Abstract
Photocatalytic removal of nitrate in wastewater has attracted wide attention because of its simple operation and environmental protection. However, the preparation of photocatalysts with high efficiency and high nitrogen selectivity is still a challenge. In this paper, TiO2 is grown in situ on Ti3C2 MXene by a simple calcination method and modified with silver particles. The presence of Ti3C2 reduces the recombination rate of photogenerated electrons and generates more photogenerated electrons. At the same time, the silver particles also increase the photoelectron density and further improve the carrier separation of the catalyst. Due to its unique structure and optical properties, the prepared photocatalyst shows an excellent nitrate removal rate under a high-pressure mercury lamp. At 500 mgN/L, the nitrate removal rate reaches 96.1%, and the nitrogen selectivity reaches 92.6%. Even after 5 cycles, the prepared photocatalyst still maintains a high nitrate photocatalytic removal efficiency (89%). The electron transfer path is verified by density functional theory calculations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excessive nitrate levels in wastewater can lead to the eutrophication of lakes and rivers (Stevens and Gowing 2004; Zhao et al. 2021). Excessive nitrate in drinking water enters the human body and will be converted into nitrite, leading to hypoxia and poisoning of the human body. It can also lead to the blue baby syndrome, methemoglobinemia, and even cancers such as esophageal cancer and gastric cancer (Stevens and Gowing 2004; Zhang et al. 2020a). Therefore, the world health organization recommends that the concentration of nitrate in water should not exceed 11.3mgN/L (Karen et al. 2010;Loganathan et al. 2013). The concentration of nitrate in drinking water in the USA is set not to exceed 10mgN/L (Khan and Aspalding 2004). The most commonly used methods for nitrate removal in industry are ion exchange, reverse osmosis, biological method, and bimetallic catalysts. But ion exchange and reverse osmosis only serve to concentrate and transfer nitrate, and the subsequent treatment of the resulting high concentration nitrate wastewater remains a serious challenge (Barrabés and Sá 2011). The removal of nitrate by biological method takes a long time, and the reaction conditions are strict, resulting in biological sludge (Li et al. 2009). Bimetallic catalysts catalyze nitrate to produce nitrogen is a green method, but the reaction requires hydrogen as a reducing agent, hydrogen storage and transportation is a serious challenge for industrial applications (Gao et al. 2019). In recent years, researchers have found that photocatalysis can effectively remove nitrate from wastewater. After studying various semiconductors, such as titanium dioxide (Kim and Pak 2019; Krasae and Wantala 2016; Zhu et al. 2018), cadmium sulfide (Fang et al. 2008; Sittinun Tawkaew et al. 2001), barium tetroxide, and zinc sulfide (Bourkeb and Wbaaloudj 2021), Chu et al. (2013) and Lucchetti et al. (2017) found that titanium dioxide due to its good stability and being non-toxic and a low-cost semiconductor in the photocatalytic removal of nitrate process has a great potential. However, due to its fast complexation of broad-growth charges and relatively wide bandgap, titanium dioxide still suffers from quantum efficiency and low visible light utilization (Hirayama and Kamiya 2014; Xiong et al. 2018). Single photocatalyst has a high photoelectron-hole recombination ratio, which affects the reaction efficiency of photocatalysis. The use of cocatalyst can effectively promote the separation of photogenerated electrons and holes (Zhao et al. 2020).
MXene is a new type of 2D transition metal carbide or carbonitride, which is produced by HF etching the Al-containing layer of MAX ceramics (Sreedhar and Noh 2021). Since it was first reported by Naguib et al. (2011), its graphene-like structure, hydrophilic layered surface, and significant conductivity properties have been widely considered as good candidates for energy storage, electrochemical sensors, and pollutant purification (Zhao et al. 2017). In the field of photocatalysis, MXene has many advantages that make it an efficient cocatalyst for photocatalysts. First, MXene has hydrophilic properties, which facilitate the adsorption of water molecules by photocatalysts, thus promoting the water separation reaction of photocatalysis (Khadidja et al. 2021). Secondly, MXene has a large number of hydrophilic functional groups (such as -OH) on the surface, which can be combined with most semiconductor photocatalysts and have a strong interaction with semiconductor photocatalysts (Hong et al. 2020). The Gibbs free energy of hydrogen adsorption of MXene is close to zero, which is conducive to the reduction of H+ to H2, and has a high activation energy of hydrogen precipitation. In addition, Ti3C2 MXene highlights specific surface areas and inhibits charge carrier recombination during photocatalytic activity (Naguib et al. 2014). Surprisingly, the carrier separation is enhanced when titanium dioxide is in close contact with Ti3C2 (Feng et al. 2021). In conclusion, Ti3C2 MXene is very beneficial in photocatalytic applications (Quyen et al. 2021). In addition to the addition of cocatalyst, there are the following methods to improve the separation of photogenerated electron-hole pairs in TiO2: combining with another semiconductor (Sivakumar et al. 2013) or a point-based material (Wu et al. 2002), such as graphene, doping transition metal ions (Rathinavelu et al. 2013) or noble metal elements (Tian et al. 2015), or depositing metal nanoparticles on semiconductor surface (Zhang et al. 2009). A certain sacrificial agent is added to the reaction (Shi et al. 2011).
Hence, this paper presents a method to grow TiO2 nanoparticles in situ on MXene surface by simple calcination and finds that the loading of TiO2 nanoparticles can be tuned by adjusting the calcination temperature. TiO2/Ti3C2 supported with silver particles was prepared as an efficient photocatalyst for nitrate removal. Under the irradiation of a high-pressure mercury lamp, the removal effect of the catalyst on nitrate was investigated and its mechanism was discussed.
