Abstract
The present work provides an insight into the development of biochemical adaptations in mung beans against ozone (O3) toxicity. The study aims to explore the O3 stress tolerance potential of mung bean genotypes under exogenous application of growth regulators. The seeds of twelve mung bean genotypes were grown in plastic pots under controlled conditions in the glasshouse. Six treatments, control (ambient ozone level 40–45 ppb), ambient O3 with ascorbic acid, ambient ozone with silicic acid, elevated ozone (120 ppb), elevated O3 with ascorbic acid (10 mM), and elevated ozone with silicic acid (0.1 mM) were applied. The O3 fumigation was carried out using an O3 generator. The results revealed that ascorbic acid and silicic acid application decreased the number of plants with foliar O3 injury symptoms in different degrees, i.e., zero, first, second, third, and fourth degrees; whereas 0–4 degree symptoms represent, no symptoms, symptoms occupying < 1/4, 1/4–1/2, 1/2–3/4, and > 3/4 of the total foliage area, respectively. Application of ascorbic acid and silicic acid also prevented the plants from the negative effects of O3 in terms of fresh as well as dry matter production, leaf chlorophyll, carotenoids, soluble proteins and ascorbic acid, proline, and malondialdehyde (MDA) contents. Overall, silicic acid application proved more effective in reducing the negative effects of O3 on mung bean genotypes as compared to that of the ascorbic acid. Three mung bean genotypes (NM 20–21, NM-2006, and NM-2016) were identified to have a better adaptive mechanism for O3 toxicity tolerance and may be good candidates for future variety development programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global climate change; an essential ecological concern is associated with many factors like temperature rise, alterations in precipitation patterns, and rising levels of greenhouse gases (Xu et al. 2021). Amongst greenhouse gases, the ever-increasing level of tropospheric O3 is causing significant losses in the productivity of crops as compared to any other air pollutant (Ren 2021). The highly oxidative gas O3 has established damaging effects on both plant growth and human health (Pires et al. 2014; Nuvolone et al. 2018). The O3 concentration has become doubled in the Northern Hemisphere since the preindustrial period (Yeung et al. 2019) and is currently increasing at a rate of 0.5–2% per year due to changes in the release of precursor compounds from industrial activities (Saunier and Blande 2019). Tropospheric O3 level is predicted to rise by a further 20% over the next 50 years (Prather et al. 2003). Rising O3 is also a hidden danger for food security and is believed to result in global yield losses of up to 16% depending upon crop species (Sharps et al. 2021).
The problem of climate change linked with rising greenhouse gas levels is particularly serious in developing countries like Pakistan (Hussain et al. 2020). Moreover, O3 damage is expected to expand more widely and efficiently in densely populated areas of Indo-Pakistan (Khan et al. 2016). Studies involving the O3 effects have mainly been focused on cereals and legumes in this (Indo-Pak) region (Tiwari and Agrawal 2009). Despite the fact that developing countries have a minimum contribution to greenhouse gas emissions, these are more threatened by climate change than developed countries. Hence, mitigation strategies to combat O3 pollution are of more importance to these regions (Sofia et al. 2020). The mitigating strategies may include exploration of adoptive germplasm, increasing tolerance to O3 pollution, uncovering plant tolerance mechanisms, nutrient management in plants under global change scenario, and exogenous supply of plant growth-promoting substances (Iriti et al. 2003; Soares et al. 2019).
Exogenous application of growth regulators to improve plant performance under unfavorable conditions has gained considerable attention from scientists (Khan et al. 2020). Many chemical compounds are known to provide protection to plants from O3 injury (Chaudhary and Rathore 2020). These include antioxidants, growth regulators, anti-senescent agents, and pesticides (Daripa et al. 2016). Significant progress has been reported regarding the use of protective chemicals in reducing the toxic effects of ozone during the last 2 decades (Tiwari and Agrawal 2009).
Silicic acid is found effective for plant growth and yield parameters when applied as a foliar spray at very minute quantities (Jardin 2015). It is cost-effective as well as eco-friendly and may act as a physical barrier for toxic elements after depositing the cell wall of different plant tissues (Muneer and Jeong 2015). Moreover, ascorbic acid is an influential antioxidant, which eradicates the stress-induced reactive oxygen species (ROS) and is recommended for improvement in crop plants, particularly under unfavorable environments (Bellini and Tullio 2019).
Mung bean {Vigna radiate (L.) Wilczek} is a preferred legume crop across Asia and other continents due to the presence of easily digestible proteins (Rani et al. 2018). In Pakistan, mung bean is grown on an estimated area of 127,500 ha. Keeping in view the rising global O3 concentration, it is time to consider the options for increasing the tolerance of crops to this abiotic stress. We need to explore or develop crop varieties with high productivity potential, suitable for predicted future climate changes. Hence, the present study has been conducted to investigate the relative performance of mung bean genotypes under rising O3 levels and to find out whether and up to what extent the applied silicic acid and ascorbic acid can ameliorate the negative effects of O3 on mung bean plants.
