Abstract
As emerging pollutants, direct and indirect adverse impacts of micro(nano)plastics (MPs/NPs) are raising an increasing environmental concern in recent years due to their poor biodegradability and difficulty in recycling. MPs/NPs can act as carriers of bacteria, viruses, or pollutants (such as heavy metals and toxic organic compounds), and may potentially change the toxicity and bioavailability of pollutants. Ingested or attached MPs/NPs can also be transferred from low-trophic level organisms to high-nutrient organisms or even the human body through the food chain transfer process. This article reviews the emerging field of micro- and nanoplastics on organisms, including the separate toxicity and toxicity of compound after the adsorption of organic pollutants or heavy metals, as well as possible mechanism of toxicological effects and evaluate the nano- and microplastics potential adverse effects on human health. The inherent toxic effects MPs/NPs mainly include the following: physical injury, growth performance decrease and behavioral alteration, lipid metabolic disorder, induced gut microbiota dysbiosis and disruption of the gut’s epithelial permeability, neurotoxicity, damage of reproductive system and offspring, oxidative stress, immunotoxicity, etc. Additionally, MPs/NPs may release harmful plastic additives and toxic monomers such as bisphenol A, phthalates, and toluene diisocyanate. The vectors’ effect also points out the potential interaction of MPs/NPs with pollutants such as heavy metals, polycyclic aromatic hydrocarbons, organochlorine pesticides, polychlorinated biphenyls, perfluorinated compounds, pharmaceuticals, and polybrominated diphenyl ethers. Nevertheless, these potential consequences of MPs/NPs being vectors for contaminants are controversial.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Micro(nano)plastics, as emerging contaminants, attract the increasing interest of researchers due to their accumulation and biological persistence in environment and undoubtedly wide distribution in all the compartments causing ineluctable human exposure. The term of microplastics (MPs) was first proposed by Thompson et al. (2004), generally with the size range of 100 nm to 5 mm in diameters (Alimi et al., 2018). Microplastics in the environment are a kind of compound pollutants with uneven spatial distribution owing to their unique physical and chemical properties (such as size, shape, color, weathered, and density) and multiple dynamic characteristics because of continuous changes of various environmental conditions in nature, and the chemical stability and high heterogeneity of these compounds which make the characterization and environmental analysis unreliable (Browne et al. 2011). In the human body, the inhaled microplastic fibers (polyester) were taken up by lung tissue and may be related to tumors (Pauly et al. 1998). The disperse blue dyes of polyester and acrylic fibers had been shown to cause dermatitis (Pratt and Taraska. 2000). The bioavailability of release monomers, dispersive dyes, mordants, and plasticizers from manufacture and sorbed contaminants from sewage is likely to be greater from fibers of polyester and acrylic, compared to the more hydrophobic microplastics (e.g., polyethylene, polypropylene) that have more heterogenic atoms (Browne et al. 2008). Nanoplastics (NPs) are the particles unintentionally produced (i.e., from the degradation and the manufacturing of the plastic objects) of size range from 1 to 1000 nm and with a colloidal behavior (Gigault et al. 2018). The presence of microplastics with size of 1.6 μm confirmed the possibility of the decomposition of microplastics into nanoplastics (Galgani et al. 2010). The clear evidence for the toxicological potential of microplastics (nanoplastics) is complex and multifaceted, encompassing primarily physical damage, chemical, biological, and ecological toxicity. Microplastics ingested by microorganisms can decrease ingestion rates (Kaposi et al. 2014; Sıkdokur et al. 2020), cut the internal organs of the organism, and lead to intestinal inflammation (Provencher et al. 2010). NPs can be transferred through the digestive tract of organisms to the circulatory and immune system, even visceral and tissue cells, and affect innate immune system (Elizalde-Velázquez et al. 2020).
Aside from causing adverse implications per se, micro(nano)plastics (MPs/NPs) can release additives and monomers to the organisms or biota, which represent more toxic sources of pollution than the MPs (or NPs) themselves (Wardrop et al. 2016; Liu et al. 2020). Moreover, MPs/NPs can act as carriers of chemical pollutants, and transfer pollutants to organisms through enrichment, transport, diffusion, and biological absorption, posing a great threat to aquatic and terrestrial organisms. The toxicological interaction between microplastics, nanoplastics, and other environmental pollutants is of great concern. MPs/NPs are well-known to interact with heavy metals (Zhou et al. 2019; Khan et al. 2015; Luís et al. 2015; Rochman et al. 2014), polycyclic aromatic hydrocarbons (Oliveira et al. 2013; Avio et al. 2015; Kleinteich et al. 2018; Karami et al. 2016; Ma et al. 2016), organochlorine pesticides (Ogata et al. 2009), polybrominated diphenyl ethers (PBDEs) (Wardrop et al. 2016) and pharmaceuticals (Fonte et al. 2016; Guilhermino et al. 2018), etc. Contaminants initially adsorbed on the surface of microplastics are released when microplastics are ingested and remain in the human body with lower pH, higher temperatures, and the presence of digestive surfactants. Furthermore, among these contaminants, some are toxic, mutagens, endocrine disruptors, and bio-amplifiers transferred through nutrients (Cole et al. 2011). A recent study found that pigmented microplastic fragments (5–10 µm in size) were detected in human placenta samples collected from six consenting women with uneventful pregnancies by Raman microspectroscopy (Ragusa et al. 2021), which may trigger immune responses (Wright and Kelly. 2017) and the release of toxic contaminants causing adverse pregnancy and fetus outcomes (Jiang et al. 2020). In addition to adversely affecting individual organisms, ingested or adhering MPs/NPs can be transferred from low-nutrient organisms to high-nutrient organisms and even humans through food chain transfer processes, causing a potential menace to organisms and human health (Catarino et al. 2018). Nevertheless, to date, the impact of MPs/NPs on environmental pollution and human health remains controversial. This may be that the dose effectiveness or time effectiveness concerning MPs/NPs has not yet been explored. Moreover, the sorption behaviors of bacteria, viruses, or chemicals on MPs/NPs have a bearing on plastic size, types, and other chemical or physical properties as well as ambient environment (Endo et al. 2005; Kang et al. 2021; Moresco et al 2021; Mughini-Gras et al. 2021). However, together with poor biodegradability and ubiquitousness, research of nano- and microplastics influence in this field is realistic and urgent. Although there have been few reports of direct relevance to humans, this review brings together increasing evidence that exposure to nano- or microplastics can directly or indirectly interfere with various biological functions of organisms. In this study, toxic effects of nano- and microplastics on organisms are reviewed, including the separate toxicity and toxicity of compound after the adsorption of organic pollutants, heavy metals or pathogens, and the potential adverse effects of MPs/NPs on human health, as well as possible mechanism of toxicological effects from the perspectives of laboratory exposure, field observation, or model simulation.
The inherent toxic effects of microplastics/nanoplastics
The accumulation of ingested or adhered microplastics in organisms will lead to various physical and chemical damages, and ultimately may result in unpredictable risks to the ecosystem. Exposed microplastics can cause change of predation behavior, obstruction of the digestive tract, and induced satiety, which may lead to decreased growth and development and even survival rates (Wright et al. 2013; Luís et al. 2015). For mussels (Mytilus edulis), microplastics may affect their growth and development, and have immunotoxicity (Canesi et al. 2015; Détrée and Gallardo-Escárate, 2018), genotoxicity (Avio et al. 2015) and neurotoxicity (Avio et al. 2015; Magni et al. 2018). After a period of exposure to water containing PS, the filtration rate decreased (Wegner et al. 2012) and energy expenditure increased by 25% in mussels (Van Cauwenberghe et al. 2015). In addition, exposure to microplastics induced a significant increase of granulocytes then a strong inflammatory response, and caused the lysosomal membrane less stability as the exposure time increased in mussel (von Moos et al. 2012). PE and PS could damage mussels’ immune system and the stability of lysosomal membrane, and have toxic effects of immune system and nervous system (Avio et al. 2015). For fish common goby Pomatoschistus microps (P. microps), poor predatory performance was found when exposed to microplastics (Fonte et al. 2016). The accumulation of microplastics in medaka can also lead to liver glycogen depletion and intrahepatic fat vacuoles (Rochman et al. 2013a). However, it has also been reported that goldfish (Carassius auratus) can quickly excrete microplastics, so that microplastics will not accumulate in their gut (Grigorakis et al. 2017), suggesting that microplastics have different effects on fish. Nevertheless, the presence of micro(nano)plastics may benefit organisms. With the presence of microplastics, algae (Raphidocelis subcapitata) in the exposed medium grew more (Canniff and Hoang 2018). Microplastics had a negative effect on the reproduction of cladoceran Daphnia magna at low algal concentrations, while a positive effect at high algal concentrations (Ogonowski et al. 2016). Li et al. (2020a, b) reported low concentrations of nanoplastics (5 mg/L) may enhance the viability of juvenile shrimp, whereas high concentrations (10, 20, 40 mg/L) had inhibitory and/or toxic effects.
Nanoplastics can be absorbed by living organisms and accumulated in the body and transferred along the food chain. Nanoplastics can penetrate through cell membranes and get into the circulatory system and lymphatic tissue system of the organisms, leading to stunted growth and development, decreased reproductive capacity, genetic toxicity, immunotoxicity, decreased survival rate, increased mortality, etc. The algal species Pseudokirchneriella subcapitata (Nolte et al. 2017), Chlorella spp., and Scenedesmus spp. (Bhattacharya et al. 2010) can adsorb nanoplastics on their surface due to electrostatic interaction. This hindered the absorption and utilization of carbon dioxide and photons, thereby decreasing the photosynthetic efficiency and thus inhibiting algal growth (Bhattacharya et al. 2010). Toxicity to organisms largely relied on the particle size and surface chemical properties of the nanoplastics. Nanoplastics with positive charge have more significant effect on normal physiological activities of cells than those with negative charge. Nanoplastics can lead to oxidative damage though decreasing antioxidant capacity and/or increasing reactive oxygen species (ROS) production (Bhattacharya et al. 2010). The toxicity of polystyrene nanoplastics (Nano-PS) is variable but is related with feeding behavior (Wegner et al. 2012; Cedervall et al. 2012), oxidative stress, neurotoxicity, immunotoxicity, and energy imbalance in aquatic species (Liu et al. 2021, 2018; Pitt et al. 2018a, 2018b; Veneman et al. 2017). Previous data have shown that Nano-PS (34–40 nm) can accumulate in zebrafish embryos under environmental exposures or maternal transplantation and affect the developing heart, the antioxidant system, and the locomotor activity of the larvae (Pitt et al. 2018a, 2018b). Nano-PS can have longer intestinal residence time than polystyrene microparticles (Ward and Kach, 2009). Polystyrene microplastics (PS-MPs, 45 μm) exhibited no obvious effects; however, Nano-PS (50 nm) caused neurotoxicity to zebrafish (Danio rerio) larvae regarding the locomotor activity (Chen et al. 2017b). Exposure of Nano-PS can cause zebrafish (Danio rerio) embryos accumulate nanoplastics mainly in lipid-rich areas like the yolk sac, and then transfer to the digestive organs, heart, and brain (Pitt et al. 2018b). Exposing primary producers such as algae to Nano-PS can cause the nutrient transfer of nanoplastics to fish and crustaceans (Chae et al. 2018), and Nano-PS can also be inherited to offspring (Brun et al. 2017; Pitt et al. 2018a). Plastics with particle size can influence their tissue distribution pattern in mice. Xu et al. (2021) reported that in mice exposed to 1 mg/day 100-nm Nano-PS by oral gavage once a day for 28 days, Nano-PS were highly detected in the stomach, small intestine, and large intestine, and were less detected in other tissues with the order of testis > brain > kidney > lung. Compared to Nano-PS, PS-MPs were mainly detected in the liver, gut, kidney, and testis (Jin et al., 2021; Deng et al., 2017). Nano-PS could penetrate blood–brain barrier of the adult fish medaka (Oryzias latipes) and mice and reach the brain (Kashiwada 2006; Xu et al. 2021). Nano-PS can affect the feeding behavior and lipid metabolism of top consumers through the aquatic food chain from algae through zooplankton to Crucian carp (Carassius carassius) (Cedervall et al. 2012). Overview of the literature involved in the inherent toxic effects of micro- and nanoplastics in vivo are shown in Table 1.