Experimental details
Sample preparation
Materials
Aluminum carbon titanite, hydrofluoric acid, silver nitrate, sodium borohydride, potassium nitrate, sodium potassium dodecylate, formic acid, and sodium hydroxide were purchased from Sinopagic Chemical Reagent Co. LTD, without further treatment. All the water used for reagents was deionized.
Preparation of Ti3C2
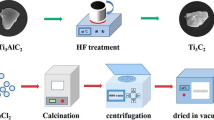
Ti3C2 powder was prepared by the reaction of hydrofluoric acid with Ti3AlC2. 1.2 g of Ti3AlC2 powder was slowly added into 21 mL, 48% hydrofluoric acid and stirred at room temperature with a magnetic stirrer at 500 r/min for 36 h to etch the aluminum layer, and finally an accordion-like solid powder was obtained. The black precipitate obtained was centrifuged and washed with deionized water until its supernatant was neutral. The obtained solid was dried in an oven at 80°C for 12 h to obtain Ti3C2 powder (Huang et al. 2020).
Preparation of TiO2/Ti3C2 composite
TiO2/Ti3C2 composite was prepared by simple calcination. 0.5g dried and ground Ti3C2 was weighed and put into a tubular furnace at a heating rate of 10°C/min. TiO2/Ti3C2 composite was obtained by calcination at 250 °C, 350 °C, 450 °C, 550 °C, and 650 °C respectively for 1h, which was recorded as tTTiO2, which t stands for calcination temperatures, such as 250 °C, 350 °C, 450 °C, 550 °C, or 650 °C.
Preparation of Ag/TiO2/Ti3C2
Ag/TiO2/Ti3C2 catalyst was prepared by simple chemical reduction method (Zhang et al. 2003). 0.5g TiO2/Ti3C2 powder was weighed and put into 10mL water. AgNO3 (0.008g, 0.016g, 0.024g, 0.032g, 0.04g) was added to the solution as Ag source, and then 0.005g sodium dodecyl sulfate was added as dispersant, and stirred for 30 min. Then, 0.05g solid sodium borohydride was dissolved in 10mL 1M NaOH solution, and the solution was added drop by drop into TiO2/Ti3C2 solution, stirred for 30 min, centrifuged, cleaned with deionized water, and dried to obtain Ag/TiO2/Ti3C2 solid, denoted as nTTA, which n represents the mass fraction of loaded silver, such as 1%, 2%, 3%, 4%, or 5%.
The synthesis process of TTA is shown in Scheme 1.
Characterization
The crystal structure of the samples was characterized by X-ray diffraction (XRD) (Ultima IV, Japan). Scanning electron microscopy (JEOL JSM-5600L SEM) and transmission electron microscope (TEM, JEM-2010) were used to observe the morphology, microstructure, and elemental analysis of the samples. BET analysis of the samples was performed using Micromeritics (ASAP 2020). The pore size distribution of mesoporous materials was analyzed by using Barrett-Joyner-Halenda (BJH) model. BaSO4 was used as the background to test the UV-visible diffuse reflectance spectrum (UV-VIS DRS) of the sample using Shimadzu ULTRAVIOLET visible spectrophotometer (UV 3600, Shimadzu). The surface chemical composition was analyzed by X-ray photoelectron spectroscopy (XPS) using Mg Kα radiation.
Photoelectrochemical measurements
Use 200mL, 0.1M Na2SO4 solution as an electrolyte solution, use the standard three-electrode system, use sample as working electrode, platinum as counter electrode, and Ag/AgCl (saturated KCl) as a reference electrode. Before the start of the test, 2 mg of the sample was added to 0.5 mL of water, 0.5 mL of ethanol, and 20 μL of Nafion, and dispersed by ultrasound. The suspension was smeared on FTO glass with a fixed area of 0.5 cm2. Finally, the FTO glass coated with the suspension was placed in an oven at 160 °C overnight to obtain a working electrode.
Photocatalytic denitrification performance
The photocatalytic reduction of nitrate was carried out in a 250mL borosilicate reactor. The light source is a 250W high-pressure mercury lamp (the main wavelength is about 365 nm). The initial nitrate concentration is 500 mgN/L (by weight of nitrogen). There is no treatment of dissolved oxygen. Twenty milligrams of the catalyst was added to 50 mL water, and the solution was stirred in the dark for 30 min to achieve the adsorption-desorption equilibrium of nitrate nitrogen. Then, 40 mM formic acid solution was added as a cavity cleaner (Soares et al. 2014). Turn on the condensate so that the reaction always takes place at room temperature during the photocatalytic process. In the photocatalytic reaction process, the liquid is extracted from the reactor regularly, the concentration of nitrate is detected by using the lightning ion concentration detector (PXS-270), the nitrite and ammonium are detected by using a spectrophotometer (UV722), and the total nitrogen is tested by using an ultraviolet spectrophotometer (UV-3600). Since nitrite, ammonia, and nitrogen are the main products in the photocatalytic process, NO and N2O by-products can be ignored, and organic nitrogen will not be generated. Therefore, the selectivity of N2 is calculated according to the following formula:
where S(N2) represents the selectivity of N2, C(NO3−)0 represents the initial concentration of nitrate nitrogen content, and C(NO3−)t, C(NO2−)t, and C(NH4+)t represents the nitrogen content of each ion after t time reaction.
