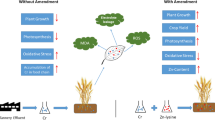
Abstract
Contamination of land and aquatic ecosystems with heavy metals (HMs) is a global issue having the persistent potential to damage the quality of food and water. In the present study, Tagetes erecta L. plants were used to assess their potential to uptake HMs from wastewater. Plants were grown in soil for 20 days and then transplanted in hydroponic system containing Hoagland nutrient solution. After more than 15 days of growth, plants were then subjected to wastewater from tannery and surgical industries in different concentrations ranging from 25 to 100% in combination of citric acid (5 and 10 mM). After 6 weeks of treatment, plants were collected and segmented into roots, stem, and leaves for characterizing the morphological properties including plant height, roots length, fresh and dry mass of roots, stem, and leaves. For evaluation of the effect of wastewater on the plants, photosynthetic pigments; soluble proteins; reactive oxygen species (ROS); antioxidant enzymes SOD, POD, CAT, and APX; and metal accumulation were analyzed. Application of industrial wastewater revealed a significant effect on plant morphology under wastewater treatments. Overall growth and physiological attributes of plant decreased, and metal accumulation enhanced with increasing concentration of wastewater. Similarly, the production of ROS and antioxidant enzymes were also increased. Chlorophyll, protein content, and enzyme production enhanced with CA (5 and 10 mM) mediation; however, ROS production and EL were reduced. Metals analysis showed that the maximum accumulation of Pb was in roots, while Cr and Ni in the stem which further increased under CA mediation. Overall, the metal accumulation ability was in the order of Pb > Ni > Cr under CA.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapid urban sprawl and industrialization are leading to increased heavy metal (HM) pollution in soil and water bodies (Borah et al. 2020; Qin et al. 2020). Among HMs, a few are biologically essential in minor quantities; hence, they have the potential to accumulate especially in the food chain (Kumar et al. 2019; Sidana et al. 2020). Heavy metals have natural geological persistence, but anthropogenic activities are the major sources including industrial effluents, mining, depositions of waste, and use of wastewater for irrigational purposes (Kamali et al. 2019; Ahsan et al. 2019). Excess of HMs in the soil damages the microbial activity and plant roots and affects the uptake of nutrients and nitrogen (N) fixation ability of plants. Higher accumulation enhances the uptake and accumulation of HMs by plants and reduces the overall growth of plants (Gjorgieva Ackova 2018; Jaiswal et al. 2018). Accumulation beyond the threshold of HMs in food chain is toxic to ecosystem and human health. Heavy metal ingestion via food and water can cause damage to the kidney and circulatory system and disturbance of the digestive and nervous systems (Engwa et al. 2019; Rai et al. 2019). Freshwater scarcity is a global issue whereas treatment of wastewater endures high cost particularly in developing countries. Industries are key source of economic activities in the developing countries. Usually, wastewater from industrial units is discharged after initial treatments into water bodies, drains, and soils (Ilyas et al. 2019). Industrial liquid effluents are used for crop irrigation purposes in several parts of the world (Rashid et al. 2018; Haroon et al. 2019). In industrial cities of Pakistan, the release of liquid wastewater effluents from the tannery and surgical industries is causing drastic damage to the environment as it consists of a range of toxic HMs (Hou et al. 2017). A wide range of chemicals are used in the tanning process and effluent comprises many pollutants ranging from salts to expensive chrome. In tannery effluent, chromium (Cr) is generally present in the form of Cr3+ and Cr 6+ along with aluminum (Al), cadmium (Cd), lead (Pb), suspended solids (SS), dissolve solids (DS), settable solids, organic matter, nitrogen, sulphide, sulphates, chlorides, oil, and grease (Gill et al. 2022; Ali et al. 2022). Surgical industries are metal intensive, and a range of important HMs are used in various processes (Spry 2005). Numerous toxic HMs including iron (Fe), Cr, copper (Cu), nickel (Ni), cobalt (Co), and Cd are reported in effluents from surgical industries (Thind et al. 2020; Ahmad et al. 2020). Release of tannery and surgical effluents into the environment has the potential to cause negative impacts on water bodies, agriculture, and ultimately human health (Cory and Kling 2018; Shammi et al. 2019).
Plants not only fulfill the essential need for food, energy, oxygen, and aesthetics, but are also natural controller of environmental pollutants (Hasanuzzaman et al. 2020). Plants have the capacity to degrade, stabilize, and uptake a variety of pollutants from polluted environment (Muthusaravanan et al. 2018; Rostami and Azhdarpoor 2019). As some metals are essential for plants, so plants also have the ability to extract and accumulate HMs in various parts depending upon the species. Plants can reduce the effect and concentration of HMs from the polluted sites through a different process of phytoremediation (Hassan et al. 2019; Ullah et al. 2018).
In recent times, the treatment of industrial wastewater by plants grown hydroponically is being carried out along with various biochemical amendments as physical and chemical methods are less feasible for effective HM removal (Promratrak 2017). Hydroponic system provides the ideal conditions and supplements for the growth of plants. A number of techniques are identified for hydroponic culture such as circulating and non-circulating solution, solid media culture, and aeroponics (Son et al. 2020). Limitless crops such as lettuce, tomatoes, carrots, celery, watercress, eggplants, beans, parsley, wild radish, leeks, strawberries, melons, fragrant and restorative plants, and so forth can be grown in hydroponics (Nalwade and Mote 2017). Hydroponics culture method is effective and feasible for wastewater treatment however, there are many hurdles associated with soil management (Kale et al. 2015). Plants can be grown in hydroponics and aquaponics for the treatment of wastewater (Delaide et al. 2016). System is much like wetlands used for the treatment of organic and inorganic pollutants.
Ornamental plants along with aesthetic preferences can degrade and accumulate pollutants (Liu et al. 2018). Ornamental plants have been widely used for phytomanagement of aquatic and terrestrial environments (Shao et al. 2019; Shyamala et al. 2019). In comparison to other plants, ornamental plants are most suitable for the extraction of HMs as there is no risk of food chain contamination (Lajayer et al. 2019). Numerous ornamental plants are studied for phytoremediation of HMs including Calendula officinalis L., Alcea rosea L. (Liu et al. 2008), Amaranthus caudatus L. (Cay 2016), Cyperus alternifolius L., Euonymus japonicus L., Cordyline fruticosa L. (Younis et al. 2015), Consolida ajacis L. (Anum et al. 2019), and Asteraceae (Ramírez et al. 2020).
Tagetes erecta is a terrestrial ornamental plant, formerly studied for its potential to uptake Pb grown in hydroponic media (Bardiya-Bhurat et al. 2017). Various types of marigold plants are identified as effective species for the phytoremediation of HMs. American marigold (T. erecta) are effective for the phytoremediation of arsenic (As) metal in soil and aquatic system (Ramana et al. 2009). Studies demonstrate that T. erecta can accumulate Cr (Murti and Maryani 2020; Coelho et al. 2017), Cu (Castillo et al. 2011), Zn (Liu et al. 2010), Cd and Pb (Aghelan et al. 2021; Shah et al. 2017a), Ni (Sathya et al. 2020), and Cd, Pb, and Zn (Madanan et al. 2021). Studies depicted that chemical amendment especially organic acid can improve the concentration and accumulation of HMs in marigold (Aghelan et al. 2021; Saffari and Saffari 2020; Hannan et al. 2021). Citric acid improves the growth, biomass, photosynthesis, and gas exchange in numerous cultivars of marigold. CA reduces stress on plants by improving nutrient uptake and improves metal detoxification (Aghelan et al. 2021).