Block the feeding organs and digestive tract, reduce growth performance, and alter behavior
Exposure to MPs (or NPs) can obstruct the feeding organs and digestive tract (AU et al. 2015; Besseling et al. 2014; Blarer and Burkhardt-Holm 2016; Nasser and Lynch 2016; Rehse et al. 2016; Huang et al. 2020), decrease the feeding rate (Nasser and Lynch 2016), or disturb the feeding process directly (AU et al. 2015; Blarer and Burkhardt-Holm 2016), reduce filtration rate (Oliveira et al. 2018; Sıkdokur et al. 2020), and change predatory performance (Yin et al. 2018), leading to energy deficiency and developmental anomaly (e.g., growth, molting) (Cole et al. 2019), reduced activities or reproductive capacity (AU et al. 2015; Besseling et al. 2014; Casado et al. 2013; Rehse et al. 2016), and even individual death (AU et al. 2015; Besseling et al. 2014; Blarer and Burkhardt-Holm 2016; Casado et al. 2013). After exposure to PE microplastics (PE-MPs), the Mediterranean mussel Mytilus galloprovincialis’ filtration rate was decreased with the concentration increase of PE-MPs (Abidli et al. 2021). However, studies with the cladoceran Daphnia magna (D. magna), a non-selective filter feeder, have shown that in exposure to 25, 50, and 100 mg/L PE-MPs (63–75 μm), no significant effect on survival and reproduction in of D. magna was found although the gut was filled with PE microbeads (Canniff and Hoang 2018).
In mammals, microplastics are currently thought to be unable to penetrate deep into the organs and therefore only cause localized inflammatory responses, although negative surface charges and smaller sizes can cause increased uptake. A 28-day study in male mice showed that PS-MPs did not cause histologically detectable damage or inflammatory responses, and did not interfere with human macrophage model’s differentiation and activation (Stock et al. 2019).
The potential influence of microplastics exposure on lipid metabolism
In addition to physical damage, the accumulation of microplastics can also lead to liver glycogen consumption, lipid vacuoles, and lipid accumulation in fish (Rochman et al. 2013a; Lu et al. 2016). When brine shrimp Artemia Parthentica larvae were exposed to 10 μm PS-MPs at a concentration of 10 particles/mL for 24 h, a greater abundance of lipid droplets appeared among the epithelia (Wang et al. 2019). In the gut tissue of fish, zebrafish (Danio rerio) and European sea bass (Dicentrarchus labrax), similar histopathological changes (vacuolation of enterocytes) may occur when chronically exposed to microplastics through ingestion (Qiao et al. 2019; Pedà et al. 2016). For male mice, oral administration of PS-MPs can cause inflammation and lipids droplets in livers (Deng et al. 2017) and reduce liver mass and cause lipid disorders (hepatic TG and TC decreased) (Lu et al. 2018a; Deng et al. 2017).
Adverse effects on the gut epithelium and intestinal microbiota
Exposure of nano- and microplastics can disrupt the intestinal microbiota and key intestinal functions include dysbiosis and destruction of intestinal epithelial permeability. Lu et al. proved that 0.5-μm and 50-μm PS-MPs with a concentration of 1000 μg/L for 5 weeks can reduce the secretion of intestinal mucus and change the composition of the intestinal microbiota (Lu et al. 2018a). Exposure to PS-MPs reduced the activities of digestive enzymes in the intestinal tract, stimulated the expression of intestinal immune responses, and induced imbalance of the intestinal microbiota of juvenile guppy (Poecilia reticulata) (Huang et al. 2020). Microplastics can alter the diversity and composition of the gut microflora of Chinese mitten crabs Eriocheir sinensis (Liu et al. 2019). Wang et al. (2021c) studied Nano-PS toxicity in intestines of juvenile orange-spotted groupers Epinephelus coioides and found that Nano-PSs at exposure concentrations of 300 and 3000 μg/ml, respectively, for 14 days decreased the specific growth rate; reduced the activities of digestive enzymes lipase, trypsin, and lysozyme; altered microbial community composition; and increased harmful bacteria (Vibrio and Aliivibrio).
Inhibition of brain AChE activity indicates neurotoxicity
Significant microplastic-induced inhibition of brain acetylcholinesterase (AChE) activity was found in fish, suggesting an adverse effect on cholinergic neurotransmission, therefore, potentially on neurological and neuromuscular function (Avio et al. 2015; Luís et al. 2015; Oliveira et al. 2013, 2018; Barboza et al. 2018a; Miranda et al. 2019). Polyethylene microplastics (1–5 μm) caused the AChE activity inhibition by 22% in common goby Pomatoschistus microps (Oliveira et al. 2013). Bivalve Corbicula fluminea exposed to microplastics showed inhibition of cholinesterase (CHE) activity, indicating neurotoxicity (Guilhermino et al. 2018; Oliveira et al. 2018). Microplastics can cause juvenile European seabass (Dicentrarchus labrax) neurotoxicity by inducing brain AChE inhibition and lipid peroxidation (Barboza et al. 2018a).
Impacts on reproduction and offspring
The main impacts of MP exposure include reproductive system damage, decrease in the number and quality of oocytes and gametes, fertility, testicular spermatogenesis, sperm quality, sperm speed, testosterone level, and offspring quality (Martínez-Gómez et al. 2017; Sussarellu et al. 2016; Sharifinia et al. 2020; Deng et al. 2021). Nano-PS could cross rats’ placental barrier and deposit in fetal placenta, fetal liver, lungs, heart, kidney, and brain and reduce fetal and placental weights (7 and 8%, respectively) and increase number of fetal reabsorptions after an acute maternal pulmonary exposure to 20-nm Nano-PS during late-stage pregnancy (Fournier et al. 2020). Exposure to 100 · ml−1 1-μm and 10-μm microplastics to larvae of Pacific oyster (C. gigas) for 8 days had no significant effect on growth, however caused reproductive damage of adult Pacific oysters (C. gigas) (Cole and Galloway 2015). Adult oysters (C. gigas) were exposed to virgin PS-MPs (2 and 6 μm, 0.023 mg·L−1) for 2 months in one reproductive cycle, and oocyte diameter, number, and sperm speed decreased significantly, and larval production and larval development of progenies from exposed parents reduced (Sussarellu et al. 2016). PS or PE-MPs could reduce the sperm number and quality and testosterone level, and induce abnormal testicular spermatogenesis in mouse testis (Deng et al. 2021; Jin et al. 2021; Hou et al. 2021). Smaller-sized PE MP fragments (17.23 ± 3.43 μm) decreased the number of offspring in D. magna when compared with MP beads (39.54 ± 9.74 μm) (An et al. 2021). Maternal mice PS-MP exposure (0.5 and 5 μm with 100 and 1000 μg/L in drinking water) during gestation had no significant effect on growth of F1 offspring, but caused blood and liver lipid disorder, and changed amino acid metabolism and carnitine metabolism homeostasis, and 5-µm MPs were more pronounced (Luo et al. 2019).
Effects on oxidative stress and lipid oxidative damage
Microplastics induced oxidative stress in some species (Oliveira et al. 2013; Avio et al. 2015; Barboza et al., 2018a, 2018b; Wan et al. 2019; Wang et al. 2021c). Marine fish European seabass (Dicentrarchus labrax) exposed to microplastics showed induction of oxidative stress in both the liver and gills, and lipid oxidation in muscle and brain (Barboza et al. 2018a, 2018b). Polyethylene microplastics (40–48 μm) induced oxidative damage (LPO) in mussels (Mytilus galloprovincialis) at low concentrations (1 and 10 μg/L) and significantly decrease of LPO in 1000 μg/L treated group of females, and inactivation of antioxidant systems (CAT and GST) in the ampholytic digestive glands at high concentrations (100 and 1000 μg/L) (Abidli et al. 2021). Su et al. demonstrated that microplastics can serve as an external stressor on dinoflagellate Cladocopium goreaui (C. goreaui), with enhancing oxidative stress though increasing the activity of superoxide dismutase and caspase 3, and reducing the activity of glutathione S-transferase (Su et al. 2020). Juvenile gilthead seabream Sparus aurata exposed to virgin and weathered MPs showed the activation of antioxidant enzymes (Rios-Fuster et al. 2021). PS-MPs induced endoplasmic reticulum (ER) stress via mitochondrial ROS production in the mice (Wang et al. 2021c).
Adverse impacts on immune system
In mussels Mytilus galloprovincialis, MPs can cause blood cell immunotoxicity, lysosomal membrane instability, and reduced phagocytic activity (von Moos et al. 2012; Avio et al. 2015; Détrée and Gallardo-Escárate, 2018). High-density polyethylene (HDPE, < 80 μm) plastic particles can be enriched in the digestive system of mussels, leading to blood flow granulocytosis, instability of lysosomal membrane, and inflammation of the body’s immune system after only 3 h of exposure (von Moos et al. 2012). For crabs Eriocheir sinensis, low concentrations (0.040 and 0.4 mg/L) or short periods of exposure to microplastics can enhance immune enzymes’ activities, while negative effects were found in high concentrations (4 and 40 mg/L) or long periods of exposure on all aspects of innate immunity (Liu et al. 2019). Nano-PS particles can activate complement system in fish (Veneman et al.2017; Elizalde-Velázquez et al. 2020), and can also be phagocytized by neutrophils and macrophages and activated macrophages, and enhance the recruitment of neutrophils to release interferon gamma and interleukin 1 beta (Brun et al. 2018; Lu et al. 2018a, b). Alterations in the innate immune response in adult fish were found after exposure to nanoplastics through the ingestion of species from a lower trophic level; the gene expression of neutrophil cytosolic factor (ncf), macrophage stimulating 1 (mst1), and complement component 3 (c3) were downregulated in the kidney and liver (Elizalde-Velázquez et al. 2020).
Leaching of plastic additives and toxic monomers
Microplastics can also leach out chemicals such as residual toxic monomers and plastic additives, which can have toxic effects on human health and the environment. Plastic additives are chemical compounds that are used primarily as stabilizers, plasticizers, flame retardants, pigments, and antioxidants. It has been reported that plastic particles may contain up to 4% by weight as additives, such as plasticizers (Andrady and Neal 2009). Different types of plastics, depending on their purpose, use different types of additives, such as plasticizers, which increase the flexibility, durability, and tensile properties of the plastic polymer. Bisphenol A (BPA), the monomer of polycarbonate (PC), is a stabilizer for other polymers, and can be released from MPs, and has carcinogenic, teratogenic, mutagenic, and reproductive effects on organisms (Hu et al. 2021; Seachrist et al. 2016; Harnett et al. 2021). The chemical substances leaching from polyvinyl chloride (PVC) and polyurethane could produce acute toxic effects (immobility) for Daphnia magna (Lithner et al. 2009). Plasticizers do not chemically bind to the polymer and therefore can leach from the plastics (OECD 2004). Phthalates, as a kind of endocrine-disrupting chemicals, account for 95% of all plasticizers (OECD 2004) and are widely spread in the environment today, some of which may cause developmental and/or reproductive toxicity concerns even at environmental concentrations (Zhang et al. 2021). Plasticizers butylbenzyl phthalate and dibutyl phthalate (DBP) can disrupt the antioxidant system of human erythrocytes that causes eryptosis (Sicińska 2018; Sicińska et al. 2020). Monomer toluene diisocyanate reacting with polyether or polyester to form polyurethane usually along with catalysts (Harper and Petrie 2003) is an allergenic, has very high acute toxicity, and is environmentally hazardous with long-term effects (Ott 2002; Zhuang et al. 2020). The leachates of polymer PS and HDPE fluff were more toxic to sea urchin embryos than the virgin materials or the aging materials themselves. The raw material microplastics may release unknown chemicals, such as residual toxic monomers or additives, which seems to be the main cause of the seen sea urchin embryo toxicity (Martínez-Gómez et al. 2017).
