Abstract
There is ambiguous evidence that high-fructose diet can induce toxicity in different organ systems but its endocrine disrupting effects by abnormal changes in female reproductive organs is poorly evidenced. This study aimed to address the reproductive safety of high fructose diet through clinical, biochemical, hormonal, histopathological, and immunohistochemical analysis. For this purpose, 5–6 weeks mature female Wistar rats were divided in three groups and each five animals/group exposed to standard chow + water + HFCS-55, standard chow + water + sucrose 75%w/v and standard chow + water for 90 days. Remarkable increase in most lipid profile factors and total body weights of HFCS-55 fed rats and sucrose fed rats were detected in similar pattern compared to control. At the same time, a battery of differential signs and symptoms in HFCS-fed groups including squamous metaplasia in the uterine tissue and ovarian congestion, significant increase in FSH and LH levels, meaningful decreased serum testosterone and 17β-estradiol levels, and strong androgen receptor expression in ovaries and uterine of HFCS group of animals were recorded compared to other two study groups. These thought-provoking signs and signals of fructose induced reproductive toxicity in this model emphasis the contribution of HFCS-55 to deteriorated ovarian and endometrial health and increased risk primary ovarian insufficiency (POI) in women.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last four decades, severe changes in human nutritional patterns and higher consumption of a high sugar-rich diet have been associates dramatically with increased incidence of hyperuricemia, dyslipidemia, hypertension, insulin resistance, and overweight/obesity (Akarca-Dizakar et al. 2020). General rising prevalence of obesity is highly associated with an increased risk of endocrine disruption as evolving worldwide health crisis in adults and children (Zhou et al. 2021). A recent animal study on rats shows a strong association between early exposure to a high-sucrose diet and deteriorated ovarian health (de Melo et al. 2021). A higher incidence of total adiposity and truncal subcutaneous fat accumulation has alerted women to health problems with clusters of hormonal dysregulation, leading to ovarian dysfunction (Ishikawa et al. 2018). Prospective cohort studies indicate that routine consumption of sugar-sweetened beverages (SSBs) is closely associated with changes in hepatic metabolism and microbiome changes (Bhat et al. 2021), weight gain, obesity, metabolic syndrome (Semnani-Azad et al. 2020; Sadowska and Rygielska 2019; Collison et al. 2009), and increased risk of Polycystic ovarian syndrome (PCOS) (Rizk and Thackray 2020) over time. Moreover, neonatal exposure to SSBs may affect infant growth, predispose them to obesity phenotypes, alter body composition (Michael et al. 2017), and increase the risk of ovarian toxic reactions in later stages of life.
Nowadays, fructose has become the primary agent in sugar-sweetened beverages and sweets in many countries (Goncalves et al. 2019). Food industries prefer to use commercial high-fructose-corn syrup 55% (HFCS-55) based on its sweetness, palatability, taste enhancement properties (DeChristopher et al. 2020), and taste maintaining properties of the beverages and food products (Chenxia Dai et al. 2020). Routine consumption of HFCS-sweetened beverages in breastfeeding mothers increases the concentration of fructose in breast milk (Paige et al. 2018), and fluids from PCOS patients showed significantly higher fructose levels than healthy women regardless of whether the follicles were mature or immature. Based on this concept of female susceptibility to fructose-induced changes, we decided in the present work to find any possible associations between HFCC-55 long-term consumption and any change in serum sex hormone levels, ovarian and uterine histopathological changes, and expression levels of sex steroid receptors by immunohistochemical method. We decided here to focus on HFCS-55 in females based on supporting evidence on the sex-specific effect of fructose in women compared to men after high‐fructose exposure as well as the ubiquitous exposure of human populations to fructose through prepackaged foods, breakfast cereals, baked goods, dairy desserts, soft drinks, and juice beverages (Zargaraan et al. 2016). We compared the hormonal effects of HFCS-55 in initial concentration (55%) with sucrose syrup at 75% using OECD 408 guidelines.
