Abstract
Regulating soil pH becomes a crucial practice to alleviate cadmium (Cd) contamination. However, little is known about the threshold of soil pH for the safe production of rice at various soil Cd levels. In this paper, the relationships between soil pH values and the contents of available Cd extracted by calcium chloride (CaCl2-Cd) in neutral and acidic soils were studied by mandatory acidification with H+ addition or neutralization with lime at various soil Cd levels. The results showed that the soil CaCl2-Cd contents dramatically decreased with increasing soil pH, and a logarithmic function could well describe the relations of soil CaCl2-Cd contents and soil pH at constant total Cd (CaCl2-Cd model). The Cd contents in rice grain (grain-Cd) in relation to soil CaCl2-Cd was further established through modified rice pot experiments. A model for the prediction of Cd content in rice grains (grain-Cd model) was set up, though which the grain-Cd content could be predicted based on soil pH and total Cd content. 122 data pairs of rice grain-Cd contents obtained at various soil total Cd contents and pH were employed from the literature to verify the reliability of the established model, approximately 95.08% of those data favorably located within the 1:1 line ± 0.5 unit area of the grain-Cd model. Notably, this model can be applied to determine the thresholds of soil pH at a specific Cd pollution level. For instance, to achieve a rice grain-Cd contents matching the Chinese national food safety limit of 0.2 mg kg−1, the soil pH thresholds were estimated to be 5.05, 5.70, and 6.02 at soil Cd contents of 0.3, 0.6, and 0.8 mg kg−1, respectively. In addition, the established model can also be used to estimate the health risk from rice in broad regions with various soil pH values and Cd contents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) ubiquitously exists in the environment, and human activities greatly accentuate its environmental burden, thus causing serious pollution, especially soil pollution, which endangers human health through the food chain (Godt et al. 2006) due to its high toxicity to humans (Xiao et al. 2018) and high mobility and bioavailability in soils (Liu et al. 2019). As early as 1974, the United Nations Environment Program and the International Labor Health Committee on Heavy Metals designated Cd a prior pollutant. Human exposure to Cd causes multiple adverse effects, including renal and hepatic dysfunction, testicular damage, and cancers (Chaney 2015; Stohs et al. 2001).
Rice (Oryza sativa L.) is the staple food for more than half of the world’s population (Kosolsaksakul et al. 2014; Meharg et al. 2013). Cd can be readily taken up by and accumulate to a high level in rice grains (Song et al. 2015; Wang et al. 2020). Rice grains may accumulate excessive Cd while maintaining normal growth under soil Cd stress, especially when planted in acidic soil with a low pH (Chen et al. 2018; Meng et al. 2018). Rice consumption has become one of the most important ways for human Cd exposure in many parts of the world including China (Li et al. 2017; Pastorelli et al. 2018; Suwatvitayakorn et al. 2020). Thus, the safe production of rice has aroused great attention in both the scientific community and the public.
Cd uptake and accumulation in rice depends not only on the total Cd contents in soils but also on its availability in soils. The bioavailability of soil Cd is governed by a series of soil property parameters, such as pH value, organic matter contents, and cation exchange capacity (CEC) (Bai et al. 2020; Guo et al. 2018b; Li et al. 2014), among which pH is the most important factor determining the forms of soil Cd present in soil and the corresponding bioavailability. The changes in soil pH may result in a new balance among various forms of soil Cd and thus an alteration in Cd availability (Guo et al. 2018b). Numerous studies have demonstrated that soil acidification activates soil Cd, thereby increasing its availability and enhancing its accumulation in crops such as rice (Chen et al. 2018), while increasing soil pH through the application of alkaline materials could effectively suppress the availability of soil Cd, thereby reducing its accumulation in crops such as rice grains (Chen et al. 2018; Meng et al. 2018). Thus, soil pH regulation becomes an effective countermeasure for ensuring food safety in Cd-contaminated soils. However, to achieve rice products with Cd contents matching the food safety requirement, to what extent should the pH of acidic soils with different Cd pollution levels be enhanced at a feasible cost, or to what extent should measures be taken if acidification occurs in neutral soil? These questions are fundamental to proper soil pH regulation. As such, a predictive model for determining the pH threshold of soil aimed at safe rice production needs to be established.
At present, many attempts have been made to establish models for the prediction of Cd contents in the edible parts of crops in different regions (Gu et al. 2019; Novotna et al. 2015; Romkens et al. 2009; Wen et al. 2020; Xiao et al. 2020). By extending the Freundlich equation, Romkens et al. (2009) obtained the relationship model between rice grain-Cd and soil available Cd, pH, and CEC, which provided guidance for rice production in Taiwan. Gu et al. (2019) selected soil properties including pH, TOC, CaO, Mn, Mo, and S as independent variables to predict heavy metal content in rice grains. Although these models can be used locally, they usually needs too many soil parameters, which takes too much work and time to determine the relevant soil parameters before planting, thus are not cost-and-time effective. Moreover, most of the established models are failed in the prediction of soil pH threshold at different soil Cd levels or Cd threshold at a specific soil pH. Therefore, a simpler and more effective prediction model needs to be established, which could be possible based on a full understanding of the relations betweed Cd in rice grains, soil Cd availability, and pH.