Materials and methods
Seeds of twelve available approved mung beans {Vigna radiate (L.) Wilczek} genotypes, NM-28, NM 13–1, NM19-19, NM 20–21, NM121-25, NM-51, NM-54, NM-92, NM-98, NM-2006, NM-2011, and NM-2016 were obtained from Nuclear Institute for Agriculture and Biology (NIAB), Faisalabad, Pakistan. The experiment was conducted in soil-filled pots under controlled conditions of a glasshouse at NIAB Faisalabad with six treatments and three replicates. The seed samples were sterilized with 0.2% HgCl2 before sowing. The study was conducted in a split-plot design with three replications of each experimental unit. After 3 weeks of germination, ascorbic acid (10 mM) and silicic acid (0.1 mM) were sprayed with the addition of 0.1% tween-20 as surfactant. Tween-20 is a nonionic hydrophilic surfactant that helps in the wetting of the leaf surface. The aqueous solution applied to the leaf surface alone would have fallen off and not adhered to the surface, whereas tween-20 helps in spreading the spray uniformly and sticking to the surface (Hassan et al. 2021). The plants were then exposed to elevated O3 levels (120 ppb) using an O3 generator (AOT-MD-500 model) for 4 h per day except for three treatments; control (O3 untreated ones), ambient O3 with ascorbic acid, and ambient O3 with silicic acid. The level of O3 was monitored in the glasshouse using an O3 meter UV photometry O3 analyzer (model: 0342e) and was maintained for 15 days (Table 4 provided in the supplementary file). Normal air was considered a control treatment.
The O3 damages were measured on the basis of percent injury to mature fully enlarged leaves. The foliage injury was calculated according to the equation used by Chang and Yu (2001) as follows:
where N0, N1, N2, N3, and N4 were the numbers of leaves with zero, first, second, third, and fourth degree symptoms of ozone injury, respectively; whereas 0–4 degree symptoms represent no symptoms, symptoms occupying < 1/4, 1/4–1/2, 1/2–3/4, and > 3/4 of the total foliage area, respectively. The injury was rated by visual estimation. The percentage of the injured area on each leaf was recorded according to a 0–100% scale in 5% increments as adopted by Madkour and Laurence (2002).
The harvested shoots and roots of all subjects were washed with tap water, blotted with brown paper towels, and dried at 70 °C for 72 h. The dry weight was recorded using an electric balance. Chlorophyll a, b, and total chlorophyll contents were determined using the method described by Arnon (1949). The optical density of acetone extracted samples was recorded at 645 and 663 nm using a spectrophotometer (Hitachi U-2001, Japan). Total carotenoid contents were calculated using the formula of Arnon (1949).
Protein estimation was made by the Bradford method (Bradford 1976). The absorbance of Bradford reagent treated samples was noted at 595 nm using the spectrophotometer (Hitachi U-2001, Japan). Total soluble sugars were measured following the Yemm and Willis (1954) method. The ethanolic extracted samples were treated with anthrone reagent, boiled and cooled. The absorbance was noted at 625 nm using a spectrophotometer (Hitachi U-2001, Japan).
MDA contents were calculated following the method of Cakmak and Horst (1991). Total phenolic contents (TPC) were calculated using the Folin–Ciocalteu reagent with some modifications of the Wolfe et al. (2003) method. Ascorbic acid was determined using the protocol of Mukherjee and Choudhuri (1983). For ascorbic acid estimation, TCA (6%) extracted material was treated with 2, 4-dinitrophenyl hydrazine (2%) and one drop of 10% thiourea. The mixture was boiled in a water bath, cooled and treated with sulfuric acid (80%). Absorbance was recorded at 530 nm using a spectrophotometer (Hitachi U-2001, Japan) with 6% TCA as blank. Leaf proline contents were determined following the method of Bates et al. (1973). The absorbance of the reaction solution was taken at 520 nm.
Statistical analysis
The collected data were subjected to statistical analysis to test the significance of differences among mean values using CoStat software version 6.303. Logarithmic transformations were carried out for data normalization, where necessary, prior to analysis. The relationships between various analyzed variables (Fig. 4) and principal component analysis (Fig. 5) were carried out using RStudio software.
Results
The shoot fresh weight value of all mung bean genotypes declined significantly under high tropospheric O3 levels as compared to control. Foliar application of ascorbic acid and silicic acid significantly improved (P ≤ 0.05) shoot fresh weight at ambient O3 level by 53 and 57%, respectively, while these also improved shoot fresh weight by 22 and 24%, respectively, in elevated O3 stressed plants as compared to O3 untreated plants. Results indicated that mung bean genotypes, NM 20–21, NM 2006, and NM 2016 showed a minimum reduction in shoot fresh weight under O3 stress (Fig. 1).