Material and methods
Study subjects
High fructose corn syrup 55 (HFCS-55)
HFCS-55% was kindly provided and certified by Zar Fructose Co., Ltd., Tehran, Iran, on March 2020, under the code number ZFQS04 with a confirmed analytical sheet. According to the analytical sheet, 100 mL of HFCS-55 provides 71.72 g of carbohydrate (fructose and glucose); the estimated energy of 100 mL of HFCS-55 is 286.9 kcal.
Sucrose syrup 75%
Sucrose (saccharose) was purified and crystallized with over 99.7°Z polarization and certified by Merck Millipore Co., Ltd., Germany, under the CAS number 57–50-1 with an accessible analysis sheet on March 2020. Sucrose comprises one glucose molecule, and one molecule of fructose joined together. It is a disaccharide, a molecule composed of two monosaccharides: glucose and fructose. Sucrose produces naturally in plants, from which table sugar refines. Sucrose is the most abundant disaccharide and the primary product of photosynthesis. Sucrose syrup 75% was prepared by dissolving 75 g of sucrose with properties mentioned above in distilled water and adjusting up to 100 mL. We prepared 100 mL of sucrose syrup 75%, providing about 280–290-kcal energy. Table 1 describes the composition of the rat’s standard diet in the present study.
Experimental animals and housing conditions
This study was carried out in a group of 15 mature, regularly cycling female Wistar rats, aged 5–6 weeks, with initial body weights of 165–180 g, purchased from the Pasture Institute of Iran. Every five female rats were housed together in one standard cage in a 12-h light/dark cycle (07:00–19:00), 23 ± 2 °C temperature, relative humidity of 30–70%, 8–15 times/h air change with access to tap water, and standard diet ad libitum. The cage cleaning schedule, air filtration and recirculation, health checks, and facility maintenance followed the 1-week adaptation and acclimation period.
The ethics committee of Islamic Azad University approved this study (IR. IAU. TMU REC.1399.216). The animal care protocol was consistent with the committee’s guidelines for the care of animals, following the Standard Operating Procedures of the Ministry of Health and Medical Education of Iran for the Care and Use of Laboratory Animals and the Canadian Council of Animal Care (CCAC) Guidelines for Care and Use of Experimental Animals.
Repeated dose oral toxicity study
The practical model of the experiment was conducted based on OECD 408 toxicology guideline.
After 10 days adaptation period, healthy female rats were randomly divided into three groups (5 rats/group) and labeled as Fo (HFCS-55), So (Sucrose 75%), and Co (control) groups. They had free access to HFCS–water (HFCS group), sucrose-water (sucrose group), or deionized water (control group). To calculate the necessary daily calorie of animals, we measured and recorded body weights once a day. Daily energy intakes are calculated based on the calories levels in Table 1 and adjusted to the same levels based on the calorie levels obtained from ingested fructose or sucrose and standard rodents chow (3.34 kcal/g).
General clinical observations once a day, simultaneously (s) each day were inspected for the level of water and food intake and signs of morbidity and mortality.
Detailed clinical observations, including changes in the skin, fur, eyes, mucous membranes, the occurrence of secretions and excretions and autonomic activity (e.g., lacrimation, pupil size, unusual respiratory pattern), changes in gait, posture, and response to handling, the presence of clonic or tonic movements, and bizarre behavior, were checked by us.
On day 90, overnight fasted rats were sacrificed after blood collections by heart puncture under light carbon dioxide anesthesia, and whole blood was drawn for further biochemical analysis.
Biochemical analysis
The collected blood samples were centrifuged at 1500 g for 15 min at 4 °C to obtain serums, and then, the 17β-estradiol were isolated and kept at − 80 °C until further analysis. The levels of glycemic factors were measured using an auto-analyzer (HITACHI 917 / OLYMPUS AU640 & COBAS INTEGRA). Other than the above factors, the lipid profile of animals was evaluated using serum concentration of total cholesterol, HDL, LDL, and ratios using spectrophotometric enzyme assay kits in Bahar Toxicology Laboratory in Tehran.
Hormonal assay
In order to determine the variations in serum concentrations of FSH, LH, testosterone, 17β-estradiol, and progesterone, tail blood samples were collected according to the standard protocol on days 15, 30,45,60, 75, and 90 of the study, and the serum was separated by cold centrifuge and collected in microtubes. Hormone levels were compared with control using the chemiluminescence immunoassays (CLIA) method using Cobas E411 from Roche Company with serial number 15D3-16.
