Abstract
Adsorption is a promising technology for removing several contaminants from aqueous matrices. In the last years, researchers worldwide have been working on developing composite adsorbents to overcome some limitations and drawbacks of conventional adsorbent materials, which depend on various factors, including the characteristics of the adsorbents. Therefore, it is essential to characterize the composite adsorbents to describe their properties and structure and elucidate the mechanisms, behavior, and phenomenons during the adsorption process. In this sense, this work aimed to review the main methods used for composite adsorbent characterization, providing valuable information on the importance of these techniques in developing new adsorbents. In this paper, we reviewed the following methods: X-Ray diffraction (XRD); spectroscopy; scanning electron microscopy (SEM); N2 adsorption/desorption isotherms (BET and BJH methods); thermogravimetry (TGA); point of zero charge (pHPZC); elemental analysis; proximate analysis; swelling and water retention capacities; desorption and reuse.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Emerging contaminants (ECs) can be classified as pharmaceuticals, personal care products (PPCPs), pesticides, some dyes, and plasticizers, among others, and have drawn the attention of the scientific community in recent years due to their wide detection in aqueous matrices worldwide (Dotto and McKay 2020; Nguyen et al. 2020). The presence of ECs in water resources can occur through industrial processes, hospitals, effluent treatment plants, excretion through urine and feces of humans and animals, and incorrect disposal in bathroom sinks or as solid waste (Sousa et al. 2018).
These contaminants have been detected in wastewater (Guillossou et al. 2021), surface water (Rivera-Jaimes et al. 2018), and even drinking water (Ebele et al. 2020), generally in ng μg L−1 concentrations. It is noteworthy that the effects of ECs on living beings are still not well understood. However, studies show toxic potential, oxidative stress, reduced growth of cyanobacteria, reduced growth, and reproduction of mosquitoes (Nieto et al. 2017; Fatma et al. 2020; Streit et al. 2021), and reduced or stimulated locomotor activity in zebrafish (Ladu et al. 2015; Tran et al. 2017).
The use of adequate techniques to remove these contaminants from aqueous matrices is a current demand and can directly contribute to scientific, technological, environmental, and public health development. Adsorption has stood out for this purpose compared to conventional methods (Karimi-Maleh et al. 2021). However, the adsorption efficiency is directly related to the material used, which must have desirable characteristics, such as easy acquisition, low cost, chemical, mechanical and thermal stability, good adsorption capacity, fast adsorption kinetics, high selectivity, good physicochemical characteristics, and texture, in addition to the possibility of reuse or regeneration (Dotto and McKay 2020; Rodrigues 2020). Thus, the addition of other compounds to the matrix of a base material results in obtaining the known as “composite adsorbents,” which have gained prominence in the scientific community due to the appeal of cost reduction and increased adsorption efficiency, aiming at the possibility of replacing conventional activated carbon, expanding the possibilities of applications (Zhou et al. 2019; Rigueto et al. 2021a).
Regarding the specific characteristics of the adsorbent, the addition of certain compounds can be carried out for several purposes, including increasing the surface area and activating or binding sites, which play a vital role in the adsorption of organic and inorganic contaminants (Manyangadze et al. 2020; Yaah et al. 2021). Furthermore, it is possible to use hydrothermal and mechanically stable materials in environments with higher temperatures, also enabling the potential for specific cooling of the system (Askalany et al. 2017; Rigueto et al. 2021b) and adequate porosity, taking into account the adsorbates of interest (Manyangadze et al. 2020).
Therefore, it is essential to characterize composite adsorbents during all the stages of production and after their application to prove whether the addition of one or more compounds in the developed material was achieved or not. In this context, the present work aims to review the main techniques utilized to characterize composite adsorbents, addressing the principles and importance of these analyses and compiling promising results reported in recent studies.
Main techniques used for composite adsorbents characterization
X-ray diffraction
X-ray diffraction (XRD) is a non-destructive technique, considered a powerful analytical tool, commonly applied to determine atomic spacing, chemical composition, and crystal structure of different materials, including composite adsorbents (Bunaciu et al. 2015; Kohli and Mittal 2019; Sharma et al. 2019; Zhang et al. 2019b). X-ray wavelengths are of the same magnitude order as the interatomic spacing of crystalline solids (Kaliva and Vamvakaki 2020). Thus, XRD can show the material nature, classifying it as crystalline or amorphous (Vishwakarma and Uthaman 2020).
XRD is based on the diffraction phenomenon, which results from the radiation scattered by a regular set of diffusion centers, whose spacing is of the same magnitude order as the radiation wavelength. Atoms are the scattering centers for X-rays, and scattering occurs from the interaction of an electromagnetic radiation photon with an orbital electron in the atom (Bunaciu et al. 2015).
XRD demonstrates the crystal structure of crystalline/semi-crystalline materials from X-ray scattering, which generates defined diffraction patterns capable of qualitatively illustrating the atomic arrangements within the crystal lattice (Rajeswari et al. 2020). XRD is based on Bragg’s law (Eq. 1), where an investigated material is submitted to X-rays for a detector’s further interpretation of XRD peaks (Kohli and Mittal 2019; Patel and Parsania 2018). This law is satisfied when the interaction of the incident rays with the sample produces constructive interference and a diffracted ray (Bunaciu et al. 2015).
where n: integer; λ: X-rays wavelength (Å); d: interplanar spacing generating the diffraction; θ: diffraction angle.
According to Bragg’s law, the pathway difference between adjacent X-ray beams is some integer (n) of radiation wavelengths (λ). Furthermore, d is the spacing between adjacent crystal planes, θ is the scattering angle (known as Bragg’s angle), and 2θ is the experimentally measured diffraction angle (Bunaciu et al. 2015). Therefore, the interlayer spacing of the investigated materials can be determined using Bragg’s law from the given 2θ values (Ashiq et al. 2019). This law relates the wavelength of electromagnetic radiation to the diffraction angle and the lattice spacing in a crystalline sample, then diffracted X-rays are detected, processed, and counted (Bunaciu et al. 2015).
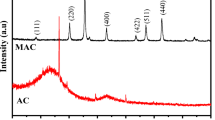
The interpretation of XRD patterns is commonly performed by analyzing the intensity (y-axis of a plot; unit: a.u.) of reflection peaks at 2θ (x-axis of a plot; unit: degree) generated from the diffractometer. The degrees obtained from the peaks corresponding to orientation planes show the material’s crystalline structure. Authors usually compare the differences between original materials and the proposed composite in the composite adsorbents production context. Based on the reflection peaks, it is possible to identify the main components of the material. Ngwenya et al. (2019) report that a peak around 2θ = 20° is a typical diffraction peak of amorphous materials.
Conversion of these diffraction peaks allows compound identification since each has a set of unique d-spacings. This identification is generally realized by comparing d-spacings with standard reference patterns (Bunaciu et al. 2015). XRD can show a lot of material parameters, such as structures, phases, preferred crystal orientations, average grain size, crystallinity, strain, and crystal defects (Kohli and Mittal 2019). Sample preparation can be critical for XRD analysis, but it depends on the material, XRD equipment, and desired results (Bish and Reynolds 1989). Monocrystalline, polycrystalline, or powder materials (randomly oriented fine particles) can be analyzed using X-ray diffraction (Kohli and Mittal 2019).
XRD presents several advantages such as (1) it is a non-destructive and fast technique with easy sample preparation;( 2) it presents high accuracy for d-spacing determinations; (3) it can be done in situ; (4) allows characterizing single crystal, poly, and amorphous materials; (5) thousands of materials have standards available for comparison and identification (Bunaciu et al. 2015; Kohli and Mittal 2019). In addition, XRD is useful in determining the crystallinity degree (Eq. 2), which considers the crystalline and amorphous contents in the analyzed sample (Patel and Parsania 2018).
where Xc: crystallinity degree; Ic: crystalline intensity; Ia: amorphous intensity.
XRD technique presents several potential uses, such as forensic science, geological applications, corrosion analysis, microelectronics, glass, and the pharmaceutical industry (Ahmadian et al. 2020). In addition, XRD is commonly used for composite adsorbents characterization to show the material’s structure at an intermolecular level (Ahmadian et al. 2020; Rigueto et al. 2021c). The greater the crystallinity of the adsorbent, the greater its physical resistance; however, its water retention capacity tends to decrease.
By adding zeolite into chitosan, Oshagbemia et al. (2020) verified an increase in chitosan crystallinity, which resulted in higher thermal stability, but decreased flexibility. Wang et al. (2019) studied different Si/Mg ratios. They found that at a molar ratio of 2:1 and a pH of 12, palygorskite and its associated minerals were restructured, generating material with better adsorptive properties for removing antibiotics and dyes.
XRD can be used to analyze materials’ mineral phase transformation and suggest whether the materials were synthesized during the composite adsorbents production (Kim et al. 2020). Furthermore, XRD can indicate the adsorbents’ recyclability potential when applied to verify if the material structure has changed after its recovery (Ahmadian et al. 2020). Besides the crystallinity of the composite adsorbents, XRD is also capable to shows or indicating (1) the main components of several materials; (2) adsorptive affinity; (3) immobilization of acids in the composites; (4) chemical interactions between the materials; (5) ions oxidation; (6) crystal structure modification of composites, among others (Chen et al. 2020; Edathil et al. 2020; Faraj et al. 2020; Jawad et al. 2020; Soliman et al. 2020; Zhang et al. 2020; Zhou et al. 2020).
Through XRD, Arumugam et al. (2019) indicated that intermolecular forces of attraction strongly bound the coir pith-activated carbon and chitosan. XRD analysis results are commonly compared with other characterization methods, especially scanning electron microscopy (SEM), energy dispersive X-ray (EDX), and Fourier Transform Infrared Spectroscopy (FTIR), to support and more clearly understand the material’s structure and composition (Song et al. 2019; Wang et al. 2020).
The crystal structure of various materials can be modified by increasing the temperature (Zhang et al. 2019a). Ashiq et al. (2019) observed that when municipal solid waste was heated to a temperature > 450 °C, the composite derived from this waste and biochar showed amorphous characteristics. During the studies of composite adsorbent production, Chen et al. (2020) and Wang et al. (2019) verified that at 300 °C and 180 °C, respectively, the crystalline structure of their materials was not significantly altered.
Therefore, XRD is an indispensable technique for producing and characterizing composite adsorbents. In addition to proving the presence of compounds by analyzing the material crystalline structure, XRD is commonly related to other characterization methods to better understand the properties, behavior in adsorption processes, and potential applications of the new composites.
Spectroscopy
Spectroscopy assesses the interaction between the molecule and electromagnetic radiation, in which these radiations are generated by an oscillation of the electrical charge and magnetic field of each atom (Yadav 2005). Electromagnetic radiation can be visible, ultraviolet, infrared, X-ray, microwave, and others (Pavia et al. 2009). Thus, spectrophotometric measurements depend on the properties of each material, and these can be through total reflectance, transmittance, absorbance, emittance, scattering, and fluorescence (Germer et al. 2014; Silva et al. 2020).
There are different spectroscopy techniques in this context: atomic absorption, nuclear magnetic resonance, atomic emission, molecular electronics, vibrational, and mass (Mendham et al. 2000). Regarding the application of spectroscopy techniques in the analysis of adsorbent materials, these are used to identify the composition of materials and understand structural change and production and bioproduction processes (Spiridon et al. 2011; Villar et al. 2012; Kowalczuk and Pitucha 2019). One of the spectroscopy methods commonly reported in the studies of adsorbent materials is Fourier transform infrared spectroscopy (ftir).