For this purpose, the variation tendency of soil available Cd during acidification of neutral soil and neutralization of acidic soils with various Cd pollution extents was studied in a simulation experiment selecting typical paddy soils in Chongqing, China, as the research object. The relationship between soil pH and Cd availability was then established, which supplied a basis for the model construction. Meanwhile, the relationship between grain-Cd in rice and CaCl2-Cd was built by modified pot experiments. Following up, a model was set up for predicting Cd contents in rice grains (grain-Cd) at various soil pH and Cd pollution level. In addition, a literature survey was also performed based on the main scientific databases, such as Web of Science, for verification of the accuracy, representativeness, and applicability of the model. The soil pH threshold at a specific Cd level or the Cd threshold at a specific soil pH for ensuring safe rice production was discussed finally. This established model could be applied to formulate soil protection guidelines and assess the health risk of rice products at various soil pH and Cd pollution levels.
Materials and methods
Soil samples
Two types of soil samples were used in this study, neutral purple paddy soil (NPPS) and acidic purple paddy soil (APPS), which were the main paddy soils in Southwest China. The soil samples were collected from the plow layer (0–20 cm) of farmlands in Hechuan District (29°48′51.92″N, 106°24′43.92″E) and Jiangjin District (28°59′47.71″N, 105°53′20.22″E) in Chongqing, China, respectively. Soil samples were collected by a clean wooden shovel in October 2019. Soil samples were air-dried, homogenized, and manually crushed to pass through a 2-mm sieve prior to use. The basic properties of the tested soils are shown in Table 1.
Preparation of artificial Cd polluted soils
According to the “risk control standard for soil pollution in agricultural land of soil environmental quality” (GB 15,618–2018), Cd solution of CdCl2·1/2H2O was spiked to the testing soils to reach four soil Cd concentrations of 0, 0.5, 1.5, and 3 mg kg−1. Then, the spiked soils were mixed and incubated at room temperature for 45 days at a constant soil moisture of 20% (w/w). After incubation, the soil samples were air-dried and passed through a 2-mm sieve again. The measured soil Cd contents were 0.39, 0.78, 1.83, and 3.42 mg kg−1 in the NPPS and 0.40, 0.82, 1.79, and 3.38 mg kg−1 in the APPS, respectively.
Soil acidification/neutralization
The required H+/OH− to generate a gradient of soil pH 4.5–7 was predetermined by incubation experiments. For NPPS, 0, 10, 20, 30, 45, and 60 mmol kg−1 H+ were added to the soil for artificial forced acidification. For APPS, 0, 10, 20, 30, and 40 mmol kg−1 OH− were added to the soil for neutralization. H+ and OH− were added in the form of dilute HCl and Ca(OH)2, respectively. Deionized water was added by the weighing method every day to ensure that the soil moisture contents were maintained at 20%. In addition, soil was thoroughly mixed every day to ensure that HCl or Ca(OH)2 reacted adequately with soils. Soil pH was monitored periodically. Incubation was terminated until the soil pH tended to be constant for 3 consecutive weeks. The values of soil pH after incubation are shown in Table S1.
Pot experiment
Modified rice pot experiment was carried out from April to August 2021, for understanding the Cd accumulation in rice grains as influenced by soil available Cd and pH and for the quantification of their relations.
The rice variety “Yixiang you 2115” widely planted in areas in the middle and upper reaches of the Yangtze River was used in this experiment. APPS soil samples containing 0.4 mg kg−1 Cd prepared in “Soil acidification/neutralization” were used; their pH values were neutralized to be pH 5.495, 5.720, 6.015, 6.180, and 6.635. Each 4 kg prepared soil was weighted into a PVP pot; basal fertilizers (150 mg N kg−1, 100 mg P2O5 kg−1, and 100 mg K2O kg−1) were applied as CO(NH2)2, K2HPO4, and KCl to the soil, and thoroughly mixed up with the soil. The upper diameter, lower diameter, height, and volume of the pot are 214 mm, 191 mm, 210 mm, and 5 L, respectively. All pots were kept flooded with tap water for 20 days prior to rice plantation. In order to make the experiment closer to the field conditions, the PVP pots were placed in a plot of rice-planting field, and the edge of the PVP pot was 10 cm higher than the water surface of the plot. Then, uniform seedlings of tested rice cultivar were transplanted into each pot (two plants per pot). The water level was maintained at approximately 3 cm above soil surface throughout the rice-growing period. Each treatment was replicated 3 times. Rice and soil samples were collected at maturity stages (105 days after transplantation) for analyses.