Effect of ascorbic acid and silicic acid on shoot fresh weight (a), shoot dry weight (b), root fresh weight (c), and root dry weight (d) of mung bean varieties under elevated O3 levels. T0 = ambient O3 level 40–45 ppb, T1 = ambient O3 + ascorbic acid, T2 = ambient O3 + silicic acid, T3 = elevated O3 level 120 ppb, T4 = elevated O3 level + ascorbic acid, T5 = elevated O3 level + silicic acid
Analysis of variance of data showed that root fresh weight of O3-treated plants decreased in mung bean genotypes used in the study, but the interaction between O3 level, varieties, and treatments was not found significant (Table 1). Foliar application of silicic acid and ascorbic acid increased root fresh weight up to 54 and 42%, respectively, in elevated O3 stressed plants while 64 and 62% in O3 unstressed plants (Fig. 1).
Shoot dry weight and root dry weight of all mung bean genotypes were decreased significantly under elevated O3. Foliar application of ascorbic and silicic acid under elevated O3 levels increased the shoot dry weight (P < 0.01) up to 32 and 41%, as compared to untreated plants (50 and 51%), respectively (Fig. 1). But the interaction between O3 level, varieties, and treatments was not found significant on root dry weight. The silicic acid foliar application was found to be more effective in improving the biomass of mung bean genotypes than that of ascorbic acid application.
Elevated O3 levels produced a remarkable decrease in chlorophyll a and b contents of mung bean genotypes. Results revealed that mung bean variety NM-51 and NM-98 showed a decrease in chlorophyll a and b contents, respectively. However, foliar application of ascorbic acid and silicic acid exhibited more significant improvement (P < 0.001) in chlorophyll a and chlorophyll b contents at an ambient level in NM-54 and NM-2011varieties, while genotype NM- 51 and NM 13–1 showed maximum improvement in chlorophyll a and chlorophyll b content under elevated O3 level, respectively (Fig. 2). Total chlorophyll contents were decreased in mung bean genotypes under a higher level of O3 treatment. Mean values of the attribute in all subjects indicated that NM-2016 produced maximum, while NM- 13–1 produced minimum total chlorophyll contents under O3 stress. Foliar application of both growth regulators increased significantly (P ≤ 0.001) total chlorophyll contents under O3 stress effectively (Fig. 2).
Effect of ascorbic acid and silicic acid on chlorophyll a (a), chlorophyll b (b), total chlorophyll (c), and carotenoids content (d) of mung bean varieties under elevated O3 levels. T0 = ambient O3 level 40–45 ppb, T1 = ambient O3 + ascorbic acid, T2 = ambient O3 + silicic acid, T3 = elevated O3 level 120 ppb, T4 = elevated O3 level + ascorbic acid, T5 = elevated O3 level + silicic acid
All the mung bean genotypes displayed differential responses against elevated O3 levels regarding carotenoid accumulation. Elevated O3 decreased the carotenoid contents in NM 13–1, NM 51, and NM 28, while all other genotypes depicted an increase in carotenoid contents. Both growth regulators helped to increase (P < 0.01). Carotenoid contents (20%) in O3 stressed and unstressed (43 and 33%) plants (Fig. 2). The silicic acid application was found to be more effective in improving the pigment contents of mung bean genotypes than that of the ascorbic acid application.
Exposure of mung bean plants to elevated O3 levels increased the foliar injury on the upper surface leaves. The foliar injury became more severe as plants grew older. Severe injury symptoms under elevated O3 stress were observed in the variety NM-51. The foliar injury symptoms in variety ranged between 36.45%. Foliar application of both growth regulators significantly (P < 0.001) proved to be helpful in reducing the O3-induced injury symptoms. Ascorbic acid and silicic acid application resulted in less injury in variety NM-92 (18.24%) and NM-2006 (17.33%), respectively, at ambient O3 levels, while NM-2016 (20.48%) and NM-2006 (20.83%) varieties showed less injury by ascorbic acid and silicic acid application under elevated O3 levels (Table 2).
Exogenous application of ascorbic acid and silicic acid enhanced the soluble protein contents in O3 treated plants as compared to untreated plants (Fig. 3), but the interaction between O3 level, varieties, and treatments was not found significant. Total soluble sugar contents decreased by 37% under O3 stress than that of O3 untreated plants. Ascorbic acid and silicic acid foliar application increased significantly (P ≤ 0.001) total soluble sugar contents by 33 and 29% in plants exposed to elevated O3 levels, while 56 and 45% in ambient O3 level exposed plants, respectively. Mung bean genotype NM-2016 displayed higher total soluble sugar contents, while NM-98 showed lower sugar contents as compared to all other genotypes (Fig. 3).
