Abstract
Water pollution by recalcitrant compounds is an increasingly important problem due to the continuous introduction of new chemicals into the environment. Choosing appropriate measures and developing successful strategies for eliminating hazardous wastewater contaminants from industrial processes is currently a primary goal. Electroplating industry wastewater involves highly toxic cyanide (CN), heavy metal ions, oils and greases, organic solvents, and the complicated composition of effluents and may also contain biological oxygen demand (BOD), chemical oxygen demand (COD), SS, DS, TS, and turbidity. The availability of these metal ions in electroplating industry wastewater makes the water so toxic and corrosive. Because these heavy metals are harmful to living things, they must be removed to prevent them from being absorbed by plants, animals, and humans. As a result, exposure to electroplating wastewater can induce necrosis and nephritis in humans and lung cancer, digestive system cancer, anemia, hepatitis, and maxillary sinus cancer with prolonged exposure. For the safe discharge of electroplating industry effluents, appropriate wastewater treatment has to be provided. This article examines and assesses new approaches such as coagulation and flocculation, chemical precipitation, ion exchange, membrane filtration, adsorption, electrochemical treatment, and advanced oxidation process (AOP) for treating the electroplating industry wastewater. On the other hand, these physicochemical approaches have significant drawbacks, including a high initial investment and operating cost due to costly chemical reagents, the production of metal complexes sludge that needs additional treatment, and a long recovery process. At the same time, advanced techniques such as electrochemical treatment can remove various kinds of organic and inorganic contaminants such as BOD, COD, and heavy metals. The electrochemical treatment process has several advantages over traditional technologies, including complete removal of persistent organic pollutants, environmental friendliness, ease of integration with other conventional technologies, less sludge production, high separation, and shorter residence time. The effectiveness of the electrochemical treatment process depends on various parameters, including pH, electrode material, operation time, electrode gap, and current density. This review mainly emphasizes the removal of heavy metals and another pollutant such as CN from electroplating discharge. This paper will be helpful in the selection of efficient techniques for treatment based on the quantity and characteristics of the effluent produced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water’s importance in terms of the environment is now widely recognized all around the world. Due to the water contamination and shortage of water, the substantial environmental load is growing, and the loss of natural water supports because its scarcity is becoming more prevalent nowadays (Carolin et al. 2017). Only less than 1% of freshwater is readily accessible for humans, and a majority of the water on the planet is saltwater from seas or oceans, which covers almost 97% of the water (Jin et al. 2016). By 2025, more than three billion people on the planet will lack access to safe water, with over one-third of this population living in water-stressed regions; by 2050, it is predicted to be two-thirds of the population, according to the WHO (World Health Organization) (Bankole et al. 2019). The rising population has resulted in unplanned habitations giving growth in wastewater discharge without any prior treatment into the rivers/streams, degrading the quality of natural water causing water pollution (Hosseini et al. 2016). Electroplating industries, metallurgical industries, mining operations, power generation facilities, wastewater including heavy metals, and polluted organic pollutants have been released into the atmosphere over the last two decades, particularly in developing countries (Chen et al. 2013). Furthermore, since 1947, industrial electroplating wastewater purification has been viewed as a large-scale protest due to its chemical composition, environmental impacts, and lack of convenient and legal regulations governing its release into the environment (Bankole et al. 2019). In the 1950s, the very first wastewater treatment technology was developed and implemented in industry. Electroplating wastewater contains a significant amount of heavy metal, cyanide-complex, and the complicated composition of effluents and may also include BOD/COD, DS, SS, TS, and turbidity. The electroplating industry wastewater contains about 29% of toxic and hazardous wastes. The concentrations of these toxic metal ions are much higher than the permissible levels. As a result, effluents from the surface finishing and plating industries must be properly treated before being released to avoid causing further environmental damage. Most of them are patents, including the electroplating wastewater and metal recovery system, as reported by Izdebski (1975), Morton (1997), and Adiga (2000). The health effect of exposure to electroplating wastewater, including kidney failure, thyroid dysfunction, sleeplessness, tiredness, rheumatoid arthritis, affects the circulatory system and neural system, causes gastrointestinal mucosa irritation, and leads to lung cancer. The electroplating industry wastewater includes heavy metals such as arsenic (As), cobalt (Co), copper (Cu), chromium (Cr), mercury (Hg), iron (Fe), nickel (Ni), zinc (Zn), and sometimes lead (Pb), cadmium (Cd), and as well as acids, alkalis, and toxic CN (Calero and Bl 2014).
The implementation of an electroplating process incorporates the electrodeposition of a thin metal over another metal. In this process, the electricity passes through an electrolytic bath, dissolving and depositing the metal at the anode and cathode. Pollution is caused by the usage of various chemicals and metals salt. Several series of processes are done during the electroplating process, which involves alkaline cleaning, plating, acid pickling, and rinsing, which create a large amount of untreated wastewater (Babu et al. 2009). This process with waste effluents treatment facilities is carried out in a particular sequence, as shown in Fig. 1. It is also important to note that waste acid and other coating elements contributed significantly to the treatment process complexity. Electroplating wastewater contains a variety of inorganic and organic species, considering turbidity, dissolved oxygen (DO), COD, BOD, total dissolved solids (TDS), total suspended solids (TSS), phosphate, sulfate, phosphate, CN complexes, and metal ions (Husain et al. 2014; Martin-Lara et al. 2014; Bankole et al. 2017). The primary characteristics of the wastewater generated by the electroplating industry are shown in Table 1.
The main goal of this study is to review the essential opportunities applicable for the removal of electroplating industry contaminants and thus provide a good starting point for future researchers who want to fill research gaps in the field and enhance the advanced wastewater treatment approaches. Although few similar review papers address the treatment of electroplating wastewater involving heavy metals despite its importance as a significant water contaminant, they all seem to be overly generic, including other metal ions or focusing on an individual treatment approach. Electroplating wastewater treatment employs a variety of techniques such as coagulation-flocculation (Xiao et al. 2021), chemical precipitation (Fu et al. 2021), ion-exchange (Zhang et al. 2021), membrane filtration (Njoya et al. 2021), adsorption (Rajivgandhi et al. 2021), and electrochemical treatment (Sahu et al. 2014; Liu et al. 2021; Wang et al. 2021).
Heavy metals in electroplating industry wastewater
Heavy metals such as Fe, Cu, Cr, Hg, Cd, Ni, Pb, Zn, and many more having a very high concentration are released from the electroplating industry wastewater. Heavy metals have a density of more than five times that of water (Al-saydeh et al. 2017). It is made up of elements having atomic weights ranging from 63.5 to 200.6 (Carolin et al. 2017). Heavy metal levels in the environment are rising, posing a major threat to human health, living resources, and ecosystems. Toxic heavy metals are released from various sources; however, specific industrial sectors are the most polluting. Due to the enormous number of unified firms and their geographical dispersion, the plating or metal finishing industry is significant among these industrial sectors. The process used in electroplating production is the most environmentally adverse industrial process; a considerable amount of wastewater is generated containing heavy metal ions. Because of the severe toxicity of heavy metals, careful consideration has been given. To decrease the risk of toxic substances affecting humans and the environment, wastewater rules were enacted. Table 2 lists the maximum contaminated level (MCL) standards for heavy metals and associated toxicities (Hosseini et al. 2016; Rajoria et al. 2021).
Non-biodegradable pollutants pose significant health and environmental risk, and secondary procedures cannot be used to remove these wastes. Advanced wastewater treatment processes such as ion-exchange, adsorption, membrane separation, and electrochemical approaches can be employed to remove these resistant pollutants.
Conventional methods for industrial wastewater treatment
Due to inhibitory properties, pollutants in wastewater can be removed by applying a high removal enforcement technology. The appropriate protocols are used for the clearance of water pollutants. In order to minimize pollution emission, water management, and energy consumption, industries must deal with a variety of issues. As a result, several treatment methods were created to ensure environmental safety, which has grown into an important research topic. Coagulation-flocculation, precipitation, adsorption, membrane filtration, ion exchange, electrodialysis, electro-flotation, electro-coagulation, electro-oxidation, and advanced oxidation process (AOP) are the most demanding and assuring techniques applicable for reducing the industrial effluent (Yadav et al. 2021). Several patents have been found regarding this type of processes (Kang 2003; Fresnel 2015; Li and Lichun 2017; Chongwu Guo 2020). Figure 2 shows the flow diagram of different wastewater treatment methods. Although all of the above methods can be used to treat wastewater, many aspects must be considered when choosing the best treatment method, including removal performance, efficiency, adaptability, and safety. Most treatment procedures are ineffective and expensive if metal ions concentrations in wastewater are less than 1–100 mg/L (Al-Qodah and Al-Shannag 2017). Fast, clean, cheap, and environmentally friendly approaches should be applied, considered the best removal method. Heavy metals as electroplating effluents are degraded using a series of techniques for proper treatment.
Coagulation/flocculation technique
Coagulation is a method in which insoluble or suspended particles are combined into big aggregates to generate coagulants. Coagulants such as aluminum, ferric chloride, ferrous sulfate, and others are often employed in traditional wastewater treatment processes to help in the removal of wastewater particulate contaminants through charge neutralization of particles. Figure 3 shows how coagulation-flocculation has been used to treat drinking water and decontaminate industrial effluents (Yadav et al. 2021). It is also effective for metal ions with concentrations of smaller than 100 ppm or greater than 1000 ppm. This process effectively removes the heavy metal when the pH ranges from 11.0 to 11.5, the same as chemical precipitation. This process cannot entirely remove the effluents in industrial wastewater, and sludge is also produced because of the coagulants used (Chang and Wang 2007). As a result, coagulation or flocculation should be integrated with other wastewater treatment procedures such as precipitation or casual reduction. For example, Bojic et al. (2009) used a micro-alloyed aluminum composite to investigate an integrated strategy, i.e., a spontaneous reduction-coagulation process to eliminate Zn(II) and Cu(II) metal from wastewater. The flow rate, pH, and metal ions concentration are the operating parameters that were investigated to achieve the higher removal capacity. Tao et al. (2016) studied the effect of Nano coagulants like AgNPs on heavy metal concentration and can reduce the TOC in the wastewater. The advantages of the coagulation-flocculation process include settling suspended materials in a short time interval and superior dewatering properties. In contrast, the disadvantages include sludge generation and high operational costs due to chemical requirements. Table 3 shows the outcomes of specific research projects that used coagulation-flocculation to remove effluents from wastewater.
Chemical precipitation technique
Chemical methods have become widely used in various processes in recent decades of electroplating industry development due to their mature techniques and low investment. Chen et al. (2009a) show the general flow of chemical precipitation methods in Fig. 4. It is an appropriate method for treating electroplating industry wastewater, including cyanide, heavy metals, etc. Although chemical treatment of electroplating wastewater is fundamentally an ultimate treatment strategy, it can only minimize the quantity of treatment discharged and cannot completely heal the problem (Lu and Wu 2020). In this process, chemicals react with heavy metals in wastewater, leading to the formation of insoluble precipitates. In the modern electroplating industry, the chemical precipitation of heavy metals using lime, sulfide, and caustic soda is the most extensively used treatment process, which occupied pH arrangement to necessary conditions to minimize metal ions solubility in the effluent (Ku and Jung 2001).
Hydroxide precipitation and sulfide precipitation are two types of processes used in the chemical precipitation technique. Cr, Cu, Zn, Cd, and Mn are easily removed by applying the chemical precipitation technique (Bilal et al. 2013). Most of them are patents, including the removal of the Cu complex reported by Zhou (2013). The chemical precipitation method has advantages like a simple operation, low-cost precipitant agents, and minimal initial investment due to their being easily accessible. At the same time, the disadvantages include that it generates a huge quantity of sludge that has to be treated further to extract metals, ineffective with the low concentration of metal ions, and sludge clearing issues, all of which have long-term negative environmental consequences (Barakat 2011; Fu and Wang 2011). Table 4 shows the outcomes of specific research work that used chemical precipitation to remove effluents from wastewater.