Recovery studies
A commercial rat serum pool (catalog no. M5905; Sigma Chemical Co., St. Louis, MO) was spiked with various hormone concentrations, and percent recovery and parallelism to the assay standard curve were determined. For each assay, the serum pool was spiked either with hormones across the assay range or vehicle to determine endogenous hormonal levels in the pool. Samples were run in duplicate, and each assay was repeated to confirm the results. Hormonal recovery from each spike was determined by subtracting hormone values from spiked hormone samples in vehicle-spiked controls.
Necropsy and Histopathological studies
During the necropsy study, the reproductive organs were dissected, including the uterus and ovaries. Before weighting the intact organs, they were rinsed with normal saline to remove fat and debris that may interfere with weighting and sectionizing. Organs were fixed in a 10% formalin solution at the next step.
To remove the water, the desired tissue was dehydrated with degrees of alcohol (30, 50, 70, 80, 90, and absolute alcohol). To strengthen the fixed tissues, they were placed in paraffin blocks, and finally, 5 micron thin sections were prepared with a microtome. Subsequently, multiple sections from each block were prepared at 5-mm diameters and stained with hematoxylin and eosin (H&E) for microscopical evaluations. The sections were examined under the light microscope (Olympus BX-51; Olympus, Tokyo, Japan) by an expert animal pathologist and scored.
Immunohistochemical assay of sex hormone receptors
As previously described (Bidgoli et al. 2011), dewaxed and rehydrated tissue sections were subjected to antigen retrieval using a microwave oven and boiling citrate buffer (pH = 6.0). Endogenous peroxidase activity and nonspecific binding sites were blocked by incubating sections with 0.3% hydrogen peroxide in methanol for 30 min and 3% BSA for 60 min, respectively. Sections were then incubated for 30 min at room temperature with an androgen receptor monoclonal antibody (Clone AR441, Dakocytomation) that recognizes the nuclear expression of androgen receptors in reproductive tissues. The results were visualized using the Opti View DAB detection kit based on the manufacturer’s instruction with necessary modifications. Sections were also counterstained with Meyer’s hematoxylin. A section in which incubation with the primary antibody was omitted was used as a negative control in each series. The ideal staining conditions were established in our preliminary experiments. Staining was considered negative only after careful examination of the entire tissue section. All samples were assessed and scored by two independent pathologists. Histoscores of androgen receptors were assessed according to Intensity and Allred scores (P + I). In each case, a histoscore with a potential range of 0–300 was calculated as follows:
-
No immunoreactive cell: negative (0)
-
≤ 1% of cells are immunoreactive: weak positive (1 +)
-
1–10% of cells are immunoreactive: intermediate positive (2 +)
-
11–33% of cells are immunoreactive: strong positive (3 +)
-
34% of cells are immunoreactive: very strong positive (4 +)
The same staining and scoring method was performed for detecting nuclear expression of estrogen receptor(ER) alpha using ER-alpha monoclonal antibody (Clone 105, Dakocytomation) (Bidgoli et al. 2010).
Statistical analysis
Parametric values were expressed as means ± standard deviation (SD). In this analysis, three different groups (two treatments and control) were compared at the same time using one-way ANOVA. We used a post hoc test whenever analysis of variance revealed a significant difference between three sample means. We used the Student’s t-test to compare the difference between the two sample means. The level of significance was set at P < 0.05. All statistical methods were performed by SPSS version 21.
Results
Clinical effects
Without any sign of morbidity and mortality, detailed clinical observations we made every day did not change in all three study groups. As described before, these observations included changes in the skin, fur, eyes, mucous membranes, secretions, and autonomic activity, changes in gait, posture, and response to handling, as well as the presence of clonic or tonic movements, stereotypes, or bizarre behavior like bringing up the tails, abnormal or ataxic gaits, drowsiness, and any change in stool and urine colors.
Total body weight
As described in Table 2, the mean ± SD body weight was significantly increased in the HFCS-55 fed group compared to the control (267.62 ± 4.61 g vs. 230.52 ± 0.84 g, p = 0.0094). A similar change was recorded in the sucrose syrup fed group compared to control (275.82 ± 6.6237 vs. 230.52 ± 0.84 g, p = 0.0003).