DFT calculation method
In this work, the density functional theory (DFT) calculation was performed by the Vienna Ab-initio Simulation Package (VASP) code, in which a plane wave basis set was used (Meng et al. 2017). The exchange and correlation interactions were modeled using the generalized gradient approximation (GGA) and the Perdew-Burke-Ernzerhof (PBE) functional (Zhang et al. 2020b). Grimme’s semi-empirical DFT was introduced in the computations to guarantee a better description of the electron interaction in a long range. The Vanderbilt ultrasoft pseudopotential was used with a cutoff energy of 450 eV (Bredas and Lhouk 2014). Geometric convergence tolerances were set for a maximum force of 0.02 eV/A° and a maximum energy change of 10−5 eV/ato. Density mixing electronic minimization was implemented and the self-consistent field (SCF) tolerance was set to the high accuracy of 10-5 eV/atom for energy convergence.
Result and discussion
The morphology and element distribution of the samples were analyzed by scanning electron microscope (SEM) and energy-dispersive X-ray spectrometer (EDS) (Mao et al. 2021). As shown in Fig. 1a and b, the etched Ti3C2 has a hierarchical accordion-like structure and is smooth. The surface of TiO2/Ti3C2 after calcination becomes rough, which is due to the formation of TiO2 nanoparticles on the surface (Fig. 1c). In addition, with the increase of the calcination temperature, the color of TiO2/Ti3C2 became whiter and whiter (Fig. S1), and the TiO2 particles formed on the surface became more and more dense, like a crispy pot (Fig. 1d, Fig. S2a–d). In this paper, TTiO2 calcined at 550°C was used for the subsequent study of supporting Ag. After loading Ag, the morphology of the sample did not change significantly (Fig. 1e). The sample was tested by TEM. Figure 1f shows the clear layered structure of Ti3C2, which also indicates that the transformation of Ti3C2 into TiO2 starts from the surface of Ti3C2 and extends to the core of Ti3C2. Taking 3%TTA as an example, After Ag was loaded, Ag particles were attached to the surface of 550TTiO2 (Fig. 1g). It can be seen that TiO2 is located on the surface of Ti3C2, and Ag particles are supported on the surface of TiO2. The lattice and fringe spacing were determined by high-resolution transmission electron microscopy (TEM). The lattice fringes of Ag, TiO2, and Ti3C2 are visible, indicating the successful preparation of TTA (Fig. 1h) (He et al. 2021). Among them, the lattice spacing of 0.24 nm, 0.35 nm, and 0.27 nm is attributed to the (101) face of Ag, the (101) planes of anatase TiO2, and the (0110) planes of Ti3C2, respectively. In addition, the elemental analysis spectrum of TTA was measured. It can be seen from Fig. 1i–l that Ag is uniformly distributed on 550TTiO2.
The X-ray diffractograms of all samples are shown in Fig. 2. After HF acid etching, the diffraction line located at 39° of (104) plane disappeared in Ti3C2 compared with Ti3AlC2, indicating that HF acid etched off the Al layer and Ti3AlC2 transformed into Ti3C2 (Yu et al. 2019). After HF etching, the Ti3C2 layer spacing increased and the 004 plane underwent a smaller angle shift, which also proved the successful etching of Ti3C2 (Li 2019). After calcination at 250 °C, the cleanliness of Ti3C2 increased and the diffraction lines were enhanced. In addition, the appearance of TiO2 anatase diffraction lines was also observed, indicating the generation of TiO2. With the increase of the calcination temperature, the content of TiO2 also increased, the crystallinity of TiO2 increased, the defect of the sample decreased, and the intensity of the TiO2 diffraction line gradually increased. Shrinkage of surface defects reduces the possibility of electron-hole recombination (Fig. 2a). In addition, Ti3C2 diffraction lines appeared at 35° and 43° in the samples calcined at 250 to 650 °C, confirming the formation of TiO2/Ti3C2 composite materials. Moreover, with the increase of calcination temperature, the intensity of the Ti3C2 diffraction line gradually decreases, which proves that Ti3C2 transforms into TiO2. At 650 °C, the peak of Ti3C2 completely disappeared, proving that Ti3C2 was completely converted to TiO2 (Fig. 2b) (Hou et al. 2021). It is proved that the yield of TiO2 can be easily adjusted by changing the calcination temperature. With the increase of Ag loading, Ag peaks gradually appeared at 44°, 64°, and 78° (Fig. 2c and d) (Jia et al. 2016).