Ion-exchange technique
Ion-exchange is a wastewater treatment separation process that can eliminate a large amount of metal ions. This technique replaces the ions with another for wastewater treatment, resulting in high-quality treated water that may be reused. As a response, ion-exchange resins of various sorts have been utilized to treat wastewater containing mixed metal ions as well as inorganic and organic contaminants (Bisht and Agarwal 2017). Synthetic resins are the most extensively approved of all the materials used in ion-exchange techniques. This resin can eliminate almost all heavy metals from a solution. Temperature, pH, initial concentration, and retention time all influence the uptake of heavy metal ions by ion-exchange resins (Gode and Pehlivan 2006). Several patents for this method have been identified (Etzel 1980; Katoh 1985). In 1995, ion exchange resin (MIEX) was used to remove natural organic materials; this resin is regarded as the first ion exchange resin (Ambashta and Sillanpaa 2010). Figure 5 shows the cation exchange resin in column ion-exchange tests to remove metal ions (Yadav et al. 2021).
Cavaco et al. (2007) tested the performance of ion exchange resins in the removal of Cr (III) from untreated wastewater. Overall, the chelating resin (Diaion CR11) appears promising for electroplating effluent treatment. According to the findings, Zewail and Yousef (2015) developed a batch conical air spouted tank for Ni and Pb removal from wastewater utilizing strong cation exchange resins (Ambserjet 1200 Na). They also look at how other factors like contact time, superficial air velocity, and the initial concentration of heavy metal ions affect the proportion of heavy metal removed. They were able to remove 99% of Pb and 98% of Ni, respectively, under optimal conditions. Alyüz and Veli (2009) examined the elimination of Ni and Zn from aqueous solutions using Dowex HCR S/S cation exchange resin. Dowex HCR S/S type resins can reduce residual heavy metal concentrations below discharge limits because they are sodium-based and have excellent cation exchange capabilities. The influences of pH, resin dosage, and contact time on the removal process were investigated using batch shaking adsorption tests. The most efficient elimination of Ni and Zn was found to be at pH 4 and 6, respectively. Ni and Zn adsorb to equilibrium in 90 min and 120 min, respectively. They found that under ideal conditions, Ni and Zn were removed from aqueous solutions at a rate of more than 98%. Besides the great use of the ion-exchange process, it also raises the operational cost, and on a large scale, it cannot be appropriate for wastewater treatment. The limitations of this method are high resin cost, slow operation rate, regenerations of resins, high capital, and operational cost. Ion exchange is also a nonselective mechanism that is extremely sensitive to the pH of the solution (Barakat 2011). The results from some research work using the ion-exchange method to remove heavy metals from wastewater are shown in Table 5.
Membrane filtration technique
Membrane filtration is a pressure-driven process of separation that has been used in wastewater treatment. Membrane filtration has several advantages over other traditional techniques, including high separation performance, energy savings, the ability to scale up quickly, high efficiency in recovering heavy metal ions, and being environmentally friendly (Zhu et al. 2014). Besides some advantages, it also has some disadvantages, such as the operational cost is high due to membrane fouling, costly membrane sheets for high removal of heavy metals, and low permeate flux (Malaviya 2011). It depends upon the different variety of membrane various membrane filtration techniques were employed. Heavy metals can be removed via membrane filtering techniques like ultra-filtration (UF), reverse osmosis (RO), and nano-filtration (NF), which are all dependent on particle size retention. On the basis of pore size (0.05 to 0.1 microns) and molecular weight of the separating compounds (1000–100,000 Da), UF uses a permeable membrane to separate heavy metals, macromolecules, and suspended particles from inorganic solution. Water can pass through the membrane in RO, a pressure-driven membrane process, while heavy metals are retained. Due to the progressively stringent environmental legislation, RO has been designed with a membrane pore size in the range of (0.0005 to 0.001 microns). NF membrane has tiny pores and membrane surface charge, which rejected the bigger neutral solutes or salts and permitted to pass the charged solutes smaller than the membrane pores. Figure 6 illustrates the various membrane technologies and their ability to remove multiple species, including heavy metal ions. Basic parameters such as pH, temperature, pressure, feed concentration, membrane configuration, and size affect membrane performance (Saeid et al. 2016).
Juang and Shiau (2000) published some major research on removing Cu(II), Co(II), Ni(II), and Zn(II) ions from aqueous solutions applying chitosan enhanced membrane filtration. The ultra-filters were made using amicon-generated cellulose YM10 and YM30. With an initial Cu(II) concentration of 79 mg/L and Zn(II) concentration of 81 mg/L and pH ranging from 8.5 to 9.5, Cu (II) ions were removed 100%, and Zn(II) ions were removed 95%. Due to its greater coordination with chitosan, Cu (II) removal is more competent than other metals. Their studies revealed that chitosan increased metal removal by 6–10 times as compared to using a membrane alone. Mohammadi et al. (2009) investigated the effectiveness of RO technology in removing Cr from synthetic wastewater samples prepared in the same way as electroplating industrial effluent. To measure the efficiency of Cr removal, researchers used a pH 6 to 7, an applied pressure of 200 psi, a temperature of around 25ºC, and a feed Cr concentration of 10 mg/L. The efficiency of Cr removal was as high as 99%under the optimized conditions. Qin et al. (2002) examined the RO technique to treat spent rinse water from metal plating to meet the standards for reuse as alkaline rinse water. Due to proper ultrafiltration (UF) coupling with RO membrane, appropriate UF pre-treatment might reduce RO membrane fouling and boost RO membrane flux by 30–50%. At permeate conductivities below 45S/cm, Ni, nitrate, and TOC concentrations were 0.01, 2.1, and 3.0 ppm, respectively, with conductivity rejections of over 97%, 99.8%, 95%, and 87%. However, Cimen (2015) employed reverse osmosis with AG, SWHR, SG, and SE membranes to study chromium removal from wastewater. The amount of chromium rejected was determined by the membrane type, operating pressure, pH, and feed water concentration. The membrane's rejection capacity increased in the following order: AG > SWHR > SG > SE. Low Cr (VI) concentrations of 50–100 mg/L produced good results. Using the AG membrane, 91% removal efficiency and permeate flux were achieved. pH 3, 100 mg/L concentration, and 20 bar pressure were shown to be the optimal conditions for chromium rejection from wastewater utilizing AG membrane. Wei et al. (2013) studied the removal of heavy metals from real electroplating effluent using thin-film composite NF hollow-fiber membranes. The concentrations of Cr, Cu, and Ni ions in the retentate were 5.72, 5.66, and 5.63 times greater, respectively, than their original feed values. Under pressure (0.4 MPa) and pH 2.31, the removal efficiency for Cr, Cu, and Ni ions was 95.76%, 95.33%, and 94.99%, respectively. Table 6 shows that, compared to UF and NF as membrane filtration, RO is more effective in removing heavy metals from wastewater.
Adsorption technique
Adsorption is a method for removing heavy metals from wastewater that is frequently employed. Adsorption is a mass transfer process in which a material is transported from the liquid phase to a solid's surface and is bound by physical and chemical interactions (Babel 2015). This procedure forms an adsorbate coating on the adsorbents' surfaces. The adsorbents have a high adsorption capacity and a large interfacial area. Desorption can be used to reverse the adsorption process and recreate the adsorbents. Adsorbents can be made from natural materials, agricultural waste, and industrial by-products. The most effective adsorbents in the adsorption process are activated carbon (AC), carbon nanotubes (CNT), and low-cost adsorbents, including clay, biomass, peat, and so on. All of these adsorbents are reviewed further below. This technique adsorption has significant economic and environmental benefits, including cheap operating costs, easy availability, profitability, and high productivity Agustiono et al. (2006) analyzed several papers on the removal of heavy metals Cr(III), Cu(II), Cd(II), Cr (VI), Ni(II), and Zn(II) from electroplating wastewater using a variety of low-cost adsorbents made from natural materials, agricultural residues, or industrial by-products.
Furthermore, Alslaibi et al. (2013) employed microwaved olive stone activated carbon to extract Cd from an aqueous solution. The interaction and relationship between operating factors (i.e., radiation power, radiation time, and impregnation ratio) were investigated using central composite design (CCD) and response surface methodology (RSM). When the radiation power and impregnation ratios are 565 W and 1.87, respectively, they found that 7.0 min of contact time is required to achieve 96.25% Cd(II) elimination and 86.05% olive stone activated carbon (OSAC) yield. For treating heavy metal-contaminated water, Guo et al. (2010) employed poultry litter as a precursor material in the manufacture of AC. They accepted that commercial activated carbons made from bituminous coal and coconut shell had much higher adsorption affinity and capacity for most heavy elements than poultry litter-based activated carbon. They also noticed that nutrient and metal ion emissions from litter-derived carbon did not constitute a risk of secondary water contamination. In a study conducted, Kongsuwan et al. (2009) demonstrated the potential of employing AC extracted from eucalyptus bark and processed using the phosphoric acid activation method to manage heavy metals in low-strength wastewater. After that, the AC was used to sorption of Cu(II) and Pb(II) ions. The greatest sorption capacities for Cu(II) and Pb(II) were 0.45 and 0.53 mmol/g, respectively, at the ideal pH for sorption of 5. Accordingly to Jiang et al. (2010), heavy metal ions such as Cd(II), Pb(II), Cu(II), and Ni(II) may be eliminated from real wastewater using kaolinite clay from Longyan, China. The adsorption of metal ions onto kaolinite clay is influenced by a number of factors, but the solution pH has a substantial impact. The selectivity sequence for adsorption of these metals was Pb(II) > Cd(II) > Ni(II) > Cu(II), while desorption of Cd(II) and Cu(II) was easier than Pb(II) and Ni(II). The absorption is rapid, with maximum adsorption taking place in less than 30 min. The amount of Pb(II) in the water was lowered by this clay from 160.00 to 8.00 mg/L. A variety of sorbents have already been investigated by a number of researchers. Some of the potential low-cost sorbents include bark/tannin-rich materials, lignin, chitin/chitosan, dead biomass, seaweed/algae/alginate, xanthate, zeolite, clay, ash, modified wool, peat moss, leaf mold, iron-oxide-coated sand, bone gelatin beads, and modified cotton. The results of altering the pH, contact time, initial metal concentration, adsorbent, and dosage as adsorption parameters are shown in Table 7.
Electrochemical techniques
The electrochemical technique was initially used to treat wastewater in England in 1889 (Wang et al. 2007b). These electrochemical approaches proved to be a great alternative to the standard physicochemical electroplating wastewater treatment techniques (Chen et al. 2009b; Rodríguez R et al. 2009). Electrochemical treatment, in comparison, is a promising approach that provides some benefits over other methods for remediation of recalcitrant pollutants, such as no requirement of further chemical reagents, low-cost operation, high selectivity at ambient temperature, and pressure, and vigorous achievement and eco-friendly. Electrocoagulation (EC), electrodeposition, electrofloatation (EF), electrodialysis (ED), and electrooxidation (EO) are the instantly applied techniques for reducing industrial effluents. Several patents have been found regarding this kind of electrochemical processes (Huang et al. 1989).