FTIR is a vibrational spectroscopy technique using energy in the infrared range, specifically in the region between 4000 and 400 cm−. This value is expressed in wavenumber, in which a dipole moment must occur during the molecular vibration to absorb the incident radiation. Thus these vibrations appear in the infrared spectrum (Mendham et al. 2000; Pavia et al. 2009). This method is used to identify pure substances, impurities, and composition of different materials, having different applications, being these in the pharmaceutical, medicinal, polymeric, food, environmental, and also in the control of counterfeit materials (Nabers et al. 2015; Malek et al. 2019; Poiana et al. 2015).
FTIR consists of an incoming infrared light beam divided into two individual beams from an optical beam splitter. Both will have a difference in the optical path given by a fixed and a mobile mirror, with a subsequent combination of these again using an optical combiner, generating interference (Stuart 2004; De Leon et al. 2014; Valand et al. 2020). Standard infrared detectors can measure this interference signal, obtaining an optical path difference function, also known as Michelson interferogram, with the unit in centimeters (Stuart 2004). The original spectrum of the interferogram is obtained by converting it by employing a fast Fourier transform (FTT), obtaining its unit in cm−1, also known as wave number (Gauglitz and Vo-Dihn 2003; Haas and Mizaikoff 2016).
The radiation sources used in infrared spectrophotometers can be from Nichrome filament, filaments with zirconium oxides, thorium, and cerium bonded by a binder (Nernst Source) and from a silicon carbide cylinder (Globar Source); both devices are heated to 1200–2000 °C, which emits infrared radiation (Mendham et al. 2000). In this equipment, due to the high absorption of infrared radiation by glass and quartz, the sample cells and diffraction grating must be made from inorganic salts, such as sodium chloride or potassium bromide (Mendham et al. 2000). In the case of liquid materials that can evaporate, closed cells are used, and when the solvent used is water, the cell preparation must be calcium or barium fluoride (Pavia et al. 2009).
The spectra obtained in FTIR allow identifying functional groups, types of bonds, and molecular conformations; however, the technique has the peculiarity of using non-aqueous samples due to the strong water absorption bands, making it used for organic compounds (Mendham et al. 2000). The method is considered qualitative, but it can also be semi-quantitative, which allows direct measurements of adsorbed compounds from a standard calibration curve of known concentrations; it is often used when it is not possible to quantify compounds that do not absorb in the UV/visible region or need costly analytical procedures (Morhardt et al. 2014; Rytwo et al. 2015).
The main techniques derived from FTIR are Fourier transform infrared-attenuated total reflectance (FTIR-ATR), Fourier transform infrared photoacoustic spectroscopy (FTIR-PAS), Fourier transform infrared image spectroscopy (coupled optical microscope), Fourier transform infrared microspectroscopy and Fourier transform infrared nano spectroscopy, with emphasis on the latter, mainly in the biomedical area of materials science (Amenabar et al. 2017).
FTIR has been used to characterize the material composition and assess whether there have been changes after physical and chemical treatments or the formation of composite materials, comparing the surface spectra of treated and untreated materials (Kowalczuk and Pitucha 2019). The purpose of transforming materials is to improve thermal, mechanical, chemical, and regeneration properties so that the new material is viable in terms of costs and sustainability (Dotto and McKay 2020).
Rigueto et al. (2021b) used FTIR to evaluate the structure of commercial gelatin granules recovered from tannery waste. They observed no changes in the amide bands between the two types of gelatin studied, suggesting similar structures. Therefore, there was no degradation of proteins in the gelatin extraction process. Other studies applied FTIR to assess whether all parts were incorporated into a composite. For example, Zhao et al. (2019) produced modified cellulose hydrogels (MCC) with acrylamide (AM) and acrylic acid (AA) and verified an introduction of AA and AM in the MCC composite based on the spectra with and without the addition of the AA and AM components, comparing the clusters of each.
Another technique in composite adsorbents is the visualization of functional groups that may have favored adsorption, comparing the spectra of materials before and after adsorption. For example, Jawad et al. (2020) obtained a similar situation when comparing the spectra of modified chitosan material (CHS) with a covalent crosslinker (epichloridine, ECH) and zeolite (ZN) before and after adsorption of the dyes methylene blue and reactive red 120. In the spectra, Si–O, –OH, –NH2, CO, and CH symmetrical and asymmetrical stretching vibrations and NH, Al–O–Si, and Si–O–Si flexural vibrations, besides COC and CO stretching vibrations, which indicate the reaction between the hydroxyl groups of CHS with ECH, forming covalent bonds, using the opening of the ECH epoxide ring and the release of a chlorine atom. Furthermore, hydrogen bonds were evidenced in the chemical interaction between ZL and CHS. Thus, the compared spectra present a similar profile, with minimal displacements of some bands, showing that several functional groups helped in dye adsorption.
Scanning electron microscopy
Scanning electron microscopy (SEM) is a fundamental instrumental analysis for scientific research, as it allows knowing the surface morphology of materials and their chemical composition (Zhou et al. 2007). However, SEM can not be considered a non-destructive analytical technique, as in some cases, it can alter important or non-important characteristics of the sample (Inkson 2016).
The SEM image is formed by capturing the signals produced by the electron beam applied to the sample. When electrons are applied to the sample, they reach its surface and penetrate, producing a band of primary excitation. Their size depends on the energy of the electron beam and the density of atoms on the surface. The volume and depth of electron penetration increase with increasing beam energy and decrease with an increasing atomic number of the material. A high acceleration voltage will result in deeper penetration, leading to a loss of surface information (Zhou et al. 2007).
By focusing a beam of primary electrons on the sample, secondary electrons and backscattered electrons are produced (Inkson 2016). Secondary electrons interact with the sample by scanning its surface with an energy between 3 and 5 eV and are attracted to a detector (Zhou et al. 2007). The detector signal is synchronized with the beam in the sample, and the signal intensity is used to modulate the image pixel (Inkson 2016). The backscattered electrons have an energy of 50 eV and allow the formation of a surface relief contrast and a contrast concerning the atomic number of the material’s chemical elements. This trend is because these electrons retain about 60 to 80% of their initial energy. Therefore, the nucleus has more positive charges when interacting with elements with higher atomic numbers, causing the backscattered signal to increase (Vernon-Parry 2000).
For samples of non-conductive composites or poor energy conductors, a coating usually made of carbon, gold, or platinum is required, as the electrostatic charge occurs due to a difference in the current of primary electrons from negative charges and the output of secondary and backscattered electrons (Inkson 2016). In addition, the resolution limit of SEM is defined by the wavelength of the source’s illumination because when exceeding the resolution limit, a blurred image is obtained (Zhou et al. 2007).
The information produced by SEM images verifies the composite surface for composite adsorbents, indicating the material’s porosity. Still, it is inappropriate that only this technique is used to reach this conclusion. BET isotherm analysis, methylene blue number, and iodine number can be used to confirm SEM observations. For example, De Rossi et al. (2020) verified that their composite presented a surface with spheroid morphology and deformities. Furthermore, it was possible to observe a difference concerning pure yeast, which has a smooth surface. The authors concluded that the material was porous, supported by the methylene blue number analysis.
By comparing chitosan and alginate composites with and without activated charcoal from leather residues, Melara et al. (2021) found that the addition of activated charcoal made the surface of the composites rougher. The rougher surface gives the material irregularities that can be more favorable to the diffusion of the contaminant through the adsorbent particle. This information was confirmed by the specific surface area analysis of BET and the adsorption capacity of the composites. Thus, the authors concluded that the composite with a rougher surface had a greater surface area and adsorption capacity. Besides, Yang et al. (2021) demonstrated an improvement in the mechanical properties of cellulose and chitosan hydrogel by observing a thicker structure through SEM.
There are variations of scanning electron microscopes, which allow many extra functions working on the same principle as secondary and backscattered electrons. One such variation is field-emission scanning electron microscopy (FESEM), which results in high-resolution images (Gao et al. 2019; Lütke et al. 2020). Another technique is to couple an energy dispersive spectrometer (SEM–EDS) to SEM, which allows for identifying and quantifying the elements of the periodic table (except for H, He, and Li) present on the surface of a composite (Newbury and Ritchie 2013).
Both variations are useful for the characterization of composite adsorbents. They allow us to prove the presence of the material’s components and quantify them, which is important to further understand the adsorption mechanisms. Wang et al. (2015) used SEM–EDS to characterize iron oxide biochar from biomass and hematite. They observed that hematite particles were welded to the coal’s surface and could map the presence of carbon, oxygen, and iron in the composite. Likewise, Khan et al. (2016) confirmed by SEM–EDS the presence of copper, oxygen, and carbon in chitosan/copper oxide composite spheres on the sample surface.
The limitation of SEM analysis is the use of a vacuum environment, which allows the analysis of solid samples that need to be coated with gold or carbon if they are not good conductors. Still, it allows the surface of materials to be characterized with a high resolution, allowing physical characteristics such as fractures, particle size, coating thickness, and chemical characteristics to be determined using EDS. Therefore, SEM has become an indispensable analysis for the characterization of adsorbents, allowing the surface to be known and its results to be integrated with other analyses to determine the potential or limitations of new adsorbents.
N2 adsorption–desorption isotherms (BET and BJH methods)
Gas adsorption is an important technique for the characterization of porous materials. BET is the technique proposed by Brunauer et al. (1938), which is based on the adsorption of N2 in its liquid state at 77 K, where physical forces adsorb the gas on the adsorbent particles. N2 adsorbed in different pressure ranges causes a change in the output composition registered by a detector. As the sample is heated, the nitrogen is desorbed. Then peak areas representing the desorbed mass can be observed (Bardestani et al. 2019), making it possible to quantify the surface area of the adsorbent and pore size distribution (Sing 2001) without destroying the sample (Sing 1985).
Adsorption of gas under high pressure can form adsorbed multimolecular layers. For example, non-polar molecules adsorbed on the surface of an adsorbent induce dipoles in the first layer, which induce a dipole in the next layer, and so on. However, the adsorption from the second layer is practically insignificant. Therefore, the molecules interact vertically (Brunauer et al. 1938).
BET theory suggests that adsorption occurs on a uniform surface and that the sites have the same adsorption energy. At low pressures (p/p0 0.0–0.2), adsorption occurs on the external surface of the adsorbent particle and on the micropores, which are more energetic locations. With the increase in pressure (p/p0 0.4–0.95), the formation of the second and third adsorption layers occurs, with condensation occurring in the mesopores and (p/p0 > 0.95) in the macropores (Bergaya and Lagaly 2013). As the pressure increases, the probability of a molecule being adsorbed to an already adsorbed molecule increases. Because of this, the second layer starts its formation before completing the first one. Therefore, layers are not limited (Rouquerol et al. 2007; Lowell and Shields 2013).
The BET theory is applied to quantify gas adsorption. This theory indicates that the adsorption energy is independent of the adsorption sites and that the adsorbate molecules interact only vertically (Bardestani et al. 2019). The linearized application of the BET equation is represented in Eq. 3.
where na is the amount adsorbed at the relative pressure p/p0, nam is the adsorption capacity in the monolayer, and C is exponentially related to the adsorption enthalpy in the first layer (Sing 1985).