Due to the calcination of Ti3C2, the surface composition of Ti3C2 changes. To further confirm the changes of surface composition and chemical states of the samples, XPS characterization was carried out. The samples were Ti3C2, 550TTiO2, 650TTiO2, and 3%TTA. Obviously, the binding energy peaks of the Ti element were observed in all four samples (Fig. 3a). Eight peaks can be seen in the high-resolution Ti 2p, as shown in Fig. 3b. 454.9 eV, 460.8 eV, 455.9 eV, 461.5 eV, 457.5 eV, 463.5 eV, 459 eV, and 465.7 eV, respectively, correspond to the Ti - C 2p3/2, Ti - C 2p1/2, Ti2 + 2p3/2, Ti2 + 2p1/2, Ti3+ 2p1/2, Ti3+ 2p1/2, TiO2 2p3/2, and TiO2 2p1/2. Consistent with previous reports (Shah et al. 2016). Compared with Ti calcined at 550 °C and 650 °C for 1h, only the main binding energy peaks of TiO2 2p3/2 and TiO2 2p1/2 at 459 eV and 464.4 eV were observed in the calcined samples, but there was no binding energy peak of Ti3C2 (Fig. 3c), which was not consistent with the XRD description. This is because XPS can only detect the components of the surface layer of the sample, that is, the components with a depth of less than 5nm, making it difficult for XPS to detect Ti3C2 located in the interior. As shown in Fig. S3 a, the XPS spectra of C1s of Ti3C2 have peaks at 281.9 eV, 284.7 eV, 286.4 eV, and 288.7 eV, corresponding to C-Ti-Txa, C-C, CO-, and COO-, respectively (Halim et al. 2016). These are attributed to Ti3C2 and amorphous carbon. In the sample calcined at 550 °C for 1h, its C spectrum has four peaks. Compared with the peak of Ti3C2, the peak at 281.9 eV of the sample calcined at 550 °C decreases in intensity, indicating that a part of Ti3C2 is converted into TiO2, while the sample calcined at 650 °C does not have the peak of C-Ti-Ox at 281.9 eV (Ghassemi et al. 2014). It is proved that Ti3C2 is completely converted to TiO2, which is consistent with XRD results (Fig. S3b–c). For the XPS peak of O1s, with the increase of calcination temperature, the peak intensity of C-Ti-(OH)x and C-Ti-Ox decreases, while the peak intensity of Ti-O increases, proving that Ti3C2 transforms to TiO2 (Fig. S3d–f). For Ag-loaded samples (Fig. 3d–f), silver XPS showed two peaks at 367.58 eV and 373.58eV, corresponding to Ag 3d5/2 and Ag 3d3/2, respectively. The bimodal separation values of Ag 3d5/2 and Ag 3d3/2 are 6 eV, which is consistent with the most common values reported in the literature (Nila et al. 2019).
The optical bandgap energy (Eg) for the sample can be obtained by UV-vis spectra, corresponding to the intercept of the (αhν)1/2 curve. As shown in Fig. 4b, Eg values of 1%TTA, 2%TTA, 3%TTA, 4%TTA, and 5%TTA were 3.18 eV, 3 eV,3.2 eV, 3.17 eV, and 3.16 eV, respectively. The detailed band structure of the sample can be obtained by testing the Motshott curve and shown in Fig. 4c. The X-intercept of the Mott-Schottky diagram represents the conduction potential E(CB) of the prepared N-type semiconductor (Baldini et al. 2020). The E(fb) value is estimated from the line segment of the X-intercept of the Mott-Schottky diagram. According to different reference electrodes, the conduction potential E(CB) can be calculated by E(fb). The results are shown in Table S1. Therefore, in combination with the bandgap energy measured by UV-VIS spectroscopy, valence band potential E(VB) can be obtained by formula (2) :
As shown in Fig. 4d, the arrangement diagram of the energy band structure of TTA was obtained through calculation (Fernández-Domene et al. 2016).
To study the separation and transfer of photogenerated electrons, EIS tests were performed on the samples (Zhang et al. 2020c). As shown in Fig. 5a, the impedance arc radius of the Nyquist plots (TTA) decreased with the increase of Ag content, indicating that the Ag loading can reduce the resistance of charge transfer and enhance charge separation in the system. To further confirm carrier separation, transient photocurrent response tests were performed (Fig. 5b). The photoresponse of photocatalyst shows transient stable photocurrent when the lamp is turned on, and returns to dark current state quickly when the lamp is turned off. The photocurrent density of TTA increased first and then decreased with the increase of silver content, and reached the maximum value at 3%TTA. Through the analysis of electrochemical impedance and photogenerated current spectrum, it is concluded that silver particles and Ti3C2 can effectively capture and conduct photogenerated electrons and inhibit the recombination of charge carriers. N2 isotherm adsorption-desorption curve is shown in Fig. 5c and Fig. S4. For TTiO2, as the calcination temperature increases, the specific surface area of the sample increases, proving that more and more TiO2 are generated. As silver loading increases, specific surface area decreases. According to Brunauer-Deming-Deming-Teller (BDDT) classification, the adsorption isotherms of the samples were class iv isotherms, indicating the presence of mesopores (2–50nm) (Low et al. 2014).
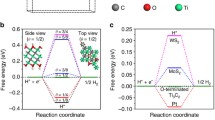
To verify the electron transfer path of samples, density functional theory (DFT) calculation was carried out for some samples, such as Fig. S5 (TiO2 (101), Ti3C2(001)) (Chen et al. 2020; Meng et al. 2018). Obviously, the alternating arrangement of Ti and C atomic layers forms the layered structure of Ti3C2, and the calculated bandgap is TiO2 (3.17 eV) and TTiO2 (−0.0305 eV). It can be speculated that band overlap leads to the disappearance of the bandgap. The total and partial state densities of Ti3C2, TiO2, and Ag are shown in Figure 6. In order to further explain the electron transfer path, the Fermi energy levels of Ag, TiO2, and Ti3C2 are calculated by DFT, which are 1.662eV, 9.149eV, and 3.542eV, respectively. The electrons should tend to transfer from the material with a high Fermi energy level to the material with a low Fermi energy level, that is, the electrons should transfer from TiO2 to Ag and Ti3C2.