Electrocoagulation
Over the previous two decades, an electro-coagulation method has been practiced to remove heavy metal ions such as As, Zn, Cr, Hg, Cd, Fe, Ni, Cu, and many others from industrial wastewater (Al-Qodah and Al-Shannag 2017). In the electro-coagulation process, coagulants are generated by electrolytic dissolution of Al or Fe ions from Al or Fe electrodes. The coagulants ions are produced at the anode, while hydrogen gas is released from the cathode to assist in the flocculation of the molecules (Fu and Wang 2011; Sharma et al. 2020a). The principle of the electro-coagulation method is shown in Fig. 7 (Zailani and Zin 2018). In contrast, to chemical coagulation, where several hours are required and adsorption on activated carbon, the electro-coagulation method attains faster removal of effluents from wastewater. However, Kamaraj et al. (2013) discussed the reduction process for Pb from aqueous solutions by electro-coagulation using magnesium as anode and galvanized iron as a cathode. With a current density of 0.8 A/dm2 and a pH of 7.0, and an energy consumption of 0.72 kWh/m3, they were able to achieve a maximum removal efficiency of 99.3% for Pb. According to Kamaraj and Ganesan (2013), direct current was used in an EC method to extract Cu from water applying magnesium as both anode and cathode. Cu optimal removal performance was obtained to be 97.8% and 97.2% at a current density of 0.025 A/dm2 and pH of 7.0, with energy consumption of 0.634 and 0.996 kWh/m3 for alternative current (AC) and direct current (DC), respectively. Furthermore, Al-Shannag et al. (2015) discussed the removal of Cu(II), Cr(III), Ni(II), and Zn(II) from metal plating wastewater by EC technique using Fe anodes. They found that using the pseudo-first-order model to evaluate the removal rates of these heavy metal ions is acceptable. The elimination efficiency rises as the EC residence time and DC density increase, according to their findings. The elimination effectiveness of heavy metal ions was 97% when using an EC treatment with a current density of 4 mA/cm2, a pH 9.56, and an EC time of 45 min. Akbal and Camcidotless (2011) investigated the removal of Cu, Cr, and Ni using Fe and Al electrodes in another investigation. The removal efficiency of heavy metal ions Cu, Cr, and Ni rise with increasing pH, current density, and conductivity. They used a Fe–Al electrode pair in an EC process to achieve 100% removal efficiency of Cu, Cr, and Ni at a current density of 10 mA/cm2 and pH 3.0, with energy and electrode consumption of 10.07 kWh/m3 and 1.08 kg/m3, respectively, and this removal efficiency increased due to hydrogen formation at the cathode and Al and Fe formation at the anode. However, Olmez (2009) used the RSM to investigate the impact of various operating parameters on the EC using stainless steel electrodes to remove Cr(VI) from industry effluent with a high concentration of 1470 mg/L. The study’s findings confirmed that RSM was an appropriate method for optimizing operating settings such as 7.4 A applied electric current, 33.6 mM electrolyte sodium chloride concentration, and EC application period of 70 min for 100% Cr(VI) removal efficiency. In contrast to the other treatment methods, electro-coagulation has many advantages such as it is fast, simple in operation, cost-effective, and environmental friendly (Ca et al. 2009).
Electrodeposition
Electrodeposition, also known as electrowinning, is a redox reaction in which positively (+ ve) charged metal ions (cations) are reduced and deposited at the cathode, and negatively (− ve) charged metal ions (anions) are oxidized at the anode. This approach is advantageous and reliable because no sludge is produced during the process, no additional reagents are required, a highly selective, cost-effective approach and no stable residues for metal recovery exist (Du et al. 2018). Organic contaminants in wastewater are destroyed at the anode while heavy metals are reduced and electroplated at the cathode during the electrodeposition process (Chang et al. 2009). An integrated methodology combining ultrasound and an electrodeposition method was employed to treat EDTA-copper wastewater. Cu removal efficiencies of 95.6% w/w follow the order of pH 3 > pH 5 > pH 7, with different voltage gradients following the order of 2.0 V/cm > 1.5 V/cm > 1.0 V/cm > 0.5 V/cm > 0.5 V/cm, showing that it is the most efficient wastewater treatment technology (Chang et al. 2009)..
Electrofloatation
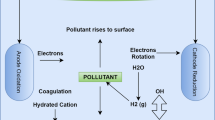
EF is a solid/liquid separation technique that incorporates the production of hydrogen and oxygen gases from water electrolysis in the form of small bubbles due to the pollutants floating to the water body's surface. EF has a variety of applications that have a lot of potential for removing heavy metals from industrial effluent. Wastewater effluents float to the surface of the liquid phase, where they can be easily separated using this EF process. In the total reaction, water electrolysis produces oxygen and hydrogen in the solution (Chen 2004):
The heavy metal particles adhere or adsorb to the oxygen and hydrogen molecules, causing the emulsified particles to be dismissed and flocs to form (Oliveira et al. 2014). The separation for the settling of the settled flocs and flotation of generated foam is carried out in the second step. In the third step, the collected pollutants are removed by filtration methods. Kolesnikov et al. (2015) investigated the effect of several surfactants on the physicochemical parameters of the dispersion phase for the removal of Cu, Ni, and Zn hydroxides at concentrations of 2, 10, 50, and 100 mg/L at pH 9.5–10.5. Anti-wear oxide electrodes were used to achieve a removal efficiency of more than 95% with a current density of roughly 0.2 A/L and a processing time of about 30 min.
On the other hand, Sun et al. (2009) discovered that employing Fe electrodes in combination with filter paper, micro, and UF bench-scale experiments could give excellent residual Ni, Fe, and turbidity results both with and without the addition of external oxygen. The residual Ni and Fe might meet the metal finishing industry’s discharge regulations with the hybridization of EF without aeration followed by microfiltration and aeration-enhanced EF followed by settling and paper filtration, according to their results. They came to the conclusion that combining an aeration-enhanced EF method with microfiltration could provide higher Ni and Fe removal results. Because this technology does not produce secondary pollution, it is particularly effective in the local water purification system.
Electrodialysis
Desalination research in the 1950s led to the development of ED (Azimi et al. 2017). ED is an ion-exchange membrane separation technique in which ionized species in a solution are transported through it while an electric potential is applied (Bruggen and Vandecasteele 2002). When ionic species in the solution pass through the cell compartments, they cross anion-exchange and cation-exchange membranes. At the same time, the anions and cations attract towards the anode and cathode, respectively (Barakat 2011). Tzanetakis et al. (2003) found some interesting results when they tested the effectiveness of ion exchange membranes for the ED of Ni(II) and Co(II) ions from a synthetic solution. Two cation exchange membranes, sulfonated polyvinylidene fluoride membrane (SPVDF) and perfluorosulfonic Nafion 117 were utilized and compared under similar working conditions. The removal efficiencies of Co(II) and Ni(II) using the perfluorosulfonic Nafion 117 membrane were 90% and 69%, respectively, with initial metal concentrations of 0.84 and 11.72 mg/L. Nataraj et al. (2007), on the other hand, constructed and operated an ED pilot plant that included a set of ion-exchange membranes and a new working system to test the rejection of Cr(VI) ions. Variations in applied potential, pH, initial Cr concentration, and flow rates were used to test the ED unit’s ability. With lower initial concentrations of less than 10 ppm, satisfactory results were obtained in meeting the maximum contaminant limit (MCL) of 0.1 mg/L for Cr.
ED has a number of benefits, including the ability to produce a highly concentrated stream for recovery and the ability to remove undesired effluents from water. Furthermore, it can be utilized to treat wastewater containing important metals like Cr and Cu. Because ED is a membrane process, it requires a clean feed, careful operation, and regular maintenance in order to avoid stack damage (Barakat 2011).
Electrooxidation
When electrochemical decomposition of CN was noticed in the late nineteenth century, an extensive study on EO for wastewater treatment was conducted (Kuhn 1971). In general, an electrochemical process comprises of redox processes occurring at both the anode (e.g., pollutant oxidation) and cathode (e.g., heavy metal reduction). The basic idea behind this technique is to take advantage of eliminating contaminants, which has long been used as a heavy metal remediation method (Nancharaiah et al. 2015). The electrochemical advanced oxidation processes (E-AOP) have raised the focus of research and application for electrochemical oxidation methods, which aim to mineralize organic molecules in process water and wastewater (Muddemann et al. 2019). Most of them are patents, including the electrolytic advance oxidation processes to treat wastewater, as reported by (Haddad 2013). Electrochemically generated oxidants can be found directly on the anode oxidants surface or indirectly through subsequent reactions with inorganic components (Botte 2017). Figure 8 illustrates the basic principle of EO. The anode (M), which involves direct charge transfer processes between the anode surface and organic pollutants in water, is the source of direct anodic oxidation or electrolysis. Only the arbitration of electrons, which can oxidize some organic contaminants at particular potentials lower than the oxygen evolution reaction (OER) potential, constitutes the mechanism (Garcia-Segura et al. 2018). However, the growth of polymeric coatings on their surface causes electrode fouling and results in poor chemical decontamination in this procedure. The indirect oxidation approach, which has an advantage over the direct oxidation method, depends on the oxygen evolution region that can be employed to overcome the issues of direct oxidation. It produces no waste and does not demand the use of oxidation catalysts in the solution. Physically adsorbed “active oxygen” (adsorbed hydroxyl radicals, •OH) or chemisorbed “active oxygen” (oxygen in the oxide lattice, MOx+1) can potentially result in direct electrooxidation of pollutants on anodes. According to a survey of research literature, the EO process is environmentally friendly and highly efficient for organic/inorganics pollutants elimination. This process was used to extract CN wastes that were exceedingly concentrated (50,000 mg/l CN−). During EO, metals can be collected at the cathode while CN ions are destroyed at the anode (Cheng et al. 2002).
On the other hand, Valiūnienė et al. (2015) used a Ti electrode covered with a 600 nm average thickness of Pt as a cost-effective anode to examine the EO process for CN ion removal from wastewater. The effective EO of a highly concentrated CN solution (2600 mg/L, or 0.1 M KCN) approaches a constant value of 3.45 V when a current density of 25 mA/cm2 is applied. The CN ions are practically eliminated (from 0.1 to 0.00016 M) when 60% current efficiency is attained, and 69 kC/L of charge is conveyed. Szpyrkowicz et al. (1998) employed stainless steel electrodes for the simultaneous EO of CN and Cu recovery. Their results displayed that at pH 13, the direct electrooxidation process was suitable and economically convenient. They also investigated reducing Cu concentration from 470 mg−1 by 79% in 1.5 h, at an energy consumption of 17 kWh/q, and recovering 335.3 mg of Cu as a pure metal electrodeposited on the cathode. In a recent study, Kazeminezhad and Mosivand (2017) examined the use of Fe sacrificial sheets in an electrolytic cell to eliminate Ni and Cu from effluent. Heavy metal concentrations were resolved using an AAS instrument. The results of the AAS demonstrated that increasing the applied voltage, electrochemical reaction duration or pH efficiently reduces the content of heavy metal contaminants in the water. It can greatly reduce Ni or Cu concentration in water when applied at 28 V for 60 min at pH 4.5. Table 8 shows the results of some study on the removal of heavy metals from industrial effluent using electrochemical methods.
Advanced oxidation processes
AOP has grown in popularity in recent years and is now widely employed to treat industrial wastewater (Korpe et al. 2019). It is a powerful treatment technique that uses hydroxyl radicals (OH•) to eliminate refractory organic pollutants effectively. The creation of hydroxyl radicals (HO•) from hydrogen peroxide (H2O2), ozone, photocatalysis, or oxidants in combination with the use of ultraviolet (UV) radiation is the basic principle of AOP. Two or more radical generators are sometimes used in combination. The HO•, on the other hand, is primarily responsible for the organic compound breakdown. It targets practically all organic complexes once it is generated. As a result of the HO• attack, the organic component is completely broken down, and AOPs reduce the pollutant concentration from a few hundred ppm to less than 5 ppb (Mohajerani et al. 2009). AOPs should, in theory, totally mineralize organic molecules to (CO2) and (H2O). The Photocatalysis AOP and electro-Fenton technique are the most commonly utilized in water treatment.