Chemical analyses
Soil pH and available Cd
The soil pH was directly determined using a pH meter (PHSJ-4F, Leici, Shanghai, China) with a soil water ratio of 1:2.5 (w/v). The available Cd in soils was extracted using a 0.01 mol·L−1 CaCl2 extraction procedure (Romkens et al. 2009). Briefly, 1 g of soil sample was mixed with 10 ml of CaCl2 solution (0.01 mol·L−1). The mixture was shaken on a reciprocal shaker at 200 rpm for 2 h at 25 °C, centrifuged at 4000 rpm for 10 min, and then filtered through a 0.45-μm membrane. The Cd contents in the filtrate were determined by a graphite furnace atomic absorption spectrophotometer (GFAAS) (Shimadzu AA6880, Japan). Certified reference materials (CRMs) for soils (GBW07428, GSS-14) provided by the National Research Center for CRM (China) were simultaneously analyzed for quality control. The Cd recovery of the reference soil ranged from 94 to 109%.
Rice grain-Cd content
The dried grains were pulverized, and the powder was digested with HNO3-HClO4 (4:1, v/v) on an electric plate for Cd determination. Cd concentrations in digestion solutions were determined by a graphite furnace atomic absorption spectrophotometer (GFAAS) (Shimadzu AA6880, Japan). Certified reference material for plants (GBW10044, GSB-22, China) were used for quality control. The Cd recovery rates of the reference rice were in the range of 92–110%. The detection limit was 0.0049 mg kg−1.
Modeling method
Since soil properties vary a lot with soil type and management, for simplicity, we tried to establish a rice grain-Cd prediction model started with the main factors—soil Cd content and pH based on our experimental results obtained at specific conditions, followed by verification by data available in literature, which may represent a more wide soil conditions. Thus, the suitability and deviation of the prediction model could be inspected if only those two main factors were taken into consideration regardless of other soil properties. As such, our modeling procedures are simplified as follows:
Based on the results of soil acidification/neutralization experiment (“Soil acidification/neutralization”), the quantitative relations of soil available Cd (CaCl2-Cd) with soil pH at different total Cd level were determined. Under the base, the dependence of rice grain-Cd on soil available Cd (CaCl2-Cd) was then examinated based on modified pot experiment. Then, a prediction model was established by combining the relations between rice grain-Cd content and available Cd and those between soil pH and total Cd content. Finally, the fitness of the model was inspected by data pairs from literature.
Assess the quality of the model
The model efficiency, the model strength, the coefficient of determination, and the model bias were used to assess the quality of the model. The success with which the models fitted the measured concentrations was evaluated by model efficiency (ME) calculated by the following equation (Novotna et al. 2015):
The model strength was determined by the mean normalized average error (MNAE) given by
and the model bias was calculated by mean normalized bias (MNB) given by
where Cmodel is the predicted concentration given by the model, Cmeasured is the measured concentration, \(\overline{C }\) is the mean of measured concentration, and n represents the number of observations.
Coefficient of determination (R2) was obtained by origin linear fitting.
The threshold of soil Cd for the prediction of rice grain risk
The rice single-factor pollution index was used to assess the risk of Cd in soil (Qu et al. 2020):
The risk degree of Cd in rice grain was evaluated by rewriting the single factor pollution index (Qu et al. 2020) to predict the degree of soil acidification or the pH value of acidic soil to achieve safe rice production. The modified formula of the rice single-factor pollution index (Eq. (4)) is as follows:
where C is the predicted Cd contents in rice grains, S is the Chinese national food safety limit of 0.2 mg kg−1 in rice, and P is the single-metal index. The risk degree can be divided into two grades: none (P < 1) and present (P ≥ 1).
Results and discussion
CaCl2-Cd model
The influence of soil pH on the content of soil CaCl2-Cd
Figure 1 shows the relationships between the CaCl2-Cd contents and soil pH during acidification in NPPS and nutrification in APPS, respectively. The variations of soil CaCl2-Cd contents as influenced by soil pH through the addition of H+ or OH− followed a similar pattern. With decreasing soil pH, CaCl2-Cd content increased significantly (Fig. 1). This tendency occurred in both the bulk soil and the soil with the addition of exogenous Cd. When the soil pH was increased/decreased by neutronization/acidification to be about 6.2 in APPS and NPPS, the soil Cd availability showed a breakthrough point as indicated by the dramatic increase/decrease of CaCl2-Cd content. Similarly, Honma et al. (2016) found that the amount of Cd dissolved in the soil changed significantly only when the soil pH was lower than 6.3, and otherwise, negligible changes occurred. The increment/decrement of soil CaCl2-Cd caused by per pH unit was largely depending on soil Cd pollution level (total Cd); larger increment/decrement occurred at higher total Cd content. At pH 6.2, when exogenous Cd of 0, 0.5, 1.5, and 3 mg kg−1 were added, the CaCl2-Cd contents were 0.023, 0.039, 0.096, and 0.220 mg kg−1 for APPS and 0.020, 0.070, 0.167, and 0.281 mg kg−1 for NPPS, respectively.