By plotting the graph of BET p/na(p0–p) on the y-axis and p on the x-axis, a straight line is obtained, and by determining the slope and intercept of the graph, the amount of N2 adsorbed can be determined (Sing 1985; Naderi 2015). If we have the capacity of the monolayer, then it is possible to determine the surface area of the adsorbent; being necessary to know the average area ofthe molecular cross-section occupied by the adsorbate molecule (for N2 at 77 K = 0.162 nm2) (Sing 1985). The total and specific surface area can be calculated according to Eqs. 4 and 5.
where As the total surface area, as the specific surface area, m adsorbent mass, and L the Avogadro constant (6.022 × 1023 mol−1) (Sing 1985).
This determination results in isotherm plots, which can be of different formats depending on the adsorbate-adsorbent and adsorbate–adsorbate interactions. The isotherm line to calculate the surface area should not occur subjectively, which may lead to misinterpretation (Rouquerol et al. 2007). Brunauer et al. (1938) suggested that the relative pressure range of 0.05 to 0.35 be used. However, according to Rouquerol et al. (2007), each adsorbent may present a range considered adequate due to characteristics such as microporosity. Thus, two assumptions are useful to select the appropriate linear portion to determine the surface area: the selected line must have a positive intercept on the p–p0 axis, thus considering only positive C values, and the term p–p0 must increase along with p/p0. If this does not occur, the selected pressure range must be reduced.
Adsorbents are porous. The pores of an adsorbent can be classified according to their diameter, macropores (d > 50 nm), mesopores (d: 50 to 2 nm), and micropores (d < 2 nm) (Sing 1985). Studies to verify the size of pores and the distribution of these sizes use the Kelvin equation, which relates the vapor pressure of a surface, such as a liquid in a capillary or pore, to the equilibrium pressure of this same liquid in a flat surface (Lowell and Shields 2013). The best-known theory is BJH (Barrett et al. 1951) which calculates the change in the thickness of the adsorbed layers by decreasing the relative pressure in desorption. By decreasing the pressure, gas desorption occurs in the larger pores with capillary condensation and reduces the thickness of the adsorbed multilayers (Bardestani et al. 2019). The commonly used form of the Kelvin equation for determining pore size is represented in Eq. 6.
where P/P0 is the relative pressure in equilibrium with a meniscus, \(\sigma\): is the surface tension of adsorbate in liquid form, VM is the molar volume of the liquid, R is the universal constant of ideal gases (8314 J mol−1 K−1), r is the radius of the meniscus formed in the mesopore, and T is the temperature (Bardestani et al. 2019).
The BJH method assumes that the pores have a cylindrical shape and that the adsorbed amount involves adsorption on the pore surface and capillary condensation of the mesopores (Bardestani et al. 2019). This behavior is because a phenomenon in the N2 adsorption isotherm can complicate the interpretation of pore size, called hysteresis. Hysteresis indicates that the gas is desorbed at a lower relative pressure than adsorbed (Lowell and Shields 2013).
Another important piece of information provided by the N2 adsorption isotherm is the total pore volume. At saturation (p/p0 = 1), the pores are filled with saturated vapor pressure. Therefore, a relative pressure above the hysteresis closure should calculate the total pore volume, usually the closest to p/p0 = 1, to include the largest pores in the analysis (Lowell and Shields 2013). Surface area and pore distribution play a fundamental role in the adsorption process. Table 1 shows specific surface area data, mean pore size distribution, and pore volume from recent works that synthesize composite adsorbents and characterize them using BET and BJH methods.
BET and BJH methods can be used to characterize organic and inorganic composite adsorbents (Table 1). Most of the works reported in Table 1 synthesized mesoporous composites. For example, Işık et al. (2021) observed an increase in the surface area from the first to the second composite, resulting in a greater extension of the adsorbent-adsorbent interactions, which improved the adsorption capacity. Besides, Melara et al. (2021) observed that a higher concentration of activated carbon (30%) increased the specific surface area and adsorption capacity of the composite. The improvement in the adsorption capacity occurred due to the greater number of active adsorption sites provided by activated carbon. In addition, the increase in the surface area reduced the steric hindrance in the active sites of chitosan, leaving them free to adsorb the pollutant molecules.
Thermogravimetry
Thermogravimetric analysis (TGA) is an experimental method in which the mass of a given sample is measured as a function of temperature or time and thus subjected to a temperature and controlled atmosphere program, in which, through adjustments to these conditions, it is possible to get different information about the sample (Wagner 2018; Saadatkhah et al. 2019). The basics of the analysis consist of a sample inserted into a “sample plate” stored in a sample holder, in which its mass is measured by a thermobalance, having its temperature defined according to a temperature program, and measured with thermocouples in contact with the sample, this heating is caused by an electric oven (De Blasio 2019).
This analysis results in a graphic curve, the TGA, in which the mass as a function of temperature or time is plotted (Wagner 2018). Another additive analysis is the first derivative of this TGA curve, named as differential thermogravimetric curve (DTG), which aims to obtain the rate of mass change (De Blasio 2019). The mass variation during the TGA analysis can be positive or negative, producing steps in the TGA curve or peaks in the DTG. When this mass variation is positive, it is a function of adsorption, oxidation, and reduction. When negative, it concerns desorption, thermal decomposition, combustion, vaporization, and sublimation phenomena (Bottom 2008).
Measurements in the TGA method can be influenced by some parameters, such as heating rate, atmosphere, sample preparation, crucible choice, instrumental effects (buoyancy and gas flow), change in physical properties during measurement (emissivity and volume), and “spit” or move effect (Bottom 2008). Temperature programs can be dynamic (constant heating rate), isothermal (constant temperature), and non-linear rate (controlled by the sample). The atmosphere can be chosen and varied during the measurement, possibly having reactive, inert, and oxidizing atmospheres (Vyazovkin et al. 2011).
Sample preparation is essential, requiring a representative sample quantity, sufficient for the method’s accuracy, altered as little as possible, and without contamination. Another essential analysis is regarding the morphology and mass of the sample due to its influence on the rate of diffusion and heat transfer (De Blasio 2019). Concerning the crucibles, they must be made of a material (alumina, platinum, and aluminum) that does not influence the reaction of the sample, and, regarding the procedure, they must be sealed and drilled immediately before the start of the measurement, so that they are “open” to the atmosphere (Bottom 2008).
The main part of the TGA analysis is the thermobalance, which has three sample loading configurations: horizontal, suspended, and upper (Saadatkhah et al. 2019). The difference between the three is related to the position of the sample holder, being horizontally at the end of the arm and without adding weight. In contrast, the arm must be rigid at the top, and the weight to keep it vertical and suspended is under the scale, being less rigid and without weight compensation (Prime et al. 2009). Another constructive requirement of the method is insulation between the balance and the oven, using purge gas to protect the balance to avoid the effect of heat radiation and corrosive decomposition products (Wagner 2018).
This technique is widely used to evaluate properties of interest in different samples, the main ones being thermal and oxidative stability, decomposition kinetics, composition in case of multi-components, stoichiometry and degree of reaction conversion, product life, and moisture content, and volatile (Mahboub et al. 2018). Regarding applying this technique for the analysis of adsorbent materials, it is to predict the thermal stability of this material and composite materials to predict whether there has been an improvement in thermal properties (Osayi et al. 2014). Thus, some studies involving composite adsorbents are presented in Table 2.
TGA analyses involving adsorbent materials indicate the thermal behavior of a material, showing its thermal stability and degradation temperature, as well as possible information on the effect of weight loss and gain at certain stages of degradation (Table 2). Starting, Wu et al. (2019) compared the thermal stability of a composite of gelatin and chitosan with the addition of different concentrations of graphene. This study observed two stages of degradation for all samples due to water evaporation (22–170 °C) and decomposition of organic matter (280–550 °C). Another observed effect was that adding larger amounts of graphene positively influences the thermal stability of the composite, decreasing weight loss in the temperature range of 22–800 °C.
Another similar effect of improving the thermal stability of the composite was reported by Eltaweil et al. (2020). They used carboxymethyl cellulose (CMC) and added graphene oxide, forming microbeads of carboxylated graphene oxide (CMC/GOCOOH). This study observed that 50% of mass loss is reached at 242.2 and 370.7 °C for CMC and CMC/GOCOOH, respectively, showing that the addition of graphene oxide effectively improved the thermal stability of the composite. In addition, it was possible through the TGA curves to the following information: the first (25–110 °C) and second (111–270 °C) stages for both compounds. The loss occurs due to water vaporization and carbon dioxide abstraction from the polymer chain. In the third (270–600 °C), there is a greater loss for the CMC composite than the CMC/GOCOOH composite, evidencing the effect of improving thermal stability.
Alver et al. (2020) evaluated the thermal resistance through TGA analysis, comparing magnetic sodium alginate (ALG) with and without the addition of rice husk (m-ALG/RH). In this study, two main losses were obtained for both materials, the first (25–200 °C) of 15%, due to water evaporation and the second (200–900 °C), due to the degradation of the two materials, with losses of 67 and 61% for ALG and m-ALG/RH, respectively, showing an improvement in thermal stability when using composite material. Therefore, the main objective of using the TGA technique in composite adsorbent materials is to evidence the effects of thermal stability and degradation of materials and thus compare whether the composite formation is effective in improving thermal resistance.
Differential scanning calorimetry (DSC) is a thermal analysis technique used to measure temperatures and heat flow related to changes in the material in terms of time and temperature (Wen et al. 2012; Meng et al. 2007). Quantitative and qualitative information is obtained according to physical and chemical changes involving endothermic or exothermic processes or variations in heat capacity (Meng et al. 2007). It is considered one of the most popular and accurate techniques for evaluating the specific heat of composite adsorbents (Soni et al. 2022). By relating the technique to TGA, one can consider a quick method for determining the thermal stability and the confirmation and estimation of the encapsulation efficiency, determination of the water content, and storage capacity of the material (Gharanjig et al. 2020).
In the heat flux DSC technique procedure, the signal from a sample cell is compared with a reference cell in an identical solution environment (Wen et al. 2012). As the cells are heated or cooled linearly, the resulting differential heat flow of the sample and reference is monitored by thermocouples connected in series (Wen et al. 2012). Thus, this energy variation makes it possible to identify and measure the thermal transitions that occur in the sample in a quantitative mode and, in this way, characterize the material for different thermal events such as transitions, melting crystallization, and more complex events (Gharanjig et al. 2020).
Thermal events can be classified as first and second-order transitions. First-order transitions show enthalpy variations, thus forming endothermic and exothermic peaks. However, the second-order transitions are characterized by the variation of the heat capacity, without enthalpy variations, and the formation of peaks in the curve, just a S-shaped shift from the baseline (Wendlandt 1986; Machado and Matos 2004).
When the sample undergoes an endothermic or exothermic transition, the heat flow of the sample must change relative to that of the reference material, thereby resulting in a peak in the curve (Gharanjig et al. 2020). Transition peaks are usually positive for exotherms and negative for endotherms (Wen et al. 2012). Endothermic events include melting, sample mass loss (vaporization of water, additives, volatile reaction, decomposition products), desorption, and reduction reactions. In addition, exothermic events can be crystallization, polymerization reactions, oxidation, oxidative degradation, and adsorption (Wendlandt 1986; Machado and Matos 2004).