Combined toxic effects of micro(nano)plastics and persistent, bioaccumulative, and toxic substances
In addition to the hazards of microplastics per se or leachates, micro(nano)plastics can also alter the form, local concentration, environmental persistence, environmental behavior, and ecological risks by adsorption and desorption of coexisting organic pollutants (Tanaka et al. 2013; Wright et al. 2013; Llorca et al. 2018) because of their small particle size (< 5 mm) and large specific surface area (Law and Thompson 2014). Toxicological interactions between MPs/NPs and persistent, bioaccumulative, and toxic substances are of grave concern, as MPs/NPs can cause a “Trojan Horse” effect by transferring other environmental pollutants to organisms and the environment that are directly exposed, or indirectly, through the long-distance transport of particulate matter and food webs (Avio et al. 2017). This vector effect also points out the potential interaction of microplastics with pollutants such as heavy metals (Ashton et al. 2010; Wen et al. 2018; Massos and Turner 2017; Kim et al. 2017; Khan et al. 2015; Kang et al. 2021), polycyclic aromatic hydrocarbons (PAHs) (Oliveira et al. 2012, 2013; Guven et al. 2018; Sørensen et al. 2020; Avio et al. 2015; Trevisan et al. 2019), organochlorine pesticides (Ogata et al. 2009), polychlorinated biphenyls (PCBs) (Engler 2012; Velzeboer et al. 2014), perfluorinated compounds (Wang et al. 2015; Bakir et al. 2014), pharmaceuticals (Wei et al. 2019; Wang et al. 2018; Li et al. 2019a, b; Xu et al. 2018a; Zhang et al. 2019), and personal care products (Wu et al. 2016), PBDEs (Wardrop et al. 2016). MPs may change these chemicals’ bioavailability, environmental behavior, and toxicity in the environment, for example, increasing their concentration to 100 times that in sediments, six orders of magnitude higher than the background concentration in surrounding seawater (Lee et al. 2014; Rochman et al. 2013b), and may modulate targets and patterns of action of plastic particles and related compounds at the molecular level. PS and PE microplastics are considered to be the plastic polymers of the highest adsorption capacity for hydrophobic organic pollutants (Sørensen et al. 2020). Black or aged MPs can absorb more pollutants that are colored or white ones (Verla et al. 2019). Toxicological interactions between micro(nano)plastics and heavy metals in vivo are shown in Table 2. Overview of in vivo studies of toxicological interactions between micro(nano)plastics and organic environmental pollutants are summarized in Table 3.
Heavy metals and MPs/NPS
Heavy metals and MPs/NPS are two types of persistent contaminants in the water environment owing to their persistence and poor biodegradability, bioaccumulation, and possible biological amplification in the food chain (Abdolahpur Monikh et al. 2013; Grigorakis et al. 2017; Gu et al. 2015; Vendel et al. 2017). MPs/NPS and heavy metals both can get into the food web (chain) by direct or indirect exposure (Hosseini et al. 2015; Vendel et al. 2017). In the field scenario, lead (Pb) and cadmium (Cd) are more abundant in microplastics than in other metallic elements (Ashton et al. 2010; Massos and Turner 2017). Akhbarizadeh et al. (2018) investigated the relationship between MPs and some metals (As, Cr, Cu, Mn, Ni, Pb, Zn, V, Fe, Se, and Hg) in different species fish muscles; a positive linear relationship was observed between MPs and metal content in shrimp scad Alepes djedaba and bartail flathead Platycephalus indicus, respectively, while the relationship between MPs and metal pollution index (MPI) in orange-spotted grouper Epinephelus coioides was negative in northeastern Persian Gulf. The desorption rate of zinc by microplastics in the intestinal tract of earthworms is much higher than that of soil, indicating that microplastics may increase metal elements’ bioavailability (Hodson et al. 2017). MPs may absorb metallic elements from the aquatic environment and the concentrations of metals absorbed on MPs were much higher than those of local estuary sediments (Rochman et al. 2014; Turner and Holmes 2015). There is also growing concern in regard to the potential role of MPs as transport carriers of heavy metals and transport of metals into fish, which may change the bioavailability and uptake route of metals in fish tissues. Silver-incubated MPs could increase the biological accumulation of silver in zebrafish (Danio rerio) intestines (Khan et al. 2015). The presence of MPs in water environment influenced the acute toxicity (96 h) of Cr(VI) on early juvenile gobies, such as enhanced oxidative damage, and reduced the predation performance in fish (Luís et al. 2015). When co-exposed to 20–200 μg L−1 PS-MPs (5 μm) and 10 μg L−1 CdCl2, the accumulation of Cd and toxic effects increased in D. rerio compared to the effect of CdCl2 exposure alone (Lu et al. 2018b). Lang et al. (2020) reported that compared with the pristine PS, the adsorption capacity of Cd2+ after aging was significantly enhanced. Aging will not only increase the specific surface area of PS, but also oxidize the functional groups on the surface of PS and form surface microcracks, resulting in a significant increase in the content of carbonyl and carboxyl groups. The adsorption process of Cd2+ by pristine PS mainly occurs on the surface, but with the continuous aging of PS, the adsorption mechanism of Cd2+ by PS is the coexistence of physics and chemistry. MP can absorb mercury from surrounding water and increase the degradation of mercury in water with increasing concentrations of microplastics, thus influencing the mercury accumulation in the European seabass (Dicentrarchus labrax) (Barboza et al. 2018a). The inhibition rate of the combination of nickel and carboxylated polystyrene (PS-COOH) on Daphnia magna was higher than that of nickel alone. PS-MPs caused a slight antagonistic effect on nickel (Ni) toxicity, while PS-COOH MPs had a slight synergistic effect with Ni (Kim et al. 2017). Exposure to nano-scale plastics (50 nm) intensified arsenic (As) toxicity of rotifer Brachionus plicatilis in marine, while As toxicity was mitigated by micro-scale ones (6 μm) (Kang et al. 2021).
However, other studies showed that microplastics had no significant effect on the bioavailability and toxicity of heavy metal copper (Davarpanah and Guilhermino 2015). Therefore, the impacts of different types of microplastics and heavy metals on different organisms may be very different. These findings indicated that MPs could not necessarily increase the bioavailability of heavy metals, which might rely on the physical and chemical properties of MPs (size, color, and concentrations) and the type and content of metals. Moreover, the long-term toxicological interactions between MPs and heavy metals are largely unclear.
Polycyclic aromatic hydrocarbons and MPs/NPS
Fish co-exposed to microplastics and PAHs have shown impaired swimming ability in young gobies (Oliveira et al. 2012). In mussels (Mytilus galloprovincialis), exposed to pyrene contaminated of polyethylene and polystyrene plastics, obvious biological accumulation of these chemicals was observed in gills, digestive gland, and blood lymphatic (Avio et al. 2015), and PE and PS microplastics can adsorb pyrene with a dose- and time-dependent relationship and augment pyrene’s bioavailability and toxicity in Mytilus galloprovincialis (Avio et al. 2015). PS-MPs had little effect on the absorption and bioaccumulation of fluoranthene, but could change its purification (detoxification) and regulate the kinetics and toxicity of fluoranthene. After purification, compared with mussels exposed to fluoranthene alone, higher concentrations of fluoranthene were detected in mussels when co-exposed to PS-MPs and fluoranthene, because the micro-plastics can weaken metabolic transformation of polycyclic aromatic hydrocarbons by inhibiting metabolic enzymes (Paul-Pont et al. 2016). The Nano-PS (50 nm) can increase the accumulation of phenanthrene and have synergistic toxic effects with phenanthrene in Daphnia magna. Meantime, Nano-PS can also inhibit the degradation of phenanthrene and its metabolites to augment the accumulation of phenanthrene in Daphnia magna (Ma et al. 2016). However, studies have found that exposure to PE microplastics (1–5 μm) can delay pyrene-induced death in marine fish P. microps juveniles and increase the concentration of pyrene metabolites in the bile of fish (Oliveira et al. 2013). The presence of microplastics can decrease the damage of toxic organic pollutants to the DNA of zebrafish gill cells. This may be due to the strong adsorption of anthracene by polystyrene particles, which can prevent anthracene from being completely desorbed on the gill surface after entering the zebrafish tissue, thereby reducing anthracene toxicity (Cai et al. 2017).
Pharmaceuticals and MPs/NPS
Some studies have reported several new types of ionizable organic pollutants (such as antibiotics) can be adsorbed on the surface of microplastics (Wei et al. 2019; Wang et al. 2018; Li et al. 2019a, b; Xu et al. 2018a, 2018b; Zhang et al. 2019; Guilhermino et al. 2018). Antibiotics with ion form of the pH 6 to 9 in natural water can also be accumulated on microplastics surface. For instance, aromatic antibiotics tetracycline and oxytetracycline containing hydroxyl and amino groups have maximum adsorption at neutral pH (with minimal electrostatic repulsion) (Xu et al. 2018b; Zhang et al. 2018). Changes in oxytetracycline adsorption on the PS-MP foams with changing pH were more prominent for the beached samples than the virgin samples owing to higher surface area, micropore area, and oxidation degree of the former (Zhang et al. 2018). The inhibition of ChE activity (32%) when bivalve Corbicula fluminea (C. fluminea) was exposed to florfenicol (1.8 and 7.1 mg/L) and ChE activity inhibition of 31% when exposed to 0.2 mg/L of microplastics was found, and no other significant alterations were observed, whereas mixtures led to feeding inhibition (57–83%), significant suppression of ChE (44–57%) and isocitrate dehydrogenase activities, and increased lipid peroxidation levels and anti-oxidant enzyme activities, suggesting that mixtures of florfenicol (low ppm range) and microplastics (ppb range) were much more toxic to C. fluminea (Guilhermino et al., 2018). PVC microplastics raised the antidepressant drug venlafaxine’s accumulation in fish mitochondria of M. anguillicaudatus, and magnified venlafaxine’s bioaccumulation factor of tissue by more than 10 times (Qu et al. 2019). The presence of PS-MPs significantly increased the bioaccumulation of roxithromycin (ROX) in the gut, gills, brain, and liver of red tilapia, whereas the inhibition of AChE activity and oxidative damage caused by ROX was alleviated in the presence of PS-MPs after 14 days of exposure (Zhang et al. 2019).
PCBs and MPs/NPS
It has been reported that discolored MPs can adsorb more PCBs than non-discolored ones (Endo et al. 2005). The adsorption capacity of PE for environmental pollutants was generally higher than that of other plastics (Alimi et al. 2018). The sorption of PCBs to Nano-PS was 1–2 orders of magnitude higher than that of PE-MPs because of greater aromaticity and surface area/volume ratio of Nano-PS (Nano-PE was not available, but the relative effect of surface adsorption and volume distribution can still be deduced by comparing Nano-PS and PE-MPs) (Velzeboer et al. 2014). Previous study showed that low doses of PS (0.074%) increased biological accumulation of PCBs with the coefficient of 1.1–3.6, the influence of which was significant for ΣPCBs and a couple of separate homologs; however, at higher doses, biological accumulation reduced when compared with the low dose, but which only PCB105 was significant (Besseling et al. 2013).
Polybrominated diphenyl ethers and MPs/NPS
The detritivorous marine amphipod Allorchestes compressa’s body tissue can take in PBDEs from the microplastics, while environmental MPs may reduce the uptake of PBDEs compared to unabsorbed free chemicals, which can transfer PBDEs into marine organisms (Chua et al. 2014). On the contrary, when rainbow fish (Melania fluviatilis) absorbed PBDEs, the accumulation of PBDEs in its tissues was greater, and low-bromine homologs were easier to accumulate in its tissues, while the high-bromine homologs were not easy to transfer to the organs due to the stronger binding power of microplastics (Wardrop et al. 2016).