Establishment of CaCl2-Cd model
Through comprehensive analyses of CaCl2-Cd data of APPS and NPPS soils, it was found that the CaCl2-Cd contents of two soils at constant Cd level had a similar logarithmic linear relationship with pH (Fig. S1a). The determination coefficient R2 values of logarithmic expressions between pH and CaCl2-Cd in soils with exogenous Cd concentrations of 0, 0.5, 1.5, and 3 mg kg−1 were 0.9130, 0.8403, 0.8967, and 0.9503, respectively. According to the fitting results, a formula for the relationship between log10(CaCl2-Cd) and soil pH was obtained:
where CdSCa is the available soil Cd expressed as CaCl2-Cd, and A and B are the intercept and slope coefficients of the logarithmic formula.
As analyzed before, the increment/decrement of soil CaCl2-Cd caused by per pH was closely related to the total Cd in the soil, which was further illustrated by the four similar trend lines showing the relations of available Cd and pH at various total Cd level (Fig. S1a). Thus, the relationship between A, B, and log10(total Cd) in Fig. S1b was obtained by taking log10(total Cd) as the abscissa, as shown in Eqs. (6) and (7):
where A and B are the coefficients in Eq. (5); B is the average slop of the four trend lines, which were − 0.56976, − 0.50705, − 0.55488, and − 0.58556 at exogenous Cd additions of 0, 0.5, 1.5, and 3 mg kg−1, respectively; and CdST is the total Cd of the soil.
Then, Eqs. (6) and (7) are substituted into Eq. (5) to obtain a model for the prediction of available Cd in soil (CaCl2-Cd model) based on soil pH and the total Cd content, as showing in Eq. (8):
Verification of the CaCl2-Cd model
To better verify the model satisfies various types of soil, 148 data pairs (Table S2) with pH range of 4–7 were collected by searching for soil, Cd, and calcium chloride on the literature search webpage. The total Cd and pH values of the collected data were substituted into Eq. (8) to obtain predicted values. The results in Fig. 2 show that the prediction results are satisfactory. Approximately 85.14% of the data pairs were within the 1:1 line ± 0.5 unit area, and 52.70% of the data pairs were within the 1:1 line ± 0.25 unit area (Fig. 2). Satisfactory results were obtained for the prediction of CaCl2-Cd concentrations in soil when the data pairs were within the 1:1 line ± 0.25 unit area with relatively better MNAE (0.364), MNB (0.128), ME (0.57), and R2 (0.85) (Table 2). Predictions of the CaCl2-Cd concentrations in all soil data pairs were not satisfactory for CaCl2-Cd model, of which ME, MNAE, MNB, and R2 were − 2.258, 0.985, 0.536, and 0.701 (Table 2), respectively. Although the CaCl2-Cd model can well predict the soil corresponding to the quoted data pairs, the difference between soils still results in some of the prediction model values deviating from the actual value.
To explore the reason why CaCl2-Cd model could not well predict CaCl2-Cd concentrations in some soils, we analyzed soil physical and chemical properties in the references. Statistics of the basic physical and chemical properties of the soil corresponding to the data pairs (Table S2) found that soil CEC, organic matter content, and organic carbon were in the range of 5.3–42.5 cmol kg−1, 2.5–52.3 g kg−1, and 5.5–65 g kg−1, respectively. Through analysis of the basic physical and chemical properties of the soil, which were represented by the data pairs inside and outside the 1:1 line ± 0.5 area of Fig. 2, it was found that CEC and OM had no obvious regular characteristics. When the SOC ranged from 11.4 to 27 g kg−1, the model can accurately predict soil CaCl2-Cd. However, the predictive values of the model tended to be greater than the measured values when the SOC level was relatively high, while a reverse trend was observed under the condition of the relatively low SOC value. Moreover, the difference between the two values increased with SOC because SOC has a negative effect on Cd in soil (Jing et al. 2020). SOC affects the mobility and availability of metals in soil in two ways. Chelating agents provided by organic matter decrease the mobility of metals and humic substances with heavy metals, establishing stable complexes (Du Laing et al. 2009; Jing et al. 2020). Both contributions decrease the extractable metals available for plant absorption in agricultural soils (Halim et al. 2015; Jing et al. 2020). Through a more stringent 1:1 line ± 0.25 area, internal and external data pairs were represented by basic physical and chemical soils. Among 71 soils with CEC data and 50 soils with OM data, 54.93% and 52.50% of the soils can be well predicted, respectively. However, there is no regular pattern. Combined with SOC, CEC, and OM, the model cannot well predict the soil with low SOC or OM, no matter what the CEC value is. In conclusion, when the soil SOC is within the range of 58.31 ~ 159.10% of the soil SOC in this study, the CaCl2-Cd concentration of most soils can be well predicted, and CEC and OM have little impact on the prediction results. In addition, most of the soils that cannot be well predicted belong to the high geological background area with low CaCl2-Cd content.