The present study is conducted to investigate the capacity of an ornamental plant for the extraction of HMs from wastewater in hydroponic system. T. erecta was grown in combined wastewater of surgical and tannery industry in aeroponic system and was examined for its role to uptake and accumulate different HMs from combined surgical and tannery liquid effluent in the presence and absence of citric acid (CA).
Material and methods
Experimentation and treatments
Seeds of T. erecta were collected from Punjab seed Company, Govt. of the Punjab Lahore. Fully mature and healthy seeds were germinated in styrofoam trays containing soil. Physicochemical characteristics of collected tannery and surgical industry wastewater were analyzed by using standard methods (Supplementary Table 1). Concentrations of HMs were analyzed by using atomic absorption spectrophotometer (Nov Aa 400 Analytik Jena Germany). After 20 days of germination, seedlings emerged from the soil and grew into young plants. Those were carefully removed from the soil, washed at normal temperature with tap water, and transferred into the hydroponic system constructed and installed in the Environmental laboratory at the Department of Environmental Science University of Gujrat, Pakistan. Hydroponic system consisted of glass container having a capacity of 5.0 L each. To provide necessary nutrients to plant, Hoagland’s nutrient solution was prepared in the laboratory and was applied to nourish the experimental plants. Plants were continuously aerated with aeration pumps. Plants were exposed to different concentrations of citric acid (CA) and combined wastewater of tannery and surgical industry after 2 weeks of transplantation into hydroponic culture. The experiment was a complete randomized design (CRD) with three replicates of each treatment. The pH of hydroponic system was monitored and maintained at 5.8–6.3 by using 1.0 M of H2SO4 and NaOH during the whole experiment. Fresh plants of T. erecta were treated with different combinations of tannery effluent (TE), surgical effluent (SE) and citric acid (CA). The TE and SE were mixed and applied as wastewater (WW). Following treatments were applied to plants: T1:CK, T2: CA5mM, T3:CA10mM, T4:WW25%, T5:WW25% + CA5Mm, T6:WW25% + CA10mM, T7:WW50%, T8:WW50% + CA5Mm, T9:WW50% + CA10mM, T10:WW75%, T11:WW75% + CA5Mm, T12:WW75% + CA10mM, T13:WW100%, T14:WW100% + CA5Mm, T15:WW100% + CA10mM. Treatments were supplied on weekly basis.
Measurements of agronomic traits
After passing the 6 weeks (42 days) of experimental treatment, the plants were harvested and evaluated for morphological and biochemical parameters. Fresh weight of the root, stem, and leaf of harvested plants was measured by analytical weighing balance. Dry weight of the root, stem, and leaf of harvested plants was also determined by drying the plants in an oven at 90 °C for 72 h till constant weight. Other parameters including the length of root, stem, and leaf count of plants under different treatments were measured by using a measuring scale and values were recorded accordingly.
Determination of chlorophyll and carotenoids content
Chlorophyll and carotenoid contents of the harvested plants were evaluated after the 6 weeks of the experiment. Chlorophyll was evaluated by using the method of Metzner et al. (1965), where the fresh leaves were nurtured at 4 °C in acetone solution (85%, v/v) under dark by continuous shaking of solution so the leaves discharged to obtain color extracts. Afterward, solution was placed at 4 °C for 10 min and 4000 × rpm for the collection of supernatants and further analyzed by using spectrophotometer (Halo DB-20/DB-20S, Dynamica Company, London, UK) at light absorbance measurement at 452.5, 644, and 663 nm for the measurement of chlorophyll and carotenoid contents. Further calculations were made using equations given by Lichtenthaler (1987).
For the measurement of chlorophyll content, equation was as follows:
Measurement of SPAD
Chlorophyll contents, in terms of soil plant analysis development (SPAD) value, were performed on second uppermost fully expanded leaf with the help of SPAD meter (SPAD-502).
Determination of protein and antioxidant enzymes
The soluble protein contents were determined following the method of Bradford (1976) by means of a standard (bovine serum albumin) and dye (Coomassie Brilliant Blue G-250) after the 6-week treatment. The fresh plants were ground using mortar and pestle under chilled condition. After that, the plants were mixed with a solution of sodium phosphate comprising polyvinylpyrrolidone 40 (2%, w/v) and 1.0 mM EDTA, which was 10 mL, and sodium phosphate and EDTA solution was buffered. The extract thus prepared previously was used for measuring the antioxidant enzymes (POD and SOD) and proteins content by centrifuging supernatant for 15 min at 4 °C and 11,000 rpm. Absorbance at 595 nm wavelength of spectrophotometer was taken to measure actual protein content, and for this purpose, 100 μL extract was mixed well and homogenized solution was made with 1.0 mL of Bradford solution. The concentration of catalase (CAT, EC 1.11.1.6) was calculated by using the protocol of Aebi (1984). For this purpose, solution was made of enzyme extract (100 μL), hydrogen peroxide (100 μL), and phosphate buffer of 2.8 mL buffer (50 mM with 2.0 mM of citric acid, pH 7.0). Due to the loss of hydrogen peroxide (ε = 39.4 mM−1 cm−1), variation in light absorbance was recorded at 240 nm which gives the amount of CAT. By using the procedure of Nakano and Asada (1981), the quantity and activity of ascorbate peroxidase (APX, EC 1.11.1.11) were assessed. For this, again a mixture of 3.0 mL was prepared which consisted of 2.7 mL phosphate buffer solution (50 mM with 2 mM citric acid, pH 7.0), 100 μL ascorbate, enzyme extract of 100 μL, and hydrogen peroxide of 100 μL (3 0 mM). The amount of APX was assessed by measuring variations in wavelength at 290 nm ensued because of its oxidation (ε = 2.8 mM−1 cm−1).
Hydrogen peroxide contents (H2O2) and malondialdehyde content
The analyzed mixture was prepared to measure H2O2 content. For this purpose, mixture was made by homogenizing the 50 mg fresh plants with buffer solution of 3.0 mL phosphate and centrifuging at 6000 × g and 4 °C for 25 min. Further, a solution of 1.0 mL of sulfuric acid (20%, v/v) and titanium sulfate (0.1%) was mixed with an extract of 3.0 mL and was again centrifuged at 6000 × g and 4 °C for 15 min. The variations in supernatants yellow color were measured by using wavelength of 410 nm and calculations were made by subsequent extermination coefficient of 0.28 μmol−1 cm−1 to measure hydrogen peroxide content.
In plant tissues, the concentration of malondialdehyde, a product of lipid peroxidation was measured by using method of reaction of thiobarbituric acid (TBA) as acknowledged by Heath and Packer (1968) laterally by minor changes added by Zhang and Kirkham (1994) and Dhindsa et al. (1981).
Assessment of electrolyte leakage
Electrolyte leakage in leaves and roots was measured by estimating the initial (EC1) and final (EC2) electrical conductivity by following the method suggested by Dionisio-Sese and Tobita (1998).