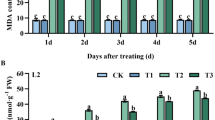
Effect of ascorbic acid and silicic acid on total soluble protein (a), total soluble sugars (b), proline (c), and MDA content (d) of mung bean varieties under elevated O3 leveld. T0 = ambient O3 level 40–45 ppb, T1 = ambient O3 + ascorbic acid, T2 = ambient O3 + silicic acid, T3 = elevated O3 level 120 ppb, T4 = elevated O3 level + ascorbic acid, T5 = elevated O3 level + silicic acid
The elevated level of O3 impacted the proline content in all twelve mung bean varieties. In variety NM- 98, a substantial increase due to O3 pollution was seen. Foliar application of ascorbic acid and silicic acid influenced this variable, but the interaction between O3 level, varieties, and treatments was not found significant (Fig. 3). MDA contents were increased by19% under O3 stress conditions. The response of all cultivars was different regarding leaf MDA contents under O3 stress with and without foliar application of growth regulators, but the interaction between O3 level, varieties, and treatments was not found significant in the study (Fig. 3). An increase in total phenolic contents (TPC) was recorded in all mung bean genotypes under O3 stress as compared to plants growing in the ambient O3 environment. Mung bean variety NM 20–21 showed the maximum value for TPC, while NM-51 showed the minimum value of TPC, but the effect of foliar application of ascorbic acid and silicic acid on TPC contents under elevated O3 levels was not found significant (Table 3). A higher value of ascorbic acid was recorded in variety NM-2006 than all other genotypes. Ascorbic acid contents of plants increased up to 27% under O3 stress as compared to the plants growing under the ambient level of O3; however, foliar application of growth regulators significantly (P ≤ 0.01) enhanced the ascorbic acid content (Table 3).
A Pearson’s correlation graph was also developed to depict a relationship between various growth and physio-biochemical parameters of different mung bean varieties under elevated O3 using the foliar application of ascorbic acid and silicic acid (Fig. 4). Leaf injury was positively correlated with each other but negatively correlated with all other morphological and physiological attributes in mung bean varieties. Although MDA contents were also negatively correlated with plant growth and biomass, photosynthetic efficiency positively correlated with leaf injury and soluble protein. This relationship depicted a close connection between plant growth and composition in mung bean varieties under O3 stress conditions.
Relationship between different variables of mung bean varieties under elevated O3 with application of ascorbic acid and silicic acid. Different abbreviations used are as follows: SFW, shoot fresh weight; SDW, shoot dry weight; TSS, total soluble sugar; AsA, ascorbic acid contents; RFW, root fresh weight; RDW, root dry weight; TC, total chlorophyll content; Carot, carotenoid content; L (1–5), leaves with 1–5% injury; L (2–25), leaves with 2–25% injury; L (>25), leaves with more than 25% injury; MDA, malondialdehyde content; SP, soluble protein
The loading plots of PCA to illustrate the effect of O3 toxicity under the application of ascorbic acid and silicic acid in various varieties of mung beans are presented in Fig. 5. In the whole database, Dim1 and Dim2 exhibited maximum contribution and occupy more than 61% of all databases. Among which, Dim1 exhibits 43.7%, and Dim2 exhibits 17.1%. All studied parameters were distributed successfully in the database, which is giving a clear indication that O3 stress causes a significant effect on the growth and physiology of all mung bean varieties. From the results, it can be indicated that leaf injuries are positively correlated; malondialdehyde, total protein, and soluble protein contents were almost neutral in the database, and shoot fresh weight, shoot dry weight, total soluble sugar, ascorbic acid content, root fresh weight, root dry weight, total chlorophyll content, and carotenoid content are negatively correlated with other studied attributes in the whole database.
Loading plots of principal component analysis (PCA) on various attributes (morphological and physiological) of different varieties of mung bean under elevated O3 with foliar application of ascorbic acid and silicic acid. Different abbreviations are as follows: SFW, shoot fresh weight; SDW, shoot dry weight; TSS, total soluble sugar; AsA, ascorbic acid contents; RFW, root fresh weight; RDW, root dry weight; TC, total chlorophyll content; Carot, carotenoid content; L (1–5), leaves with 1–5% injury; L (2–25), leaves with 2–25% injury; L (>25), leaves with more than 25% injury; MDA, malondialdehyde content; SP, soluble protein
Discussion
The significant reduction in shoot and root fresh and dry matter under elevated O3 was recorded during the present investigation and it could be related to altered partitioning of assimilates among different plant parts (Ghosh et al. 2020) due to a decrease in photosynthesis and carbon allocation (Emberson et al. 2018). The positive effects of silicic acid on plant growth and biomass production could be due to the involvement of silicon in cell division and elongation processes as well as in improvements in photosynthesis and light interception (Farooq et al. 2015). Moreover, the role of ascorbic acid as a cofactor of dioxygenases during the biosynthesis of auxins (IAA) and gibberellins (GA) had been documented (Mahmood and Dunwell 2020). Hence, enhanced plant growth in terms of biomass production as observed during the present study could be due to stimulation of IAA and GA synthesis by exogenous ascorbic acid supply, which in turn increased plant biomass production.