Prediction models for Cd in rice
The CaCl2-Cd model illustrates only Cd availability in soil and cannot directly show the Cd contents of rice grains. However, many studies have shown that there is a good correlation between the contents of Cd in rice grains and CaCl2-Cd. Cd contents in rice grains under different soil pH are presented in Fig. 3a. Grain-Cd contents declined with the increase of soil pH. When soil pH was below 5.5, grain-Cd concentrations were lower than the accepted food safety standards (0.2 mg kg−1). Figure 3b shows the relationship between CaCl2-Cd and grain-Cd, and R2 was 0.9719 by using the linear fitting. Logarithmical transformation was conducted based on the linear relationship between grain-Cd and CaCl2-Cd in order to directly predict grain-Cd contents, and as a result, Eq. (9) was obtained.
where CdG is the concentration of rice grains Cd.
Substituting Eq. (8) into Eq. (9) yields the relationship model between grain-Cd, soil total Cd, and soil pH (Eq. (10)).
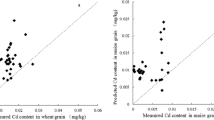
In addition, 122 data pairs for 10 papers (Table S3) were obtained through the keywords soil, cadmium, calcium chloride, and rice search. The purpose is to verify whether the prediction model can predict the cadmium content of rice in a large area. The total Cd and pH values corresponding to 122 data pairs were substituted into Eq. (10) to obtain the predicted values of Cd contents in rice grain. The results in Fig. 4b show that the prediction results are satisfactory. Approximately 95.08% of the data pairs are within the 1:1 line ± 0.5 unit area, approximately 68.85% of the data pairs are within the 1:1 line ± 0.25 unit area (Fig. 4b), and most of the data pairs are close to the 1:1 line. For all data pairs, the model quality is unsatisfactory, and few data pairs have large deviations. ME, MANf, MNB, and R2 are 0.348, 0.527, − 0.171, and 0.148, respectively (Table 2). However, after excluding the data outside the line, the quality of the model is relatively optimized and can basically accurately predict the Cd contents in rice grains. ME, MANf, MNB, and R2 are 0.678, 0.250, − 0.077, and 0.541, respectively (Table 2). In summary, the grain-Cd model based on soil pH and total Cd can satisfactorily predict Cd concentrations in rice grains in some areas. Through the data analysis of the cited literature, it is found that the prediction model cannot predict grain-Cd in areas with high geological background, such as Guangxi Province and Guizhou Province. It may be due to the low availability of cadmium in those areas, and the content of cadmium that can be transferred by rice is less. It cannot be well predicted in other regions, which may be caused by the accumulation capacity of rice varieties or the addition of passivators. But these data are basically within the 1:1 line ± 0.5. Therefore, the prediction model may be able to adapt to most areas except those with high geological background.
a Grain-Cd model validation results using reference data from Guo et al. (2018a). b Grain-Cd model validation results using reference data from 10 articles; the number of reference data was 122. All data were described as means
The data pairs in Fig. 4a are from Guo et al. (2018a) in Table S3. These data pairs are all within the 1:1 line ± 0.5. This article studied the remediation effects of hydrated lime (L), hydroxyapatite (H), and organic fertilizer (F) on lightly (DY) and moderately (YX) Cd-contaminated farmland soils through pot experiments. The two prediction curves in Fig. 4a were obtained by substituting the Cd contents of mild and moderate cadmium-contaminated soil into (Eq. (10)). The prediction curves had a good correlation with the reported YX data. The R2 between the YX Cd-contaminated soil data and the prediction curves was 0.723. However, the R2 was 0.192 for DY. When the pH is lower than 6.0, the DY data pair is far from the prediction curve. It may be the application of high doses of organic fertilizer (f) reduced soil pH and increased soil CaCl2-Cd. Therefore, grain-Cd model can be widely and accurately predict the cadmium content in rice grains of most areas, especially acidic soil and acidic soil after increasing pH.
Our model takes into account the pH and total soil Cd, which greatly simplified model application but differences in the surface properties and metal affinities of different soils, such as SOC, OM, and clay mineral contents, from being taken into account. Cd concentrations in rice grains are mainly affected by soil pH, CEC, organic matter, total Cd content, etc., among which, soil pH, and total Cd content have a significant impact on Cd uptake by rice (Rafiq et al. 2014; Romkens et al. 2011). In most literatures (Romkens et al. 2011, 2009; Wen et al. 2020), the independent variables in the prediction model of grain-Cd include soil pH and total Cd content, while other soil properties are not necessarily provided. Although soil pH and total Cd content are the most important factors, other soil properties also affect Cd availability. Li et al. (2018) evaluated the influencing factors of Cd content in rice grains based on the relative importance of random forest (RF) factors. The results showed that in three soil types, soil pH was the most important physical and chemical factor affecting Cd accumulation in rice grains, and CEC, clay, OM, and other factors also affected Cd content in rice grains. Similarly, Yu et al. (2016) evaluated the contribution of soil properties to Cd contents in rice grains based on random forest (RF) and random gradient increase (SGB), indicating that soil pH is the most important factor, and amorphous iron oxide, CEC, OM, and other factors also affect Cd contents in rice grains. Therefore, only considering pH and total Cd concentration in soil and excluding other soil properties may be the main reason for the deviation between the predicted value of the model and the actual value. However, according to the model quality assessment results and the RF results of Li et al. (2018) and Yu et al. (2016), the uncertainty of soil properties (except soil pH and total cadmium content) has little impact on the model prediction results. The model may not be valid for soil and rice in some areas, but it can be easily adopted based on soil samples collected from specific sites or areas.
