Chen J, Gao Y (2020) Effects of copper oxide nanoparticles on the growth of rice (Oryza Sativa L.) seedlings and the relevant physiological responses. Int J Environ Res Public Health 17:1260
Zafar H, Ali A, Zia M (2017) CuO nanoparticles inhibited root growth from Brassica nigra seedlings but induced root from stem and leaf explants. Appl Biochem Biotechnol 181:365–378
Zhang H, Lu L, Zhao X, Zhao S, Gu X, Du W, Wei H, Ji R, Zhao L (2019) Metabolomics reveals the “invisible” responses of spinach plants exposed to CeO2 nanoparticles. Environ Sci Technol 53:6007–6017
Funding
The study was financially supported by the Shanghai Science and Technology Commission (No. 21N21900200; 20392000300) and Shanghai Agriculture Applied Technology Development Program (No. 20180203).
Author information
Authors and Affiliations
Contributions
Y.Z. supervised and designed the research; I.H.S. designed and performed most of the experiments; M.A.M. helped in methodology and formal analysis and revised the manuscript; I.A.S. helped resources; M.A.M., M.A., and S.G. helped in data curation; L.C. and Y.Z. revised, discussed, and finalized the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, I.H., Manzoor, M.A., Sabir, I.A. et al. Phytotoxic effects of chemically synthesized copper oxide nanoparticles induce physiological, biochemical, and ultrastructural changes in Cucumis melo. Environ Sci Pollut Res 30, 51595–51606 (2023). https://doi.org/10.1007/s11356-023-26039-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26039-9