Phthalate esters & MPs/NPS
Polyethylene (PE) MPs (45–53 μm) could absorb four phthalate esters (PAEs) (DEHP > DBP > DEP > DMP) and transport PAEs into mice gut and induce aggravated intestinal inflammation and metabolic disorders (Deng et al. 2020). PAE-contaminated PE-MPs (0.4–5 μm) aggravated male mice reproductive toxicities manifested by greater alterations in sperm physiological alterations, spermatogenesis disorders, and oxidative stress in the testis (Deng et al. 2021). In contrast, adsorption to PS-MPs led to decreased bioavailability of DBP, resulting in reduced toxicity of DBP, and antagonistic toxicity between the two pollutants was observed in acute and chronic trials in marine copepod Tigriopus japonicus (Li et al. 2020a, b).
Microplastics (nanoplastics) and other toxic substances
Adsorption of Nano-PS increased the concentration of BPA in zebrafish viscera and head by 2.6 times and 2 times, respectively, and aggravated BPA neurotoxicity to central nervous system and dopaminergic system (Chen et al. 2017a). PS (or PE) microplastics increased the toxicity of organophosphorus flame retardants, and induced higher oxidative stress (greater superoxide dismutase (SOD) and catalase (CAT)) and neurotoxicity in mice (Mus musculus) (lowered AChE) (Deng et al. 2018).
Micro(nano)plastics as vectors of microorganisms or invasive substances
Micro(nano)plastics in the environment can be used as carriers for selection and transmission of animal and human pathogens such as Vibrionaceae, Flavobacteriaceae, Campylobacteraceae, and Aeromonadaceae (Frère et al. 2018; Kirstein et al. 2016; Viršek et al. 2017; He et al. 2018); invasive species; and harmful algae (Wright and Kelly. 2017; Keswani et al. 2016). Meanwhile, pathogens such as bacteria and viruses by the ingestion or direct contact with organisms through micro(nano)plastics can also cause toxicological effects (Mughini-Gras et al. 2021; Moresco et al. 2021; Wang et al. 2021a). Moreover, antibiotic resistance genes (ARGs) and antibiotic-resistant bacteria were found on microplastic surfaces (Lu et al. 2020). Micro(nano)plastics not only accelerate the spread of organisms in the environment, leading to biological invasion, but also increase the gene exchange between attached biofilm groups, leading to the transfer of pathogenic genes and ARGs (Arias-Andres et al. 2018; Mughini-Gras et al. 2021). In free-living or naturally aggregated bacteria, microplastics can increase the frequency of plasmid transfer. Microplastic biofilms serve as new hot spots for the transmission of ARGs through horizontal gene transfer (Arias-Andres et al. 2018). On microplastic particles of polyethylene, polystyrene, and polypropylene, pathogenic vibrio parahaemolyticus was detected in the North/Baltic Sea (Kirstein et al. 2016). Viršek et al. (2017) reported that 28 bacterial species were identified on the microplastics particles in the North Adriatic including Aeromonas spp. and hydrocarbon-degrading bacterial species. These studies showed that potential pathogenic microorganisms in the ocean may catch up with micro(nano)plastic particles and spread around the world through water or air currents. The potential harm caused by this is unclear and needs further scientific verification.
Nevertheless, there is controversy regarding this potential consequence, as some researchers object to MPs (or NPs) as pollutant carriers. According to Gouin et al. (2011) and Beckingham and Ghosh (2017), the transfer of organic contaminants into biological organisms by dietary MPs may be a small or limited contribution compared to other natural routes of exposure in most cases. It was reported that contaminated PE microplastics displayed a negligible vector role in terms of mercury bioaccumulation in the clams (Sıkdokur et al. 2020). The presence of polyethylene plastic microspheres in artificial sea water of a salinity of 18 ± 1 g/L test media did not significantly change the toxic effects of gold nanoparticles (ppb range) to P. microps juveniles (Ferreira et al. 2016). Therefore, MPs can introduce these harmful chemicals into organisms only when fugacity of a chemical in microplastics is greater than that in organism, which may result in an exposure risk. Moreover, the sorption behavior of chemicals on MPs is related to polymer type, particle size, color, temperature, salinity, the degree and typology of plastic aging (mechanical, UV or biological degradations), and other physical and chemical properties (Endo et al. 2005; Sørensen et al. 2020; Binda et al. 2021) and biofilms (Guan et al. 2020). In addition, the presence of other sorbents is also important. In exposures more relevant to the environment, modeling studies showed the total organic contaminant fraction or metals absorbed by plastic was much smaller than that adsorbed by other media like dissolved organic carbon and colloids in the ocean (Koelmans et al. 2016; Lohmann 2017) or freshwater environments (Guan et al. 2020). Seidensticker et al. (2017) found that the concentration of dissolved organic matter may alter the distribution of pollutants between MPs and water. In addition, the increase of MP concentration will increase the adsorption of chemical substances.
Urgency and challenges
Lack of effective indicator organisms and biomarker
Although a large number of studies have confirmed the widespread presence of micro(nano)plastics in various marine media, there is no uniform international technical method for marine micro(nano)plastics monitoring and ecological risk assessment. Seabirds and turtles have been widely used to monitor plastic debris with a size of more than 1 mm. It is also reported that mussels are suitable bioindicator for microplastic pollution because of their wide distribution, vital ecological niches, susceptibility to microplastic uptake, and close connection with marine predators and human health (Li et al. 2019a, b). However, there is a lack of effective indicator organisms for the monitoring of smaller size microplastics. Further studies are needed on efficient biomarker screening and ecological risk assessment techniques to indicate toxicological effects of micro(nano)plastics.
Effects of seafood cooking and/or processing on micro(nano)plastics toxicity
Thus far, available data on the toxicokinetics of micro(nano)plastics mainly include absorption and distribution, with less information on metabolism or excretion. There is no data on the potential effects of cooking and/or processing seafood at high temperatures on micro(nano)plastics toxicity. The toxic effect of micro(nano)plastics on human body must be first digested by digestive tract and then absorbed by intestinal wall cells to enter the circulatory system. It has been documented that ingested microplastics in Antarctic krill turned into nanoplastics through digestive fragmentation (Dawson et al. 2018); however, whether the micro(nano)plastics will be destroyed in the process of digestion, how much is the absorption and utilization rate in the process of absorption, and what factors (food matrix/digestion environment/particle size of microplastics) will lead to the decrease of its digestibility and utilization are also worthy of further study.
Lack of direct evidence of micro(nano)plastics affecting organisms in the field
Over the past decade, it is an indisputable fact that micro(nano)plastics are ubiquitous in the environment and the quantity keeps increasing rapidly. However, in the field, there have been few direct evidence that micro(nano)plastics have an impact on the organisms. Although laboratory experiments showed that micro(nano)plastics can produce a variety of toxicological effects on organisms, the real conditions and intrinsic dynamic evolution characterizing the phenomena in natural ecosystems cannot be easily reproduced at laboratory or pilot scale. For instance, unpredictable factors as climatic variations causing modifications of the current regime in sea and lakes cannot be totally investigated in lab experiments involving MPs/NPs. Therefore, the data cannot be applied to the ecological and health risk assessment of micro(nano)plastics because of the great difference between indoor research and field environment in aspects of weathering, and adsorption of organic environmental pollutants and organisms, etc. Moreover, in these laboratory experiments, the concentration of micro(nano)plastics tested was not environmentally relevant (de Sá et al. 2018; Zocchi and Sommaruga 2019), even several orders of magnitude higher than would be found in the environment.
Limitation of the methods of sample collection, separation, and identification of micro(nano)plastics
To date, only a part of the microplastics, especially nanoplastics information in the environment, are obtained due to the limitations of current sample collection, separation, and identification methods. The characterization index of microplastics in the environment, mainly including abundance, size, shape, and color, is often characterized by rough ranges or proportions. Despite some recent comments on microplastic sampling and analysis (Campanale et al 2020; Bellasi et al. 2021), it is still difficult to properly quantify dispersed particles in sediments. Due to the lack of consistent and standardized methods and protocols for evaluating and quantifying microplastics present in sediment or aqueous phase systems, appropriate comparisons between different sites are not possible, which hinders the current knowledge of the environmental and toxicological effects of microplastics. In the aqueous phase, characterization methods that only refer to the concentration of chemical pollutants are not feasible for micro(nano)plastics, and establishing a “characteristic spectrum of microplastics” may be an effective method for more comprehensive characterization of micro(nano)plastics in the environment.
Occasionality and contradiction in studies on the toxicity of micro(nano)plastics
Uncertainties remain regarding the extent of harm caused to organisms directly by ingestion of micro(nano)plastics, and over the effect they make to overall exposures to hazardous chemicals. Although current studies have reported that micro(nano)plastics will have a series of adverse effects on organisms, the toxic effects of these reports are sometimes uncertain and contradictory, and different results may be obtained in different studies and different biological tissues. Several studies have reported that microplastics caused little or no physical or chemical harm to marine biota (Koelmans et al. 2014), and had limited function as carriers of hydrophobic pollutants compared to natural phytoplankton, bacterioplankton, and yeasts (Frydkjær et al. 2017). In terms of the toxicity of microplastics to organisms, some conditions or factors such as species, the size, shape, polymer type, exposure time, and concentration of microplastics used in exposure, and environmental factors, such as food abundance (Chae and An 2020), may affect the corresponding experimental results. The toxic changes caused by the adsorption of pollutants on micro- and nanoplastics are still controversial, which depends on the chemical and physical interaction, the corresponding changes in bioavailability; the changes in intake, transport, and accumulation in organisms; and the characteristics of pollutants. When pollutants are combined with microplastics or nanoplastics, their toxicity may undergo various modification, rather than simple additive, synergistic, or competitive effects. Future research still needs to explore different biological indicators to find specific indicators that are sensitive to microplastics.
Lack of long-term and systematic toxicity studies
The current studies on microplastics or nanoplastics are mainly acute and short-term toxicity, but there is a lack of long-term and systematic toxicity studies. Therefore, there is an urgent need for future long-term and systematic toxicity studies related to micro- and nanoplastics.