Pigment determination results of the current study indicated that chlorophyll and carotenoid contents were decreased under O3 stress in all mung bean genotypes. The decrease in chlorophyll and carotenoid contents under higher O3 levels is due to damage to mesophyll cells (Maamar et al. 2015; Fatima et al. 2019). Our results also indicated the positive effects of ascorbic acid application on chlorophyll and carotenoids contents are due to the scavenging of reactive oxygen species (ROS) in leaves either by reducing their diffusion or increasing their detoxification under O3 stress (Akram et al. 2017). Similarly, the silicic acid application was more effective in enhancing chlorophyll and carotenoid contents as recorded during the current investigation and the same has been reported by Maghsoudi et al. (2015).
The foliar injury symptoms due to excessive tropospheric O3 as recorded during the current experiment had also been reported by Leung et al. (2020). The results of the present investigation also indicated purple or brown stipples, necrosis, tip or margin burns, and abscission of leaves due to elevated O3 levels (Feng et al. 2014). Visible foliar injury symptoms (chlorosis) could be due to the breakdown of chlorophyll molecules as elevated O3 induced oxidative burst in leaves and excessive accumulation of ROS leading to leaf chlorosis and necrosis (Sharps et al. 2021). Palisade mesophyll cells are the principal target of O3 damage, according to studies. Furthermore, palisade cells in plants exposed to O3 were substantially shorter than those in plants kept in filtered air. The presence of clusters of necrotized and collapsed cells in which only the remains of the organelles could be seen was verified by ultrastructural studies of mesophyll. Programmed cell death and other necrosis had occurred in some of the cells that formed the lesion. The aberrant growth of enlarged thylakoids was the most evident change produced by prolonged O3 exposure, as measured in (Faoro and Iriti 2005; Faoro and Iriti 2009). The protective role of ascorbic acid for photosynthetic pigments and other cellular components from oxidative damage as evident from the results of the current experiment is due to its detoxification capacity of ROS produced under O3 stress (Ardebili et al. 2015). Moreover, the silicic acid foliar application also increased the activities and accumulation of ROS detoxifying enzymes and antioxidants (Moradtalab et al. 2018) and hence prevented the plants from negative effects of elevated O3 in terms of foliar injury.
A decrease in total soluble sugar contents of mug beans growing under elevated O3 levels is likely to be the effect of ozone on photosynthesis, carbohydrates distribution, and gas exchange (Grulke and Heath 2020), which decreases the overall metabolic turnover in the cell. Moreover, Sanches et al. (2019) reported a decrease in carbohydrates of potato leaves at a concentration of 60 ppb O3 due to the damage to the outer membrane of the cell. The positive effects of the applied growth regulators as recorded during the present study had also been reported by other researchers. For instance, Coskun et al. (2016) reported that silicic acid applications resulted in higher soluble sugar content in plants. Likewise, Mekki et al. (2015) reported that ascorbic acid application increased the total soluble sugars that might be due to the significant increase in photosynthetic pigments, which are reflected in the photosynthesis process and led to increases in sugar contents.
The increasing trend of soluble proteins in mung beans growing under elevated O3 levels as observed during the current study had also been reported in other crops such as soybean (Ahsan et al. 2010). The silicic acid foliar application further enhanced the protein contents, which may be the result of a reduction in oxidative damage of cells caused by higher O3 levels as compared to ambient conditions (Ma and Takahashi 2002). In addition, protein contents had also been steadily increased by exogenous application of ascorbic acid that might be due to increased nitrogen; the building block of protein synthesis (Ejaz et al. 2012).
The level of O3 exposure has been demonstrated to correlate with MDA concentration, which measures the condition and integrity of a membrane by measuring the degree of lipid peroxidation. Furthermore, O3 treated plants had higher MDA levels, demonstrating that the status of membrane lipid peroxidation is related to the level of O3 exposure in plants (Iglesias et al. 2006). MDA may harm plant cells by covalently altering the thiol groups of peptides and interacting with other biological components. Moreover, most nonenzymatic lipid peroxidation products initially accumulate within membranes and can disrupt the selective permeability of the lipid bilayer (Sattler et al. 2006). The effectiveness of the exogenous application of ascorbic acid in decreasing the MDA content under O3 stress as recorded during the current investigation is due to its enhancing membrane characteristics ability and antioxidative potential (Elkeilsh et al. 2019). Similarly, silicon application had been reported to reduce MDA contents by decreasing the plasma membrane permeability of leaf cells (Hasanuzzaman et al. 2020) and hence can protect the plants from the damaging effects of O3 as indicated by the results of the present study.