According to the results of the DSC analysis, it is possible to interpret the structural and chemical transformations, as well as the physical and energetic properties suffered by the material (Meng et al. 2007; Gharanjig et al. 2020). Furthermore, it is possible to determine material purity through analysis (Brown 1979). However, it presents some limitations when referring to the interpretation of the heat flow in temperature ranges that occur simultaneously in several processes; in this way, different transitions can overlap in a single peak. On the other hand, the advantages are related to the ease of sample preparation, which can be applied to solid and liquid materials, fast analysis time, and wide temperature range (Verdonck et al. 1999; Gharanjig et al. 2020).
Point of zero charge
Given the various mechanisms that can govern adsorption, the surface charges of the adsorbent play a fundamental role. In solid–liquid adsorption, when the adsorbents are suspended in an aqueous solution, the surface electrical charge develops through the dissociation of the surface hydroxyl groups and complexation of the background electrolyte ions, where H+ and OH− are the main ions that determine the point of zero charge (pHPZC), which is the pH value where the sum of the positive and negative charges present on the surface of a solid is zero. Furthermore, the surface of the adsorbent can be considered positively charged when pH < pHpzc and negatively charged when pH > pHpzc, therefore, pHPZC interferes differently in choosing the best pH range to be used in aqueous solution assays (De Rossi et al. 2020; Khan and Sarwar 2007).
Differences in the origin of the adsorbents and experimental methods used to determine the pHPZC result in variations reported in the literature for similar adsorbents (Mahmood et al. 2011). Among the methods that can be used to determine the pHPZC of adsorbents, there are:
-
(a).
Salt addition method: Generally, NaNO3 or NaCl is used, in which the aqueous solutions have the pH adjusted from 2 to 11, with the addition of the adsorbent material, kept under stirring for a defined time (24–48 h). The initial and final pH of the solutions are measured, and then the difference between the final and initial pH (ΔpH) is calculated. Finally, the values are plotted (Mahmood et al. 2011).
-
(b).
Potentiometric titration method: Initially, two experiments are carried out, containing the adsorbent in a NaNO3 solution with a supporting electrolyte. A suspension is titrated with HNO3 and the other with NaOH. Then, after waiting 10 min for pH equilibration, titrations are carried out over a pH range of 3 to 11 to avoid solid solubilization. The pH value is checked before and during the titration, noting the volume of the titrant solution added for each pH change until it remains constant. Then, the solid surface charge as a function of pH is calculated and plotted (Davranche et al. 2003).
-
(c).
Mass titration method: The experiments are carried out under N2 atmosphere with constant ionic strength and different temperatures. Aqueous suspensions containing different weights of adsorbent are equilibrated for 24 h, and then the pH of each suspension is measured. The pHPZC is determined by the appearance of a plateau in the pH versus the mass of the adsorbent curve (Noh and Schwarz 1989).
it may be interesting to compare and validate methods and specific conditions used in the adsorption tests to choose the most suitable method to determine the pHPZC,. In this context, Mahmood et al. (2011) compared the methods described for Nickel oxide (NiO) pHPZC determination, obtaining values of 8.25, 8.53, and 8.45, respectively, by potentiometric titration, mass titration, and salt addition. The authors emphasize that the minor differences in values between the three methods decrease with increasing temperature, indicating the validity of both methodologies.
Elemental analysis
Elemental analysis or organic elemental analysis is a technique that allows determining the proximate composition of the elements carbon (C), hydrogen (H), nitrogen (N), sulfur (S), and oxygen (O) present in a sample (Silva et al. 2013, 2018). The technique can be used on various samples, including solid, liquid, volatile, and viscous (Thompson 2008).
The technique is based on the Pregl-Dumas method, which consists of the complete combustion of the sample at a high temperature, around 1000 °C, in an oxygen-rich environment to convert the elements into CO2, H2O, NOx, and SOx (Thompson 2008; Silva et al. 2013). Briefly, the gases formed are homogenized and carried by an inert, high-purity gas through a separation column, where they are quantified using a thermal conductivity or infrared radiation detector (Ma and Gutterson 1974). However, the detection system inside the analyzer can occur differently, depending on the combustion mode and sample size (Thompson 2008). Finally, the quantification of the elements occurs directly for C, H, and N, and the O content is obtained by difference (% O = 100% − % C − % H − % N) (Silva et al. 2018).
The elemental analysis aims to demonstrate the identity of the material through the proximate composition of the atoms of the molecule and by confirming the chemical structure, supporting qualitative determination (Thompson 2008; Valentini et al. 2004). Besides, it is possible to evaluate the purity of the material according to the quantitative determination performed through the mass balance resulting from the elemental analysis of the sample (Thompson 2008). Therefore, analyzing the proximate composition, it can be considered that the H/C ratio represents the aromaticity index, and the O/C ratio estimates the abundance of oxygen in the functional groups. The C/N ratio indicates the degree of nitrogen incorporation in the SH, humidification, and recalcitrant behavior (Silva et al. 2013).
According to the elemental analysis, the proximate composition nanocellulose adsorbents were synthesized with micro fibrillated cellulose (MFC) and modified with the aminosilane (APS/MFC) and the carbonated (CHA/MFC). A high amount of C and O and a low amount of H were identified in all materials analyzed. However, the presence of N was quantified only for APS/MFC, which can be indicated by the introduction of amine groups on the material’s surface. The biggest difference in elemental content was observed when comparing MFC to CHA/MFC. The carbon level was lower after modification, and the hydrogen and oxygen levels were higher due to the greater amount of calcium caused by the modification. Thus, it is related that lower concentrations of C and higher concentrations of Ca and P suggest that MFC can bind a much larger amount of CHA than its mass (Hokkanen and Sillanpää 2020). Therefore, from the elemental analysis, it was possible to identify the occurrence of changes in the chemical structure of the original material compared to the modified one, verifying the influence of the addition of each material in the proximate quantification of each element for each application.
Comparing the elemental composition obtained from the original lignite and after the water washing process, a loss of around 10% was estimated, reaching 35% of the original material. The washing process was carried out to separate and purify the original material from the recovery of the HA fraction (organic material) to form LiHA. The H/C and O/C ratios obtained from the analysis for Lignite presented values following the literature presented. Furthermore, during the alkaline extraction of the material for isolation of the LiHA fraction, a greater relative abundance of aromatics condensed in the LiHA fraction as the resulting value of the H/C ratio decreased. The O/C ratio increased possibly due to the transformation of some O groups, originally present in lignite, under alkaline conditions into hydrolyzed functional groups –COOH. The C/N ratio indicates that some structural-compositional changes occur between the nitrogen-containing subunits during the extraction procedure, but with few changes in the LiHA fraction purification process (Peuravuori et al. 2006). In summary, elemental analysis is applied to evaluate the relationship of the original material concerning the isolated fraction considering structural changes that occur during the separation and purification operations.
Proximate analysis
The proximate analysis determines the presence of different compounds and their amounts in the material of interest (Speight 2015). This analysis is performed using prescribed or standard methods, such as the American Society for Testing and Materials (Asadu et al. 2021; Ogbodo et al. 2021; Ounphikul et al. 2021). In the case of composite adsorbents, generally, the compounds measured are moisture, ash, volatile and fixed carbon, as they can affect the adsorption properties. Interferences occur mainly in adsorbents that have materials originating from thermal processes in their composition, such as charcoal (Asadu et al. 2021; Ogbodo et al. 2021).
Moisture is the amount of water contained in the material and is determined by the weight loss when the sample is oven-dried to a constant weight (Speight 2015). Adsorbent materials with high moisture contents may have a lower adsorption capacity due to blocking active sites (Ounphikul et al. 2021).
Ashes are residues that remain after burning a material, composed mainly of minerals (silica, aluminum, iron, magnesium, and calcium), oxides, and sulfates (Ahmedna et al. 2000). Ashes are often considered impurities in materials obtained by thermal processes, which can affect the pH of the material, promote repulsion to other compounds or other adverse reactions, consequently influencing the application of interest (Ahmedna et al. 2000). Furthermore, in the adsorption process, the lower the ash content, the more active the adsorbents, consequently the better the adsorption (Ahmedna et al. 2000).
Volatile matter consists of the gases and vapors (except moisture vapor) generated during material burning under strictly controlled conditions. Fixed carbon is the non-volatile solid fraction of the material (Speight 2015). Therefore, high fixed carbon content materials generally have promising adsorption properties (Asadu et al. 2021; Ogbodo et al. 2021).
There are several studies on coal production from different sources. These sources are usually composed of moisture, ash, and organic matter. For example, Asadu et al. (2021) developed a composite of coconut husks through the carbonization process followed by esterification for crude oil adsorption. The authors found that the percentage of volatile matter in the base material decreased after each treatment step (41.2 to 14.7%) and the moisture content (14.2 to 1.03%). At the same time, fixed carbon increased significantly from 39.3 to 79.6%. In crude oil adsorption experiments, the composite showed a maximum capacity of 56.322 mg/g by the Langmuir model at 343 K, demonstrating promising properties.
Ogbodo et al. (2021) also used the proximate analysis to characterize the composite adsorbents obtained from carbonized and acetylated raw banana peels. After the treatments, the volatile carbon, ash, and moisture contents decreased from 41.1 to 17.8%, 8.0 to 5.7%, and 15.8 to 2.9%, respectively. The fixed carbon content increased from 35.2 to 73.8%, showing potential characteristics for adsorption. Ounphikul et al. (2021) developed mesoporous carbon–silica composite adsorbents from molasses and silicates that presented moderate moisture contents (12.33 to 14.03% by weight) and high ash contents (42.91 to 73.48% by weight). The proximate analysis supported the authors in verifying the presence of silica and its proportion increase in the composite by the ash content.
Swelling and water retention capacities
For specific adsorbents such as spheres/beads or hydrogels, swelling can vary from twice to more than a thousand times its weight and depends on the type of crosslinker, the degree of crosslinking, and hydrophilicity of the gel. Therefore, adsorbents’ swelling behavior and structural stability are critical to their practical use in water treatment (Saber-Samandari et al. 2017; Saraydın et al. 2018). For example, the amount of adsorbent to be applied for filling fixed-bed columns in continuous processes, considering their swelling and water retention capacity.
The percentage of swelling and water retention of the adsorbents is evaluated by gravimetric analysis (Saber-Samandari et al. 2017). First, adsorbents are immersed in water for 24 h to obtain the dilation equilibrium. Then, consecutive measurements are carried out at 2 h intervals to verify the stability of the weight of the beads. Finally, after removing excess water on the surface of the swollen and balanced beads, the percentage of swelling is calculated (Eq. 7).
where: = W2 and W1 are the weights of the swollen and dried adsorbents, respectively.
Subsequently, the water retention capacity of the adsorbents is determined; for that, the swollen and balanced adsorbents are weighed and dried at 60 °C. The weight of the dry adsorbents is measured regularly for 24 h. Finally, the water retention capacity is calculated from Eq. 8.
where W2 and W3 are the weights of the swollen and deswollen adsorbents, respectively.
Rigueto et al. (2021c) reported that their beads from commercial and recovered gelatin added from carbon nanotubes (CNTs) were able to swell slightly more than twice compared to their initial weight (263.72 and 276.33%, respectively). Furthermore, the authors found that the addition of CNTs increased the water retention capacity, which is related to different types of water interactions since water can be bonded or semi-bonded to the hydrophilic groups of the CNTs, forging bridges of hydrogen. Similar behavior was described by Saber-Samandari et al. (2017). Also, studies have reported an increasing linear correlation between swelling values and dye adsorption (Saraydın et al. 2018; Shukla et al. 2012).