Conclusions
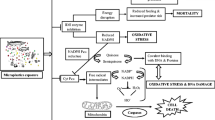
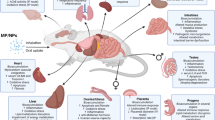
This review summarized inherent toxic effects of micro- and nanoplastics in vivo (shown in Fig. 1), which mainly include the following implications: physical injury (block the feeding organs and digestive tract), reduced growth performance and behavioral alteration, induced lipid metabolic disorder, induced gut microbiota dysbiosis and disruption of the gut’s epithelial permeability, neurotoxicity (inhibition of AChE activity), damage of reproductive system and offspring, oxidative stress, immunotoxicity, etc. We also outline the potential mechanism of toxicological effect for MPs/NPs in the body (Fig. 2). Additionally, besides hazardous consequences related to MPs/NPs themselves, plastics additives and residual and toxic monomers are diverse and complex, and the harm caused by their release cannot be ignored. Moreover, we generalize simultaneous toxic effects of micro- and nanoplastics with persistent, bioaccumulative, and toxic substances (heavy metals and organic environmental pollutants), as well as adverse effect as vectors of microorganisms, and invasive substances. Nevertheless, these potential consequences are controversial because some researchers object the idea that MPs (NPs) act as a vector for contaminants. Finally given the facts laid out above, we put forward the existing problems in the toxicity research of micro(nano)plastics and the direction of future efforts.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdolahpur Monikh F, Safahieh A, Savari A, Ronagh MT, Doraghi A (2013) The relationship between heavy metal (Cd Co, Cu, Ni and Pb) levels and the size of benthic, benthopelagic and pelagic fish species. Persian Gulf Bull Environ Contam Toxicol 90(6):691–696. https://doi.org/10.1007/s00128-013-0986-7
Abidli S, Pinheiro M, Lahbib Y, Neuparth T, Santos MM, Trigui El Menif N (2021) Effects of environmentally relevant levels of polyethylene microplastic on Mytilus galloprovincialis (Mollusca: Bivalvia): filtration rate and oxidative stress. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-021-12506-8
Akhbarizadeh R, Moore F, Keshavarzi B (2018) Investigating a probable relationship between microplastics and potentially toxic elements in fish muscles from northeast of Persian Gulf. Environ Pollut 232:154–163. https://doi.org/10.1016/j.envpol.2017.09.028
Alimi OS, Farner Budarz J, Hernandez LM, Tufenkji N (2018) Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport. Environ Sci Technol 52(4):1704–1724. https://doi.org/10.1021/acs.est.7b05559
An D, Na J, Song J, Jung J (2021) Size-dependent chronic toxicity of fragmented polyethylene microplastics to Daphnia magna. Chemosphere 271:129591. https://doi.org/10.1016/j.chemosphere.2021.129591
Andrady AL, Neal MA (2009) Applications and societal benefits of plastics. Philos Trans R Soc Lond B Biol Sci 364(1526):1977–1984. https://doi.org/10.1098/rstb.2008.0304
Ashton K, Holmes L, Turner A (2010) Association of metals with plastic production pellets in the marine environment. Mar Pollut Bull 60(11):2050–2055. https://doi.org/10.1016/j.marpolbul.2010.07.014
Arias-Andres M, Klümper U, Rojas-Jimenez K, Grossart HP (2018) Microplastic pollution increases gene exchange in aquatic ecosystems. Environ Pollut 237:253–261. https://doi.org/10.1016/j.envpol.2018.02.058
Au SY, Bruce TF, Bridges WC, Klaine SJ (2015) Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ Toxicol Chem 34(11):2564–2572. https://doi.org/10.1002/etc.3093
Avio CG, Gorbi S, Regoli F (2017) Plastics and microplastics in the oceans: from emerging pollutants to emerged threat. Mar Environ Res 128:2–11. https://doi.org/10.1016/j.marenvres.2016.05.012
Avio CG, Gorbi S, Milan M, Benedetti M, Fattorini D, d’Errico G, Pauletto M, Bargelloni L, Regoli F (2015) Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ Pollut 198:211–222. https://doi.org/10.1016/j.envpol.2014.12.021
Bakir A, Rowland SJ, Thompson RC (2014) Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ Pollut 185:16–23. https://doi.org/10.1016/j.envpol.2013.10.007
Barboza LGA, Vieira LR, Branco V, Figueiredo N, Carvalho F, Carvalho C, Guilhermino L (2018a) Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat Toxicol 195:49–57. https://doi.org/10.1016/j.aquatox.2017.12.008
Barboza LGA, Vieira LR, Branco V, Carvalho C, Guilhermino L (2018b) Microplastics increase mercury bioconcentration in gills and bioaccumulation in the liver, and cause oxidative stress and damage in Dicentrarchus labrax juveniles. Sci Rep 8(1):15655. https://doi.org/10.1038/s41598-018-34125-z
Beckingham B, Ghosh U (2017) Differential bioavailability of polychlorinated biphenyls associated with environmental particles: microplastic in comparison to wood, coal and biochar. Environ Pollut 220(Pt A):150–158. https://doi.org/10.1016/j.envpol.2016.09.033
Bellasi A, Binda G, Pozzi A, Boldrocchi G, Bettinetti R (2021) The extraction of microplastics from sediments: an overview of existing methods and the proposal of a new and green alternative. Chemosphere 278:130357. https://doi.org/10.1016/j.chemosphere.2021.130357
Besseling E, Wang B, Lürling M, Koelmans AA (2014) Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ Sci Technol 48(20):12336–43. https://doi.org/10.1021/es503001d
Besseling E, Wegner A, Foekema EM, van den Heuvel-Greve MJ, Koelmans AA (2013) Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ Sci Technol 47(1):593–600. https://doi.org/10.1021/es302763x
Bhattacharya P, Lin S, Turner JP, Ke P (2010) Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J Phys Chem C 114(39):16556–16561. https://doi.org/10.1021/jp1054759
Blarer P, Burkhardt-Holm P (2016) Microplastics affect assimilation efficiency in the freshwater amphipod Gammarus fossarum. Environ Sci Pollut Res Int 23(23):23522–23532. https://doi.org/10.1007/s11356-016-7584-2
Binda G, Spanu D, Monticelli D, Pozzi A, Bellasi A, Bettinetti R, Carnati S, Nizzetto L (2021) Unfolding the interaction between microplastics and (trace) elements in water: a critical review. Water Res 204:117637. https://doi.org/10.1016/j.watres.2021.117637
Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R (2011) Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ Sci Technol 45(21):9175–9179. https://doi.org/10.1021/es201811s
Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC (2008) Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L). Environ Sci Technol 42(13):5026–5031. https://doi.org/10.1021/es800249a
Brun NR, Beenakker MMT, Hunting ER, Ebert D, Vijver MG (2017) Brood pouch-mediated polystyrene nanoparticle uptake during Daphnia magna embryogenesis. Nanotoxicology 11(8):1059–1069. https://doi.org/10.1080/17435390.2017.1391344
Brun NR, Koch BEV, Varela M, Peijnenburg WJGM, Spaink HP, Vijver MG (2018) Nanoparticles induce dermal and intestinal innate immune system responses in zebrafish embryos. Environ Sci Nano 5(4):904–916. https://doi.org/10.1039/c8en00002f
Cai YY, Zhao JY, Li WF, Song WJ, Zhang DY, Pan XL (2017) Retention of polystyrene particles of different sizes in zebrafish gills and their effect on toxicity of anthracene to gill cells. Chin J Appl Environ Biol 23(6):1154–1158. https://doi.org/10.3724/SP.J.1145.2016.12051
Canniff PM, Hoang TC (2018) Microplastic ingestion by Daphnia magna and its enhancement on algal growth. Sci Total Environ 633:500–507. https://doi.org/10.1016/j.scitotenv.2018.03.176
Canesi L, Ciacci C, Bergami E, Monopoli MP, Dawson KA, Papa S, Canonico B, Corsi I (2015) Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Mar Environ Res 111:34–40. https://doi.org/10.1016/j.marenvres.2015.06.008
Casado MP, Macken A, Byrne HJ (2013) Ecotoxicological assessment of silica and polystyrene nanoparticles assessed by a multitrophic test battery. Environ Int 51:97–105. https://doi.org/10.1016/j.envint.2012.11.001
Catarino AI, Macchia V, Sanderson WG, Thompson RC, Henry TB (2018) Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ Pollut 237:675–684. https://doi.org/10.1016/j.envpol.2018.02.069
Cedervall T, Hansson LA, Lard M, Frohm B, Linse S (2012) Food chain transport of nanoparticles affects behaviour and fat metabolism in fish. PLoS ONE 7(2):e32254. https://doi.org/10.1371/journal.pone.0032254
Chae Y, Kim D, Kim SW, An YJ (2018) Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain. Sci Rep 8(1):284. https://doi.org/10.1038/s41598-017-18849-y
Chae Y, An YJ (2020) Effects of food presence on microplastic ingestion and egestion in Mytilus galloprovincialis. Chemosphere 240:124855. https://doi.org/10.1016/j.chemosphere.2019.124855
Chen Q, Yin D, Jia Y, Schiwy S, Legradi J, Yang S, Hollert H (2017a) Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci Total Environ 609:1312–1321. https://doi.org/10.1016/j.scitotenv.2017.07.144
Chen Q, Gundlach M, Yang S, Jiang J, Velki M, Yin D, Hollert H (2017b) Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci Total Environ 584–585:1022–1031. https://doi.org/10.1016/j.scitotenv.2017.01.156
Chua EM, Shimeta J, Nugegoda D, Morrison PD, Clarke BO (2014) Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod. Allorchestes Compressa Environ Sci Technol 48(14):8127–8134. https://doi.org/10.1021/es405717z
Cole M, Coppock R, Lindeque PK, Altin D, Reed S, Pond DW, Sørensen L, Galloway TS, Booth AM (2019) Effects of nylon microplastic on feeding, lipid accumulation, and moulting in a coldwater copepod. Environ Sci Technol 53(12):7075–7082. https://doi.org/10.1021/acs.est.9b01853
Cole M, Galloway TS (2015) Ingestion of nanoplastics and microplastics by Pacific oyster larvae. Environ Sci Technol 49(24):14625–14632. https://doi.org/10.1021/acs.est.5b04099
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62(12):2588–2597. https://doi.org/10.1016/j.marpolbul.2011.09.025
Campanale C, Savino I, Pojar I, Massarelli C, Uricchio VF (2020) A practical overview of methodologies for sampling and analysis of microplastics in riverine environments. Sustainability 12(17):6755. https://doi.org/10.3390/su12176755
Davarpanah E, Guilhermino L (2015) Single and combined effects of microplastics and copper on the population growth of the marine microalgae Tetraselmis chuii. Estuarine Costal & Shelf Science 167:269–275. https://doi.org/10.1016/j.ecss.2015.07.023
Dawson AL, Kawaguchi S, King CK, Townsend KA, King R, Huston WM, Bengtson Nash SM (2018) Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat Commun 9(1):1001. https://doi.org/10.1038/s41467-018-03465-9
Deng Y, Zhang Y, Lemos B, Ren H (2017) Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci Rep 7:46687. https://doi.org/10.1038/srep46687
Deng Y, Zhang Y, Qiao R, Bonilla MM, Yang X, Ren H, Lemos B (2018) Evidence that microplastics aggravate the toxicity of organophosphorus flame retardants in mice (Mus musculus). J Hazard Mater 357:348–354. https://doi.org/10.1016/j.jhazmat.2018.06.017
Deng Y, Yan Z, Shen R, Huang Y, Ren H, Zhang Y (2021) Enhanced reproductive toxicities induced by phthalates contaminated microplastics in male mice (Mus musculus). J Hazard Mater 406:124644. https://doi.org/10.1016/j.jhazmat.2020.124644
Deng Y, Yan Z, Shen R, Wang M, Huang Y, Ren H, Zhang Y, Lemos B (2020) Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ Int 143:105916. https://doi.org/10.1016/j.envint.2020.105916
