Abstract
This study investigated the human biomonitoring of heavy metals in the water, sediments, and tissues of mostly consumed fish species using Turkmen pregnant women’s biomarkers in winter 2019, at the Miankaleh Peninsula, north of Iran. Metal concentrations were measured in various fish organs as well as pregnant women’s blood, hair, and nail as biological indicators. For this purpose, a total of 20 water and sediment, 14 fish, and 16 human samples were collected. Inductively coupled plasma mass spectrometry (ICP-MS) was used to evaluate the concentration of Cr, Co, Cu, As, Hg, and Pb. Results showed metals with the highest concentrations as Cu and Cr in water (93.35 and 80.91 µg/l, respectively), Hg and Pb in sediment (7.40 µg/g for both), Cu and Pb in the liver (27.00 and 18.9 µg/g for C. carpio; 1414 and 31.7 µg/g for L. auratus), muscle (10.00 and 18.80 for C. carpio; 37.20 and 8.27 µg/g for L. auratus), and skin (26.40 and 9.90 for C. carpio; 10.80 and 11.74 µg/g for L. auratus). In addition, Cu, in pregnant women samples, had the highest values at 2.53 mg/l, 8.87, 36.46, and 29.04 µg/g for blood, hair, fingernail, and toenail, respectively. However, Co showed the lowest concentration in all studied samples. Fish liver and fingernail of pregnant women did reveal the highest heavy metal accumulation, whereas fish muscle and blood of pregnant women had the lowest accumulated heavy metals. The concentration of Hg in water, sediment, fish muscle, and women’s blood and hair exceeded the limits suggested by various organizations. Therefore, this study highlighted that heavy metal concentration, in particular Hg, in water, sediments, and fish is a serious risk to the health of local inhabitants who rely on fisheries products and recommended that necessary information should be provided to warn Turkmen pregnant women in consumption of Hg-contaminated fish in this area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, industrial and urban developments to improve living conditions and access to energy have led to the release of chemical pollutants, resulting in major environmental concerns (Ezemonye et al. 2019). Water is one of the most essential parts of the environment where the quality of life has been affected by various human activities (Coffie 2015). Heavy metals, in particular, pose a serious threat to the health of the ecosystem due to their high toxicity and stability (Vrhovnik et al. 2013; Ogbomida et al. 2018). Although these metals can be naturally released into aquatic environments through rocks weathering and natural processes, the main source of such metals in aquatic ecosystems are industrial activities, mining developments, and agricultural drainage as well as municipal wastewater (Walker et al. 2001; Asante 2015). Therefore, water reservoirs such as lakes, wetlands, and rivers are the main place for the restoration of heavy metals directly and indirectly (Elkady et al. 2015). Heavy metals can be accumulated through the food chain in marine organisms due to their nondegradability and stability in the water and sediment (Javed and Usmani 2016; Ullah et al. 2017; Fuentes-Gandara et al., 2018).
Metal concentration in water and sediment samples plays a crucial role in investigating the bioavailability of heavy metals, but does not provide enough information about the health status of the ecosystem. In this regard, the use of biomarkers as highly sensitive biological tools is essential in biomonitoring studies (Hadei et al. 2021). Fish are considered as one of the aquatic organisms at the highest level of the marine food chain, and they are reliable bioindicators for the heavy metal pollution monitoring in aquatic ecosystems (Varol and Sünbül 2019). Due to that, the concentration of metals in fish tissues can be much higher than the respective concentrations in the water environment (Usero et al. 2004). On the one hand, a large amount of heavy metals accumulate in various organs of fish through feeding, ion, and gas exchange on the surface of lipophilic membranes (e.g., gills), which can not only be harmful to the fish well-being but human health as a final consumer (Dadar et al. 2016; Ezemonye et al. 2019; Varol and Sünbül 2019). On the other hand, fish is considered as the main source of healthy protein for humans (37% of total animal protein) (Food 2018) and is highly recommended due to its nutritional values and high levels of essential fatty acids (e.g., omega-3), amino acids, antioxidants, vitamins, and minerals (Dadar et al. 2016; Basim and Khoshnood 2016; Fuentes-Gandara et al. 2018). Fish consumption, at least twice a week, can reduce the risk of heart attack (Kris-Etherton et al. 2002; Fallah et al. 2011). In addition, recent evidences show that fish consumption has been increased, with global per capita from 9.9 kg in 1960 to 14.4 kg in 1990 and 19.7 kg in 2013 (Bianchi et al. 2014). Due to the consumption of heavy metals contaminated fish, the amount of metals in the edible tissues is important for human health (Varol and Sünbül 2020). Besides, the overuse of this beneficial nutrient is a major path of heavy metals transmission to the human body (Ahmed et al. 2016; Basim and Khoshnood 2016).
One of the most important routes for heavy metal exposure to the human body is daily diet, and fish, despite its essential nutrients and benefits, is considered as the main source of mercury for human health (Zhuang et al. 2009). Owing to the possibility of high heavy metal concentration in contaminated fish, a serious threat may have occurred to fish consumers (Varol et al. 2017). For this, assessing the potential risk of metals to fish consumers is necessary (Garnero et al. 2020). Since heavy metals can have carcinogenic and noncarcinogenic effects on humans (Varol et al. 2017), there are several ways to assess the health risk of toxic metals received by consuming contaminated fish (Saha et al. 2016; Griboff et al. 2017). Target hazard quotient (THQ) and carcinogenic risk (CR) are suggested by the US Environmental Protection Agency to assess the potential noncarcinogenic and carcinogenic risks associated with the use of heavy metals contaminated fish (Basim and Khoshnood 2016). Many researchers have used these methods to investigate the carcinogenic and noncarcinogenic effects of heavy metals (Atique Ullah et al. 2019; Esilaba et al. 2020; Garnero et al. 2020; Kortei et al. 2020; Sadeghi et al. 2020).