Serum levels of gonadotropins and sex hormones
Table 2 compares the mean serum levels of FSH, LH, and sex hormones (17β-estradiol, progesterone, and testosterone) in female rats of the 3 study groups at day 90. Interestingly, serum 17β-estradiol level was significantly decreased in HFCS-fed animals compared to control (7.28 ± 4.55 vs. 25.12 ± 14.5, p < 0.0001) and sucrose-fed animals (7.28 ± 4.55 vs. 14.66 ± 5.7, p < 0.005). Daily HFSC-55 consumption significantly decreased serum testosterone levels (0.0294 ± 0.002 vs. 0.222 ± 0.103, p = 0.0032) compared to control and sucrose 0.0294 ± 0.002 vs. 0.1156 ± 0.102, p = 0.0056). At the same time, progesterone levels significantly increased (42.04 ± 16.74 vs. 17.046 ± 6.56, p = 0.0145) compared to control and sucrose (42.04 ± 16.74 vs. 28.79 ± 20, p = 0.0213). Significantly higher LH (0.14 ± 0.08) and FSH (0.12 ± 0.01) detected in HFCS animals compared to both control groups. In general, an upward trend in the serum concentrations of FSH, LH, and progesterone in HFCS receiving animals and a downward trend in 17β-estradiol and testosterone again in HFCS receiving animals were noted after 90 days of the administration, which were considered significant.
Uterine weight and histopathology
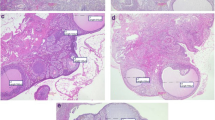
As shown in Table 2, the total weight of the uterus was significantly increased in the HFCS55-fed group (1.4115 ± 0.052 vs. 1.1975 ± 0.050, p = 0.0182) compared to the control. The same difference was detected between HFCS-fed and sucrose-fed animals (1.4115 ± 0.052 vs. 1.2102 ± 0.047, p = 0.0209). HFSC intake was accompanied by squamous metaplasia in the uterine tissue of HFCS-fed animals (Fig. 1A). The other destructive changes observed were endometrial cells with apoptosis (Fig. 1B) and endometrial apoptosis with PMN cell infiltration and inflammatory changes in lamina propia in HFCS-fed animals.
Histopathological effects of HFCS-55 in uterine and ovaries tissues in compared to control and sucrose fed animals. Photomicrographs stained with hematoxylin and eosin and showed in two magnifications. A Control and sucrose fed animals show normal uterus with endometrial glands in proliferative phase. B1 Squamous metaplasia in uterine tissue of HFCS fed animals. B2 Endometrial apoptosis with PMN cell infiltration in lamina propia in HFCS fed animals. B3 B2Endometrial cells with apoptosis in HFCS fed animals. C Normal ovarian tissue with prominent corpus luteum in control group. D Normal ovarian tissue with prominent corpus luteum in sucrose fed animals
Ovarian weight and histopathology
As shown in Table 1, the total weight of ovaries was significantly increased in HFCS55-Fed animals (0.3528 ± 0.070 vs. 0.2993 ± 0.072, p < 0.0001) compared to control. The same difference was detected between HFCS and sucrose-fed animals (0.3528 ± 0.070 vs. 0.3025 ± 0.069, p < 0.0001). Destructive changes in the ovaries accompanied HFSC intake. The control and sucrose-fed groups demonstrated typical basic ovarian structure, which usually contains corpus luteum and all developmental stages of follicles but moderate to severe congestion in HFSC fed animals’ ovarian tissue (Fig. 1B1).
Immunohistochemical expression of androgen receptor
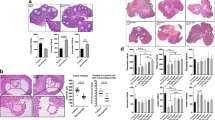
Table 2 compares the expression levels of androgen receptor (AR) based on proposition score (P), intensity score (I), and Allred score (P + I) in epithelial cells of ovary follicles and ovarian superficial epithelium. AR was overexpressed in the ovaries of HFSC-fed animals according to proposition score, AR intensity score, and Allred score. Similar to the control group, AR’s expression pattern remained unchanged in sucrose-fed animals.