Risk threshold prediction of soil Cd
The harm from Cd pollution in main crops to human health is worrying, especially in rice. A large amount of research has been conducted on the Cd pollution of rice, especially the use of lime, biochar, and other passivators to treat acidic soil to reduce the Cd concentration in rice (Chen et al. 2018; Sui et al. 2020). However, the critical threshold for adjusting soil pH with different Cd concentrations by applying lime and other regulators is still unclear. Figure 5a shows the relationship between pH and grain-Cd. Each curve represents the change in grain-Cd with pH in soils with Cd. For soils with Cd concentrations less than 0.3 mg kg−1, if the pH value is lower than 5.21, rice may be at risk and needs to be screened. For soils with Cd concentrations greater than 3 mg kg−1, the soil pH is greater than 7.28, and rice may not be at risk. In addition, to predict grain-Cd risk based on soil pH and soil total Cd, the potential risk of grain-Cd was calculated by using the rice grain-Cd model and the soil environmental quality risk control standard for soil contamination of agricultural land (GB15618-2018). The pH risk values of soils with Cd concentrations of 0.3, 0.4, 0.6, 0.8, 1.5, 2, 3, and 4 mg kg−1 in Fig. 5a are 5.05, 5.33, 5.70, 6.02, 6.61, 6.89, 7.33, and 7.65, respectively. Figure 5b shows the relationship between the soil Cd contents and grain-Cd. Each curve represents the change in Cd concentration in soil with pH. The Cd risk values of soils with pH 5.5, 6.5, and 7.5 in Fig. 5b are 0.474, 1.28, and 3.32 mg kg−1, respectively.
A fitting analysis of the data of Chen et al. (2018) found that when the soil pH was adjusted to 5.87 in 2017, the Cd concentrations of rice grains did not exceed the national standard value of 0.2 mg kg−1. Equation (10) was used to predict that the soil pH in this article needs to be adjusted to approximately 5.84 (Fig. S2). The predicted value was close to the calculated value in the paper, within the allowable error range. When the total Cd concentration of the soil is reduced to 0.38 mg kg−1, the Cd of rice grains will reach the national standard (Fig. S2b). For the soil in the paper by Guo et al. (2018a), when the pH is increased to 5.93 or the total Cd in the soil is reduced to 0.41, the Cd of rice grains will meet the national standard (Fig. S2). For dangerous acidic soils, the pH value of the soil can be adjusted according to the regulator used (such as quicklime, hydrated lime, or calcium carbonate). It is also possible to use plant extraction technology to grow high-yield crops in mines, such as southeast sedum, solanum sedum, and sedum, to reduce the total Cd concentration in the soil. Both of these techniques can be applied to our model to make predictions, accurately obtain risk thresholds and achieve precise control.
Conclusion
In this paper, a model of the relationship between total soil Cd, pH, and Cd content in rice grains (grain-Cd model) was established through indoor simulation and pot experiments. The prediction effect of Cd in rice grains in soils with similar soil properties is satisfactory, and the quality of the model is satisfactory. The model can be applied to most acidic and neutral soil environments, and the soil risk can be quickly evaluated by obtaining the total soil Cd and soil pH. This has considerable advantages in assessing and predicting pH thresholds for the risk from Cd pollution in the soil. This can enable us to estimate the risk of Cd in rice before rice planting and propose effective and economical plans for adjusting soil pH in risk areas.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article (and its supplementary information fles).