Electrolyte leakage was calculated by the following formula:
Determination of heavy metals content in plant material
Concentration of Pb, Ni, and Cr in marigold plant was measured after 6 weeks of treatment. For this purpose, plant samples were dried out at 90 °C and then converted to ash in a muffle furnace at 600 °C for 6 h. A 50-mL solution with distilled water was made by adding 3.0 mL of concentrated solution of HNO3 and HCL and ash samples were dissolved in solution. Concentration of Cr, Pb, and Ni was measured by following the method of Perkin Elmer Analyst 100 (USA). The analytical software Tallahassee, USA, was used to analyze data. Concentration of metal was calculated by using the following formula.
where
The accumulation of metal in the whole plant was calculated using the following formula (Zayed et al. 1998):
The translocation factor (TF) from root to shoot was measured by following the equation as given below
where Cshoot and Croot are metals concentration in the shoot (mgkg−1) and root of plant (mgkg−1), respectively.
Statistical analysis
Software Statistics 10.0 was used to analyze data. ANOVA (analysis for variance) and graphical representation was performed. Significant differences among mean values of treatments were measured by Tukey’s test. Mean difference was represented by small letters and values were significantly different from each other at P ≤ 0.05.
Results
Agronomic traits of T. erecta
Morphological characteristics of T. erecta showed significant reduction under HM stress. Growth and biomass of T. erecta decreased gradually along the increasing concentration of applied wastewater (Supplementary Table 2). Addition of CA led to a significant improvement in the growth and biomass of T. erecta as shown in supplementary table 1. Maximum decrease in T. erecta was observed under 100% wastewater application as compared to control plants. This comparative decrease for root length was 55–52%, plant height 49.39–35.69%, no. of leaves 51.28–47.92%, fresh weight root 56.09–53.23%, fresh weight stem 48.09–42.67%, fresh weight leaf 51.36–41.98%, dry weight root 49.37–44.92%, dry weight stem 53.99–51.23%, and dry weight leaf 65.37–48.32%. Moreover, the addition of CA 5 mM and CA 10 mM improved the root length 2.55–4.78% and 6.07–8.45%, plant height 1.80–18.94% and 3.12–31.03%, no. of leaves 7.10–19.48% and 14.81–54.12%, fresh weight root 14.09–16.08% and 24.65–32.74%, fresh weight stem 13.87–19.56% and 22.37–35.16%, fresh weight leaf 11.49–22.03% and 17.99–40.73%, dry weight root 7.86–16.07% and 16.75–27.02%, dry weight stem 10.55–14.30% and 21.34–28.59%, and dry weight leaf 8.24–44.39% and 16.60–74.03% at 0–100% combined tannery and surgical wastewater treatment, respectively as compared to respective treatments alone.
Photosynthetic pigments of T. erecta
To measure the oxidative damage by high concentration of wastewater to T. erecta, photosynthetic pigments chlorophyll (a, b, and total) and carotenoids were measured (Fig. 1). Photosynthetic pigments decreased due to increased oxidative stress with increasing wastewater (Fig. 1A–D). Maximum decrease in T. erecta was observed under wastewater application as compared to controls of chlorophyll a by 76.61–68.60%, chlorophyll b by 63.21–61.23%, total chlorophyll by 72–64.43%, and carotenoids by 79.88–76.88%, respectively. Application of CA improved the content of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids under the application of wastewater as compared to plants treated with only wastewater. Application of CA 5 mM and 10 mM increased the content of chlorophyll a by 7.10–19.48% and 14.81–54.12%, chlorophyll b by 17.44–23.54% and 19.40–36.60%, total chlorophyll by 8.04–17.80% and 9.50–39.08%, and carotenoids by 3.65–21.91% and 16.92–45.99% at 0–100% combined tannery and surgical effluent treatment as compared to respective treatments alone.
Effect of tannery and surgical wastewater and citric acid on chlorophyll a (A), chlorophyll b (B), total chlorophyll (C), and carotenoids (D) in T. erecta grown in hydroponic with increasing wastewater (25, 50, 75, and 100%) treated or not with citric acid (5 mM and 10 mM). Values are demonstrated as mean of three replicates along with standard deviation. Mean values followed by small different letters are significantly different from each other at P ≤ 0.05
Soluble proteins and SPAD value in T. erecta
To measure the oxidative stress induced by HMs on T. erecta, protein content and SPAD value was measured in T. erecta by increasing wastewater concentration (Fig. 2). Decreased content of soluble proteins and SPAD were recorded with increasing concentration of wastewater (Fig. 2A–C). Maximum decrease in T. erecta was observed under wastewater application as compared to controls of SP root by 71.41–67.74%, SP leaf by 78.27–73.32%, and SPAD by 66.89–62.57%, respectively. Application of CA improved the content of SP root, SP leaf, and SPAD under the application of wastewater as compared to plants treated with only wastewater. Application of CA 5 mM and 10 mM increased the content of SP root by 7.92–17.34% and 10.28–38.84%, SP leaf by 5.92–19.68% and 13.25–39.54%, and SPAD value by 7.85–26.30% and 12.56–36.54% at 0–100% combined tannery and surgical effluent treatment as compared to respective treatments alone.
Effect of tannery and surgical wastewater and citric acid on SPAD (A), SP in root (B), and SP in leaf (C) in T. erecta grown in hydroponic with increasing wastewater (25, 50, 75, and 100%) treated or not with citric acid (5 mM and 10 mM). Values are demonstrated as mean of three replicates along with standard deviation. Mean values followed by small different letters are significantly different from each other at P ≤ 0.05
Antioxidant enzymes activities in T. erecta
Production of major antioxidant enzymes SOD, POD, APX, and CAT were measured in roots and leaves of T. erecta to measure the effect of wastewater on antioxidant defense mechanism in the presence and absence of CA (Fig. 3). Maximum increase in T. erecta was observed under wastewater application as compared to controls for SOD root by 379.49–302.87%, SOD leaf by 1179.24–829.72%, POD root by 166.68–102.21%, POD leaf by 221.38–151.99%, APX root by 329.95–242.30%, APX leaf by 388.49–257.02%, CAT root by 161.48–158.69%, and CAT leaf by 146.51–117.95%, respectively. Application of CA along with industrial wastewater improved the activity of enzymes as compared to plants treated with only wastewater. Application of CA 5 mM and 10 mM increased the content of SOD root by 5.12–17.58% and 11.56–32.78%, SOD leaf by 6.95–54% and 13.31–85.24%, POD root by 2.73–19% and 4.40–39.53%, POD leaf by 2.78–13.02% and 5.72–36.12%, APX root by 6.18–11.435 and 12.89–42.18%, APX leaf by 2.0–18.75% and 5.03–43.71%, CAT root by 3.21–8.42% and 8.77–12.69%, and CAT leaf by 3.27–13.33% and 5.79–22.99% at 0–100% combined tannery and surgical effluent treatment as compared to respective treatments alone.