Photochemical advanced oxidation process
Photocatalysis is a type of photochemical AOP that has a lot of potential due to its ease of application, inexpensive, high degradation rate, low toxicity, and high stability. Semiconductor photocatalysis, first invented in 1972, is a new wastewater treatment process. Semiconductor photocatalysis, which uses UV-irradiated titanium dioxide (TiO2) to detoxify toxic materials in the aqueous phase, is already a well-established topic of research (Xu et al. 2006; Meichtry et al. 2007). It has received a lot of interest as a possible solution for wastewater treatment and environmental protection. The electronic structure and photoelectric characteristics of TiO2 are responsible for its catalytic activity. The band theory can be used to describe the photocatalytic reaction concept (Robert et al. 2004). TiO2 has a valence band and a conduction band, with a bandgap energy of 3.2 eV. When the surface of TiO2 is irradiated with light equal to or greater than the bandgap energy of TiO2, the surface is stimulated, resulting in the production of a hole-electronic pair with oxidation and reduction abilities. Expression is the same as Eq. (2).
The produced h+ can oxidize OH- and H2O on the TiO2 surface to OH, and OH can almost totally oxidize all pollutants deposited on the TiO2 surface. Any metal ions with a reduction potential greater than the edge of the TiO2 conduction band can theoretically be reduced by e-. The photocatalytic mechanism of TiO2 semiconductor is shown in Fig. 9 (Jiang et al. 2012). The series of reactions involved in the photocatalytic process are as follows:
Several semiconductor photocatalysts have been examined and reported, like titanium dioxide (Tryba et al. 2019), zinc oxide (Shen et al. 2008), tungsten trioxide (Yu et al. 2008), and cadmium sulfide (Di et al. 2009). The application research of TiO2 photocatalytic degradation of organic contaminants has been an interesting subject since the late 1960s. Various findings show that TiO2 is highly promising than the other semiconductor photocatalysts for applications in air purification, water decontamination, adsorption of contaminants (Tanzifi et al. 2018), and treatment of wastewater (Shahrezaei et al. 2012) due to its properties like low cost, non-corrosivity, high chemical resistance, and antioxidant ability. PC has proven its applications in various environmental fields like removal of aqueous pollutants and metal removal or/and recovery. Organics and inorganics (heavy metal ions, CN-containing waste, NO2-containing waste, and so on) can be destroyed simultaneously using this method (Schrank et al. 2002). Photocatalytic reduction and photocatalytic oxidation are the most common mechanisms for the elimination of inorganic contaminants. Cr(VI), Hg(II) and Pb(II) are currently the focus of additional study on photocatalytic remediation of metal ions contaminants in effluent (Kabra et al. 2008; Luo et al. 2017) Eq. (8) depicts the photocatalytic pathway for heavy metals (Mn+ denotes metal oxide, and M denotes the photocatalysis product).
Cyanide (mainly free cyanogen root) is highly hazardous and is primarily produced by the electroplating industry. In specific fields, cyanide emissions have recently grown. Photocatalysis with TiO2 may effectively convert poisonous CN− to CO2 or CO32− and harmless N2. The reaction is written as follows: (9).
The sol–gel method was used to make a new photocatalyst, TiO2 doped with neodymium (Nd), which was used to reduce Cr(VI) photo-catalytically under UV irradiation (Rengaraj et al. 2007). According to the findings, adding Nd (III) to TiO2 catalysts increases the photocatalytic reaction of Cr(VI) reduction substantially. On the TiO2 surface, Nd ions serve as electron accumulation sites. Charge carriers can be routed more efficiently towards favorable reduction and oxidation events rather than recombination reactions due to the better separation of electrons and holes on the modified TiO2 surface. The inclusion of sacrificial electron donors, such as formic acid aids photocatalytic reduction. Cr(VI) adsorbed on the surface of TiO2 particles was found to be nearly completely photo-reduced. A novel anodization-based immobilization technique was applied and tested to overcome the limitations of powder TiO2 (Yoon et al. 2009). An immobilized TiO2 electrode was employed to convert dangerous Cr(VI) to non-toxic Cr(III) in an aqueous solution under UV irradiation. The anodized samples were annealed in an oxygen stream at temperatures ranging from 450 to 850 °C after being anodized with 0.5% hydrofluoric acid. Photocatalytic Cr(VI) reduction was found to be helpful under acidic conditions, with 98% of the Cr(VI) being reduced after 2 h at pH 3 (Yoon et al. 2009).
Electro-Fenton process
The electro-Fenton method is one of the (E-AOP), and it has been found to be energy efficient for wastewater treatment as an improved Fenton process. The interaction of polycarboxylic acid with hydrogen peroxide (H2O2) was found in the 1890s by Fenton H.J.H., who observed a substantial promotion in the existence of ferrous ions (Fe2+). An oxidant, usually H2O2, and a catalyst, generally Fe in the form of Fe2+ are used in the Fenton process, also known as dark Fenton. The oxidation of Fe2+ results in the formation of •OH. Simultaneously, in the electro-Fenton approach, the strong •OH radicals created by the catalytic breakdown of electrogenerated H2O2 in the treated solution facilitate the destruction of organic pollutants (Asgari et al. 2016). Adding a redox couple to the system causes H2O2 to decompose into highly reactive •OH radicals (Aramyan 2017). For the electro-Fenton procedure, the Fe2+/Fe3+ couple produces the best results (Pimentel et al. 2008).
The process can be propagated by regenerating Fe2+ by chemical or electrochemical processes (Eqs. (11)–(14)) (Umar et al. 2010).
Anodic oxidation, cathodic reduction, neutralization, and electrodeposition may be included in the reaction mechanism, with the metal complex elimination procedure employing electro-Fenton displayed in Fig. 10.
As a result, electro-Fenton offers several distinct advantages: high removal efficiency, simplified reactor structure, increased wastewater organic degradability, continuous generation of H2O2 from O2 reduction or Fe2+ from the Fe anode, lowering treatment costs, the minimization of secondary pollution, the requirement to adjust current (A) and voltage (V) throughout the electrolysis process (Xu et al. 2021). The fact that this procedure happens in acidic media and that Fe removal is necessary are the key applicative limitations. The electro-Fenton process highly depends on electrode materials, and considerable work has gone into selecting appropriate conductive materials to optimize metal complex removal (Burgos-Castillo et al. 2018; An et al. 2019). Carbon felt cathodes were changed by doping with pyridinic N (Zhou et al. 2020). This alteration produced a large number of active sites by producing near-ring defects in the heterogeneous electro-Fenton process for purification of Ni(II)-EDTA by replacing C˭C groups with nitrogen, which greatly reduced H2O2 consumption and raised the utilization ratio.
However, the electro-Fenton process have some advantages, such as electro-Fenton's reagent is inexpensive, the procedure is simple to set up and maintain, the activation of H2O2 does not require any energy, short reaction time among all AOP. Some drawbacks include electrode material resistance, high energy consumption needs, low current efficiency, and Fe2+ is used at a faster rate than it is regenerated. As a result, further investigation is necessary on 3D electrode materials because they offer significant benefits in terms of improving current efficiency and lowering energy consumption (Hou et al. 2015; Peng et al. 2015).
Comparison of electroplating wastewater treatment technologies
Various techniques applied for waste streams generated from the electroplating industry have been considered and are equally important for detecting industrial effluents. Overall, each treatment process has its own set of benefits and drawbacks. The pros and cons of the different convectional and advanced treatments studied in this research are summarized in Table 9.
Status of “zero emission” of electroplating wastewater
Designing alternative industrial wastewater treatment solutions involving hazardous and non-biodegradable organic substances that cannot be fully oxidized as a traditional approach has been a major concern. Most of these physiochemical approaches (i.e., chemical precipitation, coagulation, flocculation, etc.) have proved unsatisfactory due to the high complexity of the industrial effluents. Due to the inadequacy of physiochemical techniques, economic, legal, social, and environmental demands have increased to adopt the excellent technology at reduced prices and seek “zero discharge.” Today, “zero emissions” has evolved into a problem that we are deeply concerned about. Finally, various companies have performed substantial research into what technologies may be used to achieve “zero-emission” of electroplating effluent (Figs. 11 and 12). To achieve zero discharge, wastewater reuse, metal salt recycling, and other challenges must be addressed first (Lu and Wu 2020).
Future prospective
The development of efficient and ecologically friendly wastewater treatment systems is being aided by rising environmental concern and social awareness of the health issues and ecosystem impacts of industrial contaminants. Most techniques with proper operation can lead to answerable for the electroplating wastewater treatment. Recovery of heavy metal ions and effective treatment of electroplating industry effluents is not feasible economically. Before the selection of treatment method, a complete analysis of any particular plant and wastewater generated.
More research study is still required for each technique to develop it and make it more appropriate. Inventive methods are needed to advance cheap, readily available, superior, and long-lasting membranes for membrane filtration. As for electro-dialysis, the improvement of new designs is necessary to progress the separation efficiency. Because most of the literature evaluated in this work is limited to an initial estimation of removal efficiency, it is critical to pursue further investigations at the pilot-plant size. As a result, the authors believe that more research is needed to establish feasible technology at a range of scales for applications at various locations and scales around the world and better understand the industrial effluent rejection phenomenon. More research into these methods should focus on testing them with real wastewater and operating them in a continuous mode to allow for progressive scaling up. Batch treatment systems have fewer industrial uses than continuous treatment systems. The majority of literature studies are done in batch systems; hence there is a need to develop continuous systems.
The electrode material chosen is important since it impacts the selectivity and efficiency of the electrochemical process. In other words, anode materials are the essential part of the electrode, as they regulate the with which pollutants are oxidized. More research on electrocatalytic anodic materials is needed to concentrate on lowering the initial costs of electrode purchase.
The future of wastewater treatment implies a combination of numerous process and advanced treatment methods (i.e., AOP). As a result, hybrid procedures or a combination of modern electrochemical techniques and other chemical/biological methods are urgently needed, where a reasonable compromise between acceptable economic cost, high removal efficiency, and environmental responsibility can be reached.
Conclusions
Electroplating wastewater treatment for the elimination of industrial effluents such as heavy metals has seen significant performance in recent years and witnessed vast advancement in applications and technologies. Generally, a vast amount of research has been carried out by a more significant number of researchers to remove metals ions from industrial wastewater by applying many various technologies. Nowadays, one major challenge is finding the most cost-effective, efficient, and appropriate method for removing hazardous contaminants from water bodies. Most conventional techniques (e.g., chemical precipitation, coagulation, and flocculation) have proven ineffective due to the high complication of industrial wastewater composition. Due to the inadequacy of traditional approaches, economic, social, legal, and environmental demands have increased to apply the best technology at reduced prices and seek “zero discharge.” Each technique has its own benefits/drawbacks, with different removal efficiencies as well as characteristics influencing the removal process. The variable parameters altered removal efficiencies of Cd, Mn, Ni, Fe, and As from 47 to 99%, while complete removal of some metals (i.e., Cu, Pd, Cr, and Zn) which was examined from different technique results and their experimental condition. The removal efficiency of heavy metals (47–100%) and reducing their dosage to standard limits under optimum conditions were achieved using RO, UF, electroflotation, and electrocoagulation.
The following conclusions could be drawn from the various techniques which are discussed in the review:
-
1.
Chemical precipitation is a simple and cost-effective way to treat industrial effluent. It has drawbacks, such as the operational cost being high due to the sludge disposal. This approach is extensively used when heavy metal concentrations are high, but it is inadequate when metal ions concentrations are low. While the coagulation/ flocculation process has a high removal efficiency, it also produces secondary contaminants, which transfer the harmful compounds into the environment. During this treatment, the formation of sludge appears, which must be handled finally.
-
2.
An ion-exchange process is another method for treating industrial wastewater. This technology has a minimal maintenance cost and produces an excellent flow rate of treated water. However, ion exchange has some benefits, such as fast kinetics and high treatment capacity. Still, this process has many problems, such as high resins cost and resins regeneration requirement due to its fouling.