TTiO2 calcined at 550 °C was used as the carrier and supported with different contents of Ag to carry out photocatalytic reactions of nitrate. The photocatalytic reduction capacity of the catalysts with different Ag contents for nitrate was 3%TTA > 4%TTA > 5%TTA > 2%TTA > 1%TTA. When the Ag loading does not exceed 3%, it can be seen that with the increase of Ag loading, the nitrate removal rate also increases. But, when the silver loading exceeds 3%, the nitrate removal rate decreases rapidly with the increase of silver loading. The reason may be that there is too much silver on the surface of TiO2, so that the silver particles agglomerate, thus reducing the number of active sites. After 4 h of reaction time, 3% TTA showed the highest nitrate removal rate (96.1%) (Fig. 7a). At the same time, kinetic curve fitting was carried out (Mao and X-p 2017). As shown in Fig. 7b, it was found that the reaction conforms to the reaction kinetic model (-ln(C/C0)= Kt), where C0 represents the initial concentration of nitrate, C represents the concentration of nitrate after t reaction time, and K represents the reaction rate constant. 3%TTA shows the highest photocatalytic dynamic performance. The kinetic rate constant is the highest, which is three times that of 1%TTA, as shown in Fig. S2. Subsequently, 3%TTA was used for further study. The photocatalytic removal of nitrate (500mg/L) with 3%TTA was studied, and the nitrate removal rate reached 96.1% and nitrogen selectivity reached 92.6% within 4h (Fig. 7c). Nitrate is first reduced to N2, NO2−, and NH4+ in the photocatalytic removal process (Challagulla et al. 2017). Nitrite, acting as an intermediate, accumulates in the initial stage and is subsequently converted to NH4+ and N2. Although some NO3− and NO2− are overreduced to form NH4+, the residual concentration of NH4+ is very low. Cyclic experiments showed that 3%TTA had strong stability, and the nitrate removal rate was still 89% after 5 rounds of experiments (Fig. 7d).
a Photocatalytic reduction of low concentration nitrate (C0 =500 mgN/L) over different photocatalysts, b kinetic curves fit by first-order kinetic equation, c NO3−, NO2−, NH4+, and N2 selectivity during the process of photocatalytic denitrification (C0 =500 mgN/L), d the cycling experiment of 3% TTA for photocatalytic reduction of nitrate (C0 =500 mgN/L)
The photocatalytic mechanism of TTA is shown in Scheme.2. Under light irradiation, electrons on the valence band (VB) of TiO2 nanocrystals are excited to the conduction band (CB), generating photogenerated electrons and holes (Fuxiang Zhang et al. 2004a) (Eq. (2)). The photogenerated electrons further migrate to Ti3C2. This process allows charge separation, protects the charge carriers from rapid compounding, and maximizes the electron lifetime (Zhang et al. 2004b). The electron absorption effect of Ag nanoparticles promotes the transfer of photogenerated electrons from the conduction band (CB) of TiO2 to Ag nanoparticles, facilitating the electron-hole separation. The electrons generated by TiO2 can react directly with NO3− to generate NO2−, NH4+, and N2 (Eqs. (4)–(8)). Because Eθ(NO2−/NH4+)=0.9V, Eθ(NO3−/NO2−)=0.94V, Eθ(NO3−/NH4+)=1.2V, Eθ(NO3−/N2)=1.25V, Eθ(NO2−/N2)=1.45V are contained in the energy band of the prepared TiO2. The holes generated by TiO2 can react with the hole cleaner HCOOH to generate CO2− (Eq. (3)). CO2− has a strong reducing ability (Eθ(CO2/CO2−) =−1.81V), and can directly reduce NO3− to N2 (Eθ(NO3−/N2)=1.25V), NO2− (Eθ(NO2−/N2)=1.45V) or NH4+ (Eθ(NO3−/NH4+)=1.2V) (Eqs.(9)–(10)).
Conclusion
In summary, the layered Ti3C2 was successfully prepared by the etching method, and the TiO2/Ti3C2 carrier was prepared by simple calcination, and the effect of calcination temperature on TiO2/Ti3C2 was explored. A chemical reduction method was used to support Ag particles on TiO2/Ti3C2. The effect of silver loading on the performance of photocatalytic nitrate removal was explored. Proposed related mechanisms. Because of the existence of Ti3C2 and Ag, the photoinduced electrons quickly migrate to Ti3C2 and Ag, thereby promoting the separation of electrons and holes and improved the catalyst activity, so that the removal rate of nitrate reaches 96.1%. Nitrogen selectivity reaches 92.6%. It provides a new method for the removal of nitrate.
Data availability
Not applicable.