Effect of tannery and surgical wastewater and citric acid on SOD in root (A), SOD in leaf (B), POD in root (C), POD in leaf (D), APX in root (E), APX in leaf (F), CAT in root (G), and CAT in leaf (H) in T. erecta grown in hydroponic with increasing wastewater (25, 50, 75, and 100%) treated or not with citric acid (5 mM and 10 mM). Values are demonstrated as mean of three replicates along with standard deviation. Mean values followed by small different letters are significantly different from each other at P ≤ 0.05
ROS content in T. erecta
To measure the oxidative stress induced by HMs on T. erecta, reactive oxygen species MDA, H2O2, and EC were measured in the root and leaf of plants. MDA, H2O2, and EC were increased in T. erecta along with the increasing concentration of wastewater (Fig. 4). Maximum increase in T. erecta was observed under wastewater application as compared to controls for EL in root by 535.38–356.52%, EL in leaf by 366.19–191.85%, MDA in root by 218.20–153.17%, MDA in leaf by 183.06–158.15%, H2O2 in the root by 232.52–155.16%, and H2O2 in leaf by 386.68–220.61%, respectively. Application of CA decreased the content of ROS in plants except for control. Addition of CA decreased the EL in root by 13.21–4.13% and 22.62–9.05%, EL in leaf by 10.82–3.77% and 17.25–8.01%, MDA in root by 8.69–1.44% and 17.73–5.83%, MDA in leaf by 11.61–2.63% and 17.06–6.80%, H2O2 in root by 8.57–2.40% and 14.11–5.80%, and H2O2 in leaf by 9.18–3.36% and 16.38–8.69% at 25–100% combined tannery and surgical effluent treatment as compared with respective treatment without CA application.
Effect of tannery and surgical wastewater and citric acid on MDA in root (A), MDA in leaf (B), H2O2 in root (C), H2O2 in leaf (D), EC in root (E), and EC in leaf (F) in T. erecta grown in hydroponic with increasing wastewater (25, 50, 75, and 100%) treated or not with citric acid (5 mM and 10 mM). Values are demonstrated as mean of three replicates along with standard deviation. Mean values followed by small different letters are significantly different from each other at P ≤ 0.05
Heavy metal concentration and accumulation in T. erecta
Concentration and accumulation of HMs (Cr, Ni, and Pb) were increased in T. erecta along with the increasing concentration of wastewater (Tables 1, 2, and 3). Results showed that uptake and accumulation of Pb, Ni, and Cr in T. erecta was increased in the presence and absence of CA. The concentration of Pb increased in roots by 260.94–217.57%, in stem by 291.04–240.89%, and in leaf by 308.85–254.03%, while accumulation increased in root by 114.93–105.34%, stem by 100.67–98.38%, and leaf by 114.71–58.29%. The increase in concentration of Ni was by 293.19–192.66% in roots, by 330.90–216.46% in stem, and by 330.68–216.46% in leaf, while accumulation of Ni increased by 134.14–89.10% in roots, by 121.60–84.22% in stem, and by 125.18–66.62% in leaf. The concentration of Cr increased by 155.71–118.50% in roots, by 329.88–253.31% in stem, and by 371.91–292.72% in leaf, while accumulation of Cr increased in roots by 138.84–116.19%, in stem by 120.56–105.58%, and in leaf by 137.83–82.52% at 25–100% wastewater application respectively. Application of CA improved the concentration and accumulation of HMs as compared to plants treated with only wastewater. Application of CA 5 mM and 10 mM increased the concentration of Pb in roots by 13.31–4.97% and 10.20–28.97%, in stem by 15.02–4.09% and 32.83–9.17%, and in leaf by 15.92–4.81% and 32.27–9.95%, while accumulation of Pb increased in roots by 22.42–11.52% and 24.46–44.56%, in stem by 18.63–11.60% and 39.62–27.72%, and in leaf by 49.93–15.01% and 89.89–36.58% at 25–100% of wastewater respective to those treatments without CA. Application of CA 5 mM and 10 mM increased the concentration of Ni in root by 22.47–2.42% and 40.04–4.23%, in stem by 29.74–2.84% and 44.24–5.93%, and in leaf by 11.68–3.72% and 25.07–7.87%, while accumulation of Ni increased in root by 34.39–18.84% and 63.90–32.28%, in stem by 38.78–11.80% and 63.92–26.43%, and in leaf by 50.06–14.11% and 87.97–35.37% at 25–100% of wastewater respective to those treatments without CA. Application of CA 5 mM and 10 mM increased the concentration of Cr in root by 13.31–4.97% and 28.97–9.62%, in stem by 15.02–4.09% and 32.83–9.17%, and in leaf by 15.92–4.81% and 32.27–9.95%, while accumulation of Cr increased in root by 24.34–12.41% and 50.79–24.45%, in stem by 22.77–13.68% and 50.65–29.28%, and in leaf by 51.80–17.87% and 91.80–47.05% at 25–100% of wastewater respective to those treatments without CA. The T. erecta showed translocation factor (TF) of all HMs > 1 under all applied treatments with and without CA (Fig. 5).
Discussion
Ornamental plants have potential to uptake and accumulate HMs without any toxic effects to soil (Liu et al. 2018; Lajayer et al. 2019). Various species of marigold are reported to accumulate HMs (Madanan et al. 2021; Aghelan et al. 2021; Sun et al. 2018; Ali et al. 2019a) and T. erecta grown in wastewater can remediate HMs. Some researchers have reported that T. erecta can be efficient for metal hyperaccumulation due to natural capacity to uptake and tolerate HMs (Farooq et al. 2020; Sun et al. 2018; Sathya et al. 2020). Addition of CA improved the growth, biomass, and metal accumulation in T. erecta (Sinhal, et al. 2010; Sathya et al. 2020). Focus of the present study was mainly to investigate the effect of combined tannery and surgical wastewater on T. erecta alone and in combination with CA.
Agronomic traits
Increasing concentration of wastewater (0, 25, 50, 75, and 100%) reduced the growth and biomass accumulation in T. erecta (ST 1). Primarily growth of plants in presence of HMs is reduced due to inhibition of cell division at root tips of plants. Growth of plant is reduced due to disturbance of enzymes, chlorophyll, ROS, nutrients uptake, protein synthesis, and electrical conductivity which imbalances hormonal activity and membrane permeability. HMs damage cell division and cell elongation of seedlings which reduces overall root length (Shah et al. 2017b). Study shows that the suppression and elongation of growth cells due to irreversible Cd-induced damage to proton pump was the reason of reduction in growth of T. erecta (Shah et al. 2017a). Reduced dry biomass accumulation shows the phytotoxicity of metals on the plant. Similar results were reported by Ali and Chaudhury (2016) in T. erecta treated with EDTA. Fresh and dry weight, root, and stem length were decreased at all concentrations of Ni in T. erecta as reported by Bardiya-Bhurat et al. (2017). Some enzymes become active in presence of HMs. These cells and enzymes start to digest food and thus plant growth is inhibited. Some studies reported increase in wet and dry biomass accumulation of plants in presence of metals due to the struggle to uptake nutrients from soil as reported by Acharya and Sharma (2014). Present study shows that CA (5 and 10 mM) can increase the growth and biomass accumulation in T. erecta. Aghelan et al. (2021) also reported the increase in dry weight of Amaranthus caudatus L. and T. erecta under the application of CA, EDTA and AA.