-
3.
A membrane separation process is another extensively utilized wastewater treatment method. The recovery of heavy metal ions with high efficiency is possible with this technology; however, the disadvantages of this process include high membrane cost, membrane fouling, process complexity, as well as high operating costs, which have limited their use in heavy metal removal.
-
4.
Furthermore, the adsorption technique for heavy metal removal is a relatively new practice. It has proven to be a great approach for reducing metal contamination. Furthermore, greater research into low-cost adsorption processes is needed to increase the large-scale utilization of non-conventional adsorbents. The usage of low-cost adsorbents can provide benefits such as cost reduction and increased heavy metal removal efficiency. Activated carbon's high cost prevents it from being used in adsorption. Activated carbons are expensive, and the regeneration process still has some issues. They can only remove a few micrograms of metal ions per gram of activated carbon. Overall, the two most important characteristics in determining the most likely adsorbent for heavy metal removal from wastewater are accessibility and price.
-
5.
One of the most often utilized approaches is the electrochemical method, which is used to remove toxic effluents from contaminated wastewater and, according to a recent literature review, has emerged as a promising alternative to traditional methods of pollution treatment. Electrochemical treatment with electroplating wastewater showed complete removal of Cu, Cr, Zn, and Ni, while Ni and Pb removal ranged between 95 and 99%. Other metals which were present in lower concentrations were also effectively removed. This method is beneficial, including producing less sludge, having a high separation selectivity; it is regarded as quick and yields good reduction yields. This electrochemical treatment employs electrical energy to remove contaminants from water, reusing without chemicals. Furthermore, due to the short lifetime of electrode material, the utilization of electrochemical treatment in wastewater is limited.
-
6.
AOP is now applied for the treatment of industrial wastewater because of its benefits, which include a strong oxidizer, rapid reaction rates, highly efficient, no secondary pollutant generation, and non-selective oxidation, which allows the treatment of several contaminants at the same time. For the elimination of harmful organic materials and heavy metals, it is a highly recommended approach. This technique has the potential to lower pollutant concentrations from hundreds of parts per million (ppm) to a few parts per billion (ppb). Furthermore, AOP systems utilizing H2O2 should be carefully controlled for residual H2O2 as it can have negative effects on subsequent treatment steps. However, by carefully designing the system, excess residual H2O2 and any related repercussions can be avoided.
Although all of the above techniques can be based on some parameters such as pH, initial metal concentration, wastewater component, environmental impact, and economic parameters such as capital investment and operational costs, it is necessary to highlight that selecting the most appropriate treatment methods, the overall treatment performance compared to other technologies are all important considerations. Furthermore, plant flexibility, reliability, technological accessibility, and cost-effectiveness are important factors to consider when choosing the most appropriate and cost-effective treatment system for removing pollutants and protecting the environment.
References
Abu H, Moussab H (2004) Removal of heavy metals from wastewater by membrane processes : a comparative study. Desalination 164:105–110
Adiga MR (2000) Plating waste water treatment and metals recovery system. US Pat US006162361A
Agustiono T, Chan GYS, Lo W, Babel S (2006) Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci Total Environ 366:409–426. https://doi.org/10.1016/j.scitotenv.2005.10.001
Ahmed JK, Ahmaruzzaman M (2016) A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J Water Process Eng 10:39–47. https://doi.org/10.1016/j.jwpe.2016.01.014
Ahn K, Song K, Cha H, Yeom I (1999) Removal of ions in nickel electroplating rinse water using low-pressure nanofiltration. Desalination 122:77–84
Aimin Li, Lichun FU et al (2017) Electroplating waste water treatment method. US Pat US9708202B2 2:
Akbal F, Camcidotless S (2011) Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination 269:214–222. https://doi.org/10.1016/j.desal.2010.11.001
Al-alawy AF (2017) Theoretical and Experimental Study of Nanofiltration and Reverse Osmosis Membranes for Removal of Heavy Metals from Wastewater. Int J Sci Res 6:778–788. https://doi.org/10.21275/art20178885
Al-Qodah Z, Al-Shannag M (2017) Heavy metal ions removal from wastewater using electrocoagulation processes: A comprehensive review. Sep Sci Technol 52:2649–2676. https://doi.org/10.1080/01496395.2017.1373677
Al-saydeh SA, El-naas MH, Zaidi SJ (2017) Copper removal from industrial wastewater : A comprehensive review. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2017.07.026
Al-Shannag M, Al-Qodah Z, Bani-Melhem K et al (2015) Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chem Eng J 260:749–756. https://doi.org/10.1016/j.cej.2014.09.035
Al Aji B, Yavuz Y, Koparal AS (2012) Electrocoagulation of heavy metals containing model wastewater using monopolar iron electrodes. Sep Purif Technol 86:248–254. https://doi.org/10.1016/j.seppur.2011.11.011
Alslaibi TM, Abustan I, Ahmad MA, Foul AA (2013) Cadmium removal from aqueous solution using microwaved olive stone activated carbon. J Environ Chem Eng 1:589–599. https://doi.org/10.1016/j.jece.2013.06.028
Alyüz B, Veli S (2009) Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J Hazard Mater 167:482–488. https://doi.org/10.1016/j.jhazmat.2009.01.006
Ambashta RD, Sillanpää M (2010) Water purification using magnetic assistance : A review. J Hazard Mater 180:38–49. https://doi.org/10.1016/j.jhazmat.2010.04.105
An J, Li N, Wang S et al (2019) A novel electro-coagulation-Fenton for energy efficient cyanobacteria and cyanotoxins removal without chemical addition. J Hazard Mater 365:650–658. https://doi.org/10.1016/j.jhazmat.2018.11.058
Aramyan SM (2017) Advances in Fenton and Fenton based oxidation processes for industrial effluent contaminants control-a review. Int J Environ Sci Nat Resour 2:. https://doi.org/10.19080/ijesnr.2017.02.555594
Argun ME, Dursun S, Ozdemir C, Karatas M (2007) Heavy metal adsorption by modified oak sawdust : Thermodynamics and kinetics. J Hazard Mater 141:77–85. https://doi.org/10.1016/j.jhazmat.2006.06.095
Asgari G, Feradmal J, Poormohammadi A et al (2016) Taguchi optimization for the removal of high concentrations of phenol from saline wastewater using electro-Fenton process. Desalin Water Treat 57:27331–27338. https://doi.org/10.1080/19443994.2016.1170635
Ayub S, Siddique AA, Khursheed MS et al (2020) Removal of heavy metals (Cr, Cu, and Zn) from electroplating wastewater by electrocoagulation and adsorption processes. Desalin Water Treat 179:263–271. https://doi.org/10.5004/dwt.2020.25010
Azimi A, Azari A, Rezakazemi M, Ansarpour M (2017) Removal of heavy metals from industrial wastewaters: a review. ChemBioEng Rev 4:37–59. https://doi.org/10.1002/cben.201600010
Azmi AA, Jai J, Zamanhuri NA, Yahya A (2018) Precious Metals Recovery from Electroplating Wastewater: A Review. Mater Sci Eng 358. https://doi.org/10.1088/1757-899X/358/1/012024
Babu BR, Bhanu SU, Meera KS (2009) Waste minimization in electroplating industries: a review. J Environ Sci Heal - Part C Environ Carcinog Ecotoxicol Rev 27:155–177. https://doi.org/10.1080/10590500903124158
Bakar AFA, Halim AA, Hanafiah MM (2015) Optimization of coagulation-flocculation process for automotive wastewater treatment using response surface methodology. Nat Environ Pollut Technol 14:567–572
Bankole MT, Abdulkareem AS, Mohammed IA et al (2019) Selected heavy metals removal from electroplating wastewater by purified and polyhydroxylbutyrate functionalized carbon nanotubes adsorbents. Sci Rep 9:1–19. https://doi.org/10.1038/s41598-018-37899-4
Bankole MT, Abdulkareem AS, Tijani JO et al (2017) Chemical oxygen demand removal from electroplating wastewater by purified and polymer functionalized carbon nanotubes adsorbents. Water Resour Ind 18:33–50. https://doi.org/10.1016/j.wri.2017.07.001
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4:361–377. https://doi.org/10.1016/j.arabjc.2010.07.019
Bharti V (2020) Hexavalent chromium reduction from real electroplating wastewater by chemical precipitation. Bull Chem Soc Ethiop 34:67–74
Bilal M, Shah JA, Ashfaq T et al (2013) StartWaste biomass adsorbents for copper removal from industrial wastewater–A review. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2013.07.071
Bisht R, Agarwal M (2017) Methodologies for removal of heavy metal ions from wastewater : an overview. Interdiscip Environ Rev 18:124–142. https://doi.org/10.1504/IER.2017.10008828
Blue LY, Van AMA, Matlock M, Ã DAA, (2008) Low-level mercury removal from groundwater using a synthetic chelating ligand. Water Res 42:2025–2028. https://doi.org/10.1016/j.watres.2007.12.010
Bojic AL, Bojic D, Andjelkovic T (2009) Removal of Cu 2 + and Zn 2 + from model wastewaters by spontaneous reduction – coagulation process in flow conditions. J Hazard Mater 168:813–819. https://doi.org/10.1016/j.jhazmat.2009.02.096
Botte GG (2017) Electrochemical technologies for water treatment, management, and efficiency. Electrochem Soc Interface 26:53–61. https://doi.org/10.1149/2.F04172if
Bouguerraa W, Barhoumib A, Ibrahimb N et al (2015) Optimization of the electrocoagulation process for the removal of lead from water using aluminium as electrode material. Desalin Water Treat 37–41. https://doi.org/10.1080/19443994.2015.1015308
Bruggen B Van Der, Vandecasteele C (2002) Distillation vs . membrane filtration : overview of process evolutions in seawater desalination. des 143:207–218
Burgos-Castillo R, Sillanpää M, Brillas E, Sirés I (2018) Removal of metals and phosphorus recovery from urban anaerobically digested sludge by electro-Fenton treatment. Sci Total Environ 644:173–182. https://doi.org/10.1016/j.scitotenv.2018.06.337
Ramakrishnaiah PCR (2012) Hexavalent chromium removal from industrial wastewater by chemical precipitation method. Int J Eng Res Appl 2:599–603
Ca P, Mart F, Jim C et al (2009) Technical and economic comparison of conventional and electrochemical coagulation. J Chem Technol Biotechnol 702–710. https://doi.org/10.1002/jctb.2102
Calero M, Bl G (2014) New treatment of real electroplating wastewater containing heavy metal ions by adsorption onto olive stone. J Clean Prod 81:120–129. https://doi.org/10.1016/j.jclepro.2014.06.036