Desorption and reuse
In addition to producing composite adsorbents with improved characteristics, the possibility of reusing materials produced for more cycles has also been an objective made possible in recent studies since the disposal of adsorbents is a problem that encompasses economic and environmental aspects. Therefore, a longer life cycle of composites can reduce the need to produce new adsorbents (Kulkarni and Kaware 2014).
The technique used for desorption of the studied adsorbate must be defined to perform the regeneration tests, which used an eluent solution in most reported studies. Subsequently, tests of the adsorption and desorption cycles are performed, where data can be plotted as a function of adsorption capacity (mg g−1) or removal (%).Eqs. 9 and 10 are used to determinate the adsorption capacity and removal percentage, respectively.
where: q is the amount of contaminant adsorbed per gram of adsorbent in the cycle (mg g−1), C0 and C are the concentrations of the initial and final aqueous solutions, respectively (mg L−1), V is the volume of the solution (L), and m is the weight of the adsorbent (g).
where C0 is the initial concentration (mg L−1), and Cads is the final concentration after the adsorption cycle (mg L−1).
The desorption/regeneration analysis is performed to complete the first cycle. First, the adsorbent containing the adsorbed contaminant is placed in contact with the eluent solution under defined conditions, and after determining the concentrations, the regeneration is calculated, according to Eq. 11.
where mdes is the amount of contaminant adsorbed in the desorption cycle (mg), and mads is the amount of contaminant adsorbed in the adsorption cycle (mg).
Table 3 presents recent studies reporting composite adsorbent materials, including the adsorbate, eluent used in desorption, adsorption/desorption cycles feasible for use, and adsorption efficiencies.
According to Table 3, most articles report the possibility of recycling the materials produced in up to 10 cycles. The eluents used vary according to the contaminant to be desorbed. The regeneration method’s selection depends on this operation’s purpose (Shah et al. 2013). For example, suppose the recovery of adsorbate is desired. In that case, physical, thermal, or pressure methods are more suitable, and if this is not desired, then regeneration oxidative or chemical may be used. On an industrial scale, steam generation can be used for the desorption and regeneration of adsorbents. However, if the adsorbate has hydrophilic characteristics, it must be recovered by separation techniques such as distillation. On the other hand, hydrophobic compounds can be separated from condensed water using gravity alone, thus increasing the possibilities and benefits of steam generation in industries (Shah et al. 2013).
Cuccarese et al. (2021) employed thermal recovery compared to solvent washing for the desorption of diclofenac from expanded thermoplastic graphite. The authors reported that after 4 cycles, the thermal desorption reached about 100% efficiency since the adsorbed diclofenac is released by heating the entire material. In addition, all sites available for adsorption interact with other molecules in a new cycle. Another important factor related to material reuse is how it is separated at the end of the process. Thus, metal compounds, such as Fe3O4 have been added to the composite matrix in preparing composite adsorbents, favoring material separation by applying an external magnetic field (Tam et al. 2020).
In general, the reuse of adsorbents is a current and emerging need. Therefore, advances in technologies that allow the achievement of composite adsorbents with improved characteristics and extended life cycle can contribute to obtaining more sustainable adsorbents to remove several contaminants present in water and wastewater, aiming to provide more effective and eco-friendly treatments.
Conclusions and remarks
This paper reviewed the main methods used for composite adsorbents characterization, such as X-ray diffraction, spectroscopy, scanning electron microscopy, N2 adsorption–desorption isotherms (BET and BJH), thermogravimetry, point of zero charge, elemental analysis, proximate analysis, swelling and water retention capacities, desorption, and reuse.
These techniques present valuable information about composite adsorbents, including crystalline structure (X-ray diffraction), the composition of materials and structural change according to production processes (spectroscopy), surface morphology (scanning electron microscopy), surface area and pore size distribution (BET and BJH), weight loss of material over temperature (thermogravimetry), superficial charge (point of zero charge), the proximate composition of C, H, N, S, and O (elemental analysis), determination of moisture, ashes, volatile and fixed carbon (proximate analysis), additional analysis aimed at practical applications (swelling and water retention capacities), and life cycle (desorption and reuse).
The revised methods are essential for the characterization of composite adsorbents to determine the best production process, its experimental conditions, the used additives and their proportions to be added (in the case of composites), making it possible to evaluate such influences on the characteristics of the adsorbents, such as the ability to adsorption, kinetics, strength or integrity of the material and its reuse potential. Furthermore, these techniques can be useful to explain the mechanisms involved in adsorption processes, which may vary according to the chemical structure of the contaminant of interest. Finally, the characterization methods described can be considered a “right-hand” to obtain potential adsorbent materials applicable for removing several emerging contaminants from aqueous matrices.
Finally, we point out that the sustainable and environment-friendly appeal, it is highly recommended that studies on the development and application of adsorbents include the adsorption/desorption cycle and how to properly dispose the composite adsorbents after the end of their life cycle (containing pollutants in their composition). In addition, life cycle assessments can collaborate to prove the feasibility of these new materials for industrial scale applications.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmadian M, Anbia M, Rezaie M (2020) Sulfur dioxide removal from flue gas by supported CuO nanoparticle adsorbents. Ind Eng Chem Res 59(50):21642–21653. https://doi.org/10.1021/acs.iecr.0c05629
Ahmedna M, Marshall WE, Rao RM (2000) Granular activated carbons from agricultural by-products: preparation, properties, and application in cane sugar refining. LSU Agricultural Experiment Station Reports 456. http://digitalcommons.lsu.edu/agexp/456
Alver E, Metin AÜ, Brouers F (2020) Methylene blue adsorption on magnetic alginate/rice husk bio-composite. Int J Biol Macromol 154:103–113. https://doi.org/10.1016/j.ijbiomac.2020.02.330
Amenabar I, Poly S, Goikoetxea M, Nuansing W, Lasch P, Hillenbrand R (2017) Hyperspectral infrared nanoimaging of organic samples based on Fourier transform infrared nanospectroscopy. Nat Commun 8:14402. https://doi.org/10.1038/ncomms14402
Arumugam TK, Krishnamoorthy P, Rajagopalan NR, Nanthini S, Vasudevan D (2019) Removal of malachite green from aqueous solutions using a modified chitosan composite. Int J Biol Macromol 128:655–664. https://doi.org/10.1016/j.ijbiomac.2019.01.185
Asadu CO, Anthony EC, Elijah OC, Ike IS, Onoghwarite OE, Okwudili UE (2021) Development of an adsorbent for the remediation of crude oil polluted water using stearic acid grafted coconut husk (Cocos nucifera) composite. Appl Surf Sci 6:100179. https://doi.org/10.1016/j.apsadv.2021.100179
Ashiq A, Sarkar B, Adassooriya N, Walpita J, Rajapaksha AU, Ok YS, Vithanage M (2019) Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media. Chemosphere 236:124384. https://doi.org/10.1016/j.chemosphere.2019.124384
Askalany AA, Henninger SK, Ghazy M, Saha BB (2017) Effect of improving thermal conductivity of the adsorbent on performance of adsorption cooling system. Appl Therm Eng 110:695–702. https://doi.org/10.1016/j.applthermaleng.2016.08.075
Bardestani R, Patience GS, Kaliaguine S (2019) Experimental methods in chemical engineering: specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can J Chem Eng 97(11):2781–2791. https://doi.org/10.1002/cjce.23632
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73(1):373–380
Benhalima T, Ferfera-Harrar H (2019) Eco-friendly porous carboxymethyl cellulose/dextran sulfate composite beads as reusable and efficient adsorbents of cationic dye methylene blue. Int J Biol Macromol 132:126–141. https://doi.org/10.1016/j.ijbiomac.2019.03.164
Bergaya F, Lagaly G (2013) Handbook of clay science, vol 5, 2nd edn. Hardcover
Bish DL, Reynolds RC (1989) Sample preparation for x-ray diffraction. Modern Powder Diffr 73–100. https://doi.org/10.1515/9781501509018-007
De Blasio C (2019) Thermogravimetric Analysis (TGA). In: De Blasio C (Ed) Fundamentals of biofuels engineering and technology, Green Energy and Technology, pp 91–102. https://doi.org/10.1007/978-3-030-11599-9_7
Bottom R (2008) Thermogravimetric Analysis. In: Gabbott P (ed) Principles and applications of thermal analysis, Blackwell Publishing Ltd, pp. 88–118. https://doi.org/10.1002/9780470697702.ch3
Brown ME (1979) Determination of purity by differential scanning calorimetry (DSC). J Chem Educ 56(5):310. https://doi.org/10.1021/ed056p310
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60(2):309–319. https://doi.org/10.1021/ja01269a023
Bunaciu AA, UdriŞTioiu EG, Aboul-Enein HY (2015) X-ray diffraction: instrumentation and applications. Crit Rev Anal Chem 45(4):289–299. https://doi.org/10.1080/10408347.2014.949616
Chauhan M, Saini VK, Suthar S (2020) Ti-pillared montmorillonite clay for adsorptive removal of amoxicillin, imipramine, diclofenac-sodium, and paracetamol from water. J Hazard Mater 399:122832. https://doi.org/10.1016/j.jhazmat.2020.122832
Chen Y, Ma Y, Lu W, Guo Y, Zhu Y, Lu H, Song Y (2018) Environmentally friendly gelatin/β-cyclodextrin composite fiber adsorbents for the efficient removal of dyes from wastewater. Molecules 23(10):1–17. https://doi.org/10.3390/2Fmolecules23102473
Chen H, Zheng K, Zhu A, Meng Z, Li W, Qin C (2020) Preparation of bentonite/chitosan composite for bleaching of deteriorating transformer oil. Polymers 12(1):60. https://doi.org/10.3390/polym12010111060
Cuccarese M, Brutti S, De Bonis A, Teghil R, Mancini IM, Masi S, Caniani D (2021) Removal of diclofenac from aqueous solutions by adsorption on thermo-plasma expanded graphite. Sci Rep 11(1):1–15. https://doi.org/10.1038/s41598-021-83117-z
Davranche M, Lacour S, Bordas F, Bollinger JC (2003) An easy determination of the surface chemical properties of simple and natural solids. J Chem Educ 80(1):76. https://doi.org/10.1021/ed080p76
De Leon A, Tiu B, Mangadlao J, Pangilinan K, Cao P, Advincula R (2014) Applications of Fourier transform infrared (FTIR) imaging. In: Gauglitz G, Moore DS (eds) Handbook of Spectroscopy. Wiley Online Library Hoboken, Weinheim, pp 1179–1200. https://doi.org/10.1002/9783527654703.ch31
De Rossi A, Rigueto CVT, Dettmer A, Colla LM, Piccin JS (2020) Synthesis, characterization, and application of Saccharomyces cerevisiae/alginate composites beads for adsorption of heavy metals. J Environ Chem Eng 8(4):104009. https://doi.org/10.1016/j.jece.2020.104009
Dotto GL, McKay G (2020) Current scenario and challenges in adsorption for water treatment. J Environ Chem Eng 8:103988. https://doi.org/10.1016/j.jece.2020.103988
Ebele AJ, Oluseyi T, Drage DS, Harrad S, Abdallah MAE (2020) Occurrence, seasonal variation and human exposure to pharmaceuticals and personal care products in surface water, groundwater and drinking water in Lagos State, Nigeria. Emerg Contam 6:124–132. https://doi.org/10.1016/j.emcon.2020.02.004
Edathil AA, Kannan P, Banat F (2020) Adsorptive oxidation of sulfides catalysed by δ-MnO2 decorated porous graphitic carbon composite. Environ Pollut 266:115218. https://doi.org/10.1016/j.envpol.2020.115218
El-Kousy SM, El-Shorbagy HG, El-Ghaffar MAA (2020) Chitosan/montmorillonite composites for fast removal of methylene blue from aqueous solutions. Mater Chem Phys 254:123236. https://doi.org/10.1016/j.matchemphys.2020.123236
Eltaweil AS, Elgarhy GS, El-Subruiti GM, Omer AM (2020) Carboxymethyl cellulose/carboxylated graphene oxide composite microbeads for efficient adsorption of cationic methylene blue dye. Int J Biol Macromol 154:307–318. https://doi.org/10.1016/j.ijbiomac.2020.03.122
Faraj SS, Alkizwini RS, Al Juboury MF (2020) Simulate permeable reactive barrier by using a COMSOL model and comparison with the Thomas, Yoon-Nelson and Clark models for CR dye remediation by composite adsorbent (sewage and waterworks sludge). Water Sci Technol 82(12):2902–2919. https://doi.org/10.2166/wst.2020.500
Farhadi S, Sohrabi MR, Motiee F, Davallo M (2021) Organophosphorus Diazinon pesticide removing from aqueous solution by zero-valent iron supported on biopolymer chitosan: RSM optimization methodology. J Polym Environ 29(1):103–120. https://doi.org/10.1007/s10924-020-01855-z
Fatma S, Asif N, Ahmad R, Fatma T (2020) Toxicity of NSAID drug (paracetamol) to nontarget organism-Nostoc muscorum. Environ Sci Pollut Res 27:35208–35216. https://doi.org/10.1007/s11356-020-09802-0
Fissaha HT, Torrejos REC, Kim H, Chung WJ, Nisola GM (2020) Thia-crown ether functionalized mesoporous silica (SBA-15) adsorbent for selective recovery of gold (Au+3) ions from electronic waste leachate. Microporous Mesoporous Mater 305:110301. https://doi.org/10.1016/j.micromeso.2020.110301
Gao Z, Ma W, Huang S, Hua P, Lan C (2019) Deep learning for super-resolution in a field emission scanning electron microscope. Ai 1(1):1–9. https://doi.org/10.3390/ai1010001
Gauglitz G, Vo-Dihn T (2003) Handbook of spectroscop. Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim. https://doi.org/10.1002/3527602305
Gedam AH, Dongre RS (2016) Activated carbon from Luffa cylindrica doped chitosan for mitigation of lead (II) from an aqueous solution. RSC Adv 6(27):22639–22652. https://doi.org/10.1039/c5ra22580a
Germer TA, Zwinkels JC, Tsai BK (2014) Introduction. In: Germer TA, Zwinkels JC, Tsai BK (Ed). Experimental methods in the physical sciences, Elsevier, pp 1–9. https://doi.org/10.1016/B978-0-12-386022-4.00001-7
Gharanjig H, Gharanjig K, Hosseinnezhad M, Jafari SM (2020) Differential scanning calorimetry (DSC) of nanoencapsulated food ingredients. Characterization of Nanoencapsulated Food Ingredients 295–346. https://doi.org/10.1016/b978-0-12-815667-4.00010-9.