References
Bai H, Luo M, Wei S, Jiang Z, He M (2020) The vital function of humic acid with different molecular weight in controlling Cd and Pb bioavailability and toxicity to earthworm (Eisenia fetida) in soil. Environ Pollut 261. https://doi.org/10.1016/j.envpol.2020.114222
Chaney RL (2015) How Does Contamination of Rice Soils with Cd and Zn Cause High Incidence of Human Cd Disease in Subsistence Rice Farmers. Curr Pollut Rep 1(1):13–22. https://doi.org/10.1007/s40726-015-0002-4
Chen H, Zhang W, Yang X, Wang P, McGrath SP, Zhao F-J (2018) Effective methods to reduce cadmium accumulation in rice grain. Chemosphere 207:699–707. https://doi.org/10.1016/j.chemosphere.2018.05.143
Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack FMG (2009) Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci Total Environ 407(13):3972–3985. https://doi.org/10.1016/j.scitotenv.2008.07.025
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, Groneberg DA (2006) The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol. https://doi.org/10.1186/1745-6673-1-22
Gu Q, Yang Z, Yu T, Ji J, Hou Q, Zhang Q (2019) Application of ecogeochemical prediction model to safely exploit seleniferous soil. Ecotoxicol Environ Saf 177:133–139. https://doi.org/10.1016/j.ecoenv.2019.03.084
Guo F, Ding C, Zhou Z, Huang G, Wang X (2018a) Effects of combined amendments on crop yield and cadmium uptake in two cadmium contaminated soils under rice-wheat rotation. Ecotoxicol Environ Saf 148:303–310. https://doi.org/10.1016/j.ecoenv.2017.10.043
Guo F, Ding C, Zhou Z, Huang G, Wang X (2018b) Stability of immobilization remediation of several amendments on cadmium contaminated soils as affected by simulated soil acidification. Ecotoxicol Environ Saf 161:164–172. https://doi.org/10.1016/j.ecoenv.2018.05.088
Halim MA, Majumder RK, Zaman MN (2015) Paddy soil heavy metal contamination and uptake in rice plants from the adjacent area of Barapukuria coal mine, northwest Bangladesh. Arab J Geosci 8(6):3391–3401. https://doi.org/10.1007/s12517-014-1480-1
Honma T, Ohba H, Kaneko-Kadokura A, Makino T, Nakamura K, Katou H (2016) Optimal soil Eh, pH, and water management for simultaneously minimizing arsenic and cadmium concentrations in rice grains. Environ Sci Technol 50(8):4178–4185. https://doi.org/10.1021/acs.est.5b05424
Jing F, Chen X, Wen X, Liu W, Hu S, Yang Z, Guo B, Luo Y, Yu Q, Xu Y (2020) Biochar effects on soil chemical properties and mobilization of cadmium (Cd) and lead (Pb) in paddy soil. Soil Use Manag 36(2):320–327. https://doi.org/10.1111/sum.12557
Kosolsaksakul P, Farmer JG, Oliver IW, Graham MC (2014) Geochemical associations and availability of cadmium (Cd) in a paddy field system, northwestern Thailand. Environ Pollut 187:153–161. https://doi.org/10.1016/j.envpol.2014.01.006
Li D, Li W, Lu Q, Li Y, Li N, Xu H, Ren Z, Zhang Y, Wang J (2018) Cadmium bioavailability well assessed by DGT and factors influencing cadmium accumulation in rice grains from paddy soils of three parent materials. J Soils Sediments 18(7):2552–2561. https://doi.org/10.1007/s11368-018-1950-2
Li L, Wu H, van Gestel CAM, Peijnenburg WJGM, Allen HE (2014) Soil acidification increases metal extractability and bioavailability in old orchard soils of Northeast Jiaodong Peninsula in China. Environ Pollut 188:144–152. https://doi.org/10.1016/j.envpol.2014.02.003
Li T, Chang Q, Yuan X, Li J, Ayoko GA, Frost RL, Chen H, Zhang X, Song Y, Song W (2017) Cadmium transfer from contaminated soils to the human body through rice consumption in southern Jiangsu Province, China. Environ Sci-Process Impacts 19(6):843–850. https://doi.org/10.1039/c6em00631k
Liu B, Wu C, Pan P, Fu Y, He Z, Wu L, Li Q (2019) Remediation effectiveness of vermicompost for a potentially toxic metal-contaminated tropical acidic soil in China. Ecotoxicol Environ Saf 182. https://doi.org/10.1016/j.ecoenv.2019.109394
Meharg AA, Norton G, Deacon C, Williams P, Adomako EE, Price A, Zhu Y, Li G, Zhao F-J, McGrath S, Villada A, Sommella A, De Silva PMCS, Brammer H, Dasgupta T, Islam MR (2013) Variation in rice cadmium related to human exposure. Environ Sci Technol 47(11):5613–5618. https://doi.org/10.1021/es400521h
Meng J, Zhong L, Wang L, Liu X, Tang C, Chen H, Xu J (2018) Contrasting effects of alkaline amendments on the bioavailability and uptake of Cd in rice plants in a Cd-contaminated acid paddy soil. Environ Sci Pollut Res 25(9):8827–8835. https://doi.org/10.1007/s11356-017-1148-y
Novotna M, Mikes O, Komprdova K (2015) Development and comparison of regression models for the uptake of metals into various field crops. Environ Pollut 207:357–364. https://doi.org/10.1016/j.envpol.2015.09.043
Pastorelli AA, Angeletti R, Binato G, Mariani MB, Cibin V, Morelli S, Ciardullo S, Stacchini P (2018) Exposure to cadmium through Italian rice (Oryza sativa L.): Consumption and implications for human health. J Food Compos Anal 69:115–121. https://doi.org/10.1016/j.jfca.2018.02.005