Photosynthetic pigments and carotenoids content
Study revealed that with increasing dose of wastewater (25, 50, 75, and 100%), the color of leaves was less green due to inhibition in photosynthetic activity except in CK and under the application of CA (Fig. 1). Heavy metals damage pigments such as chlorophyll, carotenoids, and xanthophyll. Decrease in chlorophyll content is the regular symptom of metal stress as they reduce the rate of photosynthesis and absorbance of green lights by plants. Reduction in photosynthetic activity in plants is due to inhibition of enzymes such as aminolaevulinic acid dehydrates (Sharma et al. 2020). Damage to chlorophyll content in T. erecta in presence of Pb and Cd was reported by Shah et al. (2017b). Bardiya-Bhurat (2017) reported the decrease in chlorophyll content from 1 to 0.85 mg/g with increasing dose from 100 to 250 mmol of Pb and Ni in T. erecta. Excessive production of ROS reduced carotenoid content. Carotenoids play the role of guard and protect chlorophyll, membrane, and genetic code of plant from ROS. Reduction in content of carotenoids is a symptom of metal toxicity in plants. At high concentration, these metals activate mechanism to destroy carotenoids (Zhang et al. 2020; Parmar et al. 2013). Decrease in content of carotenoids was reported by Goswami and Das (2016) in C. officinalis under Cu-induced stress (Lajayer et al. 2019). Reduction in content of carotenoids under Cr toxicity was also reported by Oliveira (2012).
Soluble protein and SPAD value
A decrease in protein and SPAD content with increasing metal concentration is found in different treatments to T. erecta (Fig. 2). High concentration of HMs degraded proteins by inducing structural changes through denaturation and fragmentation of proteins (Shah et al. 2017a). Reduced protein content in plants is due to HM-induced lipid peroxidation and fragmentation. Protein synthesis reduces due to the production of reactive oxygen species (ROS) with increasing wastewater (25, 50, 75, and 100%) (Kaur 2016). In presence of Pb, the concentration of free amino acids is increased in plant. Production of free amino acids is attributed to the production of protease enzyme in many plants which is responsible for the degradation of proteins in the presence of HMs (Seneviratne et al. 2019). Same results were reported by Shah et al. (2017b) in T. erecta when grown in lead-contaminated soil. Reduction in protein content in T erecta in presence of Cd was also reported by Shah et al. (2017a). Protein content in presence of Cd is reduced due to its complex bonding peptides which form complexes such as g-glutamic acid-cysteine. Such complex bonds reduce the amount of free peptides and synthesis of proteins is inhibited (Shah et al. 2017b; Gomes et al. 2012). Addition of chelating agents such as CA can improve the protein and carotenoids content in T. erecta (Aghelan et al. 2021).
Antioxidant enzymes activity
Plants are naturally gifted to overcome the oxidative stress induced in plants due to biotic and abiotic factors (Kumar et al. 2020). Heavy metals alone and in combination with CA increased the antioxidant activity in T. erecta as reported in the results (Fig. 3). Similar results are reported by Bardiya-Bhurat et al. (2017) in T. erecta in the presence of Pb and Ni. In response to oxidative stress, antioxidant enzymes are produced in plants which protect plant in harsh circumstances. Most important enzymes are POD, SOD, CAT, and APX. Synthesis of these enzymes is reduced with increasing metal stress. Plants are damaged due to instability in ROS and antioxidant enzymes (Bhaduri and Fulekar 2012). Farooq et al. (2020) reported the increase in activity of SOD, CAT, APX, and POD by 150%, 79%, 97%, and 60%, respectively in marigold (Calendula calypso) when exposed to Cd 100 mg/kg of soil. SOD is reported as the most important to overcome the effect of ROS. SOD converts H2O2 into molecular oxygen and water and reduces the toxicity of H2O2 (Rehman et al. 2019). POD is part of lignin biosynthesis and source of physical blockage of H2O2 toxicity (Anjum et al. 2015). As a result of this activity, the damage to cell membrane was reduced to Calendula calypso as reported by Farooq et al. (2020). CAT controls the excessive production of OH free radical and degradation of H2O2 under metal-induced stress in C. officinalis. Activity of APX also increases under Cd-induced stress along with the stress of SOD. SOD alone cannot detoxify the metal-induced stress; the production of other enzymes is necessary for combating metal stress (Saffari and Saffari 2020). The increase in activity of antioxidant enzymes was increased in Alternanthera bettzickiana L. in combined application of Pb and AA. The activity of AA reduces the production of MDA in A. bettzickiana significantly.
Production of reactive oxygen species
Abiotic stress exerts ROS which are hyperactive in nature and can damage proteins, carbohydrates, and nucleic acid and cause irreversible damage to cells (Hasanuzzaman et al. 2019). It is due to the oxidation of redox-active HMs. ROS is produced at low concentration during normal metabolic process. But synthesis of ROS increases when plants are exposed to stress. Production of ROS causes damage to biomolecules including DNA, membrane damage, and poor synthesis of proteins (Parmar et al. 2013). Increase in the production of MDA and H2O2 was reported by many researchers. Production of H2O2 and MDA was increasing in C. officinalis with increasing concentration of Cd but along with the application of CA and TA the production of H2O2 was reduced as a result of mitigation of metal stress (Saffari and Saffari 2020). Increased production of MDA represents the increasing toxicity of metals to plant as reported by Latif et al. (2020). Loss of membrane integrity and increase in conductivity of electrolyte leakage are the results of Pb-induced stress in ornamental plants as reported by song et al. (2020). The results we got are similar with the studies of Ehsan et al. (2014), Song et al. (2020), and Afshan et al. (2015). Sidhu et al. (2018) also reported the similar finding for H2O2 and MDA in Coronopus didymus L. exposed to Ni. Application of exogenous CA reduced the content of ROS in T. erecta. Farid et al. (2020) reported the decrease in content of EL under the application of CA in sunflower.
Heavy metal concentration and accumulation
Heavy metal Pb, Ni, and Cr were observed to be accumulated in various parts of T. erecta on applying various concentrations of wastewater (25, 50, 75, and 100%) and CA as shown in the results (Tables 1, 2, and 3). Plants can uptake and accumulate HMs depending upon the plant species, type of metal, and many other factors. According to Madanan et al. (2021), T. erecta can accumulate HMs such as Pb, Cd, and Zn in different concentrations and suggested it as hyperaccumulator. Shah et al. (2017a) also reported the accumulation of Cd and Pb in T. erecta when exposed to contaminated soil. Chelating agents improve the metal accumulation as reported by many researchers.
Sinhal et al. (2010) reported the accumulation of HMs, i.e., Zn, Cu, Pb, and Cd in T. erecta in presence of EDTA. EDTA increased the accumulation and translocation of Zn and Cd in T. erecta sp. as reported by Ali and Chaudhury (2016). Other chemical amendments including CA and SA are reported by Aghelan et al. (2021) in different species of marigold. Bardiya-Bhurat et al. (2017) reported the accumulation of Ni and Pb by T. erecta in hydroponics. Hydroponic analysis shows that T. erecta can accumulate and tolerate stress induced by Pb and Ni due to natural defense mechanism. Antioxidant enzymes are produced in T. erecta to overcome the stress induced by reactive oxygen species under metal-induced stress. Similarly, Sun et al. (2018) reported the tolerance and accumulation capacity of T. erecta cultivars for different HMs. Coelho et al. (2017) reported the accumulation potential of T. erecta for Cr in hydroponics. Cr was mainly accumulated in roots of the plant. Plant grown in Cr-contaminated soil and water cannot inhibit the uptake of Cr.