Figure 2 compares AR expression among study groups. AR expression was mild to negative in epithelial cells of ovary follicles and superficial ovarian epithelium of animals in control and sucrose groups (Fig. 1A2). However, AR expression in epithelial cells of ovary follicles was strong (3 +) and moderate (2 +) in the superficial ovarian epithelium (Fig. 1B1 and B2) and ovarian superficial (Fig. 1C epithelium of HFCS fed animals showed a similar pattern of AR expression in Sucrose fed animals.
Immunohistochemical expression of androgen receptor and estrogen receptor in ovarian tissues of Wistar rats. Photomicrographs of sections A and B show negative/mild expression of AR in epithelial cells of ovary follicles and ovarian superficial epithelium in control sucrose groups (× 100). C1 (× 100), C2–C4 (× 400) show strong (3 +) expression of AR in epithelial cells of ovary follicles and ovarian superficial epithelium in HFCS-55 fed animals. Photomicrographs of D, E, and F1–F4 show mild to moderate expression of estrogen receptor in epithelial cells of ovary follicles and ovarian superficial epithelium of all study groups in similar pattern
Uterine expression of androgen receptor (AR)
Mild nuclear expression of AR in the endometrial epithelium of HFCS fed animals (Fig. 3C2) and moderate nuclear expression of AR in the endometrial epithelium of sucrose-fed animals (Fig. 3C3) were detected.AR expression in the endometrial epithelium of the control group was strong (Fig. 3C4).
Immunohistochemical expression of androgen receptor and estrogen receptor in the uterine of Wistar rats. Photomicrographs of sections A and B show negative/mild expression of AR in endometrial epithelium of animals in control and sucrose group. C1–C4 shows moderate to strong nuclear expression of AR in in endometrial epithelium of HFCS fed animals. Photomicrographs of D, E, and F1–F4 show negative/mild expression of estrogen receptor in endometrial epithelium of animals of all study groups in similar pattern
Immunohistochemical expression estrogen receptor
Table 2 compares the expression levels of estrogen receptor (ER) based on proposition score (P), intensity score (I), and Allred score (P + I) in epithelial cells of ovary follicles and ovarian superficial epithelium. ER expression pattern was practically similar in the ovaries (Fig. 2) and (Fig. 3) uterine of the study groups.
Lipid profile
As shown in Tables 3 and 4, all lipid profile factors in HFCS-55 fed rats significantly increased compared to control and sucrose groups except HDL-cholesterol. Out of different lipid profile-related factors, LDL-cholesterol and LDL/HDL ratio raised significantly in HFCS-55 fed animals (p = 0.008), not in the sucrose group. Other factors changed in the following manner:
-
Triglyceride (TG mg/dL): serum level of TG in HFCS-55 was significantly higher than Control (p < 0.0001) and Sucrose (p = 0.0034)
-
Serum level of total cholesterol raised significantly in both treatment groups compared to the control, but the difference was higher in HFCS-55(p = 0.0095) than sucrose (p = 0.045)
-
Non-HDL cholesterol also increased significantly in both treatment groups compared to the control, but the difference was again higher in HFCS-55(p = 0.0021) than sucrose (p = 0.0062)
-
Cholesterol/HDL ratio was increased in both groups, but the level in HFCS-55 fed animals was significantly higher (p = 0.0165)
Glycemic factors
As shown in Tables 3 and 4, out of different glycemic factors, FBS increased in both study groups. However, the mean (SD) was significantly higher in HFCS-55 compared to control (125 ± 17.21918 vs. 79 ± 15.033, p = 0.002), and the difference with sucrose was not meaningful (p = 0.6302).
Discussion
In this long-term 90-day experimental study, some reproductive factors were significantly changed in female rats after feeding with an HFCS-55-enriched regular chow diet compared to a standard chow diet or sucrose-enriched chow diet. These meaningful changes were observed in a comparable pattern of obesity, hyperglycemia, and hyperlipidemia in both fructose and sucrose-fed animals. Nevertheless, differential changes in serum FSH, LH, testosterone, and 17β-estradiol levels, massive increased organ weights, and extensive AR overexpression in ovaries and uterine of HFCS-55 fed animals indicate a close association between dietary fructose and the development of hyper-gonadotropic hypoestrogenism signs. Scientists refer to this unexplained feature as “primary ovarian insufficiency” (POI) (Marzieh Rostami Dovom et al. 2019), a growing disease with an increased risk of idiopathic infertility, metabolic syndrome and impaired reproductive function according to human studies (Tsiligiannis et al. 2019; Rossetti et al. 2017) (Table 5).