References
Baldini E, Palmieri T, Dominguez A, Rubio A, Chergui M (2020) Giant exciton Mott density in anatase TiO_{2}. Phys Rev Lett 125:116403. https://doi.org/10.1103/PhysRevLett.125.116403
Barrabés NSá J (2011) Catalytic nitrate removal from water, past, present and future perspectives. Applied Catalysis B: Environmental 104:1–5. https://doi.org/10.1016/j.apcatb.2011.03.011
Bourkeb K, Wbaaloudj O (2021) Facile electrodeposition of ZnO on graphitic substrate for photocatalytic application: degradation of antibiotics in a continuous stirred-tank reactor. Journal of Solid State Electrochemistry. https://doi.org/10.1007/s10008-021-05045-2
Bredas J, Lhouk KN (2014) DFT elucidation of materials properties. Acc Chem Res 47:3207. https://doi.org/10.1021/ar5003889
Stevens CJ, Gowing DJ (2004) Impact of nitrogen deposition on the species richness of grasslands. SCIENCE 303:1876–1879. https://doi.org/10.1126/science.1094678
Challagulla S, Tarafder K, Ganesan Rroy S (2017) All that glitters is not gold: a probe into photocatalytic nitrate reduction mechanism over noble metal doped and undoped TiO2. The Journal of Physical Chemistry C 121:27406–27416. https://doi.org/10.1021/acs.jpcc.7b07973
Chen L, Ye X, Chen S, Ma L, Wang Z, Wang Q, Hua N, Xiao X, Cai S, Liu X (2020) Ti3C2 MXene nanosheet/TiO2 composites for efficient visible light photocatalytic activity. Ceramics International 46:25895–25904. https://doi.org/10.1016/j.ceramint.2020.07.074
Chu S, Wang Y, Guo Y, Feng J, Wang C, Luo W, Fan X, Zou Z (2013) Band structure engineering of carbon nitride: in search of a polymer photocatalyst with high photooxidation property. ACS Catalysis 3:912–919. https://doi.org/10.1021/cs4000624
Fang Y-M, Sun J-J, Wu A-H, Su X-LChen G-N (2008) Catalytic electrogenerated chemiluminescence and nitrate reduction at CdS nanotubes modified glassy carbon electrode. 555-560. https://doi.org/10.1021/la802650e
Feng X, Yu Z, Sun Y, Long R, Shan M, Li X, Liu Y, Liu J (2021) Review MXenes as a new type of nanomaterial for environmental applications in the photocatalytic degradation of water pollutants. Ceramics International 47:7321–7343. https://doi.org/10.1016/j.ceramint.2020.11.151
Fernández-Domene RM, Sánchez-Tovar R, Sánchez-González S, García-Antón J (2016) Photoelectrochemical characterization of anatase-rutile mixed TiO2 nanosponges. International Journal of Hydrogen Energy 41:18380–18388. https://doi.org/10.1016/j.ijhydene.2016.08.012
Gao J, Jiang B, Ni C, Qi Y, Zhang Y, Oturan N, Oturan MA (2019) Non-precious Co3O4-TiO2/Ti cathode based electrocatalytic nitrate reduction: preparation, performance and mechanism. Applied Catalysis B: Environmental 254:391–402. https://doi.org/10.1016/j.apcatb.2019.05.016
Ghassemi H, Harlow W, Mashtalir O, Beidaghi M, Lukatskaya MR, Gogotsi Y, Taheri ML (2014) In situ environmental transmission electron microscopy study of oxidation of two-dimensional Ti3C2and formation of carbon-supported TiO2. Journal of Materials Chemistry A 2. https://doi.org/10.1039/C4TA02583K
Halim J, Cook KM, Naguib M, Eklund P, Gogotsi Y, Rosen J, Barsoum MW (2016) X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Applied Surface Science 362:406–417. https://doi.org/10.1016/j.apsusc.2015.11.089
He J, Wu P, Chen L, Li H, Hua M, Lu L, Wei Y, Chao Y, Zhou S, Zhu W, Li H (2021) Dynamically-generated TiO2 active site on MXene Ti3C2: boosting reactive desulfurization. Chemical Engineering Journal 416. https://doi.org/10.1016/j.cej.2021.129022
Hirayama J, Kamiya Y (2014) Combining the photocatalyst Pt/TiO2 and the nonphotocatalyst SnPd/Al2O3 for effective photocatalytic purification of groundwater polluted with nitrate. ACS Catalysis 4:2207–2215. https://doi.org/10.1021/cs5003564
Hong L-f, Guo R-t, Yuan Y, Ji X-y, Li Z-s, W-g L Z-dP (2020) Recent progress of two-dimensional MXenes in photocatalytic applications: a review. Materials Today Energy 18. https://doi.org/10.1016/j.mtener.2020.100521
Hou Z, Chu J, Liu C, Wang J, Li A, Lin T, François-Xavier CP (2021) High efficient photocatalytic reduction of nitrate to N2 by Core-shell Ag/SiO2@cTiO2 with synergistic effect of light scattering and surface plasmon resonance. Chemical Engineering Journal 415. https://doi.org/10.1016/j.cej.2021.128863
Huang K, Li C, Li H, Ren G, Wang L, Wang W, Meng X (2020) Photocatalytic applications of two-dimensional Ti3C2 MXenes: a review. ACS Applied Nano Materials 3:9581–9603. https://doi.org/10.1021/acsanm.0c02481
Jia C, Yang P, Li J, Huang B, Matras-Postolek K (2016) Photocatalytic activity evolution of different morphological TiO2 shells on Ag nanowires. ChemCatChem 8:839–847. https://doi.org/10.1002/cctc.201501045
Karen R, Burow BTN, Rupert MG, Dubrovsky NM (2010) Nitrate in groundwater of the United States, 1991−2003. Environ. Sci. Technol. 44:4988–4997. https://doi.org/10.1021/es100546y
Khadidja MF, Fan J, Li S, Li S, Cui K, Wu J, Zeng W, Wei H, Jin H-G, Naik N, Chao Z, Pan D, Guo Z (2021) Hierarchical ZnO/MXene composites and their photocatalytic performances. Colloids and Surfaces A: Physicochemical and Engineering Aspects 628. https://doi.org/10.1016/j.colsurfa.2021.127230