Metals mostly accumulate in the roots initially and then move to other parts of the plant. Less accumulation of metals in aerial parts is due to various reasons such as accumulation of metals in plasma membranes, binding with negatively charged proteins, precipitation of metals with salts, etc. (Arias et al. 2010). Similar findings were reported by Sinhal et al. (2010) for metal accumulation, protein, chlorophyll, and wet and dry weight in T. erecta in presence of CA and EDTA.
Role of citric acid in alleviating metal toxicity
It is obvious from the previous studies that citric acid can improve plant growth and provide protection to plants from overproduction of ROS along with the increase in uptake of metals as it acts as chelating agent. The tolerance ability of plant is improved against the metal-induced stress under the application of CA (Afshan et al. 2015; Farid et al. 2020). Present study shows that the application of CA increases the morphological parameters of plant growth solely and along with the wastewater. CA improved growth and metal accumulation in T. erecta as reported by Sinhal et al. (2010). Aghelan et al. (2021) also reported the increase in metal uptake and accumulation in ornamental plants Amaranthus caudatus L. and T. erecta grown in Pb-contaminated soils along the application of chelating agents, i.e., CA, SA, and EDTA. Similar findings were also reported by Farid et al. (2017) and Sallah-Ud-Din et al. (2017).
Conclusions
The present study revealed that physiological and biochemical parameters of cultivated plant were affected significantly with increasing concentration of applied wastewater. Overall, the traits were affected; however, fresh and dry weight, chlorophyll, and protein content in T. erecta decreased with increasing quantity of wastewater. Generation of ROS and antioxidant enzymes (COD, POD, APX, and CAT) were increased with increasing level of wastewater. Study further shows that HM uptake significantly affects T. erecta at all the experimental levels of applied wastewater. Citric acid used as a chelator which improved plant growth, chlorophyll, and protein content, and enhanced the activities of antioxidant enzymes. Application of CA reduced production of ROS in T. erecta. Application of citric acid is effective for alleviating metal-induced stress and for improving metal uptake in plant. The study concludes that T. erecta might be a suitable candidate for the treatment of tannery and surgical wastewater containing HMs and can tolerate stress induced by Pb, Ni and Cr.
Data availability
Not applicable.
References
Acharya S, Sharma DK (2014) Study on the effects of heavy metals on seed germination and plant growth on Jatropha curcas. Int J Agric Sci Res 3(3):31–34
Aebi H (1984) Catalase in vitro methods. Enzymol 105:121–126
Afshan S, Ali S, Bharwana SA, Rizwan M, Farid M, Abbas F, Abbasi GH (2015) Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ Sci Pollut Res 22(15):11679–11689
Aghelan N, Sobhanardakani S, Cheraghi M, Lorestani B, Merrikhpour H (2021) Evaluation of some chelating agents on phytoremediation efficiency of Amaranthus caudatus L. and Tagetes patula L. in soils contaminated with lead. J Environ Health Sci Eng 19(1):503–514
Ahmad R, Ali S, Abid M, Rizwan M, Ali B, Tanveer A, Ahmad I, Azam M, Ghani MA (2020) Glycinebetaine alleviates the chromium toxicity in Brassica oleracea L. by suppressing oxidative stress, and modulating the plant morphology and photosynthetic attributes. Environ Sci Pollut Res 27:1101–1111
Ahsan MA, Satter F, Siddique MABA, Kbor MA, Ahmed S, Shajahan M, Khan R (2019) Chemical and physicochemical characterization of effluents from the tanning and textile industries in Bangladesh with multivariate statistical approach. Environ Monit Assess 191(9):1–24
Ali SY, Chaudhury S (2016) EDTA-enhanced phytoextraction by tagetes sp and effect on bioconcentration and translocation of heavy metals. Environ Process 3(4):735–746
Ali S, Mfarrej MFB, Hussain A, Akram NA, Rizwan M, Wang X, Maqbool A, Nafees M, Ali B (2022) Zinc fortification and alleviation of cadmium stress by application of lysine chelated zinc on different varieties of wheat and rice in cadmium stressed soil. Chemosphere 295:133829
Ali H, Khan E, Ilahi I (2019a) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem 6730305 https://doi.org/10.1155/2019/6730305
Anjum SA, Tanveer M, Hussain S, Bao M, Wang L, Khan I, Shahzad B (2015) Cadmium toxicity in Maize (Zea mays L.): consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ Sci Pollut Res 22(21):17022–17030
Anum S, Khan SM, Chaudhary HJ, Ahmad Z, Afza R (2019) Phytoremediation of nickel polluted ecosystem through selected ornamental plant species in the presence of bacterium Kocuria rhizophila. Bioremdiat J 23(3):215–226
Arias JA, Peralta-Videa JR, Ellzey JT, Ren M, Viveros MN, Gardea-Torresdey JL (2010) Effects of Glomus deserticola inoculation on Prosopis: enhancing chromium and lead uptake and translocation as confirmed by X-ray mapping, ICP-OES and TEM techniques. Environ Experi Bot 68(2):139–148
Bardiya-Bhurat K, Sharma S, Mishra Y, Patankar C (2017) Tagetes erecta (marigold) a phytoremediant for Ni-and Pb-contaminated area: a hydroponic analysis and factors involved. Rendiconti Lincei 28(4):673–678
Bhaduri AM, Fulekar MH (2012) Antioxidant enzyme responses of plants to heavy metal stress. ReV Environ Sci BioTech 11(1):55–69
Borah P, Kumar M, Devi P (2020) Types of inorganic pollutants: metals/metalloids acids, and organic forms. Inorg Pollut Water. Chapter # 2: Elsevier, pp17–31
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of proteindye binding. Anal Biochem 72:248–254
Castillo OS, Dasgupta-Schubert N, Alvarado CJ, Zaragoza EM, Villegas HJ (2011) The effect of the symbiosis between Tagetes erecta L. (marigold) and Glomus intraradices in the uptake of Copper (II) and its implications for phytoremediation. New biotech 29(1):156–164
Cay S (2016) Enhancement of cadmium uptake by Amaranthus caudatus, an ornamental plant, using tea saponin. Environ MonitAssess 188(6):1–8
Coelho LC, Bastos ARR, Pinho PJ, Souza GA, Carvalho JGT, Coelho VA, Faquin V (2017) Marigold (Tagetes erecta): The potential value in the phytoremediation of chromium. Pedosphere 27(3):559–568
Cory RM, Kling GW (2018) Interactions between sunlight and microorganisms influence dissolved organic matter degradation along the aquatic continuum. Limnol Oceanogr 3(3):102–116
Delaide B, Goddek S, Gott J, Soyeurt H, Jijakli MH (2016) Lettuce (Lactuca sativa L. var. Sucrine) growth performance in complemented aquaponic solution outperforms hydroponics. Water 8(10):467
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Ehsan S, Ali S, Noureen S, Mahmood K, Farid M, Ishaque W, Shakoor MB, Rizwan M (2014) Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol Environ Safe 106:164–172