Many environmental toxicants are characterized as endocrine-disrupting chemicals (EDCs), affect the hypothalamus–pituitary–thyroid axis, and interfere at any developmental stage of follicle growth (Bidgoli et al. 2022). Evidence from this study strongly hypothesizes that dietary fructose could act possibly similarly to potent endocrine-disrupting chemicals (EDC) in the female reproductive system by inducing ovarian congestion and androgen receptor dysregulation but its direct effect on follicle growth should be assessed in subsequent studies. Detailed evidence of androgen receptor overexpression in both ovary and endometrial tissues of HFCS-55-fed animals in parallel to testosterone deficiency is good evidence which was associated with ovarian congestion in this group of animals, but further studies on sex hormone-binding globulin (SHBG) and free androgen index (FAI) (Santoro and Randolph 2011) in animal and epidemiological studies seem necessary to confirm the critical role of nutritional fructose overconsumption on reproductive women health. We also predict overexpression of ER in a more extended study design according to significant 17β-estradiol deficiency in the present study and progesterone receptor downregulation based on overproduction of progesterone in this pilot study setting.
In the context of normal endometrial glands in the proliferative phase in control and sucrose-fed animals, the incidence of squamous metaplasia in the uterine tissue of HFCS-fed animals and apoptotic changes confirms the hormonal properties of HFCS-55. However, estrogenic or antiestrogenic properties are a controversial issue because the incidence of squamous metaplasia in the female has been reported only one time in Sprague–Dawley rats in a multigenerational reproductive and chronic toxicity model by ethinyl estradiol following the incidence of uterine lesions showed by atypical focal hyperplasia and (Delclos 2009). The exact role of fructose on 17β-estradiol secretion, its binding capacity as a ligand to hormonal receptors, should be assessed in the subsequent studies based on estrus cycles in regular 3-day intervals and conception potentials of follicles, a program which we could not perform in the current setting due to technical limitations.
Endometrial metaplasia caused by angiogenesis is under the control of endometrial endothelial cells, which is under the control of estradiol. These preliminary results could synthesize the hypothesis that fructose may affect endometrial angiogenesis by disturbing some functional properties of the cells, similar to BPA and estrogenic compounds. Nevertheless, fructose-induced changes in human endometrial endothelial cell (HEEC) activities need specific mediators, and this essential issue should be assessed on endometrial tissue in the subsequent studies. Through animal studies, typically mature male rats with fully developed hypothalamic-pituitary–gonadal (HPG) axis and baseline serum androgen-estrogen levels are susceptible to a high-fructose diet (Tkachenko et al. 2020). We showed a similar pattern in the female rat with extraordinary weight gain and expectable metabolic changes.
Obesity observed in a study group was an etiologic factor that disrupted normal folliculogenesis through the increased production of IL-10 in visceral fat. However, in the context of regular expression of ER in fructose-fed animals, the contribution of other factors, e.g., adipokines, inflammatory factors, and IL-10 in fructose-induced endometrial changes, is another viewpoint for subsequent studies based on a present concept.
According to the science of recent years, a certain amount of androgens through androgen receptors is essential for normal ovarian function. At the same time, its high levels are associated with polycystic ovary syndrome (PCOS), and its low levels are associated with POI. Over the years, there have been many studies on androgen receptor function as a nuclear transcriptional regulator (Marzieh et al. 2021). However, new evidence confirms the role of androgen as an essential mediator through epigenetic modulation involving both the nuclear and extra-nuclear androgen signaling. Association between dietary fructose and androgen-mediated epigenetic changes further reproductive toxic changes is another issue for following studies.