Proline levels in O3 contaminated mung bean leaves increased considerably in the current study because proline helps to stabilize subcellular structures (e.g., membranes and proteins), scavenge free radicals, and buffer cellular redox potential under stress circumstances (Ashraf and Foolad 2007). However, Hemmati et al. (2018) observed that the application of ascorbic acid-induced tolerance to stress reduced the reactive oxygen species by increasing antioxidant enzyme activity. Silicon foliar application increased proline levels in plants as compared to those treated with O3 stress because proline is considered a biochemical indicator of stress and an osmotic regulator (Mauad et al. 2016). The enhancement in total phenolic contents in mung bean genotypes due to O3 stress pointed toward a link between increased phenolic content and impaired growth. It had been suggested that the allocation of carbon in O3 exposed plants was changed toward the formation of phenolics (Wang et al. 2018). Furthermore, phenolics also act as antioxidants and show protective properties against ROS (Phuyal et al. 2020). The increase in total phenolics due to foliarly applied ascorbic could be explained by the fact that ascorbic acid activates some kind of gene regulatory mechanism to stimulate the functioning of nonenzymatic antioxidants (Halimeh et al. 2013). Similarly, silicon application had been found to improve secondary metabolism by promoting the formation of phenolic compounds and structural functions (Filha et al. 2011).
The results obtained from the present study establish differential responses of all twelve mung bean genotypes with the foliar application of two different growth regulators under high O3 levels (120 ppb). Four genotypes of mung bean (NM 28, NM 13–1, NM-51, and NM-98) were found to be sensitive; five genotypes (NM 19–19, NM 121–25, NM-54, NM-92, and NM-2011) were moderately sensitive, while three genotypes (NM 20–21, NM-2006, and NM-2016) tolerated O3 stress. Foliar application of ascorbic acid and silicic acid remunerated the negative effects of O3 pollution by restructuring the metabolic flux in terms of reduced visible injury, growth maintenance, and an upturn of antioxidant profile. The application of 0.1 mM silicic acid was found to be more operative than 10 mM ascorbic acid in reducing the negative effects of O3 damage in the subject genotypes of mung bean. The O3 stress tolerance behavior of the signposted genotypes (NM 20–21, NM-2006, and NM-2016) may be explored at a molecular level in the future and could be used as blood in varietal development programs of mung beans in the country.
References
Ahsan N, Nanjo Y, Sawada H, Kohno Y, Komatsu S (2010) Ozone stress-induced proteomic changes in leaf total soluble and chloroplast proteins of soybean reveal that carbon allocation is involved in adaptation in the early developmental stage. Proteomics 10(14):2605–2619
Akram NA, Shafiq F, Ashraf M (2017) Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Science 8:613
Ardebili ZO, Ardebili NO, Jalili S, Safiallah S (2015) The modified qualities of basil plants by selenium and/or ascorbic acid. Turk J Bot 39(3):401–407
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris Plant Physiol 24(1):1
Ashraf MFMR, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59(2):206–216
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Bellini E, De Tullio MC (2019) Ascorbic acid and ozone: novel perspectives to explain an elusive relationship. Plants 8(5):122
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83(3):463–468
Chang YS, Yu MR (2001) Correlation between ozone resistance and relative chlorophyll fluorescence or relative stomatal conductance of bedding plants. Bot Bull Acad Sin 42:265–272
Chaudhary IJ, Rathore D (2020) Relative effectiveness of ethylenediurea, phenyl urea, ascorbic acid and urea in preventing groundnut (Arachis hypogaea L) crop from ground level ozone. Environ Technol Innov 19:100963
Coskun D, Britto DT, Huynh WQ, Kronzucker HJ (2016) The role of silicon in higher plants under salinity and drought stress. Front Plant Sci 7:1072
Daripa A, Bhatia A, Ojha S, Tomer R, Chattaraj S, Singh KP, Singh SD (2016) Chemical and natural plant extract in ameliorating negative impact of tropospheric ozone on wheat crop: a case study in a part of semiarid north west india. Aerosol Air Qual Res 16(7):1742–1756
Du Jardin P (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic 196:3–14
Ejaz B, Sajid ZA, Aftab F (2012) Effect of exogenous application of ascorbic acid on antioxidant enzyme activities, proline contents, and growth parameters of Saccharum spp. hybrid cv. HSF-240 under salt stress. Turk J Biol 36(6):630–640