Engwa GA, Ferdinand PU, Nwalo FN, Unachukwu MN (2019) Mechanism and health effects of heavy metal toxicity in humans. Poison. in the modern world-new tricks for an old dog. 10: 70-90
Farid M, Ali S, Rizwan M, Ali Q, Abbas F, Bukhari SAH, Wu L (2017) Citric acid assisted phytoextraction of chromium by sunflower; morpho-physiological and biochemical alterations in plants. Ecotoxicol Environ Safe 145:90–102
Farid M, Ali S, Rizwan M, Yasmeen T, Arif MS, Riaz M, Ayub MA (2020) Combined effects of citric acid and 5-aminolevulinic acid in mitigating chromium toxicity in sunflower (Helianthus annuus L.) grown in Cr spiked soil. Pak J Agric Sci 57(2):477–488
Farooq A, Nadeem M, Abbas G, Shabbir A, Khalid MS, Javeed HMR, Akhtar G (2020) Cadmium partitioning physiological and oxidative stress responses in Marigold (Calendula calypso) grown on contaminated soil: implications for phytoremediation. Bull Environ Cont Toxicol 105(2):270–276
Gill RA, Kanwar MK, dos Reis AR, Ali B (2022) EDITORIAL: Heavy metal toxicity in plants: recent insights on physiological and molecular aspects. Front Plant Sci 12:830682
Gjorgieva AD (2018) Heavy metals and their general toxicity on plants. Plant Sci Today 5(1):15–19
Gomes MP, Marques TCLLSM, Carneiro MMLC, Soares ÂM (2012) Anatomical characteristics and nutrient uptake and distribution associated with the Cd-phytoremediation capacity of Eucalyptus camaldulenses Dehnh. J Soil Sci Plant Nutrients 12(3):481–496
Goswami S, Das S (2016) Copper phytoremediation potential of Calandula officinalis L. and the role of antioxidant enzymes in metal tolerance. Ecotoxicol Environ Safe 126:211–218
Hannan F, Huang Q, Farooq MA, Ayyaz A, Ma J, Zhang N, Ali B, Deyett E, Zhou WJ, Islam F (2021) Organic and inorganic amendments for the remediation of nickel contaminated soil and its improvement on Brassica napus growth and oxidative defense. J Hazard Mater 416:125921
Haroon B, Ping A, Pervez A, Faridullah F, Irshad M (2019) Characterization of heavy metal in soils as affected by long-term irrigation with industrial wastewater. J Water Reuse Desalin 9(1):47–56
Hasanuzzaman M, Bhuyan MHM, Anee TI, Parvin K, Nahar K, Mahmud JA, Fujita M (2019) Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 8(9):384
Hasanuzzaman M, Bhuyan MHM, Raza A, Hawrylak-Nowak B, Matraszek-Gawron R, Nahar K, Fujita M (2020) Selenium toxicity in plants and environment: biogeochemistry and remediation possibilities. Plants 9(12):1711
Hassan MU, Chattha MU, Khan I, Chattha MB, Aamer M, Nawaz M, Khan TA (2019) Nickel toxicity in plants: reasons toxic effects, tolerance mechanisms, and remediation possibilities—a review. Environ Sci Pollut Res 26(13):12673–12688
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: i kinetics and stoichiometry of fatty acid peroxidation. Archives of biochemistry and biophysics 125(1):189–198
Hou D, O’Connor D, Nathanail P, Tian L, Ma Y (2017) Integrated GIS and multivariate statistical analysis for regional scale assessment of heavy metal soil contamination: a critical review. Environ Pollut 231:1188–1200
Ilyas M, Ahmad W, Khan H, Yousaf S, Yasir M, Khan A (2019) Environmental and health impacts of industrial wastewater effluents in Pakistan: a review. Environ Health Rev 34(2):171–186
Jaiswal A, Verma A, Jaiswal P (2018) Detrimental effects of heavy metals in soil, plants, and aquatic ecosystems and in humans. J Environ Pathol Toxicol Oncol 37(3):183-197
Kale RA, Lokhande VH, Ade AB (2015) Investigation of chromium phytoremediation and tolerance capacity of a weed, Portulaca oleracea L. in a hydroponic system. Water Environ J 29(2):236–242
Kamali M, Suhas DP, Costa ME, Capela I, Aminabhavi TM (2019) Sustainability considerations in membrane-based technologies for industrial effluents treatment. Chem Eng J 368:474–494
Kaur L (2016) Tolerance and accumulation of lead by fenugreek. J Agric Ecol 1:22–34
Kumar S, Prasad S, Yadav KK, Shrivastava M, Gupta N, Nagar S, Malav LC (2019) Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches-A review. Environ Res 179:108792
Kumar N, Singh H, Sharma SK (2020) Antioxidants: responses and importance in plant defense system. Sustainable Agric Era of Climate Change. Springer, Cham, pp 251–264
Lajayer BA, Moghadam NK, Maghsoodi MR, Ghorbanpour M, Kariman K (2019) Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: mechanisms and efficiency improvement strategies. Environ Sci Pollut Res 26(9):8468–8484
Latif U, Farid M, Rizwan M, Ishaq HK, Farid S, Ali S, Wijaya L (2020) Physiological and biochemical response of Alternanthera bettzickiana (Regel) G. Nicholson under acetic acid assisted phytoextraction of lead. Plants 9(9):1084
Lichtenthaler HK (1987) Chlorophyll and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:331–382
Liu JN, Zhou QX, Sun T, Ma LQ, Wang S (2008) Identification and chemical enhancement of two ornamental plants for phytoremediation. Bull Environ Cont Toxicol 80(3):260–265
Liu J, Zhou Q, Wang S (2010) Evaluation of chemical enhancement on phytoremediation effect of Cd-contaminated soils with Calendula officinalis L. Int J Phytorem 12(5):503–515
Liu J, Xin X, Zhou Q (2018) Phytoremediation of contaminated soils using ornamental plants. Environ Rev 26(1):43–54
Madanan MT, Shah IK, Varghese GK, Kaushal RK (2021) Application of Aztec marigold (Tagetes erecta L.) for phytoremediation of heavy metal polluted lateritic soil. Environ Chem Ecotoxicol 3:17–22
Murti VM, Maryani (2020) Anatomical responses of marigold (Tagetes erecta L.) roots and stems to batik wastewater. AIP Conf Proc. AIP Publishing LLC, 2260(1): 030018-1
Metzner H, Rau H, Senger H (1965) Untersuchungenzur synchronisierbaketieinzel-nerpigmentmangel-mutation von chlorella. Planta 65:186–194
Muthusaravanan S, Sivarajasekar N, Vivek JS, Paramasivan T, Naushad M, Prakashmaran J, Al-Duaij OK (2018) Phytoremediation of heavy metals: mechanisms, methods and enhancements. Environ Chem Lett 16(4):1339–1359
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nalwade R, Mote T (2017) Hydroponics farming. Int. Conf. on Trends in Electronics and Informatics. IEEE, 645–650
Oliveira H (2012) Chromium as an environmental pollutant: insights on induced plant toxicity. J Bot 2:1372–1382
Parmar P, Dave B, Sudhir A, Panchal K, Subramanian RB (2013) Physiological, biochemical and molecular response of plants against heavy metals stress. Int J Current Res 5(1):80–89
Promratrak L (2017) The effect of using LED lighting in the growth of crops hydroponics. Int J Smart Grid Clean Energy 6(2):133–140
Qin G, Niu Z, Yu J, Li Z, Ma JY, Xiang P (2020) Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere. 267: 129–205