Conclusion
Our results in the present setting emphasize the role of excessive dietary fructose on the development of hyper-gonadotropic hypoestrogenism signs and early cessation of ovarian function associated with hyperlipidemia, hyperglycemia, and massive histopathological changes in ovaries and uterine of exposed females. There are deficits in our understanding of this condition and, subsequently, the long-term health consequences of dietary fructose on women’s health. However, this factor clearly emphasizes the role of dietary fructose as a potent endocrine-disrupting factor and an underlying etiologic factor. Our knowledge of long-term fructose-induced genetic and epigenetic changes and the clinical consequences explicitly relating to women with POI is limited. Most data on this subject emerged from subsequent studies based on present findings.
Data availability
All data and materials are available upon journal request.
References
Akarca-Dizakar SÖ, Erdoğan D, Peker T, CoşkunAkçay N, Türkoğlu I, Eşmekaya MA, Ömeroğlu S (2020) Effects of co-administered melatonin, fructose and bisphenol A (BPA) on rat epididymis and sperm characteristics. Biotech Histochem 95(1):18–26
Bhat SF, Pinney SE, Kennedy KM, McCourt CR, Mundy MA, Surette MG, Sloboda DM, Simmons RA (2021) Exposure to high fructose corn syrup during adolescence in the mouse alters hepatic metabolism and the microbiome in a sex-specific manner. J Physiol 599(5):1487–1511
Bidgoli SA, Karimi M, Asami Z, Baher H, DjamaliZavarhei M (2011) Association between testicular Aryl hydrocarbon Receptor levels and idiopathic male infertility: a case-control study in Iran. Sci Total Environ 409(18):3267–3273
Bidgoli SA Ahmadi R, Zavarhei MD (2010) Role of hormonal and environmental factors on early incidence of breast cancer in Iran. Sci Total Environ 408 (19)- pp. 4056–61.
Bidgoli SA, Arabshahi P, Ramezan Y (2022) Ovarian toxicity of plant-derived edible oils: a 28 days hormonal and histopathological study in Wistar rat. Environ Sci Pollut Res Int 29(6):9153-9163. https://doi.org/10.1007/s11356-021-13279-w
Chenxia Dai TM, Hai J, Xiao Y, Li Y, Zhao J, Qiu H, Bo Xu (2020) A novel glucose isomerase from Caldicellulosiruptor bescii with great potentials in the production of high-fructose corn syrup. Biomed Res Int 2020:1871934
Collison KS, Saleh SM, Bakheet RH, Al-Rabiah RK, Inglis AL, Makhoul NJ, Maqbool ZM, Zaidi MZ, Al-Johi MA, Al-Mohanna FA (2009) Diabetes of the liver: the link between nonalcoholic fatty liver disease and HFCS-55. Obesity (silver Spring) 17(11):2003–2013
de Melo GB, Soares JF, Costa TCL, Benevides ROA, Vale CC, Paes AMA, Gaspar RS (2021) Early exposure to high-sucrose diet leads to deteriorated ovarian health. Front Endocrinol (lausanne) 12:656831
DeChristopher LR, Auerbach BJ, Tucker KL (2020) High fructose corn syrup, excess-free-fructose, and risk of coronary heart disease among African Americans- the Jackson Heart Study. BMC Nutr 6(1):70
Goncalves MD Lu C, Tutnauer J, Hartman TE, Hwang SK, Murphy CJ, Pauli C, Morris R, Taylor S, Bosch K, Yang S, Wang Y, Van Riper J, Lekaye HC, Roper J, Kim Y, Chen Q, Gross SS, Rhee KY, Cantley LC, Yun J (2019) High-fructose corn syrup enhances intestinal tumor growth in mice. Science 363 (6433)1345–1349
Ishikawa H, Hashimoto S, Ohashi K (2018) Maternal fructose intake disturbs ovarian 17β-estradiol synthesis in rats. Life Sci 202:117–123
Marzieh Rostami Dovom, Mahsa Noroozzadeh, Nariman Mosaffa, Azita Zadeh–Vakili, Abbas Piryaei, Fahimeh Ramezani Tehrani (2019) Induced premature ovarian insufficiency by using D galactose and its effects on reproductive profiles in small laboratory animals: a systematic review. J Ovarian Res. - Vol. 12. - p. 96.