Elkeilsh A, Awad YM, Soliman MH, Abu-Elsaoud A, Abdelhamid MT, El-Metwally IM (2019) Exogenous application of β-sitosterol mediated growth and yield improvement in water-stressed wheat (Triticum aestivum) involves up-regulated antioxidant system. J Plant Res 132(6):881–901
Emberson LD, Pleijel H, Ainsworth EA, Van den Berg M, Ren W, Osborne S, Büker P (2018) Ozone effects on crops and consideration in crop models. Eur J Agron 100:19–34
Faoro F, Iriti M (2005) Cell death behind invisible symptoms: early diagnosis of ozone injury. Biol Plant 49(4):585–592
Faoro F, Iriti M (2009) Plant cell death and cellular alterations induced by ozone: key studies in Mediterranean conditions. Environ Pollut 157(5):1470–1477
Farooq MA, Saqib ZA, Akhtar J (2015) Silicon-mediated oxidative stress tolerance and genetic variability in rice (Oryza sativa L.) grown under combined stress of salinity and boron toxicity. Turk J Agric For 39(5):718–729
Fatima A, Singh AA, Mukherjee A, Dolker T, Agrawal M, Agrawal SB (2019) Assessment of ozone sensitivity in three wheat cultivars using ethylenediurea. Plants 8(4):80
Feng Z, Sun J, Wan W, Hu E, Calatayud V (2014) Evidence of widespread ozone-induced visible injury on plants in Beijing, China. Environ Pollut 193:296–301
Filha MX, Rodrigues F, Domiciano G, Oliveira H, Silveira P, Moreira W (2011) Wheat resistance to leaf blast mediated by silicon. Australas Plant Pathol 40(1):28–38
Ghosh A, Agrawal M, Agrawal S B (2020) Examining the effectiveness of biomass-derived biochar for the amelioration of tropospheric ozone-induced phytotoxicity in the Indian wheat cultivar HD 2967. J Hazard Mater 124968. https://doi.org/10.1016/j.jhazmat.2020.124968
Grulke NE, Heath R (2020) Ozone effects on plants in natural ecosystems. Plant Biol 22:12–37
Halimeh R, Mahlagh G, Maryam P, Pazoki A (2013) Effect of drought interactions with ascorbate on some biochemical parameters and antioxidant enzymes activities in Dracocephalum moldavica L. Middle East J Sci Res 13:522–531
Hasanuzzaman M, Bhuyan M, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9(8):681
Hassan A, Amjad SF, Saleem MH, Yasmin H, Imran M, Riaz M, Alyemeni MN (2021) Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J Biol Sci 28(8):4276–4290
Hemmati K, Ebadi A, Khomari S, Sedghi M (2018) Influence of ascorbic acid and 24-epibrassinolide on physiological characteristics of pot marigold under water-stress condition. J Plant Interact 13(1):364–372
Hussain M, Butt AR, Uzma F, Ahmed R, Irshad S, Rehman A, Yousaf B (2020) A comprehensive review of climate change impacts, adaptation, and mitigation on environmental and natural calamities in Pakistan. Environ Monit and Assess 192(1):1–20
Iglesias DJ, Calatayud Á, Barreno E, Primo-Millo E, Talon M (2006) Responses of citrus plants to ozone: leaf biochemistry, antioxidant mechanisms and lipid peroxidation. Plant Physiol Biochem 44(2–3):125–131
Iriti M, Rabotti G, De Ascensao A, Faoro F (2003) Benzothiadiazole-induced resistance modulates ozone tolerance. J Agric Food Chem 51(15):4308–4314
Khan MA, Khan JA, Ali Z, Ahmad I, Ahmad MN (2016) The challenge of climate change and policy response in Pakistan. Environ Earth Sci 75(5):412
Khan N, Bano AM, Babar A (2020) Impacts of plant growth promoters and plant growth regulators on rainfed agriculture. PLoS ONE 15(4):e0231426
Ma JF, Takahashi E (2002) Soil, fertilizer, and plant silicon research in Japan. Elsevier, Amsterdam
Leung F, Pang J, Tai AP, Lam T, Tao DK, Sharps K (2020) Evidence of ozone-induced visible foliar injury in Hong Kong using Phaseolus vulgaris as a bioindicator. Atmosphere 11(3):266
Maamar B, Maatoug M, Iriti M, Dellal A (2015) Physiological effects of ozone exposure on De Colgar and Rechaiga II tomato (Solanum lycopersicum L.) cultivars. Environ Sci Pollut Res 22(16):12124–12132
Madkour SA, Laurence JA (2002) Egyptian plant species as new ozone indicators. Environ Pollut 120(2):339–353
Maghsoudi K, Emam Y, Ashraf M (2015) Influence of foliar application of silicon on chlorophyll fluorescence, photosynthetic pigments, and growth in water-stressed wheat cultivars differing in drought tolerance. Turk J Bot 39(4):625–634
Mahmood AM, Dunwell JM (2020) 2-oxoglutarate-dependent dioxygenases: a renaissance in attention for ascorbic acid in plants. PloS One 15(12):e0242833
Mauad M, Crusciol CAC, Nascente AS, Grassi H, Lima GPP (2016) Effects of silicon and drought stress on biochemical characteristics of leaves of upland rice cultivars. Rev Cienc Agron 47:532–539