Guillossou R, Roux JL, Goffin A, Mailler R, Varrault G, Vulliet E, Morlay C, Nauleau F, Guérin S, Rocher V, Gaspéri J (2021) Fluorescence excitation/emission matrices as a tool to monitor the removal of organic micropollutants from wastewater effluents by adsorption onto activated carbon. Water Res 190:116749. https://doi.org/10.1016/j.watres.2020.116749
Haas J, Mizaikoff B (2016) Advances in mid-infrared spectroscopy for chemical analysis. Annu Rev Anal Chem 9:45–68. https://doi.org/10.1146/annurev-anchem-071015-041507
Hokkanen S, Sillanpää M (2020) Nano- and microcellulose-based adsorption materials in water treatment Adv Water Treat 1–83. https://doi.org/10.1016/b978-0-12-819216-0.00001-1
Hoseinzadeh H, Hayati B, Ghaheh FS, Seifpanahi-Shabani K, Mahmoodi NM (2021) Development of room temperature synthesized and functionalized metal-organic framework/graphene oxide composite and pollutant adsorption ability. Mater Res Bull 142:111408. https://doi.org/10.1016/j.materresbull.2021.111408
Inkson BJ (2016) Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) for materials characterization. Mater Characterization Using Nondestr Eval (NDE) Methods 17–43. https://doi.org/10.1016/B978-0-08-100040-3.00002-X
Işık B, Kurtoğlu AE, Gürdağ G, Keçeli G (2021) Radioactive cesium ion removal from wastewater using polymer metal oxide composites J Hazard Mater 403. https://doi.org/10.1016/j.jhazmat.2020.123652
Işık B, Uğraşkan V (2021) Adsorption of methylene blue on sodium alginate–flax seed ash beads: Isotherm, kinetic and thermodynamic studies. Int J Biol Macromol 167:1156–1167. https://doi.org/10.1016/j.ijbiomac.2020.11.070
Jawad AH, Abdulhameed AS, Abdallah R, Yaseen ZM (2020) Zwitterion composite chitosan-epichlorohydrin/zeolite for adsorption of methylene blue and reactive red 120 dyes. Int J Biol Macromol 163:756–765. https://doi.org/10.1016/j.ijbiomac.2020.07.014
Kaliva M, Vamvakaki M (2020) Nanomaterials characterization. In: Kaliva M, Vamvakaki M (Ed.), Polymer Science and Nanotechnology, Elsevier, pp 401–433. https://doi.org/10.1016/B978-0-12-816806-6.00017-0
Karimi-Maleh H, Ayati A, Davoodi R, Tanhaei B, Karimi F, Malekmohammadi S, Orooji Y, Fu L, Sillanpää M (2021) Recent advances in using of chitosan-based adsorbents for removal of pharmaceutical contaminants: A review J Clean Prod 125880. https://doi.org/10.1016/j.jclepro.2021.125880
Kasap S, Nostar AE, Oztürk I (2019) Investigation of MnO2 nanoparticles-anchored 3D-graphene foam composites (3DGF-MnO2) as an adsorbent for strontium using the central composite design (CCD) method. New J Chem 43(7):2981–2989. https://doi.org/10.1039/c8nj05283b
Khan MN, Sarwar A (2007) Determination of points of zero charge of natural and treated adsorbents. Surf Rev Lett 14:461–469. https://doi.org/10.1142/S0218625X07009517
Khan SB, Ali F, Kamal T, Anwar Y, Asiri AM, Seo J (2016) CuO embedded chitosan spheres as antibacterial adsorbent for dyes. Int J Biol Macromol 88:113–119. https://doi.org/10.1016/j.ijbiomac.2016.03.026
Kim J, Lee K, Seo BK, Hyun JH (2020) Effective removal of radioactive cesium from contaminated water by synthesized composite adsorbent and its thermal treatment for enhanced storage stability. Environ Res 191:110099. https://doi.org/10.1016/j.envres.2020.110099
Kohli R, Mittal KL (2019) Methods for Assessment and Verification of Cleanliness of Surfaces and Characterization of Surface Contaminants. In: Kohli R, Mittal KL, Developments in Surface Contamination and Cleaning, v. 12, Elsevier. https://doi.org/10.1016/C2017-0-03847-8
Kowalczuk D, Pitucha M (2019) Application of FTIR method for the assessment of immobilization of active substances in the matrix of biomedical materials. Mater 12:2972. https://doi.org/10.3390/2Fma12182972
Kulkarni S, Kaware J (2014) Regeneration and recovery in adsorption-a review. Int J Innov Sci Eng Technol 1:61–65
Ladu F, Mwaffo V, Li J, Macrì S, Porfiri M (2015) Acute caffeine administration affects zebrafish response to a robotic stimulus. Behav Brain Res 289:48–54. https://doi.org/10.1016/j.bbr.2015.04.020
Lessa EF, Nunes ML, Fajardo AR (2018) Chitosan/waste coffee-grounds composite: an efficient and eco-friendly adsorbent for removal of pharmaceutical contaminants from water. Carbohydr Polym 189:257–266. https://doi.org/10.1016/j.carbpol.2018.02.018
Lowell S, Shields JE (2013) Powder surface area and porosity (Vol. 2). Springer Science & Business Media
Lütke SF, Oliveira MLS, Silva LFO, Cadaval TRS Jr, Dotto GL (2020) Nanominerals assemblages and hazardous elements assessment in phosphogypsum from an abandoned phosphate fertilizer industry. Chemosphere 256:127138. https://doi.org/10.1016/j.chemosphere.2020.127138
Ma TS, Gutterson M (1974) Organic elemental analysis. Anal Chem 46(5):437–451. https://doi.org/10.1021/ac60341a032
Machado LDB, Matos JR (2004) Differential thermal analysis and differential scanning calorimetry. In: Canevarolo Junior SV (ed) Polymer characterization techniques, Artliber, pp 229–261
Mahboub MJD, Dubois JL, Cavani F, Rostamizadeh M, Patience GS (2018) Catalysis for the synthesis of methacrylic acid and methyl methacrylate. Chem Soc Rev 47(20):7703–7738. https://doi.org/10.1039/C8CS00117K
Mahmood T, Saddique MT, Naeem A, Westerhoff P, Mustafa S, Alum A (2011) Comparison of different methods for the point of zero charge determination of NiO. Ind Eng Chem Res 50(17):10017–10023. https://doi.org/10.1021/ie200271d
Malek MA, Nakazawa T, Kang HW, Tsuji K, Ro CU (2019) Multi-modal compositional analysis of layered paint chips of automobiles by the combined application of ATR-FTIR imaging, Raman Microspectrometry, and SEM/EDX. Molecules 24:1381. https://doi.org/10.3390/molecules24071381
Manyangadze M, Chikuruwo NHM, Chakra CS, Narsaiah TB, Radhakumari M, Danha G (2020) Enhancing adsorption capacity of nano-adsorbents via surface modification: A review. S Afr J Chem Eng 31(1):25–32. https://doi.org/10.1016/j.sajce.2019.11.003
Melara F, Machado TS, Alessandretti I, Manera C, Perondi D, Godinho M, Piccin JS (2021) Synergistic effect of the activated carbon addition from leather wastes in chitosan/alginate-based composites. Environ Sci Pollut Res 28:48666–48680. https://doi.org/10.1007/s11356-021-14150-8
Mendham J, Denney RC, Barnes JD, Thomas MJK (2000) Vogel’s quantitative chemical analysis, 6th edn. Prentice Hall, New York
Meng F, Schricker S, Brantley W, Mendel D, Rashid R, Fields H Jr, Vig K, Alapati S (2007) Differential scanning calorimetry (DSC) and temperature-modulated DSC study of three mouthguard materials. Dent Mater 23(12):1492–1499. https://doi.org/10.1016/j.dental.2007.01.006
Morhardt C, Ketterer B, Heißler S, Franzreb M (2014) Direct quantification of immobilized enzymes by means of FTIR ATR spectroscopy – A process analytics tool for biotransformations applying non-porous magnetic enzyme carriers. J Mol Catal b: Enzym 107:55–63. https://doi.org/10.1016/j.molcatb.2014.05.018
Nabers A, Ollesch J, Schartner J, Kötting C, Genius J, Haußmann U, Klafki H, Wiltfang J, Gerwert K (2015) An infrared sensor analyzing label-free the secondary structure of the Abeta peptide in presence of complex fluids. J Biophotonics 9:224–234. https://doi.org/10.1002/jbio.201400145
Naderi M (2015) Surface Area: Brunauer-Emmett-Teller (BET). Progr Filtr Sep 585–608. https://doi.org/10.1016/B978-0-12-384746-1.00014-8
Nagpal M, Kakkar R (2020) Facile synthesis of mesoporous magnesium oxide–graphene oxide composite for efficient and highly selective adsorption of hazardous anionic dyes. Res Chem Intermed 46(5):2497–2521. https://doi.org/10.1007/s11164-020-04103-0
Newbury DE, Ritchie NWM (2013) Is scanning electron microscopy/energy dispersive X-ray spectrometry (SEM/EDS) quantitative? Scanning 35(3):141–168. https://doi.org/10.1002/sca.21041
Nguyen DT, Tran HN, Juang RS, Dat ND, Tomul F, Ivanets A, Woo SH, Hosseini-Bandegharaei A, Nguyen VP, Chao HP (2020) Adsorption process and mechanism of acetaminophen onto commercial activated carbon. J Environ Chem Eng 8(6):104408. https://doi.org/10.1016/j.jece.2020.104408
Ngwenya S, Guyo U, Zinyama NP, Chigondo F, Nyamunda BC, Muchanyereyi N (2019) Response surface methodology for optimization of Cd (II) adsorption from wastewaters by fabricated tartaric acid-maize tassel magnetic hybrid sorbent. Biointerface Res Appl Chem 9(4):3996–4005. https://doi.org/10.33263/BRIAC94.996005
Nieto E, Corada-Fernández C, Hampel M, Lara-Martín PA, Sánchez-Argüello P, Blasco J (2017) Effects of exposure to pharmaceuticals (diclofenac and carbamazepine) spiked sediments in the midge, Chironomus riparius (Diptera, Chironomidae). Sci Total Environ 609:715–723. https://doi.org/10.1016/j.scitotenv.2017.07.171
Noh JS, Schwarz JA (1989) Estimation of the point of zero charge of simple oxides by mass titration. J Colloid Interface Sci 130(1):157–164. https://doi.org/10.1016/0021-9797(89)90086-6