de Sá LC, Oliveira M, Ribeiro F, Rocha TL, Futter MN (2018) Studies of the effects of microplastics on aquatic organisms: what do we know and where should we focus our efforts in the future? Sci Total Environ 645:1029–1039. https://doi.org/10.1016/j.scitotenv.2018.07.207
Détrée C, Gallardo-Escárate C (2018) Single and repetitive microplastics exposures induce immune system modulation and homeostasis alteration in the edible mussel Mytilus galloprovincialis. Fish Shellfish Immunol 83:52–60. https://doi.org/10.1016/j.fsi.2018.09.018
Elizalde-Velázquez A, Crago J, Zhao X, Green MJ, Cañas-Carrell JE (2020) In vivo effects on the immune function of fathead minnow (Pimephales promelas) following ingestion and intraperitoneal injection of polystyrene nanoplastics. Sci Total Environ 735:139461. https://doi.org/10.1016/j.scitotenv.2020.139461
Endo S, Takizawa R, Okuda K, Takada H, Chiba K, Kanehiro H, Ogi H, Yamashita R, Date T (2005) Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: variability among individual particles and regional differences. Mar Pollut Bull 50(10):1103–1114. https://doi.org/10.1016/j.marpolbul.2005.04.030
Engler RE (2012) The complex interaction between marine debris and toxic chemicals in the ocean. Environ Sci Technol 46(22):12302–12315. https://doi.org/10.1021/es3027105
Ferreira P, Fonte E, Soares ME, Carvalho F, Guilhermino L (2016) Effects of multi-stressors on juveniles of the marine fish Pomatoschistus microps: gold nanoparticles, microplastics and temperature. Aquat Toxicol 170:89–103. https://doi.org/10.1016/j.aquatox
Fonte E, Ferreira P, Guilhermino L (2016) Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquat Toxicol 180:173–185. https://doi.org/10.1016/j.aquatox.2016.09.015
Fournier SB, D’Errico JN, Adler DS, Kollontzi S, Goedken MJ, Fabris L, Yurkow EJ, Stapleton PA (2020) Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part Fibre Toxicol 17(1):55. https://doi.org/10.1186/s12989-020-00385-9
Frydkjær CK, Iversen N, Roslev P (2017) Ingestion and egestion of microplastics by the Cladoceran Daphnia magna: effects of regular and irregular shaped plastic and sorbed phenanthrene. Bull Environ Contam Toxicol 99(6):655–661. https://doi.org/10.1007/s00128-017-2186-3
Frère L, Maignien L, Chalopin M, Huvet A, Rinnert E, Morrison H, Kerninon S, Cassone AL, Lambert C, Reveillaud J, Paul-Pont I (2018) Microplastic bacterial communities in the Bay of Brest: influence of polymer type and size. Environ Pollut 242(Pt A):614–625. https://doi.org/10.1016/j.envpol.2018.07.023
Galgani F, Fleet D, van Franeker J, Katsanevakis S, Maes T, Mouat J, Oosterbaan L, Poitou I, Hanke G, Thompson R (2010) Marine Strategy Framework directive-Task Group 10 Report marine litter do not cause harm to the coastal and marine environment. Report on the identification of descriptors for the Good Environmental Status of European Seas regarding marine litter under the Marine Strategy Framework Directive, Office for Official Publications of the European Communities
Gigault J, Halle AT, Baudrimont M, Pascal PY, Gauffre F, Phi TL, El Hadri H, Grassl B, Reynaud S (2018) Current opinion: what is a nanoplastic? Environ Pollut 235:1030–1034. https://doi.org/10.1016/j.envpol.2018.01.024
Gouin T, Roche N, Lohmann R, Hodges G (2011) A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplastic. Environ Sci Technol 45(4):1466–1472. https://doi.org/10.1021/es1032025
Grigorakis S, Mason SA, Drouillard KG (2017) Determination of the gut retention of plastic microbeads and microfibers in goldfish (Carassius auratus). Chemosphere 169:233–238. https://doi.org/10.1016/j.chemosphere.2016.11.055
Guan J, Qi K, Wang J, Wang W, Wang Z, Lu N, Qu J (2020) Microplastics as an emerging anthropogenic vector of trace metals in freshwater: significance of biofilms and comparison with natural substrates. Water Res 184:116205. https://doi.org/10.1016/j.watres.2020.116205
Guven O, Bach L, Munk P, Dinh KV, Mariani P, Nielsen TG (2018) Microplastic does not magnify the acute effect of PAH pyrene on predatory performance of a tropical fish (Lates calcarifer). Aquat Toxicol 198:287–293. https://doi.org/10.1016/j.aquatox.2018.03.011
Gu YG, Lin Q, Wang XH, Du FY, Yu ZL, Huang HH (2015) Heavy metal concentrations in wild fishes captured from the South China Sea and associated health risks. Mar Pollut Bull 96(1–2):508–512. https://doi.org/10.1016/j.marpolbul.2015.04.022
Guilhermino L, Vieira LR, Ribeiro D, Tavares AS, Cardoso V, Alves A, Almeida JM (2018) Uptake and effects of the antimicrobial florfenicol, microplastics and their mixtures on freshwater exotic invasive bivalve Corbicula fluminea. Sci Total Environ 622–623:1131–1142. https://doi.org/10.1016/j.scitotenv.2017.12.020
Harnett KG, Moore LG, Chin A, Cohen IC, Lautrup RR, Schuh SM (2021) Teratogenicity and toxicity of the new BPA alternative TMBPF, and BPA, BPS, and BPAF in chick embryonic development. Curr Res Toxicol 2:399–410. https://doi.org/10.1016/j.crtox.2021.11.001
Harper CA, Petrie EM (2003) Plastic materials and processes. A Concice Encyclopedia. John Wiley & Sons Inc, Hoboken
He L, Wu D, Rong H, Li M, Tong M, Kim H (2018) Influence of nano- and microplastic particles on the transport and deposition behaviors of bacteria in quartz sand. Environ Sci Technol 52(20):11555–11563. https://doi.org/10.1021/acs.est.8b01673
Hodson ME, Duffus-Hodson CA, Clark A, Prendergast-Miller MT, Thorpe KL (2017) Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ Sci Technol 51(8):4714–4721. https://doi.org/10.1021/acs.est.7b00635
Hosseini M, Nabavi SM, Nabavi SN, Pour NA (2015) Heavy metals (Cd Co, Cu, Ni, Pb, Fe, and Hg) content in four fish commonly consumed in Iran: risk assessment for the consumers. Environ Monit Assess 187(5):237. https://doi.org/10.1007/s10661-015-4464-z
Hou B, Wang F, Liu T, Wang Z (2021) Reproductive toxicity of polystyrene microplastics: in vivo experimental study on testicular toxicity in mice. J Hazard Mater 405:124028. https://doi.org/10.1016/j.jhazmat.2020.124028
Huang JN, Wen B, Zhu JG, Zhang YS, Gao JZ, Chen ZZ (2020) Exposure to microplastics impairs digestive performance, stimulates immune response and induces microbiota dysbiosis in the gut of juvenile guppy (Poecilia reticulata). Sci Total Environ 733:138929. https://doi.org/10.1016/j.scitotenv.2020.138929
Hu X, Biswas A, Sharma A, Sarkodie H, Tran I, Pal I, De S (2021) Mutational signatures associated with exposure to carcinogenic microplastic compounds bisphenol A and styrene oxide. NAR Cancer 3(1):zcab004. https://doi.org/10.1093/narcan/zcab004
Jiang B, Kauffman AE, Li L, McFee W, Cai B, Weinstein J, Lead JR, Chatterjee S, Scott GI, Xiao S (2020) Health impacts of environmental contamination of micro- and nanoplastics: a review. Environ Health Prev Med 25(1):29. https://doi.org/10.1186/s12199-020-00870-9
Jin H, Ma T, Sha X, Liu Z, Zhou Y, Meng X, Chen Y, Han X, Ding J (2021) Polystyrene microplastics induced male reproductive toxicity in mice. J Hazard Mater 401:123430. https://doi.org/10.1016/j.jhazmat.2020.123430
Kang HM, Byeon E, Jeong H, Lee Y, Hwang UK, Jeong CB, Yoon C, Lee JS (2021) Arsenic exposure combined with nano- or microplastic induces different effects in the marine rotifer Brachionus plicatilis. Aquat Toxicol 233:105772. https://doi.org/10.1016/j.aquatox.2021.105772
Kaposi KL, Mos B, Kelaher BP, Dworjanyn SA (2014) Ingestion of microplastic has limited impact on a marine larva. Environ Sci Technol 48(3):1638–1645. https://doi.org/10.1021/es404295e
Keswani A, Oliver DM, Gutierrez T, Quilliam RS (2016) Microbial hitchhikers on marine plastic debris: human exposure risks at bathing waters and beach environments. Mar Environ Res 118:10–19. https://doi.org/10.1016/j.marenvres.2016.04.006
Khan FR, Syberg K, Shashoua Y, Bury NR (2015) Influence of polyethylene microplastic beads on the uptake and localization of silver in zebrafish (Danio rerio). Environ Pollut 206:73–79. https://doi.org/10.1016/j.envpol.2015.06.009
Kim D, Chae Y, An YJ (2017) Mixture toxicity of nickel and microplastics with different functional groups on Daphnia magna. Environ Sci Technol 51(21):12852–12858. https://doi.org/10.1021/acs.est.7b03732
Kirstein IV, Kirmizi S, Wichels A, Garin-Fernandez A, Erler R, Löder M, Gerdts G (2016) Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar Environ Res 120:1–8. https://doi.org/10.1016/j.marenvres.2016.07.004
Kleinteich J, Seidensticker S, Marggrander N, Zarfl C (2018) Microplastics reduce short-term effects of environmental contaminants. Part II: Polyethylene Particles Decrease the Effect of Polycyclic Aromatic Hydrocarbons on Microorganisms. Int J Environ Res Public Health 15(2):287. https://doi.org/10.3390/ijerph15020287
Koelmans AA, Bakir A, Burton GA, Janssen CR (2016) Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies. Environ Sci Technol 50(7):3315–3326. https://doi.org/10.1021/acs.est.5b06069
Koelmans AA, Besseling E, Foekema EM (2014) Leaching of plastic additives to marine organisms. Environ Pollut 187:49–54. https://doi.org/10.1016/j.envpol.2013.12.013
Lang M, Yu X, Liu J, Xia T, Wang T, Jia H, Guo X (2020) Fenton aging significantly affects the heavy metal adsorption capacity of polystyrene microplastics. Sci Total Environ 722:137762. https://doi.org/10.1016/j.scitotenv.2020.137762
Law KL, Thompson RC (2014) Microplastics in the seas. Science 345(6193):144–145. https://doi.org/10.1126/science.1254065
Lee H, Shim WJ, Kwon JH (2014) Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci Total Environ 470–471:1545–1552. https://doi.org/10.1016/j.scitotenv.2013.08.023
Lee WS, Cho HJ, Kim E, Huh YH, Kim HJ, Kim B, Kang T, Lee JS, Jeong J (2019) Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos. Nanoscale 11(7):3173–3185. https://doi.org/10.1039/c8nr09321k
Li J, Lusher AL, Rotchell JM, Deudero S, Turra A, Bråte ILN, Sun C, Shahadat Hossain M, Li Q, Kolandhasamy P, Shi H (2019a) Using mussel as a global bioindicator of coastal microplastic pollution. Environ Pollut 244:522–533. https://doi.org/10.1016/j.envpol.2018.10.032
Lithner D, Damberg J, Dave G, Larsson K (2009) Leachates from plastic consumer products–screening for toxicity with Daphnia magna. Chemosphere 74(9):1195–1200. https://doi.org/10.1016/j.chemosphere.2008.11.022
Li Y, Li M, Li Z, Yang L, Liu X (2019b) Effects of particle size and solution chemistry on Triclosan sorption on polystyrene microplastic. Chemosphere 231:308–314. https://doi.org/10.1016/j.chemosphere.2019.05.116
Li Y, Liu Z, Li M, Jiang Q, Wu D, Huang Y, Jiao Y, Zhang M, Zhao Y (2020a) Effects of nanoplastics on antioxidant and immune enzyme activities and related gene expression in juvenile Macrobrachium nipponense. J Hazard Mater 398:122990. https://doi.org/10.1016/j.jhazmat.2020.122990
Li Z, Zhou H, Liu Y, Zhan J, Li W, Yang K, Yi X (2020b) Acute and chronic combined effect of polystyrene microplastics and dibutyl phthalate on the marine copepod Tigriopus japonicus. Chemosphere 261:127711. https://doi.org/10.1016/j.chemosphere.2020.127711
Liu P, Zhan X, Wu X, Li J, Wang H, Gao S (2020) Effect of weathering on environmental behavior of microplastics: properties, sorption and potential risks. Chemosphere 242:125193. https://doi.org/10.1016/j.chemosphere.2019.125193