Carolin CF, Kumar PS, Saravanan A et al (2017) Efficient techniques for the removal of toxic heavy metals from aquatic environment : A review. J Environ Chem Eng 5:2782–2799. https://doi.org/10.1016/j.jece.2017.05.029
Cavaco SA, Fernandes S, Quina MM, Ferreira M (2007) Removal of chromium from electroplating industry effluents by ion exchange resins. J Hazard Mater 144:634–638. https://doi.org/10.1016/j.jhazmat.2007.01.087
Chang J, Ellis AV, Yan C, Tung C (2009) The electrochemical phenomena and kinetics of EDTA – copper wastewater reclamation by electrodeposition and ultrasound. Sep Purif Technol 68:216–221. https://doi.org/10.1016/j.seppur.2009.05.014
Chang Q, Wang G (2007) Study on the macromolecular coagulant PEX which traps heavy metals. Chem Eng Sci 62:4636–4643. https://doi.org/10.1016/j.ces.2007.05.002
Chaudhari LB, Murthy ZVP (2010) Separation of Cd and Ni from multicomponent aqueous solutions by nanofiltration and characterization of membrane using IT model. J Hazard Mater 180:309–315. https://doi.org/10.1016/j.jhazmat.2010.04.032
Chen G (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38:11–41. https://doi.org/10.1016/j.seppur.2003.10.006
Chen Q, Luo Z, Hills C et al (2009a) Precipitation of heavy metals from wastewater using simulated flue gas : Sequent additions of fly ash, lime and carbon dioxide. Water Res 43:2605–2614. https://doi.org/10.1016/j.watres.2009.03.007
Chen Q, Yao Y, Li X et al (2018) Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J Water Process Eng 26:289–300. https://doi.org/10.1016/j.jwpe.2018.11.003
Chen S, Li C, Hsu H et al (2009b) Concentration and purification of chromate from electroplating wastewater by two-stage electrodialysis processes. J Hazard Mater 161:1075–1080. https://doi.org/10.1016/j.jhazmat.2008.04.106
Chen X, Huang G, Wang J (2013) Electrochemical Reduction / Oxidation in the Treatment of Heavy Metal Wastewater. J Metall Eng 2:161–164
Cheng SC, Gattrell M, Guena T, Macdougall B (2002) The electrochemical oxidation of alkaline copper cyanide solutions. Electrochim Acta 47:3245–3256
Chongwu Guo FL (2020) Method for integrated treatment of electroplating wastewater. US Pat 20200048125A1
Cimen A (2015) Removal of Chromium from Wastewater by Reverse Osmosis. Russ J Phys Chem 89:1238–1243. https://doi.org/10.1134/S0036024415070055
Di X, Kansal SK, Deng W (2009) Preparation, characterization and photocatalytic activity of flowerlike cadmium sulfide nanostructure. Sep Purif Technol 68:61–64. https://doi.org/10.1016/j.seppur.2009.04.007
Djaenudin WDR, Hariyadi HR (2015) Effect of Electrodeposition Reactor Type on Nickel Recovery from Electroplating Wastewater. Procedia Chem 16:155–163. https://doi.org/10.1016/j.proche.2015.12.026
Du X, Guo X, You S (2018) Precious metals recovery from electroplating wastewater : a review. IOP Conf Ser Mater Sci Eng 358. https://doi.org/10.1088/1757-899X/358/1/012024
Dubey SP, Gopal K (2007) Adsorption of chromium ( VI ) on low cost adsorbents derived from agricultural waste material : A comparative study. J Hazard Mater 145:465–470. https://doi.org/10.1016/j.jhazmat.2006.11.041
Feng Y, Yang S, Xia L et al (2019) In-situ ion exchange electrocatalysis biological coupling (i-IEEBC) for simultaneously enhanced degradation of organic pollutants and heavy metals in electroplating wastewater. J Hazard Mater 364:562–570. https://doi.org/10.1016/j.jhazmat.2018.10.068
Fresnel J-M (2015) Electrolysis method, and method and plant for the pretreatment of raw water. US Pat AU2010223782B2 3001:
Fu D, Kurniawan TA, Avtar R et al (2021) Recovering heavy metals from electroplating wastewater and their conversion into Zn2Cr-layered double hydroxide (LDH) for pyrophosphate removal from industrial wastewater. Chemosphere 271. https://doi.org/10.1016/j.chemosphere.2021.129861
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Garcia-Segura S, Ocon JD, Chong MN (2018) Electrochemical oxidation remediation of real wastewater effluents — A review. Process Saf Environ Prot 113:48–67. https://doi.org/10.1016/j.psep.2017.09.014
Gayathri R, Gopinath KP, Kumar PS (2021) Adsorptive separation of toxic metals from aquatic environment using agro waste biochar: Application in electroplating industrial wastewater. Chemosphere 262:128031. https://doi.org/10.1016/j.chemosphere.2020.128031
Gherasim CV, Mikulášek P (2014) Influence of operating variables on the removal of heavy metal ions from aqueous solutions by nanofiltration. Desalination 343:67–74. https://doi.org/10.1016/j.desal.2013.11.012
Ghosh P, Samanta AN, Ray S (2011) Reduction of COD and removal of Zn2+ from rayon industry wastewater by combined electro-Fenton treatment and chemical precipitation. Desalination 266:213–217. https://doi.org/10.1016/j.desal.2010.08.029
Gode F, Pehlivan E (2006) Removal of chromium ( III ) from aqueous solutions using Lewatit S 100: The effect of pH, time, metal concentration and temperature. J Hazard Mater 136:330–337. https://doi.org/10.1016/j.jhazmat.2005.12.021
Golder AK, Dhaneesh VS, Samanta AN, Ray S (2008) Removal of nickel and boron from plating rinse effluent by electrochemical and chemical techniques. Chem Eng Technol 31:143–148. https://doi.org/10.1002/ceat.200700330
Golder AK, Samanta AN, Ray S (2007) Removal of Cr 3 + by electrocoagulation with multiple electrodes : Bipolar and monopolar configurations. J Hazard Mater 141:653–661. https://doi.org/10.1016/j.jhazmat.2006.07.025
Guan W, Tian S, Cao D et al (2017) Electrooxidation of nickel-ammonia complexes and simultaneous electrodeposition recovery of nickel from practical nickel-electroplating rinse wastewater. Electrochim Acta 246:1230–1236. https://doi.org/10.1016/j.electacta.2017.06.121
Guan Z, Guo Y, Li S et al (2020) Decomplexation of heterogeneous catalytic ozonation assisted with heavy metal chelation for advanced treatment of coordination complexes of Ni. Sci Total Environ 732:139223. https://doi.org/10.1016/j.scitotenv.2020.139223
Guo M, Qiu G, Song W (2010) Poultry litter-based activated carbon for removing heavy metal ions in water. Waste Manag 30:308–315. https://doi.org/10.1016/j.wasman.2009.08.010
Guo Z, Zhang G, Fang J, Dou X (2006) Enhanced chromium recovery from tanning wastewater. J Clean Prod 14:75–79. https://doi.org/10.1016/j.jclepro.2005.01.005
Haddad RY (2013) Electrolytic advance oxidation processes to treat wastewater, brackish and saline water without hydrogen evolution. US Pat GB2515324A
Hegazi HA (2013) Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J 9:276–282. https://doi.org/10.1016/j.hbrcj.2013.08.004
Heredia JB, Martín JS (2009) Removing heavy metals from polluted surface water with a tannin-based flocculant agent. J Hazard Mater 165:1215–1218. https://doi.org/10.1016/j.jhazmat.2008.09.104
Hosseini SS, Bringas E, Tan NR et al (2016) Recent progress in development of high performance polymeric membranes and materials for metal plating wastewater treatment: A review. J Water Process Eng 9:78–110. https://doi.org/10.1016/j.jwpe.2015.11.005
Hou B, Han H, Zhuang H et al (2015) A novel integration of three-dimensional electro-Fenton and biological activated carbon and its application in the advanced treatment of biologically pretreated Lurgi coal gasification wastewater. Bioresour Technol 196:721–725. https://doi.org/10.1016/j.biortech.2015.07.068
Hu J, Chen C, Zhu X, Wang X (2009) Removal of chromium from aqueous solution by using oxidized multiwalled carbon nanotubes. J Hazard Mater 162:1542–1550. https://doi.org/10.1016/j.jhazmat.2008.06.058
Huang Y, Wu D, Wang X et al (2016) Removal of heavy metals from water using polyvinylamine by polymer-enhanced ultrafiltration and flocculation. Sep Purif Technol 158:124–136. https://doi.org/10.1016/j.seppur.2015.12.008
Huang Y-H et al (1989) Method of wastewater treatment by electrolysis and oxidization. US Pat US006126838A
Husain A, Javed I, Khan NA (2014) Characterization and treatment of electroplating industry wastewater using Fenton’s reagent. J Chem Pharm Res 6:622–627
Idrisb A, Hisham N, Hamidc A (1996) Total removal of heavy metal from mixed plating rinse wastewater. Desalination 106:419–422
Ilhan F, Ulucan-Altuntas K, Avsar Y et al (2019) Electrocoagulation process for the treatment of metal-plating wastewater: Kinetic modeling and energy consumption. Front Environ Sci Eng 13:1–8. https://doi.org/10.1007/s11783-019-1152-1
Islamoglu S, Yilmaz L, Ozbelge HO (2006) Development of a precipitation based separation scheme for selective removal and recovery of heavy metals from cadmium rich electroplating industry effluents. Sep Sci Technol 41:3367–3385. https://doi.org/10.1080/01496390600851665
Izdebski W (1975) Removal of heavy metal ions from plating wastes. US Pat US3869386A
Etzel VKJE (1980) Treatment of metal plating wastes with an unexpanded vermiculite cation exchange column. US Pat US4210530A
Jiang L, Wang Y, Feng C (2012) Application of photocatalytic technology in environmental safety. Procedia Eng 45:993–997. https://doi.org/10.1016/j.proeng.2012.08.271
Jiang M, Jin X, Lu X, Chen Z (2010) Adsorption of Pb ( II ), Cd ( II ), Ni ( II ) and Cu ( II ) onto natural kaolinite clay. Desalination 252:33–39. https://doi.org/10.1016/j.desal.2009.11.005
Jin W, Du H, Zheng S, Zhang Y (2016) Electrochemical processes for the environmental remediation of toxic Cr ( VI ): A review. Electrochim Acta 191:1044–1055
Juang R, Shiau R (2000) Metal removal from aqueous solutions using chitosan-enhanced membrane filtration. J Memb Sci 165:159–167
Kabay N, Arda M, Saha B, Streat M (2003) Removal of Cr (VI) by solvent impregnated resins ( SIR ) containing aliquat 336. React Funct Polym 54:103–115
Kabra K, Chaudhary R, Sawhney RL (2008) Solar photocatalytic removal of Cu(II), Ni(II), Zn(II) and Pb(II): Speciation modeling of metal-citric acid complexes. J Hazard Mater 155:424–432. https://doi.org/10.1016/j.jhazmat.2007.11.083
Kamaraj R, Ganesan P (2013) Removal of copper from water by electrocoagulation process — effect of alternating current ( AC ) and direct current ( DC ). Env Sci Pollut Res 399–412. https://doi.org/10.1007/s11356-012-0855-7
Kamaraj R, Ganesan P, Vasudevan S (2013) Removal of lead from aqueous solutions by electrocoagulation: isotherm, kinetics and thermodynamic studies. Int J Environ Sci Technol 12:683–692. https://doi.org/10.1007/s13762-013-0457-z
Kang S (2003) A water purifier using electrolysis. US Pat WO2003037802A1
Katoh S (1985) Method of treating liquid wastes containing heavy metal chelate compounds. US Pat EP0168752A2
Kazeminezhad I, Mosivand S (2017) Elimination of copper and nickel from wastewater by electrooxidation method. J Magn Magn Mater 422:84–92. https://doi.org/10.1016/j.jmmm.2016.08.049
Khelifa A, Moulay S, Naceur AW (2005) Treatment of metal finishing effluents by the electroflotation technique. Desalination 181:27–33