Ogbodo NO, Asadu CO, Ezema CA, Onoh MI, Elijah OC, Ike IS, Onoghwarite OE (2021) Preparation and characterization of activated carbon from agricultural waste (Musa-paradisiaca peels) for the remediation of crude oil contaminated water. J Hazard Mater Adv 2:100010. https://doi.org/10.1016/j.hazadv.2021.100010
Osayi JI, Iyuke S, Ogbeide SE (2014) Biocrude production through pyrolysis of used tyres. J Catal 2014:386371. https://doi.org/10.1155/2014/386371
Oshagbemia AA, Autaa M, Kovoa AS, Yahyaa MD, Abdulkareema AS, Ibrahima MA, Obayomib KS (2020) Synthesized chitosan–zeolite composite matrix for the adsorption of chromium (VI) ions. Desalin Water Treat 195:377–388. https://doi.org/10.5004/dwt.2020.25885
Ounphikul B, Chantarasombat N, Hunt AJ, Ngernyen Y (2021) A new low-cost carbon–silica composite adsorbent from a by-product of the sugar industry. Mater Today: Proc 54(5):1884–1887. https://doi.org/10.1016/j.matpr.2021.10.113
Patel JP, Parsania PH (2018) Characterization, testing, and reinforcing materials of biodegradable composites Biodegrad Biocompat Polym Compos 55–79. https://doi.org/10.1016/B978-0-08-100970-3.00003-1
Pavia DL, Lampman GM, Kriz DS, Vyvyan JR (2009) introduction to Spectroscopy: A guide for students of organic chemistry, 4th edn. Thomson Learning Academic Resource Center, Belmont
Peuravuori J, Žbánková P, Pihlaja K (2006) Aspects of structural features in lignite and lignite humic acids. Fuel Process Technol 87(9):829–839. https://doi.org/10.1016/j.fuproc.2006.05.003
Poiana MA, Alexa E, Munteanu MF, Gligor R, Moigradean D, Mateescu C (2015) Use of ATR-FTIR spectroscopy to detect the changes in extra virgin olive oil by adulteration with soybean oil and high temperature heat treatment. Open Chem 13:689–698. https://doi.org/10.1515/chem-2015-0110
Pourebrahimi S, Kazemeini M, Ganji BE, Taheri A (2015) Removal of the CO2 from flue gas utilizing hybrid composite adsorbent MIL-53(Al)/GNP metal-organic framework. Microporous Mesoporous Mater 218:144–152. https://doi.org/10.1016/j.micromeso.2015.07.013
Prime RB, Bair HE, Vyazovkin S, Gallagher PK, Riga A (2009) Thermogravimetric Analysis (TGA). In: Menczel JD, Prime RB (eds) Thermal Analysis of Polymers: Fundamentals and Applications. John Wiley & Sons, Hoboken, pp 241–317. https://doi.org/10.1002/9780470423837.ch3
Pryia SAK, Kaith BS, Tanwar V, Bhatia JK, Sharma N, Bajaj S, Panchal S (2019) RSM-CCD optimized sodium alginate/gelatin based ZnS-nanocomposite hydrogel for the effective removal of biebrich scarlet and crystal violet dyes. Int J Biol Macromol 129:214–226. https://doi.org/10.1016/j.ijbiomac.2019.02.034
Rajeswari A, Christy EJS, Gopi S, Jayaraj K, Pius A (2020) Characterization studies of polymer-based composites related to functionalized filler-matrix interface. In: Goh KL, Aswathi MK, Silva RT, Thomas S (ed) Interfaces in Particle and Fibre Reinforced Composites, Elsevier, pp 219–250. https://doi.org/10.1016/B978-0-08-102665-6.00009-1
Rasoulzadeh H, Sheikhmohammadi A, Abtahi M, Roshan B (2021) Eco-friendly rapid removal of palladium from aqueous solutions using alginate-diatomite magnano composite. J Environ Chem Eng 9(5):105954. https://doi.org/10.1016/j.jece.2021.105954
Rastegar A, Farzadkia M, Jafari JA, Esrafili A (2019) Preparation of Carbon-Alumina (C/Al2O3) aerogel nanocomposite for benzene adsorption from flow gas in fixed bed reactor. MethodsX 6:2476–2483. https://doi.org/10.1016/j.mex.2019.10.019
Rigueto CVT, Nazari MT, Massuda LA, Ostwald BEP, Piccin JS, Dettmer A (2021a) Production and environmental applications of gelatin-based composite adsorbents for contaminants removal: a review. Environ Chem Lett 19:2465–2486. https://doi.org/10.1007/s10311-021-01184-0
Rigueto CVT, Nazari MT, Rosseto M, Massuda LA, Alessandretti I, Piccin JS, Dettmer A (2021b) Emerging contaminants adsorption by beads from chromium (III) tanned leather waste recovered gelatin. J Mol Liq 330:115638. https://doi.org/10.1016/j.molliq.2021.115638
Rigueto CVT, Rosseto M, Nazari MT, Ostwald BEP, Alessandretti I, Manera C, Piccin JS, Dettmer A (2021c) adsorption of diclofenac sodium by composite beads prepared from tannery wastes-derived gelatin and carbon nanotubes. J Environ Chem Eng 9(1):105030. https://doi.org/10.1016/j.jece.2021.105030
Rivera-Jaimes JA, Postigo C, Melgoza-Alemán RM, Aceña J, Barceló D, de Alda ML (2018) Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico: Occurrence and environmental risk assessment. Sci Total Environ 613:1263–1274. https://doi.org/10.1016/j.scitotenv.2017.09.134
Rodrigues A (2020) Chemical engineering and environmental challenges cyclic adsorption/reaction technologies: materials and process together! J Environ Chem Eng 8:103926. https://doi.org/10.1016/j.jece.2020.103926
Rouquerol J, Llewellyn P, Rouquerol F (2007) Is the BET equation applicable to microporous adsorbents? Stud Surf Sci Catal 160:49–56. https://doi.org/10.1016/s0167-2991(07)80008-5
Rytwo G, Zakai R, Wicklein B (2015) The use of ATR-FTIR spectroscopy for quantification of adsorbed compounds. J Spectrosc 727595. https://doi.org/10.1155/2015/727595
Saadatkhah N, Garcia AC, Ackermann S, Leclerc P, Latifi M, Samih S, Patience GS, Chaouki J (2019) Experimental methods in chemical engineering: thermogravimetric analysis-TGA. Can J Chem Eng 98:34–43. https://doi.org/10.1002/cjce.23673
Saber-Samandari S, Saber-Samandari S, Joneidi-Yekta H, Mohseni M (2017) Adsorption of anionic and cationic dyes from aqueous solution using gelatin-based magnetic nanocomposite beads comprising carboxylic acid functionalized carbon nanotube. Chem Eng J 308:1133–1144. https://doi.org/10.1016/j.cej.2016.10.017
Sanad MMS, Farahat MM, Abdel Khalek MA (2021) One-step processing of low-cost and superb natural magnetic adsorbent: kinetics and thermodynamics investigation for dye removal from textile wastewater. Adv Powder Technol 32:1573–1583. https://doi.org/10.1016/j.apt.2021.03.013
Saraydın D, Işıkver Y, Karadağ E (2018) A Study on the correlation between adsorption and swelling for poly (Hydroxamic Acid) hydrogels-triarylmethane dyes systems. J Polym Environ 26:3924–3936. https://doi.org/10.1007/s10924-018-1257-9
Scheverín N, Fossati A, Horst F, Lassalle V, Jacobo S (2020) Magnetic hybrid gels for emulsified oil adsorption: an overview of their potential to solve environmental problems associated to petroleum spills. Environ Sci Pollut Res 27:861–872. https://doi.org/10.1007/s11356-019-06752-0
Shah IK, Pre P, Alappat BJ (2013) Steam regeneration of adsorbents: an experimental and technical review. Chem Sci Trans 2:1078–1088. https://doi.org/10.7598/cst2013.545
Sharma M, Joshi M, Nigam S, Shree S, Avasthi DK, Adelung R, Srivastava SK, Mishra YK (2019) ZnO tetrapods and activated carbon based hybrid composite: adsorbents for enhanced decontamination of hexavalent chromium from aqueous solution. Chem Eng J 358:540–551. https://doi.org/10.1016/j.cej.2018.10.031
Shukla NB, Rattan S, Madras G (2012) Swelling and dye-adsorption characteristics of an amphoteric superabsorbent polymer. Ind Eng Chem Res 51:14941–14948. https://doi.org/10.1021/ie301839z
Silva AC, Silva VE, Silva BP, Camargo PB, Pereira RC, Barra UM, Botelho AMM, Torrado PV (2013) Lignocellulosic and isotopic composition of vegetation and soil organic matter in a tropical peatland. II - humic substances and humification processes. Rev Bras Cienc Solo 37:134–144. https://doi.org/10.1590/S0100-06832013000100014
Silva RR, Lucena GN, Machado AF, Freitas GA, Matos AT, Abrahão WAP (2018) Spectroscopic and elementar characterization of humic substances in organic substrates. Comun Sci 9:264–274. https://doi.org/10.14295/cs.v9i2.2734
Silva LFO, Pinto D, Neckel A, Dotto GL, Oliveira MLS (2020) The impact of air pollution on the rate of degradation of the fortress of Florianópolis Island, Brazil. Chemosphere 251:126838. https://doi.org/10.1016/j.chemosphere.2020.126838
Sing KSW (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57:603–619. https://doi.org/10.1351/pac198557040603
Sing K (2001) The use of nitrogen adsorption for the characterisation of porous materials. Colloids Surf A 187–188:3–9. https://doi.org/10.1016/S0927-7757(01)00612-4
Soliman EM, Ahmed SA, Fadl AA (2020) Microwave-enforced green synthesis of novel magnetic nano composite adsorbents based on functionalization of wood sawdust for fast removal of calcium hardness from water samples. Water Environ Res 92:2112–2128. https://doi.org/10.1002/wer.1383
Song T, Luo W, Mu J, Cai Y, Wei J, Li H (2019) Preparation of polyacrylic-acid/palygorskite composite particles via inverse-suspension polymerization for efficient separation of Ce3+ from aqueous solution. J Colloid Interface Sci 535:371–379. https://doi.org/10.1016/j.jcis.2018.09.103