Rai PK, Lee SS, Zhang M, Tsang YF, Kim KH (2019) Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ Int 125:365–385
Ramana S, Biswas AK, Rao AS (2009) Phytoremediation of cadmium contaminated soils by marigold and chrysanthemum. Natl Acad Sci Lett 32(11/12):333–336
Ramírez A, García G, Werner O, Ros RM (2020) In vitro lead tolerance and accumulation in three Chrysanthemum cultivars for phytoremediation purposes with ornamental plants. Int J Phytorem 22(11):1110–1121
Rashid H, Arslan C, Khan SN (2018) Wastewater irrigation, its impact on environment and health risk assessment in peri urban areas of Punjab Pakistan-a review. Environ ContamRev 1:30–35
Rehman S, Abbas G, Shahid M, Saqib M, Farooq AB, Hussain M, Murtaza B, Amjad M, Naeem MA, Farooq A (2019) Effect of salinity on cadmium tolerance, ionic homeostasis and oxidative stress responses in conocarpus exposed to cadmium stress: Implications for phytoremediation. Ecotoxicol Environ Safe 171:146–153
Rostami S, Azhdarpoor A (2019) The application of plant growth regulators to improve phytoremediation of contaminated soils: a review. Chemosphere 220:818–827
Saffari VR, Saffari M (2020) Effects of EDTA, citric acid, and tartaric acid application on growth, phytoremediation potential, and antioxidant response of Calendula officinalis L. in a cadmium-spiked calcareous soil. Int Phytorem. 2(11):1204–1214
Sallah-Ud-Din R, Farid M, Saeed R, Ali S, Rizwan M, Tauqeer HM, Bukhari SAH (2017) Citric acid enhanced the antioxidant defense system and chromium uptake by Lemna minor L. grown in hydroponics under Cr stress. Environ Sci Pollut Res 24(21):17669–17678
Sathya V, Mahimairaja S, Bharani A, Krishnaveni A (2020) Influence of soil bioamendments on the availabilty of nickel and phytoextraction capability of marigold from the contaminated soil. Int J Plant Soil Sci. 31(5):1–12
Seneviratne M, Rajakaruna N, Rizwan M, Madawala HMSP, Ok YS, Vithanage M (2019) Heavy metal-induced oxidative stress on seed germination and seedling development: a critical review. Environ Geochem Health 41(4):1813–1831
Shah K, Mankad AU, Reddy MN (2017a) Cadmium accumulation and its effects on growth and biochemical parameters in Tagetes erecta L. J Pharmacogn Phytochem 6(3):111–115
Shah K, Mankad AU, Reddy MN (2017b) Lead accumulation and its effects on growth and biochemical parameters in Tagetes erecta L. Int J Life Sci Scientia Res 3(4):1142–1147
Shammi M, Rahman M, Bondad SE, Bodrud-Doza M (2019) Impacts of salinity intrusion in community health: a review of experiences on drinking water sodium from coastal areas of Bangladesh. Healthcare 7(1):50 (Multidisciplinary Digital Publishing Institute)
Shao Z, Lu W, Nasar J, Zhang J, Yan L (2019) Growth responses and accumulation characteristics of three ornamentals under copper and lead contamination in a hydroponic-culture experiment. Bull Enviro Contam Toxicol 103(6):854–859
Sharma A, Kumar V, Shahzad B, Ramakrishnan M, Singh Sidhu GP, Bali AS, Zheng B (2020) Photosynthetic response of plants under different abiotic stresses: a review. J Plant Growth Regul 39(2):509–531
Shyamala S, Manikandan NA, Pakshirajan K, Tang VT, Rene ER, Park HS, Behera SK (2019) Phytoremediation of nitrate contaminated water using ornamental plants. J Water Supp ResTech 68(8):731–743
Sidana N, Kaur H, Devi P (2020) Organic linkers for colorimetric detection of inorganic water pollutants. Inorg Pollut Water. Chapter # 8. Elsevier, 135–152
Sidhu G, Bali AS, Singh HP, Batish DR, Kohli RK (2018) Ethylenediamine disuccinic acid enhanced phytoextraction of nickel from contaminated soils using Coronopus didymus (L.) Sm. Chemosphere 205:234–243
Sinhal VK, Srivastava A, Singh VP (2010) EDTA and citric acid mediated phytoextraction of Zn, Cu, Pb and Cd through marigold (Tagetes erecta). J Environ Biol 31(3):255
Son JE, Kim HJ, Ahn TI (2020) Hydroponic systems. Plant factory. Academic Press. 20: 273–283
Song X, Zhang C, Chen W, Zhu Y, Wang Y (2020) Growth responses and physiological and biochemical changes in five ornamental plants grown in urban lead-contaminated soils. PlantEnviron Interac 1(1):29–47
Spry C (2005) Essentials of perioperative nursing. Jones and Bartlett Publishers. 3: 215–217
Sun R, Sun Q, Wang R, Cao L (2018) Cadmium accumulation and main rhizosphere characteristics of seven French marigold (Tagetes patula L.) cultivars. Int J Phytorem 20(12):1171–1178
Thind S, Hussain I, Ali S, Hussain S, Rasheed R, Ali B, Hussain HA (2020) Physiological and biochemical bases of foliar silicon-induced alleviation of cadmium toxicity in wheat. J Soil Sci Plant Nutr 20:2714–2730
Ullah A, Khan D, Khan I, Zheng S (2018) Does agricultural ecosystem cause environmental pollution in Pakistan? Promise and menace. Environ Sci Pollut Res 25(14):13938–13955
Younis A, Riaz A, Mushtaq N, Tahir Z, Siddique MI (2015) Evaluation of the suitability of sewage and recycled water for irrigation of ornamental plants. Commun Soil Sci Plant Anal 46(1):62–79
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35:785–791
Zhang H, Xu Z, Guo K, Huo Y, He G, Sun H, Sun G (2020) Toxic effects of heavy metal Cd and Zn on chlorophyll, carotenoid metabolism and photosynthetic function in tobacco leaves revealed by physiological and proteomics analysis. Ecotoxicol Environ Safe 202:110856
Zayed A, Gowthaman S, Terry N (1998) Phytoaccumulation of trace elements by wetland plants: I. Duckweed. J Environ Qual 27:715–721
Acknowledgements
This project was funded by the Higher Education Commission (HEC) of Pakistan under National Research Program for Universities (NRPU) grant No. HEC/R&D/NRPU/2017/8996. The authors, therefore, gratefully acknowledge HEC-Pakistan and University of Gujrat, Gujrat, Pakistan, for technical and financial support.
Funding
The present study was supported by the HEC funder project 2017/HEC/R&D/NRPU/8996.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Experimentation and analysis were performed by Arooj Fatima, Mujahid Farid, Sheharyaar Farid, and Muhammad Zubair. The first draft of the manuscript was written by Mujahid Farid, Muhammad Rizwan, Zaki ul Zaman Asam, Mohsin Abbas, and Arooj Fatima. All authors gave their feedback and input during the writeup, experimentation, analysis, data validation, and proofreading of the present research work. The final proofreading and revision of the article were done by Shafaqat Ali, Sheharyaar Farid, Zaki ul Zaman Asam, and Mujahid Farid.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fatima, A., Farid, M., Asam, Z.U.Z. et al. Efficacy of marigold (Tagetes erecta L.) for the treatment of tannery and surgical industry wastewater under citric acid amendment: a lab scale study. Environ Sci Pollut Res 30, 43403–43418 (2023). https://doi.org/10.1007/s11356-023-25299-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25299-9