Kim T, Kim TK, Zoh KD (2020) Removal mechanism of heavy metal (Cu, Ni, Zn, and Cr) in the presence of cyanide during electrocoagulation using Fe and Al electrodes. J Water Process Eng 33:101109. https://doi.org/10.1016/j.jwpe.2019.101109
Kobya M, Demirbas E, Dedeli A, Sensoy MT (2010) Treatment of rinse water from zinc phosphate coating by batch and continuous electrocoagulation processes. J Hazard Mater 173:326–334. https://doi.org/10.1016/j.jhazmat.2009.08.092
Kolesnikov AV, Kuznetsov VV, Kolesnikov VA, Kapustin YI (2015) The role of surfactants in the electroflotation extraction of copper, nickel, and zinc hydroxides and phosphates. Theor Found Chem Eng 49:3–11. https://doi.org/10.1134/S0040579515010042
Kongsuwan A, Patnukao P, Pavasant P (2009) Binary component sorption of Cu(II) and Pb(II) with activated carbon from Eucalyptus camaldulensis Dehn bark. J Ind Eng Chem 15:465–470. https://doi.org/10.1016/j.jiec.2009.02.002
Korpe S, Bethi B, Sonawane SH, Jayakumar KV (2019) Tannery wastewater treatment by cavitation combined with advanced oxidation process (AOP). Ultrason Sonochem 59:104723. https://doi.org/10.1016/j.ultsonch.2019.104723
Ku Y, Jung I (2001) Photocatalytic reduction of Cr(VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Res 35:135–142
Kuhn AT (1971) Electrolytic decomposition of cyanides, phenols and thiocyanates in effluent streams-a literature review. J Appl Biotechnol 21:29–34
Szpyrkowicz L, Zilio-Grandi SNKF and SR-S (1998) Electrochemical treatment of copper cyanide wastewaters using stainless steel electrodes. Water Sci Technol 38:261–268https://doi.org/10.1016/S0273-1223(98)00598-8
Lan J, Ren Y, Lu Y et al (2019) Combined microbial desalination and chemical-production cell with Fenton process for treatment of electroplating wastewater nanofiltration concentrate. Chem Eng J 359:1139–1149. https://doi.org/10.1016/j.cej.2018.11.067
Li T, Xiao K, Yang B et al (2019) Recovery of Ni(II) from real electroplating wastewater using fixed-bed resin adsorption and subsequent electrodeposition. Front Environ Sci Eng 13. https://doi.org/10.1007/s11783-019-1175-7
Li Y, Zeng X, Liu Y et al (2003) Study on the treatment of copper-electroplating wastewater by chemical trapping and flocculation. Sep Purif Technol 31:91–95
Liu Y, Liu G, Wang H et al (2021) Elongation the duration of steel anode with polypyrrole modification during the electrocoagulation treatment process of electroplating wastewater. J Environ Chem Eng 9:104969. https://doi.org/10.1016/j.jece.2020.104969
López-Maldonado EA, Zavala García OG, Escobedo KC, Oropeza-Guzman MT (2017) Evaluation of the chelating performance of biopolyelectrolyte green complexes (NIBPEGCs) for wastewater treatment from the metal finishing industry. J Hazard Mater 335:18–27. https://doi.org/10.1016/j.jhazmat.2017.04.020
Lu BJ, Wu K (2020) Electroplating wastewater treatment method and development trend analysis. Mater Sci Eng 774. https://doi.org/10.1088/1757-899X/774/1/012092
Luo S, Qin F, Ming Y et al (2017) Fabrication uniform hollow Bi2S3 nanospheres via Kirkendall effect for photocatalytic reduction of Cr(VI) in electroplating industry wastewater. J Hazard Mater 340:253–262. https://doi.org/10.1016/j.jhazmat.2017.06.044
Lyu H, Gong Y, Tang J et al (2016) Immobilization of heavy metals in electroplating sludge by biochar and iron sulfide. Environ Sci Pollut Res 23:14472–14488. https://doi.org/10.1007/s11356-016-6621-5
Mahmoud A, Hoadley AFA (2012) An evaluation of a hybrid ion exchange electrodialysis process in the recovery of heavy metals from simulated dilute industrial wastewater. Water Res 46:3364–3376. https://doi.org/10.1016/j.watres.2012.03.039
Malaviya PSA (2011) Physicochemical technologies for remediation of chromium-containing waters and wastewaters. Crit Rev Environ Sci Technol 37–41. https://doi.org/10.1080/10643380903392817
Martin-Lara MA, Blazquez G, Trujillo MC et al (2014) New treatment of real electroplating wastewater containing heavy metal ions by adsorption onto olive stone. J Clean Prod 81:120–129. https://doi.org/10.1016/j.jclepro.2014.06.036
Matlock MM, Howerton BS, Atwood DA (2002) Chemical precipitation of heavy metals from acid mine drainage. Water Res 36:4757–4764
Meas Y, Ramirez JA, Villalon MA, Chapman TW (2010) Industrial wastewaters treated by electrocoagulation. Electrochim Acta 55:8165–8171. https://doi.org/10.1016/j.electacta.2010.05.018
Meichtry JM, Brusa M, Mailhot G et al (2007) Heterogeneous photocatalysis of Cr(VI) in the presence of citric acid over TiO2 particles: Relevance of Cr(V)-citrate complexes. Appl Catal B Environ 71:101–107. https://doi.org/10.1016/j.apcatb.2006.09.002
Mohajerani M, Mehrvar M, Ein-Mozaffari F (2009) An overview of the integration of advanced oxidation technologies and other processes for water and wastewater treatment. Int J Eng 3:120–146
Mohammadi H, Gholami M, Rahimi M (2009) Application and optimization in chromium- contaminated wastewater treatment of the reverse osmosis technology of the reverse osmosis technology. Desalin Water Treat 9:229–233. https://doi.org/10.5004/dwt.2009.808
Mohsen-Nia M, Montazeri P, Modarress H (2007) Removal of Cu2+ and Ni 2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 217:276–281. https://doi.org/10.1016/j.desal.2006.01.043
Morton SG (1997) Method and apparatus for detecting and classifying contaminants in water. US Pat US005646863A
Muddemann T, Haupt D, Sievers M, Kunz U (2019) Electrochemical reactors for wastewater treatment. ChemBioEng Rev 142–156. https://doi.org/10.1002/cben.201900021
Muthukrishnan M, Guha BK (2008) Effect of pH on rejection of hexavalent chromium by nanofiltration. Desalination 219:171–178. https://doi.org/10.1016/j.desal.2007.04.054
Nancharaiah YV, Venkata Mohan S, Lens PNL (2015) Metals removal and recovery in bioelectrochemical systems: A review. Bioresour Technol 195:102–114. https://doi.org/10.1016/j.biortech.2015.06.058
Narayanan NV, Ganesan M (2009) Use of adsorption using granular activated carbon ( GAC ) for the enhancement of removal of chromium from synthetic wastewater by electrocoagulation. J Hazard Mater 161:575–580. https://doi.org/10.1016/j.jhazmat.2008.03.113
Nataraj SK, Hosamani KM, Aminabhavi TM (2007) Potential application of an electrodialysis pilot plant containing ion-exchange membranes in chromium removal. Desalination 217:181–190. https://doi.org/10.1016/j.desal.2007.02.012
Njoya O, Zhao S, Kong X et al (2021) Efficiency and potential mechanism of complete Cr(VI) removal in the presence of oxalate by catalytic reduction coupled with membrane filtration. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2021.118915
Oden MK, Sari-Erkan H (2018) Treatment of metal plating wastewater using iron electrode by electrocoagulation process: Optimization and process performance. Process Saf Environ Prot 119:207–217. https://doi.org/10.1016/j.psep.2018.08.001
De OI, Adilson J, Castro D et al (2014) Study of electroflotation method for treatment of wastewater from washing soil contaminated by heavy metals. Integr Med Res 4:109–113. https://doi.org/10.1016/j.jmrt.2014.11.004
Ölmez T (2009) The optimization of Cr ( VI ) reduction and removal by electrocoagulation using response surface methodology. J Hazard Mater 162:1371–1378. https://doi.org/10.1016/j.jhazmat.2008.06.017
Pang FM, Kumar P, Teng TT et al (2011) Removal of lead, zinc and iron by coagulation-flocculation. J Taiwan Inst Chem Eng 42:809–815. https://doi.org/10.1016/j.jtice.2011.01.009
Pang FM, Teng SP, Teng TT, Mohd Omar AK (2009) Heavy metals removal by hydroxide precipitation and coagulation-flocculation methods from aqueous solutions. Water Qual Res J Canada 44:174–182. https://doi.org/10.2166/wqrj.2009.019
Papadopoulos A, Fatta D, Parperis K et al (2004) Nickel uptake from a wastewater stream produced in a metal finishing industry by combination of ion-exchange and precipitation methods. Sep Purif Technol 39:181–188. https://doi.org/10.1016/j.seppur.2003.10.010
Peng G, Deng S, Liu F et al (2020a) Superhigh adsorption of nickel from electroplating wastewater by raw and calcined electroplating sludge waste. J Clean Prod 246:118948. https://doi.org/10.1016/j.jclepro.2019.118948
Peng G, Deng S, Liu F et al (2020b) Calcined electroplating sludge as a novel bifunctional material for removing Ni(II)-citrate in electroplating wastewater. J Clean Prod 262:121416. https://doi.org/10.1016/j.jclepro.2020.121416
Peng Q, Zhao H, Qian L et al (2015) Design of a neutral photo-electro-Fenton system with 3D-ordered macroporous Fe2O3/carbon aerogel cathode: High activity and low energy consumption. Appl Catal B Environ 174–175:157–166. https://doi.org/10.1016/j.apcatb.2015.02.031
Pimentel M, Oturan N, Dezotti M, Oturan MA (2008) Phenol degradation by advanced electrochemical oxidation process electro-Fenton using a carbon felt cathode. Appl Catal B Environ 83:140–149. https://doi.org/10.1016/j.apcatb.2008.02.011
Pociecha M, Lestan D (2010) Using electrocoagulation for metal and chelant separation from washing solution after EDTA leaching of Pb, Zn and Cd contaminated soil. J Hazard Mater 174:670–678. https://doi.org/10.1016/j.jhazmat.2009.09.103
Porto MB, Costa JM, de Almeida Neto AF (2020) Ni-W alloys and their anticorrosive properties: Ni removal efficiency from galvanic wastewater by electrodeposition. J Water Process Eng 36:101250. https://doi.org/10.1016/j.jwpe.2020.101250
Qin J, Wai M, Oo M, Wong F (2002) A feasibility study on the treatment and recycling of a wastewater from metal plating. J Memb Sci 208:213–221
Qu J, Tian X, Jiang Z et al (2020) Multi-component adsorption of Pb(II), Cd(II) and Ni(II) onto microwave-functionalized cellulose: Kinetics, isotherms, thermodynamics, mechanisms and application for electroplating wastewater purification. J Hazard Mater 387. https://doi.org/10.1016/j.jhazmat.2019.121718
Rajivgandhi G, RTV V, Raju N, et al (2021) Adsorption of nickel ions from electroplating effluent by graphene oxide and reduced graphene oxide. Environ Res 199:111322. https://doi.org/10.1016/j.envres.2021.111322
Rajoria S, Vashishtha M, Sangal VK (2021) Review on the treatment of electroplating industry wastewater by electrochemical methods. Mater Today Proc. https://doi.org/10.1016/j.matpr.2021.04.165
Rengaraj S, Venkataraj S, Yeon JW et al (2007) Preparation, characterization and application of Nd-TiO2 photocatalyst for the reduction of Cr(VI) under UV light illumination. Appl Catal B Environ 77:157–165. https://doi.org/10.1016/j.apcatb.2007.07.016
Revathi M, Ahmed Basha C, Velan M (2016) Removal of copper(II) ions from synthetic electroplating rinse water using polyethyleneimine modified ion-exchange resin. Desalin Water Treat 57:20350–20367. https://doi.org/10.1080/19443994.2015.1108234
Robert D, Piscopo A, Weber JV (2004) First approach of the selective treatment of water by heterogeneous photocatalysis. Environ Chem Lett 2:5–8. https://doi.org/10.1007/s10311-004-0063-x