Khan I, Aspalding RF (2004) Enhanced in situ denitrification for a municipal well. Water Res 38:3382–3388. https://doi.org/10.1016/j.watres.2004.04.052
Kim DE, Pak D (2019) Ti plate with TiO2 nanotube arrays as a novel cathode for nitrate reduction. Chemosphere 228:611–618. https://doi.org/10.1016/j.chemosphere.2019.04.071
Krasae N, Wantala K (2016) Enhanced nitrogen selectivity for nitrate reduction on Cu–nZVI by TiO 2 photocatalysts under UV irradiation. Applied Surface Science 380:309–317. https://doi.org/10.1016/j.apsusc.2015.12.023
Li M, Feng C, Zhang Z, Lei X, Chen R, Yang Y, Sugiura N (2009) Simultaneous reduction of nitrate and oxidation of by-products using electrochemical method. J Hazard Mater 171:724–730. https://doi.org/10.1016/j.jhazmat.2009.06.066
Li ZWY (2019) 2D early transition metal carbides (MXenes) for catalysis. Small 15:e1804736. https://doi.org/10.1002/smll.201804736
Loganathan P, Vigneswaran S, Kandasamy J (2013) Enhanced removal of nitrate from water using surface modification of adsorbents--a review. J Environ Manage 131:363–374. https://doi.org/10.1016/j.jenvman.2013.09.034
Low J, Yu J, Li Q, Cheng B (2014) Enhanced visible-light photocatalytic activity of plasmonic Ag and graphene co-modified Bi2WO6 nanosheets. Phys Chem Chem Phys 16:1111–1120. https://doi.org/10.1039/C3CP53820F
Lucchetti R, Onotri L, Clarizia L, Natale FD, Somma ID, Andreozzi R, Marotta R (2017) Removal of nitrate and simultaneous hydrogen generation through photocatalytic reforming of glycerol over “in situ” prepared zero-valent nano copper/P25. Applied Catalysis B: Environmental 202:539–549. https://doi.org/10.1016/j.apcatb.2016.09.043
Mao C-f, X-p Z R-xL (2017) Phenylpropanoic acid-based DESs as efficient extractants and catalysts for the removal of sulfur compounds from oil. Fuel 189:400–407. https://doi.org/10.1016/j.fuel.2016.10.113
Mao C, Yin K, Yang C, Dong G, Tian G, Zhang Y, Zhou Y (2021) Fe-based MOFs@Pd@COFs with spatial confinement effect and electron transfer synergy of highly dispersed Pd nanoparticles for Suzuki-Miyaura coupling reaction. J Colloid Interface Sci 608:809–819. https://doi.org/10.1016/j.jcis.2021.10.055
Meng Q, Ma J, Zhang Y, Li Z, Zhi C, Hu A, Fan J (2018) The S-functionalized Ti3C2 Mxene as a high capacity electrode material for Na-ion batteries: a DFT study. Nanoscale 10:3385–3392. https://doi.org/10.1039/C7NR07649E
Meng X, Zhang G, Li N (2017) Bi24Ga2O39 for visible light photocatalytic reduction of Cr(VI): controlled synthesis, facet-dependent activity and DFT study. Chemical Engineering Journal 314:249–256. https://doi.org/10.1016/j.cej.2016.12.090
Naguib M, Kurtoglu M, Presser V, Lu J, Niu J, Heon M, Hultman L, Gogotsi Y, Barsoum MW (2011) Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv Mater 23:4248–4253. https://doi.org/10.1002/adma.201102306
Naguib M, Mochalin VN, Barsoum MW, Gogotsi Y (2014) 25th anniversary article: MXenes: a new family of two-dimensional materials. Adv Mater 26:992–1005. https://doi.org/10.1002/adma.201304138
Nila A, Baibarac M, Udrescu A, Smaranda I, Mateescu A, Mateescu G, Mereuta P, Negrila CC (2019) Photoluminescence and structural properties of the nitrogen doped TiO2 and the influence of SiO2 and Ag nanoparticles. J Phys Condens Matter 31:375201. https://doi.org/10.1088/1361-648X/ab2692
Quyen VT, Ha LTT, Thanh DM, Le QV, Viet NM, Nham NT, Thang PQ (2021) Advanced synthesis of MXene-derived nanoflower-shaped TiO2@Ti3C2 heterojunction to enhance photocatalytic degradation of rhodamine B. Environmental Technology & Innovation 21. https://doi.org/10.1016/j.eti.2020.101286
Rathinavelu JR, Jung M-J, Im J-S, Seak-Lee Y, Palanivelu K (2013) Electrospinning of polymer-unaided TiO2 fibers and iron impregnation for sunlight-induced photo-Fenton's degradation of dyes. Environmental Engineering Science 30:653–662. https://doi.org/10.1089/ees.2012.0051
Shah SA, Habib T, Gao H, Gao P, Sun W, Green MJ, Radovic M (2016) Template-free 3D titanium carbide (Ti3C2Tx) MXene particles crumpled by capillary forces. Chem Commun (Camb) 53:400–403. https://doi.org/10.1039/C6CC07733A
Shi H, Zhang T, Wang H, Wang X, He M (2011) Photocatalytic conversion of naphthalene to α-naphthol using nanometer-sized TiO2. Chinese Journal of Catalysis 32:46–50. https://doi.org/10.1016/S1872-2067(10)60158-1
Sittinun Tawkaew YF, Yin S, Sato T (2001) Synthesis of cadmium sulfide pillared layered compounds and photocatalytic reduction of nitrate under visible light irradiation. Colloids and Surfaces A Physicochemical and Engineering Aspects 179:139–144. https://doi.org/10.1016/S0927-7757(00)00649-X
Sivakumar S, Selvaraj A, Ramasamy AK, Balasubramanian V (2013) Enhanced photocatalytic degradation of reactive dyes over FeTiO3/TiO2 heterojunction in the presence of H2O2. Water, Air, & Soil Pollution 224. https://doi.org/10.1007/s11270-013-1529-x
Soares OSGP, Pereira MFR, Órfão JJM, Faria JL, Silva CG (2014) Photocatalytic nitrate reduction over Pd–Cu/TiO2. Chemical Engineering Journal 251:123–130. https://doi.org/10.1016/j.cej.2014.04.030