Liu Z, Yu P, Cai M, Wu D, Zhang M, Chen M, Zhao Y (2019) Effects of microplastics on the innate immunity and intestinal microflora of juvenile Eriocheir sinensis. Sci Total Environ 685:836–846. https://doi.org/10.1016/j.scitotenv.2019.06.265
Liu Z, Li Y, Pérez E, Jiang Q, Chen Q, Jiao Y, Huang Y, Yang Y, Zhao Y (2021) Polystyrene nanoplastic induces oxidative stress, immune defense, and glycometabolism change in Daphnia pulex: application of transcriptome profiling in risk assessment of nanoplastics. J Hazard Mater 402:123778. https://doi.org/10.1016/j.jhazmat.2020.123778
Liu Z, Cai M, Yu P, Chen M, Wu D, Zhang M, Zhao Y (2018) Age-dependent survival, stress defense, and AMPK in Daphnia pulex after short-term exposure to a polystyrene nanoplastic. Aquat Toxicol 204:1–8. https://doi.org/10.1016/j.aquatox.2018.08.017
Llorca M, Schirinzi G, Martínez M, Barceló D, Farré M (2018) Adsorption of perfluoroalkyl substances on microplastics under environmental conditions. Environ Pollut 235:680–691. https://doi.org/10.1016/j.envpol.2017.12.075
Lohmann R (2017) Microplastics are not important for the cycling and bioaccumulation of organic pollutants in the oceans-but should microplastics be considered POPs themselves? Integr Environ Assess Manag 13(3):460–465. https://doi.org/10.1002/ieam.1914
Lu L, Wan Z, Luo T, Fu Z, Jin Y (2018a) Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci Total Environ 631–632:449–458. https://doi.org/10.1016/j.scitotenv.2018.03.051
Lu K, Qiao R, An H, Zhang Y (2018b) Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 202:514–520. https://doi.org/10.1016/j.chemosphere.2018.03.145
Lu XM, Lu PZ, Liu XP (2020) Fate and abundance of antibiotic resistance genes on microplastics in facility vegetable soil. Sci Total Environ 709:136276. https://doi.org/10.1016/j.scitotenv.2019.136276
Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, Ding L, Ren H (2016) Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol 50(7):4054–4060. https://doi.org/10.1021/acs.est.6b00183
Luo T, Zhang Y, Wang C, Wang X, Zhou J, Shen M, Zhao Y, Fu Z, Jin Y (2019) Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ Pollut 255(Pt 1):113122. https://doi.org/10.1016/j.envpol.2019.113122
Luís LG, Ferreira P, Fonte E, Oliveira M, Guilhermino L (2015) Does the presence of microplastics influence the acute toxicity of chromium(VI) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations. Aquat Toxicol 164:163–174. https://doi.org/10.1016/j.aquatox.2015.04.018
Magni S, Gagné F, André C, Della Torre C, Auclair J, Hanana H, Parenti CC, Bonasoro F, Binelli A (2018) Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci Total Environ 631–632:778–788. https://doi.org/10.1016/j.scitotenv.2018.03.075
Martínez-Gómez C, León VM, Calles S, Gomáriz-Olcina M, Vethaak AD (2017) The adverse effects of virgin microplastics on the fertilization and larval development of sea urchins. Mar Environ Res 130:69–76. https://doi.org/10.1016/j.marenvres.2017.06.016
Ma Y, Huang A, Cao S, Sun F, Wang L, Guo H, Ji R (2016) Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ Pollut 219:166–173. https://doi.org/10.1016/j.envpol.2016.10.061
Massos A, Turner A (2017) Cadmium, lead and bromine in beached microplastics. Environ Pollut 227:139–145. https://doi.org/10.1016/j.envpol.2017.04.034
Miranda T, Vieira LR, Guilhermino L (2019) Neurotoxicity, behavior, and lethal effects of cadmium, microplastics, and their mixtures on Pomatoschistus microps juveniles from two wild populations exposed under laboratory conditions-implications to environmental and human risk assessment. Int J Environ Res Public Health 16(16):2857. https://doi.org/10.3390/ijerph16162857
Moresco V, Oliver DM, Weidmann M, Matallana-Surget S, Quilliam RS (2021) Survival of human enteric and respiratory viruses on plastics in soil, freshwater, and marine environments. Environ Res 199:111367. https://doi.org/10.1016/j.envres.2021.111367
Mughini-Gras L, van der Plaats RQJ, van der Wielen PWJJ, Bauerlein PS, de Roda Husman AM (2021) Riverine microplastic and microbial community compositions: a field study in the Netherlands. Water Res 192:116852. https://doi.org/10.1016/j.watres.2021.116852
Karami A, Romano N, Galloway T, Hamzah H (2016) Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environ Res 151:58–70. https://doi.org/10.1016/j.envres.2016.07.024
Kashiwada S (2006) Distribution of nanoparticles in the see-through medaka (Oryzias latipes). Environ Health Perspect 114(11):1697–1702. https://doi.org/10.1289/ehp.9209
Nasser F, Lynch I (2016) Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. J Proteomics 137:45–51. https://doi.org/10.1016/j.jprot.2015.09.005
Nolte TM, Hartmann NB, Kleijn JM, Garnæs J, van de Meent D, Jan Hendriks A, Baun A (2017) The toxicity of plastic nanoparticles to green algae as influenced by surface modification, medium hardness and cellular adsorption. Aquat Toxicol 183:11–20. https://doi.org/10.1016/j.aquatox.2016.12.005
OECD (2004) Emission Scenario Document on Plastic Additives. Series on Emission Scenario Documents, No. 3. OECD Environmental Health and Safety Publications. Environment Directorate, Paris
Ogata Y, Takada H, Mizukawa K, Hirai H, Iwasa S, Endo S, Mato Y, Saha M, Okuda K, Nakashima A, Murakami M, Zurcher N, Booyatumanondo R, Zakaria MP, le Dung Q, Gordon M, Miguez C, Suzuki S, Moore C, Karapanagioti HK, Weerts S, McClurg T, Burres E, Smith W, Van Velkenburg M, Lang JS, Lang RC, Laursen D, Danner B, Stewardson N, Thompson RC (2009) International pellet watch: global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar Pollut Bull 58(10):1437–1446. https://doi.org/10.1016/j.marpolbul.2009.06.014
Ogonowski M, Schür C, Jarsén Å, Gorokhova E (2016) The effects of natural and anthropogenic microparticles on individual fitness in Daphnia magna. PLoS ONE 11(5):e0155063. https://doi.org/10.1371/journal.pone.0155063
Oliveira P, Barboza LGA, Branco V, Figueiredo N, Carvalho C, Guilhermino L (2018) Effects of microplastics and mercury in the freshwater bivalve Corbicula fluminea (Müller, 1774): Filtration rate, biochemical biomarkers and mercury bioconcentration. Ecotoxicol Environ Saf 164:155–163. https://doi.org/10.1016/j.ecoenv.2018.07.062
Oliveira M, Gravato C, Guilhermino L (2012) Acute toxic effects of pyrene on Pomatoschistus microps (Teleostei, Gobiidae): mortality, biomarkers and swimming performance. Ecol Ind 2012(19):206–214. https://doi.org/10.1016/j.ecolind.2011.08.006
Oliveira M, Ribeiro A, Hylland K, Guilhermino L (2013) Single and combined effects of microplastics and pyrene on juveniles (0 + group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol Ind 34:641–647. https://doi.org/10.1016/j.ecolind.2013.06.019
Ott MG (2002) Occupational asthma, lung function decrement, and toluene diisocyanate (TDI) exposure: a critical review of exposure-response relationships. Appl Occup Environ Hyg 17(12):891–901. https://doi.org/10.1080/10473220290107093
Parenti CC, Magni S, Ghilardi A, Caorsi G, Binelli A (2020) Does triclosan adsorption on polystyrene nanoplastics modify the toxicity of single contaminants. Environmental science. Nano (8). https://doi.org/10.1039/D0EN00961J
Pauly JL, Stegmeier SJ, Allaart HA, Cheney RT, Zhang PJ, Mayer AG, Streck RJ (1998) Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidemiol Biomarkers Prev 7(5):419–428 (PMID: 9610792)
Paul-Pont I, Lacroix C, González Fernández C, Hégaret H, Lambert C, Le Goïc N, Frère L, Cassone AL, Sussarellu R, Fabioux C, Guyomarch J, Albentosa M, Huvet A, Soudant P (2016) Exposure of marine mussels Mytilus spp. to polystyrene microplastics: toxicity and influence on fluoranthene bioaccumulation. Environ Pollut 216:724–737. https://doi.org/10.1016/j.envpol.2016.06.039.
Pedà C, Caccamo L, Fossi MC, Gai F, Andaloro F, Genovese L, Perdichizzi A, Romeo T, Maricchiolo G (2016) Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: preliminary results. Environ Pollut 212:251–256. https://doi.org/10.1016/j.envpol.2016.01.083
Pitt JA, Trevisan R, Massarsky A, Kozal JS, Levin ED, Di Giulio RT (2018a) Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): a case study with nanopolystyrene. Sci Total Environ 643:324–334. https://doi.org/10.1016/j.scitotenv.2018.06.186
Pitt JA, Kozal JS, Jayasundara N, Massarsky A, Trevisan R, Geitner N, Wiesner M, Levin ED, Di Giulio RT (2018b) Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat Toxicol 194:185–194. https://doi.org/10.1016/j.aquatox.2017.11.017
Pratt M, Taraska V (2000) Disperse blue dyes 106 and 124 are common causes of textile dermatitis and should serve as screening allergens for this condition. Am J Contact Dermat 11(1):30–41. https://doi.org/10.1016/s1046-199x(00)90030-7
Provencher JF, Gaston AJ, Mallory ML, O’hara PD, Gilchrist HG, (2010) Ingested plastic in a diving seabird, the thick-billed murre (Uria lomvia), in the eastern Canadian Arctic. Mar Pollut Bull 60(9):1406–11. https://doi.org/10.1016/j.marpolbul.2010.05.017
Qiao R, Deng Y, Zhang S et al (2019) Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 236:124334. https://doi.org/10.1016/j.chemosphere.2019.07.065
Qu H, Ma R, Wang B, Yang J, Duan L, Yu G (2019) Enantiospecific toxicity, distribution and bioaccumulation of chiral antidepressant venlafaxine and its metabolite in loach (Misgurnus anguillicaudatus) co-exposed to microplastic and the drugs. J Hazard Mater 370:203–211. https://doi.org/10.1016/j.jhazmat.2018.04.041
Ragusa A, Svelato A, Santacroce C, Catalano P, Notarstefano V, Carnevali O, Papa F, Rongioletti MCA, Baiocco F, Draghi S, D’Amore E, Rinaldo D, Matta M, Giorgini E (2021) Plasticenta: first evidence of microplastics in human placenta. Environ Int 146:106274. https://doi.org/10.1016/j.envint.2020.106274
Rios-Fuster B, Arechavala-Lopez P, García-Marcos K, Alomar C, Compa M, Álvarez E, Julià MM, Solomando Martí A, Sureda A, Deudero S (2021) Experimental evidence of physiological and behavioral effects of microplastic ingestion in Sparus aurata. Aquat Toxicol 231:105737. https://doi.org/10.1016/j.aquatox.2020.105737
Rehse S, Kloas W, Zarfl C (2016) Short-term exposure with high concentrations of pristine microplastic particles leads to immobilisation of Daphnia magna. Chemosphere 153:91–99. https://doi.org/10.1016/j.chemosphere.2016.02.133
Rochman CM, Hentschel BT, Teh SJ (2014) Long-term sorption of metals is similar among plastic types: implications for plastic debris in aquatic environments. PLoS ONE 9(1):e85433. https://doi.org/10.1371/journal.pone.0085433
Rochman CM, Hoh E, Kurobe T, Teh SJ (2013a) Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci Rep 3:3263–3269. https://doi.org/10.1038/srep03263
Rochman CM, Browne MA, Halpern BS, Hentschel BT, Hoh E, Karapanagioti HK, Rios-Mendoza LM, Takada H, Teh S, Thompson RC (2013b) Policy: classify plastic waste as hazardous. Nature 494(7436):169–171. https://doi.org/10.1038/494169a
Seachrist DD, Bonk KW, Ho SM, Prins GS, Soto AM, Keri RA (2016) A review of the carcinogenic potential of bisphenol A. Reprod Toxicol 59:167–182. https://doi.org/10.1016/j.reprotox.2015.09.006