Rodrigo MA, Cañizares P, Buitrón C, Sáez C (2010) Electrochemical technologies for the regeneration of urban wastewaters. Electrochim Acta 55:8160–8164. https://doi.org/10.1016/j.electacta.2010.01.053
Rodríguez R MG, Mendoza V, Puebla H, D SAM, (2009) Removal of Cr(VI) from wastewaters at semi-industrial electrochemical reactors with rotating ring electrodes. J Hazard Mater 163:1221–1229. https://doi.org/10.1016/j.jhazmat.2008.07.114
Roy S, Majumdar S, Sahoo GC et al (2020) Removal of As(V), Cr(VI) and Cu(II) using novel amine functionalized composite nanofiltration membranes fabricated on ceramic tubular substrate. J Hazard Mater 399:122841. https://doi.org/10.1016/j.jhazmat.2020.122841
Babel TAKS (2015) A Research Study on Cr ( VI ) Removal from Contaminated Wastewater Using Low- Cost Adsorbents and Commercial Activated Carbon. 2nd Reg Conf Energy Technol Towar a Clean Environ 018
Saeid S, Bringas E, Tan NR, Ortiz I (2016) Recent progress in development of high performance polymeric membranes and materials for metal plating wastewater treatment : a review. J Water Process Eng 9:78–110. https://doi.org/10.1016/j.jwpe.2015.11.005
Sahu O, Mazumdar B, Chaudhari PK (2014) Treatment of wastewater by electrocoagulation: a review. Environ Sci Pollut Res 21:2397–2413. https://doi.org/10.1007/s11356-013-2208-6
Salman RH (2019) Removal of manganese ions (Mn2+) from a simulated wastewater by electrocoagulation/ electroflotation technologies with stainless steel mesh electrodes: process optimization based on Taguchi approach. Iraqi J Chem Pet Eng 20:39–48
Sardella F, Gimenez M, Navas C et al (2015) Conversion of viticultural industry wastes into activated carbons for removal of lead and cadmium. J Environ Chem Eng 3:253–260. https://doi.org/10.1016/j.jece.2014.06.026
Schrank SG, Joseé HJ, Moreira RFPM (2002) Simultaneous photocatalytic Cr(VI) reduction and dye oxidation in a TiO2 slurry reactor. J Photochem Photobiol A Chem 147:71–76. https://doi.org/10.1016/S1010-6030(01)00626-8
Shahrezaei F, Mansouri Y, Zinatizadeh AAL, Akhbari A (2012) Process modeling and kinetic evaluation of petroleum refinery wastewater treatment in a photocatalytic reactor using TiO 2 nanoparticles. Powder Technol 221:203–212. https://doi.org/10.1016/j.powtec.2012.01.003
Sharma D, Chaudhari PK, Dubey S, Prajapati AK (2020a) Electrocoagulation treatment of electroplating wastewater: a review. J Environ Eng 146. https://doi.org/10.1061/(asce)ee.1943-7870.0001790
Sharma D, Chaudhari PK, Prajapati AK (2020b) Removal of chromium (VI) and lead from electroplating effluent using electrocoagulation. Sep Sci Technol 55:321–331. https://doi.org/10.1080/01496395.2018.1563157
Shen W, Li Z, Wang H et al (2008) Photocatalytic degradation for methylene blue using zinc oxide prepared by codeposition and sol-gel methods. J Hazard Mater 152:172–175. https://doi.org/10.1016/j.jhazmat.2007.06.082
Sun Y, Chen A, Pan SY et al (2019) Novel chitosan-based flocculants for chromium and nickle removal in wastewater via integrated chelation and flocculation. J Environ Manage 248:109241. https://doi.org/10.1016/j.jenvman.2019.07.012
Sun Y, Zhou S, Sun W et al (2020) Flocculation activity and evaluation of chitosan-based flocculant CMCTS-g-P(AM-CA) for heavy metal removal. Sep Purif Technol 241:116737. https://doi.org/10.1016/j.seppur.2020.116737
Tang SY, Qiu YR (2019) Selective separation of copper and zinc and regeneration of polymer from electroplating effluent using shear induced dissociation coupling with ultrafiltration. Korean J Chem Eng 36:1321–1327. https://doi.org/10.1007/s11814-019-0310-2
Tanzifi M, Tavakkoli Yaraki M, Karami M et al (2018) Modelling of dye adsorption from aqueous solution on polyaniline/carboxymethyl cellulose/TiO2 nanocomposites. J Colloid Interface Sci 519:154–173. https://doi.org/10.1016/j.jcis.2018.02.059
Tao W, Chen G, Zeng G et al (2016) Chemosphere In fl uence of silver nanoparticles on heavy metals of pore water in contaminated river sediments. Chemosphere 162:117–124. https://doi.org/10.1016/j.chemosphere.2016.07.043
Thakare YN, Jana AK (2015) Performance of high density ion exchange resin (INDION225H) for removal of Cu(II) from waste water. J Environ Chem Eng 3:1393–1398. https://doi.org/10.1016/j.jece.2015.01.002
Tryba B, Jafari S, Sillanpaa M et al (2019) Influence of TiO2 structure on its photocatalytic activity towards acetaldehyde decomposition. Appl Surf Sci 470:376–385. https://doi.org/10.1016/j.apsusc.2018.11.137
Tzanetakis N, Taama WM, Scott K et al (2003) Comparative performance of ion exchange membranes for electrodialysis of nickel and cobalt. Sep Purif Technol 30:113–127
Umar M, Aziz HA, Yusoff MS (2010) Trends in the use of Fenton, electro-Fenton and photo-Fenton for the treatment of landfill leachate. Waste Manag 30:2113–2121. https://doi.org/10.1016/j.wasman.2010.07.003
Valiūnienė A, Margarian VR (2015) Electrooxidation of cyanide ion on a platinized Ti electrode. React Kinet Mech Catal 115. https://doi.org/10.1007/s11144-015-0860-1
Wang C, Li T, Yu G, Deng S (2021) Removal of low concentrations of nickel ions in electroplating wastewater by combination of electrodialysis and electrodeposition. Chemosphere 263:128208. https://doi.org/10.1016/j.chemosphere.2020.128208
Wang H, Zhou A, Peng F et al (2007a) Mechanism study on adsorption of acidified multiwalled carbon nanotubes to Pb ( II ). J Colloid Interface Sci 316:277–283. https://doi.org/10.1016/j.jcis.2007.07.075
Wang J, Huang X, Kao JCM, Stabnikova O (2007b) Simultaneous removal of organic contaminants and heavy metals from kaolin using an upward electrokinetic soil remediation process. J Hazard Mater 144:292–299. https://doi.org/10.1016/j.jhazmat.2006.10.026
Wei X, Kong X, Wang S et al (2013) Removal of heavy metals from electroplating wastewater by thin-film composite nanofiltration hollow-fiber membranes. Ind Eng Chem Res 52:17583–17590. https://doi.org/10.1021/ie402387u
Xiao X, Sun Y, Liu J, Zheng H (2021) Flocculation of heavy metal by functionalized starch-based bioflocculants: Characterization and process evaluation. Sep Purif Technol 267:118628. https://doi.org/10.1016/j.seppur.2021.118628
Xu T, Zheng X, Zhou Y et al (2021) Study on the treatment of Cu2+-organic compound wastewater by electro-Fenton coupled pulsed AC coagulation. Chemosphere 280:130679. https://doi.org/10.1016/j.chemosphere.2021.130679
Xu XR, Bin LH, Gu JD (2006) Simultaneous decontamination of hexavalent chromium and methyl tert-butyl ether by UV/TiO2 process. Chemosphere 63:254–260. https://doi.org/10.1016/j.chemosphere.2005.07.062
Yadav M, Singh G, Jadeja RN (2021) Physical and chemical methods for heavy metal removal. Pollut Water Manag 377–397. https://doi.org/10.1002/9781119693635.ch15
Yan FL, Wang Y, Wang WH et al (2020) Application of biochars obtained through the pyrolysis of Lemna minor in the treatment of Ni-electroplating wastewater. J Water Process Eng 37:101464. https://doi.org/10.1016/j.jwpe.2020.101464
Ye Z, Yin X, Chen L et al (2019) An integrated process for removal and recovery of Cr(VI) from electroplating wastewater by ion exchange and reduction–precipitation based on a silica-supported pyridine resin. J Clean Prod 236:117631. https://doi.org/10.1016/j.jclepro.2019.117631
Yoon J, Shim E, Bae S, Joo H (2009) Application of immobilized nanotubular TiO2 electrode for photocatalytic hydrogen evolution: Reduction of hexavalent chromium (Cr(VI)) in water. J Hazard Mater 161:1069–1074. https://doi.org/10.1016/j.jhazmat.2008.04.057
Yu B, Zhang Y, Shukla A et al (2000) The removal of heavy metal from aqueous solutions by sawdust adsorption — removal of copper. J Hazard Mater 80:33–42
Yu X, Guo Y, Xu L et al (2008) A novel preparation of mesoporous CsxH3-xPW12O40/TiO2 nanocomposites with enhanced photocatalytic activity. Colloids Surfaces A Physicochem Eng Asp 316:110–118. https://doi.org/10.1016/j.colsurfa.2007.08.046
Zailani LWM, Zin NSM (2018) Application of electrocoagulation in various wastewater and leachate treatment-a review. IOP Conf Ser Earth Environ Sci 140. https://doi.org/10.1088/1755-1315/140/1/012052
Zeliha B, Recep C, Yılmaz E (2015) Arsenic and Boron Removal by Electrocoagulation with Aluminum Electrodes. Arab J Sci Eng. https://doi.org/10.1007/s13369-015-1922-4
Zewail TM, Yousef NS (2015) Kinetic study of heavy metal ions removal by ion exchange in batch conical air spouted bed. Alexandria Eng J 54:83–90. https://doi.org/10.1016/j.aej.2014.11.008
Zhang S, Ning S, Liu H et al (2021) Preparation of ion-exchange resin via in-situ polymerization for highly selective separation and continuous removal of palladium from electroplating wastewater. Sep Purif Technol 258:117670. https://doi.org/10.1016/j.seppur.2020.117670
Zhou B (2013) Method for Treating Wastewater Containing Copper Complex. US Pat 20130168314A1 1:2–6
Zhou H, Luo J, Chen Y (2020) Nitrogen moieties -dominated Co–N-doped nanoparticle-modified cathodes in heterogeneous-electro-Fenton-like system for catalytic decontamination of EDTA-Ni(II). Chemosphere 239:124743. https://doi.org/10.1016/j.chemosphere.2019.124743
Zhu W, Sun S, Gao J et al (2014) Dual-layer polybenzimidazole/polyethersulfone (PBI/PES) nano filtration (NF) hollow fi ber membranes for heavy metals removal from wastewater. J Memb Sci 456:117–127. https://doi.org/10.1016/j.memsci.2014.01.001
Author information
Authors and Affiliations
Contributions
Sonal Rajoria: writing—original draft, data curation, conceptualization, investigation, validation. Manish Vashishtha: supervision, visualization, investigation. Vikas K. Sangal: supervision, visualization, investigation, writing—review and editing.
Ethical approval.
Not applicable.
Corresponding authors
Ethics declarations
Consent to participate
All participants gave informed consent to participate.
Consent to publish
Not applicable.
Conflict of interest
The authors declare no competing interests.
Availability of data and materials
The datasets analyzed during the current study are available in the [Physical and Chemical Methods for Heavy Metal Removal]; Yadav M, Singh G, Jadeja RN (2021) Physical and Chemical Methods for Heavy Metal Removal. Pollut Water Manag 377–397. https://doi.org/10.1002/9781119693635.ch15; and [Electroplating Wastewater Treatment Method and Development Trend Analysis]; Lu BJ, Wu K (2020) Electroplating wastewater treatment method and development trend analysis. Mater Sci Eng 774:. https://doi.org/10.1088/1757-899X/774/1/012092].
Additional information
Responsible Editor: Weiming Zhang
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajoria, S., Vashishtha, M. & Sangal, V.K. Treatment of electroplating industry wastewater: a review on the various techniques. Environ Sci Pollut Res 29, 72196–72246 (2022). https://doi.org/10.1007/s11356-022-18643-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18643-y