Mekki BE-D, Hussien H-A, Salem H (2015) Role of glutathione, ascorbic acid and α-tocopherol in alleviation of drought stress in cotton plants. Int J Chemtech Res 8(4):1573–1581
Moradtalab N, Weinmann M, Walker F, Höglinger B, Ludewig U, Neumann G (2018) Silicon improves chilling tolerance during early growth of maize by effects on micronutrient homeostasis and hormonal balances. Front Plant Sci 9:420
Mukherjee SP, Choudhuri MA (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58(2):166–170
Muneer S, Jeong BR (2015) Proteomic analysis of salt-stress responsive proteins in roots of tomato (Lycopersicon esculentum L.) plants towards silicon efficiency. Plant Growth Regul 77(2):133–146
Nuvolone D, Petri D, Voller F (2018) The effects of ozone on human health. Environ Sci Pollut Res 25(9):8074–8088
Phuyal N, Jha PK, Raturi PP, Rajbhandary S (2020) Total phenolic, flavonoid contents, and antioxidant activities of fruit, seed, and bark extracts of Zanthoxylum armatum DC. The Sci World J. https://doi.org/10.1155/2020/8780704
Pires JCM, Souza A, Pavão HG, Martins FG (2014) Variation of surface ozone in Campo Grande, Brazil: meteorological effect analysis and prediction. Environ Sci Pollut Res 21(17):10550–10559
Prather M, Gauss M, Berntsen T, Isaksen I, Sundet J, Bey I, Stevenson D (2003) Fresh air in the 21st century? Geophys Res Lett 30(2). https://doi.org/10.1029/2002GL016285
Rani S, Schreinemachers P, Kuziyev B (2018) Mungbean as a catch crop for dryland systems in Pakistan and Uzbekistan: a situational analysis. Cogent Food Agric 4(1):1499241
Ren J (2021) Effects of O3 pollution near formation on crop yield and economic loss. Environ Technol Innov 22:101446
Sanches RFE, Silva APOD, Costa VPD, Carvalho MÂMD, Silva EAD (2019) Changes in carbohydrates accumulation in Viguiera discolor Baker in response to water deficit. Hoehnea 46(4). https://doi.org/10.1590/2236-8906-38/2019
Sattler SE, Mene-Saffrane L, Farmer EE, Krischke M, Mueller MJ, DellaPenna D (2006) Non-enzymatic lipid peroxidation reprograms gene expression and activates defense markers in Arabidopsis tocopherol-deficient mutants. Plant Cell 18(12):3706–3720
Saunier A, Blande JD (2019) The effect of elevated ozone on floral chemistry of Brassicaceae species. Environ Pollut 255:113257
Sharps K, Hayes F, Harmens H, Mills G (2021) Ozone-induced effects on leaves in African crop species. Environ Poll 268:115789
Soares JC, Santos CS, Carvalho SM, Pintado MM, Vasconcelos MW (2019) Preserving the nutritional quality of crop plants under a changing climate: importance and strategies. Plant Soil 443(1):1–26
Sofia D, Gioiella F, Lotrecchiano N, Giuliano A (2020) Mitigation strategies for reducing air pollution. Environ Sci Pollut Res 27(16):19226–19235
Tiwari S, Agrawal M (2009) Protection of palak (Beta vulgaris L. var Allgreen) plants from ozone injury by ethylenediurea (EDU): roles of biochemical and physiological variations in alleviating the adverse impacts. Chemosphere 75(11):1492–1499
Wang H, Zhang L, Ma X, Zou J, Siemann E (2018) The effects of elevated ozone and CO2 on growth and defense of native, exotic and invader trees. J Plant Ecol 11(2):266–272
Wolfe K, Wu X, Liu RH (2003) Antioxidant activity of apple peels. J Agric Food Chem 51(3):609–614
Xu S, Wang Y, Zhang W, Li B, Du Z, He X, Li M (2021) Experimental warming alleviates the adverse effects from tropospheric ozone on two urban tree species. Environ Pollution 268:115289
Yemm EW, Willis AJ (1954) The estimation of carbohydrate in the plant extract by anthrone reagent. J Biochem 57:508–514
Yeung LY, Murray LT, Martinerie P, Witrant E, Hu H, Banerjee A, Chappellaz J (2019) Isotopic constraint on the twentieth-century increase in tropospheric ozone. Nature 570(7760):224–227
Acknowledgements
We acknowledge the Chairman Department of Botany GCUF and the Nuclear Institute for Agriculture and Biology (NIAB) for helping in designing and executing the experiments.
Funding
The corresponding author is thankful to the Government College University Faisalabad Pakistan for providing funds to execute this project.
Author information
Authors and Affiliations
Contributions
Conceptualization and funding acquisition, NI; methodology and lab work, ES and MN; project administration, MN and NI; supervision and technical support, MA and MJA; original draft writing, ES and MN.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shahzadi, E., Nawaz, M., Adrees, M. et al. Elevated ozone phytotoxicity ameliorations in mung bean {Vigna radiata (L.) Wilczek} by foliar nebulization of silicic acid and ascorbic acid. Environ Sci Pollut Res 29, 69680–69690 (2022). https://doi.org/10.1007/s11356-022-20549-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20549-8