Marzieh Rostami Dovom, Razieh Bidhendi-Yarandi, Kazem Mohammad, Maryam Farahmand, Fereidoun Azizi, Fahimeh Ramezani Tehrani (2021) Prevalence of premature ovarian insufficiency and its determinants in Iranian populations: Tehran lipid and glucose study. BMC Womens Health 21:79
Michael I, Goran Ashley A, Martin Tanya L, Alderete Hideji Fujiwara, David A (2017) Fields Fructose in breast milk is positively associated with infant body composition at 6 months of age. Nutrients 9(2):146
Paige K, Berger David A, Fields Ellen W, Demerath Hideji Fujiwara, Goran Michael I (2018) High-fructose corn-syrup-sweetened beverage intake increases 5-hour breast milk fructose concentrations in lactating women. Nutrients 10(6):669
Rizk MG, Thackray VG (2020) Intersection of polycystic ovary syndrome and the gut microbiome. J Endocr Soc 5(2):bvaa177
Rossetti R, Ferrari I, Bonomi M, Persani L (2017) Genetics of primary ovarian insufficiency. Clin Genetx 91(2):183–198
Sadowska J, Rygielska M (2019) The effect of high fructose corn syrup on the serum insulin and leptin concentration, body weight gain and fat accumulation in rat. Adv Clin Exp Med 28(7):879–884
Santoro N, Randolph JF Jr (2011) Reproductive hormones and the menopause transition. Obstet Gynecol Clin North Am 38(3):455–466
Semnani-Azad Z, Khan TA, Blanco Mejia S, de Souza RJ, Leiter LA, Kendall CWC, Hanley AJ, Sievenpiper JL (2020) Association of major food sources of fructose-containing sugars with incident metabolic syndrome: a systematic review and meta-analysis. JAMA Netw Open 3(7):e209993
Tkachenko OY, Shayakhmetova GM, Matvienko AV, Kovalenko VM (2020) Reproductive disorders in male rats induced by high-fructose consumption from juvenile age to puberty. Arh Hig Rada Toksikol 71(1):78–86
Tsiligiannis S, Panay N, Stevenson JC (2019) Premature ovarian insufficiency and long-term health consequences. Curr Vasc Pharmacol 19(6):604–609
Vercellini P, Viganò P, Somigliana E, Fedele L (2014) Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 10(5):261–275
Zargaraan A, Kamaliroosta L, SeyedYagoubi A, SeyedYagoubi L, Mirmoghtadaie L (2016) Effect of substitution of sugar by high fructose corn syrup on the physicochemical properties of bakery and dairy products: a review. Nutr Food Sci Res 3(4):3–11
Zhou Y, Chi J, Lv W, Wang Y (2021) Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19). Diabetes Metab Res Rev 37(2):e3377
Acknowledgements
The authors are thankful from Zar Fructose Co., Ltd., Tehran, Iran, for providing our study samples. The authors also acknowledge Dr. Hasti Azar Abad as the veterinary pathologist of the present study for her excellent supports of histopathological studies. We acknowledge cancer Institute of Iran for supporting immunohistochemical analysis.
Author information
Authors and Affiliations
Contributions
RM performed all animal studies and lab as part of her PhD thesis. She read and approved the manuscript before submission. SAB is the main supervisor of this study who planned, designed the work, conducted, performed the statistical analysis, and provided the manuscript. RK was the second supervisor of this PhD study who suggested the main topic and contributed in planning and study design execution of HFCS-55. SS and HAA were co-advisors of this PhD program who were contributed to all parts of this study.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the ethics committee of Islamic Azad University, Tehran Medical Sciences (IAUTMU) under the number of IR. IAU. TMU REC.1399.216.
Consent to participate
Not applicable.
Consent for publication
The authors ensure that this Journal and the Publisher have the Author’s permission to publish the relevant contribution.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mirzaei, R., Bidgoli, S.A., Khosrokhavar, R. et al. Increased risk of primary ovarian insufficiency by high-fructose diet consumption: a 90-day study in female rats. Environ Sci Pollut Res 30, 7415–7426 (2023). https://doi.org/10.1007/s11356-022-22258-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22258-8