Seidensticker S, Zarfl C, Cirpka OA, Fellenberg G, Grathwohl P (2017) Shift in mass transfer of wastewater contaminants from microplastics in the presence of dissolved substances. Environ Sci Technol 51(21):12254–12263. https://doi.org/10.1021/acs.est.7b02664
Sharifinia M, Bahmanbeigloo ZA, Keshavarzifard M, Khanjani MH, Lyons BP (2020) Microplastic pollution as a grand challenge in marine research: a closer look at their adverse impacts on the immune and reproductive systems. Ecotoxicol Environ Saf 204:111109. https://doi.org/10.1016/j.ecoenv.2020.111109
Sicińska P (2018) Di-n-butyl phthalate, butylbenzyl phthalate and their metabolites induce haemolysis and eryptosis in human erythrocytes. Chemosphere 203:44–53. https://doi.org/10.1016/j.chemosphere.2018.03.161
Sicińska P, Kik K, Bukowska B (2020) Human erythrocytes exposed to phthalates and their metabolites alter antioxidant enzyme activity and hemoglobin oxidation. Int J Mol Sci 21(12):4480. https://doi.org/10.3390/ijms21124480
Sıkdokur E, Belivermiş M, Sezer N, Pekmez M, Bulan ÖK, Kılıç Ö (2020) Effects of microplastics and mercury on manila clam Ruditapes philippinarum: feeding rate, immunomodulation, histopathology and oxidative stress. Environ Pollut 262:114247. https://doi.org/10.1016/j.envpol.2020.114247
Stock V, Böhmert L, Lisicki E, Block R, Cara-Carmona J, Pack LK, Selb R, Lichtenstein D, Voss L, Henderson CJ, Zabinsky E, Sieg H, Braeuning A, Lampen A (2019) Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch Toxicol 93(7):1817–1833. https://doi.org/10.1007/s00204-019-02478-7
Su Y, Zhang K, Zhou Z, Wang J, Yang X, Tang J, Li H, Lin S (2020) Microplastic exposure represses the growth of endosymbiotic dinoflagellate Cladocopium goreaui in culture through affecting its apoptosis and metabolism. Chemosphere 244:125485. https://doi.org/10.1016/j.chemosphere.2019.125485
Sussarellu R, Suquet M, Thomas Y, Lambert C, Fabioux C, Pernet ME, Le Goïc N, Quillien V, Mingant C, Epelboin Y, Corporeau C, Guyomarch J, Robbens J, Paul-Pont I, Soudant P, Huvet A (2016) Oyster reproduction is affected by exposure to polystyrene microplastics. Proc Natl Acad Sci U S A 113(9):2430–2435. https://doi.org/10.1073/pnas.1519019113
Syberg K, Nielsen A, Khan FR, Banta GT, Palmqvist A, Jepsen PM (2017) Microplastic potentiates triclosan toxicity to the marine copepod Acartia tonsa (Dana). J Toxicol Environ Health A 80(23–24):1369–1371. https://doi.org/10.1080/15287394.2017.1385046
Sørensen L, Rogers E, Altin D, Salaberria I, Booth AM (2020) Sorption of PAHs to microplastic and their bioavailability and toxicity to marine copepods under co-exposure conditions. Environ Pollut 258:113844. https://doi.org/10.1016/j.envpol.2019.113844
Tanaka K, Takada H, Yamashita R, Mizukawa K, Fukuwaka MA, Watanuki Y (2013) Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar Pollut Bull 69(1–2):219–222. https://doi.org/10.1016/j.marpolbul.2012.12.010
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AW, McGonigle D, Russell AE (2004) Lost at sea: where is all the plastic? Science 304(5672):838. https://doi.org/10.1126/science.1094559
Trevisan R, Voy C, Chen S, Di Giulio RT (2019) Nanoplastics decrease the toxicity of a complex PAH mixture but impair mitochondrial energy production in developing zebrafish. Environ Sci Technol 53(14):8405–8415. https://doi.org/10.1021/acs.est.9b02003
Turner A, Holmes LA (2015) Adsorption of trace metals by microplastic pellets in fresh water. Environ Chem 12:600. https://doi.org/10.1071/EN14143
Van Cauwenberghe L, Claessens M, Vandegehuchte MB, Janssen CR (2015) Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ Pollut 199:10–17. https://doi.org/10.1016/j.envpol.2015.01.008
Velzeboer I, Kwadijk CJ, Koelmans AA (2014) Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ Sci Technol 48(9):4869–4876. https://doi.org/10.1021/es405721v
Vendel AL, Bessa F, Alves VEN, Amorim ALA, Patrício J, Palma ART (2017) Widespread microplastic ingestion by fish assemblages in tropical estuaries subjected to anthropogenic pressures. Mar Pollut Bull 117(1–2):448–455. https://doi.org/10.1016/j.marpolbul.2017.01.081
Veneman WJ, Spaink HP, Brun NR, Bosker T, Vijver MG (2017) Pathway analysis of systemic transcriptome responses to injected polystyrene particles in zebrafish larvae. Aquat Toxicol 190:112–120. https://doi.org/10.1016/j.aquatox.2017.06.014
Verla AW, Enyoh CE, Verla EN, Nwarnorh KO (2019) Microplastic–toxic chemical interaction: a review study on quantified levels, mechanism and implication. SN Applied Sciences 1:1400. https://doi.org/10.1007/s42452-019-1352-0
Viršek MK, Lovšin MN, Koren Š, Kržan A, Peterlin M (2017) Microplastics as a vector for the transport of the bacterial fish pathogen species Aeromonas salmonicida. Mar Pollut Bull 125(1–2):301–309. https://doi.org/10.1016/j.marpolbul.2017.08.024
von Moos N, Burkhardt-Holm P, Köhler A (2012) Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ Sci Technol 46(20):11327–11335. https://doi.org/10.1021/es302332w
Wang F, Shih KM, Li XY (2015) The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere 119:841–847. https://doi.org/10.1016/j.chemosphere.2014.08.047
Wang L, Wu WM, Bolan NS, Tsang DCW, Li Y, Qin M, Hou D (2021a) Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: current status and future perspectives. J Hazard Mater 401:123415. https://doi.org/10.1016/j.jhazmat.2020.123415
Wang Q, Huang F, Liang K, Niu W, Duan X, Jia X, Wu X, Xu P, Zhou L (2021b) Polystyrene nanoplastics affect digestive function and growth in juvenile groupers. Sci Total Environ 808:152098. https://doi.org/10.1016/j.scitotenv.2021.152098
Wang Y, Mao Z, Zhang M, Ding G, Sun J, Du M, Liu Q, Cong Y, Jin F, Zhang W, Wang J (2019) The uptake and elimination of polystyrene microplastics by the brine shrimp, Artemia parthenogenetica, and its impact on its feeding behavior and intestinal histology. Chemosphere 234:123–131. https://doi.org/10.1016/j.chemosphere.2019.05.267
Wang YL, Lee YH, Hsu YH, Chiu IJ, Huang CC, Huang CC, Chia ZC, Lee CP, Lin YF, Chiu HW (2021c) the kidney-related effects of polystyrene microplastics on human kidney proximal tubular epithelial cells HK-2 and male C57BL/6 mice. Environ Health Perspect 129(5):57003. https://doi.org/10.1289/EHP7612
Wang Z, Chen M, Zhang L, Wang K, Yu X, Zheng Z, Zheng R (2018) Sorption behaviors of phenanthrene on the microplastics identified in a mariculture farm in Xiangshan Bay, southeastern China. Sci Total Environ 628–629:1617–1626. https://doi.org/10.1016/j.scitotenv.2018.02.146
Wan Z, Wang C, Zhou J, Shen M, Wang X, Fu Z, Jin Y (2019) Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere 217:646–658. https://doi.org/10.1016/j.chemosphere.2018.11.070
Wardrop P, Shimeta J, Nugegoda D, Morrison PD, Miranda A, Tang M, Clarke BO (2016) Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish. Environ Sci Technol 50(7):4037–4044. https://doi.org/10.1021/acs.est.5b06280
Ward JE, Kach DJ (2009) Marine aggregates facilitate ingestion of nanoparticles by suspension-feeding bivalves. Mar Environ Res 68(3):137–142. https://doi.org/10.1016/j.marenvres.2009.05.002
Wegner A, Besseling E, Foekema EM, Kamermans P, Koelmans AA (2012) Effects of nanopolystyrene on the feeding behavior of the blue mussel (Mytilus edulis L.). Environ Toxicol Chem 31(11):2490–7. https://doi.org/10.1002/etc.1984
Wei X, Li M, Wang Y, Jin L, Ma G, Yu H (2019) Developing predictive models for carrying ability of micro-plastics towards organic pollutants. Molecules 24(9):1784. https://doi.org/10.3390/molecules24091784
Wen B, Jin SR, Chen ZZ, Gao JZ, Liu YN, Liu JH, Feng XS (2018) Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ Pollut 243(Pt A):462–471. https://doi.org/10.1016/j.envpol.2018.09.029
Wright SL, Thompson RC, Galloway TS (2013) The physical impacts of microplastics on marine organisms: a review. Environ Pollut 178:483–492. https://doi.org/10.1016/j.envpol.2013.02.031
Wright SL, Kelly FJ (2017) Plastic and human health: a micro issue? Environ Sci Technol 51(12):6634–6647. https://doi.org/10.1021/acs.est.7b00423
Wu C, Zhang K, Huang X, Liu J (2016) Sorption of pharmaceuticals and personal care products to polyethylene debris. Environ Sci Pollut Res Int 23(9):8819–8826. https://doi.org/10.1007/s11356-016-6121-7
Xu B, Liu F, Brookes PC, Xu J (2018a) The sorption kinetics and isotherms of sulfamethoxazole with polyethylene microplastics. Mar Pollut Bull 131(Pt A):191–196. https://doi.org/10.1016/j.marpolbul.2018.04.027
Xu B, Liu F, Brookes PC, Xu J (2018b) Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environ Pollut 240:87–94. https://doi.org/10.1016/j.envpol.2018.04.113
Xu D, Ma Y, Han X, Chen Y (2021) Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells. J Hazard Mater 417:126092. https://doi.org/10.1016/j.jhazmat.2021.126092
Yin L, Chen B, Xia B, Shi X, Qu K (2018) Polystyrene microplastics alter the behavior, energy reserve and nutritional composition of marine jacopever (Sebastes schlegelii). J Hazard Mater 360:97–105. https://doi.org/10.1016/j.jhazmat.2018.07.110
Zhang H, Wang J, Zhou B, Zhou Y, Dai Z, Zhou Q, Chriestie P, Luo Y (2018) Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: kinetics, isotherms and influencing factors. Environ Pollut 243(Pt B):1550–1557. https://doi.org/10.1016/j.envpol.2018.09.122
Zhang S, Ding J, Razanajatovo RM, Jiang H, Zou H, Zhu W (2019) Interactive effects of polystyrene microplastics and roxithromycin on bioaccumulation and biochemical status in the freshwater fish red tilapia (Oreochromis niloticus). Sci Total Environ 648:1431–1439. https://doi.org/10.1016/j.scitotenv.2018.08.266
Zhang Y, Jiao Y, Li Z, Tao Y, Yang Y (2021) Hazards of phthalates (PAEs) exposure: a review of aquatic animal toxicology studies. Sci Total Environ 771:145418. https://doi.org/10.1016/j.scitotenv.2021.145418
Zhou Y, Liu X, Wang J (2019) Characterization of microplastics and the association of heavy metals with microplastics in suburban soil of central China. Sci Total Environ 694:133798. https://doi.org/10.1016/j.scitotenv.2019.133798
Zhuang J, Cui H, Zhuang L, Zhai Z, Yang F, Luo G, He J, Zhao H, Zhao W, He Y, Sun E (2020) Bronchial epithelial pyroptosis promotes airway inflammation in a murine model of toluene diisocyanate-induced asthma. Biomed Pharmacother 125:109925. https://doi.org/10.1016/j.biopha.2020.109925
Zocchi M, Sommaruga R (2019) Microplastics modify the toxicity of glyphosate on Daphnia magna. Sci Total Environ 697:134194. https://doi.org/10.1016/j.scitotenv.2019.134194
Acknowledgements
Not applicable.
Funding
This project was funded by the Tianjin Municipal Health Bureau Science and Technology Talent Cultivation Project (RC20206).
Author information
Authors and Affiliations
Contributions
Q. Z. and Y. H. carried out collection and assembly of data, and analysis and interpretation of the data. R. J. C. and Q. L. helped to draft the manuscript. X. H. L., Q. Z., and Z. Y. Q. conceived the study, participated in its design and coordination, corrected the manuscript, and supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Q., He, Y., Cheng, R. et al. Recent advances in toxicological research and potential health impact of microplastics and nanoplastics in vivo. Environ Sci Pollut Res 29, 40415–40448 (2022). https://doi.org/10.1007/s11356-022-19745-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19745-3