Qu X, Xu W, Ren J, Zhao X, Li Y, Gu X (2020) A field study to predict Cd bioaccumulation in a soil-wheat system: application of a geochemical model. J Hazard Mater 400. https://doi.org/10.1016/j.jhazmat.2020.123135
Rafiq MT, Aziz R, Yang X, Xiao W, Rafiq MK, Ali B, Li T (2014) Cadmium phytoavailability to rice (Oryza sativa L.) grown in representative Chinese soils. A model to improve soil environmental quality guidelines for food safety. Ecotoxicol Environ Saf 103:101–107. https://doi.org/10.1016/j.ecoenv.2013.10.016
Romkens PFAM, Brus DJ, Guo HY, Chu CL, Chiang CM, Koopmans GF (2011) Impact of model uncertainty on soil quality standards for cadmium in rice paddy fields. Sci Total Environ 409(17):3098–3105. https://doi.org/10.1016/j.scitotenv.2011.04.045
Romkens PFAM, Guo HY, Chu CL, Liu TS, Chiang CF, Koopmans GF (2009) Prediction of cadmium uptake by brown rice and derivation of soil-plant transfer models to improve soil protection guidelines. Environ Pollut 157(8–9):2435–2444. https://doi.org/10.1016/j.envpol.2009.03.009
Song W-E, Chen S-B, Liu J-F, Chen L, Song N-N, Li N, Liu B (2015) Variation of Cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by species-sensitivity distribution. J Integr Agric 14(9):1845–1854. https://doi.org/10.1016/s2095-3119(14)60926-6
Stohs SJ, Bagchi D, Hassoun E, Bagchi M (2001) Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol 20(2):77–88
Sui F, Wang J, Zuo J, Joseph S, Munroe P, Drosos M, Li L, Pan G (2020) Effect of amendment of biochar supplemented with Si on Cd mobility and rice uptake over three rice growing seasons in an acidic Cd-tainted paddy from central South China. Sci Total Environ 709. https://doi.org/10.1016/j.scitotenv.2019.136101
Suwatvitayakorn P, Ko M-S, Kim K-W, Chanpiwat P (2020) Human health risk assessment of cadmium exposure through rice consumption in cadmium-contaminated areas of the Mae Tao sub-district, Tak, Thailand. Environ Geochem Health 42(8):2331–2344. https://doi.org/10.1007/s10653-019-00410-7
Wang J, Lu X, Zhang J, Ouyang Y, Wei G, Xiong Y (2020) Rice intercropping with alligator flag (Thalia dealbata): a novel model to produce safe cereal grains while remediating cadmium contaminated paddy soil. J Hazard Mater 394. https://doi.org/10.1016/j.jhazmat.2020.122505
Wen Y, Li W, Yang Z, Zhuo X, Guan D-X, Song Y, Guo C, Ji J (2020) Evaluation of various approaches to predict cadmium bioavailability to rice grown in soils with high geochemical background in the karst region, Southwestern China. Environ Pollut 258. https://doi.org/10.1016/j.envpol.2019.113645
Xiao W, Ye X, Zhang Q, Chen D, Hu J, Gao N (2018) Evaluation of cadmium transfer from soil to leafy vegetables: influencing factors, transfer models, and indication of soil threshold contents. Ecotoxicol Environ Saf 164:355–362. https://doi.org/10.1016/j.ecoenv.2018.08.041
Xiao W, Ye X, Zhu Z, Zhang Q, Zhao S, Chen D, Fang X, Gao N, Hu J (2020) Evaluation of cadmium (Cd) transfer from paddy soil to rice (Oryza sativa L.) using DGT in comparison with conventional chemical methods: derivation of models to predict Cd accumulation in rice grains. Environ Sci Pollut Res 27(13):14953–14962. https://doi.org/10.1007/s11356-020-07976-1
Yu H-Y, Liu C, Zhu J, Li F, Deng D-M, Wang Q, Liu C (2016) Cadmium availability in rice paddy fields from a mining area: the effects of soil properties highlighting iron fractions and pH value. Environ Pollut 209:38–45. https://doi.org/10.1016/j.envpol.2015.11.021
Funding
This research was financially supported by the National Natural Science Foundation of China (42177012, 41771347) and the National Key R & D Program of China (2018YFD0800600).
Author information
Authors and Affiliations
Contributions
Hanyi Liu: conceptualization, methodology, data curation, formal analysis, writing — original draft, writing — review and editing.Yun Ling: resources, writing — review and editing. Na Liu: investigation. Ying Chen: investigation. Shiqiang Wei: conceptualization, methodology, writing — review and editing, supervision, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
This manuscript does not report on or involve the use of any animal or human data or tissue.
Consent to participate
Not applicable.
Consent for publication
All authors have consented to the submission of the manuscript to the journal.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Kitae Baek
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, H., Ling, Y., Liu, N. et al. The determination of regulating thresholds of soil pH under different cadmium stresses using a predictive model for rice safe production. Environ Sci Pollut Res 29, 88008–88017 (2022). https://doi.org/10.1007/s11356-022-21751-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21751-4