Soni P, Sur A, Gaba VK (2022) Experimental Investigation of silica-gel based composite adsorbent for adsorption refrigeration system. Therm Sci Eng Prog 29:101194. https://doi.org/10.1016/j.tsep.2022.101194
Sousa JCG, Ribeiro AR, Barbosa MO, Pereira MFR, Silva AMT (2018) A review on environmental monitoring of water organic pollutants identified by EU guidelines. J Hazard Mater 334:146–162. https://doi.org/10.1016/j.jhazmat.2017.09.058
Speight JG (2015) Proximate Analysis. In: Speight JG (ed) Handbook of Coal Analisys, 2nd edn. Wiley, New York, pp 116–142
Spiridon I, Teaca CA, Bodirlau R (2011) Structural changes evidenced by FTIR spectroscopy in cellulosic materials after pre-treatment with ionic liquid and enzymatic hydrolysis. BioResources 6:400–413
Streit AFM, Collazzo GC, Druzian SP, Verdi RS, Foletto EL, Oliveira LFS, Dotto GL (2021) Adsorption of ibuprofen, ketoprofen, and paracetamol onto activated carbon prepared from effluent treatment plant sludge of the beverage industry. Chemosphere 262:128322. https://doi.org/10.1016/j.chemosphere.2020.128322
Stuart BH (2004) Infrared spectroscopy: fundamentals and applications. John Wiley & Sons, Hoboken
Tam NTM, Liu YG, Van Thom N (2020) Magnetic gelatin-activated biochar synthesis from agricultural biomass for the removal of sodium diclofenac from aqueous solution: adsorption performance and external influence. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1832483
Thirumoorthy K, Krishna SK (2020) Removal of cationic and anionic dyes from aqueous phase by Ball clay - Manganese dioxide nanocomposites. J Environ Chem Eng 8:103582. https://doi.org/10.1016/j.jece.2019.103582
Thompson M (2008) CHNS elemental analysers. Analytical methods committee technical briefs. The Royal Society of Chemistry, United Kingdown. https://www.rsc.org/images/CHNS-elemental-analysers-technical-brief-29_tcm18-214833.pdf
Tran S, Fulcher N, Nowicki M, Desai P, Tsang B, Facciol A, Chow H, Gerlai R (2017) Time-dependent interacting effects of caffeine, diazepam, and ethanol on zebrafish behaviour. Prog in Neuro-Psychopharmacol Biol Psychiatry 75:16–27. https://doi.org/10.1016/j.pnpbp.2016.12.004
Valand R, Tanna S, Lawson G, Bengtström L (2020) A review of Fourier Transform Infrared (FTIR) spectroscopy used in food adulteration and authenticity investigations. Food Addit Contam Part A 37:19–38. https://doi.org/10.1080/19440049.2019.1675909
Valentini A, Carreño NLV, Probst LFD, Leite ER, Longo E (2004) Synthesis of Ni nanoparticles in microporous and mesoporous Al and Mg oxides. Microporous Mesoporous Mater 68:151–157. https://doi.org/10.1016/j.micromeso.2003.12.021
Verdonck E, Schaap K, Thomas LC (1999) A discussion of the principles and applications of Modulated Temperature DSC (MTDSC). Int J Pharm 192:3–20. https://doi.org/10.1016/S0378-5173(99)00267-7
Vernon-Parry KD (2000) Scanning electron microscopy: an introduction. III-Vs Review 13:40–44. https://doi.org/10.1016/S0961-1290(00)80006-X
Villar A, Gorritxategi E, Aranzabe E, Fernández S, Otaduy D, Fernández LA (2012) Low-cost visible–near infrared sensor for on-line monitoring of fat and fatty acids content during the manufacturing process of the milk. Food Chem 135:2756–2760. https://doi.org/10.1016/j.foodchem.2012.07.074
Vishwakarma V, Uthaman S (2020) Environmental impact of sustainable green concrete. In: Liew MS, Nguyen-Tri P, Nguyen TA, Kakooei S (eds) Smart Nanoconcretes and Cement-Based Materials: Properties, Modelling and Applications. Elsevier, Amsterdam, pp 241–255. https://doi.org/10.1016/B978-0-12-817854-6.00009-X
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19. https://doi.org/10.1016/j.tca.2011.03.034
Wagner M (2018) Thermogravimetric Analysis. In: Wagner M (ed) Thermal Analysis in Practice. Hanser Publications, Liberty Twp, pp 162–186
Wang S, Gao B, Zimmerman AR, Li Y, Ma L, Harris WG, Migliaccio KW (2015) Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour Technol 175:391–395. https://doi.org/10.1016/j.biortech.2014.10.104
Wang W, Lu T, Chen Y, Tian G, Sharma VK, Zhu Y, Zong L, Wang A (2019) Mesoporous silicate/carbon composites derived from dye-loaded palygorskite clay waste for efficient removal of organic contaminants. Sci Total Environ 696:133955. https://doi.org/10.1016/j.scitotenv.2019.133955
Wang RF, Deng LG, Li K, Fan XJ, Li W, Lu HQ (2020) Fabrication and characterization of sugarcane bagasse–calcium carbonate composite for the efficient removal of crystal violet dye from wastewater. Ceram Int 46:27484–27492. https://doi.org/10.1016/j.ceramint.2020.07.237
Wang Y, He L, Dang G, Li H, Li X (2021) Polypyrrole-functionalized magnetic Bi2MoO6 nanocomposites as a fast, efficient and reusable adsorbent for removal of ketoprofen and indomethacin from aqueous solution. J Colloid Interface Sci 592:51–65. https://doi.org/10.1016/j.jcis.2021.02.033
Wen J, Arthur K, Chemmalil L, Muzammil S, Gabrielson J, Jiang Y (2012) Applications of differential scanning calorimetry for thermal stability analysis of proteins: qualification of DSC. J Pharm Sci 101:955–964. https://doi.org/10.1002/jps.22820
Wendlandt WW (1986) Thermal analysis. John Wiley & Sons, New York
Wu M, Chen W, Mao Q, Bai Y, Ma H (2019) Facile synthesis of chitosan/gelatin filled with graphene bead adsorbent for orange II removal. Chem Eng Res Des 144:35–46. https://doi.org/10.1016/j.cherd.2019.01.027
Yaah VBK, Ojala S, Khallok H, Laitinen T, Selent M, Zhao H, Sliz R, de Oliveira SB (2021) Development and characterization of Composite Carbon Adsorbents with Photocatalytic Regeneration Ability: Application to Diclofenac Removal from Water. Catalysts 11:173. https://doi.org/10.3390/catal11020173
Yadav LDS (2005) Organic spectroscopy. Springer Science & Business Media, Dordrecht
Yang SC, Liao Y, Karthikeyan KG, Pan XJ (2021) Mesoporous cellulose-chitosan composite hydrogel fabricated via the co-dissolution-regeneration process as biosorbent of heavy metals. Environ Pollut 286:117324. https://doi.org/10.1016/j.envpol.2021.117324
Yıldız H, Tokalıoğlu Ş, Soykan C (2021) Preparation of polyacrylonitrile/polyindole conducting polymer composite and its use for solid phase extraction of copper in a certified reference material. Spectrochim Acta A Mol Biomol Spectrosc 244:118826. https://doi.org/10.1016/j.saa.2020.118826
Zhang B, Wang X, Li S, Liu Y, An Y, Zheng X (2019a) Preferable adsorption of nitrogen and phosphorus from agricultural wastewater using thermally modified zeolite-diatomite composite adsorbent. Water 11:2053. https://doi.org/10.3390/w11102053
Zhang D, Li N, Cao S, Liu X, Qiao M, Zhang P, Zhao Q, Song L, Huang X (2019b) A layered chitosan/graphene oxide sponge as reusable adsorbent for removal of heavy metal ions. Chem Res Chin Univ 35:463–470. https://doi.org/10.1007/s40242-019-8369-1
Zhang M, Chen J, Zhao F, Zeng B (2020) Determination of fluoroquinolones in foods using ionic liquid modified Fe3O4/MWCNTs as the adsorbent for magnetic solid phase extraction coupled with HPLC. Anal Methods 12:4457–4465. https://doi.org/10.1039/D0AY01045F
Zhao B, Jiang H, Lin Z, Xu S, Xie J, Zhang A (2019) Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr Polym 224:115022. https://doi.org/10.1016/j.carbpol.2019.115022
Zhou W, Apkarian R, Wang ZL, Joy D (2007) Fundamentals of scanning electron microscopy (SEM). In: Zhou W, Wang ZL (eds) Scanning Microscopy for Nanotechnology: Techniques and Applications. Springer, New York, pp 1–40. https://doi.org/10.1007/978-0-387-39620-0_1
Zhou Y, Lu J, Zhou Y, Liu Y (2019) Recent advances for dyes removal using novel adsorbents: a review. Environ Pollut 252:352–365
Zhou RY, Yu JX, Li HX, Chi RA (2020) Removal of phosphate from aqueous solution by ferrihydrite/bagasse composite prepared through in situ precipitation method. Colloids and Surf a: Physicochem Eng Asp 603:125144. https://doi.org/10.1016/j.colsurfa.2020.125144
Funding
This study was financed partly by the National Council for Scientific and Technological (CNPq)—Projects Codes 403658/2020–9 and 140541/2021–7 and Coordination for the Improvement of Higher Education Personnel (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
Conceptualization: M. T. Nazari, J. S. Piccin, G. L. Dotto; methodology: C. V. T. Rigueto, I. Alessandretti; formal analysis and investigation: F. Melara, N. F. da Silva; writing—original draft preparation: M. T. Nazari, J. S. Piccin, L. Crestani, V. Ferrari; writing—review and editing: C. Schnorr, G. L. Dotto, J. Vieillard, L. F. O. Silva; funding acquisition: J. S. Piccin; supervision: J. S. Piccin. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nazari, M.T., Schnorr, C., Rigueto, C.V.T. et al. A review of the main methods for composite adsorbents characterization. Environ Sci Pollut Res 29, 88488–88506 (2022). https://doi.org/10.1007/s11356-022-23883-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23883-z