Sreedhar ANoh J-S (2021) Recent advances in partially and completely derived 2D Ti3C2 MXene based TiO2 nanocomposites towards photocatalytic applications: a review. Solar Energy 222:48–73. https://doi.org/10.1016/j.solener.2021.05.010
Tian F, Zhu R, Song K, Ouyang F, Cao G (2015) The effects of amount of La on the photocatalytic performance of ZnIn2S4 for hydrogen generation under visible light. International Journal of Hydrogen Energy 40:2141–2148. https://doi.org/10.1016/j.ijhydene.2014.12.025
Wu G, Katsumura Y, Muroya Y, Lin M, Morioka T (2002) Temperature dependence of carbonate radical in NaHCO3 and Na2CO3 solutions: is the radical a single anion. J. Phys. Chem 106,:2430-2437. https://doi.org/10.1021/jp013401p
Xiong Z, Lei Z, Li Y, Dong L, Zhao Y, Zhang J (2018) A review on modification of facet-engineered TiO2 for photocatalytic CO2 reduction. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 36:24–47. https://doi.org/10.1016/j.jphotochemrev.2018.07.002
Yu B, Tawiah B, Wang LQ, Yin Yuen AC, Zhang ZC, Shen LL, Lin B, Fei B, Yang W, Li A, Zhu SE, Hu EZ, Lu HD, Yeoh GH (2019) Interface decoration of exfoliated MXene ultra-thin nanosheets for fire and smoke suppressions of thermoplastic polyurethane elastomer. J Hazard Mater 374:110–119. https://doi.org/10.1016/j.jhazmat.2019.04.026
Zhang D, Qiu R, Song L, Eric B, Mo Y, Huang X (2009) Role of oxygen active species in the photocatalytic degradation of phenol using polymer sensitized TiO2 under visible light irradiation. J Hazard Mater 163:843–847. https://doi.org/10.1016/j.jhazmat.2008.07.036
Zhang F, Chen J, Zhang X, Gao W, Jin R, Guan N (2004a) Simple and low-cost preparation method for highly dispersed Pd/TiO2 catalysts. Catalysis Today 93-95:645–650. https://doi.org/10.1021/cm034306r
Zhang F, Chen J, Zhang X, Gao W, Jin R, Li N, Ga Y (2004b) Synthesis of titania-supported platinum catalyst: the effect of pH on morphology control and valence state during photodeposition. Langmuir 20:9329–9334. https://doi.org/10.1021/la049394o
Zhang F, Guan N, Li Y, Zhang X, Chen J, Zeng H (2003) Control of morphology of silver clusters coated on titanium dioxide during photocatalysis. 19:8230–8234. https://doi.org/10.1021/la034917y
Zhang H, Liu Z, Li Y, Zhang C, Wang Y, Zhang W, Wang L, Niu L, Wang P, Wang C (2020a) Intimately coupled TiO2/g-C3N4 photocatalysts and in-situ cultivated biofilms enhanced nitrate reduction in water. Applied Surface Science 503. https://doi.org/10.1016/j.apsusc.2019.144092
Zhang L, Peng C-T, Shi JLR (2020b) Surface alloying of chromium/tungsten/stannum on pure nickel and theoretical analysis of strengthening mechanism. Applied Surface Science 532. https://doi.org/10.1016/j.apsusc.2020.147477
Zhang Y, Kirk C, Robertson N (2020c) Nitrogen doping and carbon coating affects substrate selectivity of TiO2 photocatalytic organic pollutant degradation. Chemphyschem 21:2643–2650. https://doi.org/10.1002/cphc.202000492
Zhao B, Li X, Wang Y, Tan X, Qi W, Li H, Wei J, You Y, Shi W, Zhang Q (2021) Dissimilatory nitrate reduction and functional genes in two subtropical rivers. Environ Sci Pollut Res Int, China. https://doi.org/10.1007/s11356-021-15197-3
Zhao MQ, Xie X, Ren CE, Makaryan T, Anasori B, Wang G, Gogotsi Y (2017) Hollow MXene spheres and 3D macroporous MXene frameworks for Na-Ion storage. Adv Mater 29. https://doi.org/10.1002/adma.201702410
Zhao Y, Que M, Chen J, Yang C (2020) MXenes as co-catalysts for the solar-driven photocatalytic reduction of CO2. Journal of Materials Chemistry C 8:16258–16281. https://doi.org/10.1039/D0TC02979C
Zhu N, Wu Y, Tang J, Duan P, Yao L, Rene ER, Wong PK, An T, Dionysiou DD (2018) A new concept of promoting nitrate reduction in surface waters: simultaneous supplement of denitrifiers, electron donor pool, and electron mediators. Environ Sci Technol 52:8617–8626. https://doi.org/10.1021/acs.est.8b01605
Funding
This work was supported by the National Nature Science Foundation of China (21978048), Scientific Innovation Research Foundation of College Graduate in Jiangsu Province (KYCX21_0091), Fundamental Research Funds for the Central Universities (2242015k30001), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) (1107047002), and Fund Project for Transformation of Scientific and Technological Achievements of Jiangsu Province of China (BA2019054).
Author information
Authors and Affiliations
Contributions
Wanying Chen planned and carried out the experimental work. Qingzhao Yao monitored the progress of the experiment and reviewed the draft. Guomeng Dong and Yang Liu contributed to the analysis and interpretation of results. Bo Wu and Changjiang Zuo established and calculated the DFT model; Yiwei Zhang, Yuming Zhou, and Zewu Zhang provided the financial support for the project leading to this publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1690 kb)
Rights and permissions
About this article
Cite this article
Chen, W., Wu, B., Yao, Q. et al. A MXene-based multiple catalyst for highly efficient photocatalytic removal of nitrate. Environ Sci Pollut Res 29, 58149–58160 (2022). https://doi.org/10.1007/s11356-022-19616-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19616-x