In biomonitoring studies, human health risk, in terms of chemicals, is assessed using default methods of exposure and measuring pollutants in various sources. However, such methods may not be accurate enough and estimate the values less or more than the real exposure results. For example, it may examine only one of the sources or pathways of the contaminants entering the body. Nevertheless, human biological monitoring (HBM) includes all pollutants from different sources and different exposure pathways and provides a sensitive risk assessment of potential pollutants in the human body (Cherrie et al. 2006). Therefore, human biomonitoring assessment contributes to the calculation of the total amount of heavy metals entering from different sources and routes, the environmental conditions of the region, distribution of exposure to different population groups, identification of vulnerable groups, identification of highly infected areas, as well as a better estimation of health assessment (Cherrie et al. 2006; Angerer et al. 2007, 2011; Clewell et al. 2008; Louro et al. 2019; Faure et al. 2020). Biomonitoring is a direct measurement of chemicals in biological samples, such as blood, serum, urine, hair, nails, and saliva of an organism exposed to contaminants (LaKind et al. 2008; Hays and Aylward 2009; Lemos et al. 2009; Angerer et al. 2011). Studies have shown that various factors, such as age, sex, body weight, geographical factors, time of exposure, workplace, diet, smoking, sanitation, health status, or the consumption of vitamin and mineral supplements affect the bioaccumulation of heavy metals in the human body (Christensen 1995; Marrugo-Negrete et al. 2013; Alrobaian and Arida 2019; Al-Saleh 2020). However, the main focus is on Turkmen pregnant women because women are highly vulnerable during pregnancy period; in addition, development of unborn babies and fetuses are extremely sensitive to pollutants in the embryonic stage (Vahter et al. 2002).
The US National Academies of Sciences, Engineering, and Medicine in its recent report, entitled “Using the 21st Century Science to Improve Risk-Related Evaluations” (USNAS 2017), has considered human biological monitoring as an essential tool for human exposure to chemical substances (http://foodmetabolome.org). In addition, under the authority of the European Union (EU), the Horizon 2020 European Joint Program HBM4EU (2017–2021) has established more accurate evidences of human exposure to chemicals with the aim of minimizing human health risks through human biological monitoring process (Ganzleben et al. 2017). In Canada, a human biomonitoring program has been established in collaboration with the Canadian Health Measures Survey (CHMS) and the Public Health Agency of Canada and investigated 250 chemical contaminants in biological monitoring components such as blood, urine, and hair of humans aged 3 to 79 (Canada 2010, 2013, 2017; Haines et al. 2017; Eykelbosh et al. 2018).
Miankaleh International Wetland is a unique habitat for wintering migratory birds and aquatic reproduction. In addition, it plays a leading role in supplying food for local people. Despite the high rate of seafood consumption by local residents in this area, there is no comprehensive study concerning the levels of heavy metals in various human tissues. Therefore, the aim of this study was the monitoring of Cr, Co, Cu, As, Hg, and Pb metals in water and sediment samples as well as measuring heavy metals concentrations in C. carpio and L. auratus fish species to assess human health risks of heavy metals in relation to fish consumption. Moreover, for the first time, biological monitoring of heavy metals in Turkmen pregnant women’s hair, blood, fingernail, and toenail is performed in two areas named Chapaqli and Qaraesou around the Miankaleh wetland. We also attempted to investigate the sociodemographic and lifestyle factors which may change the levels of these heavy metals in pregnant women’s organs.
Material and methods
Study area
Miankaleh Peninsula, which is known as a Wildlife Refuge and Biosphere Reserve, is situated in the southeastern region of the Caspian Sea (36°50'N 053°17'E) and has a surface area of 96,678.5 hectares. Miankaleh was considered as an international wetland in the Ramsar commission, in 1975. It includes two parts, water and land environment, and as a result of the Caspian Sea water retreat, it has been separated from the bay and the sea in the form of a sandy and saline land. The average length of the wetland is about 60 km with an average width of 2.5 and 6.5 km in its downstream and upstream areas, respectively. Miankaleh Peninsula has a mean height of approximately 28–10 m below sea level, the annual rainfall is approximately 700 mm, and the average temperature is about 17 °C. Due to its unique environment, the wetland is a worthy place for migratory birds and aquatic animals to spend the winter for breeding; consequently, about half a million migratory birds migrate from Siberia to this region every year. A total of 47 species of birds, 24 species of fish, 27 genera of phytoplankton, and 5 genera of benthic invertebrates have been identified in this unique ecosystem. The presence of food, chemical, metal, and nonmetallic industrial units around the wetland as well as the location of oil reservoirs and hydrocarbon activities have affected the wetland and caused physical, chemical, and biological pollution of the wetlands and surrounding rivers (Ramsar 1975; UNESCO 1976).
Field sampling
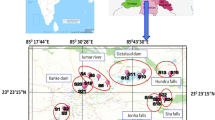
A total of 10 water and sediment sampling sites in the study area were chosen, including Qhalepayan (S1), Miankaleh center (S2), Galougah (S3), Bandar Gaz (S4), Esmailsay (S5), Qaraesou (S6), Bandar Torkman (S7), Chapaqli (S8), Khozeini channel (S9), and Ashourade (S10) (Fig. 1). Water and sediment samples were collected triplicate at each station in winter 2019. To collect the surface water and sediment samples, Ruttner Water and Ekman-Grab samplers were applied, respectively. Then, water samples were fixed with 5-ml 70% nitric acid and stored in an ice cool box so as to analyze metals, and sediment samples were preserved in plastic bags for drying and analysis. A total of 14 fish were bought from 9 fish markets of Ashourade, Bandar Torkman, Chapaqli, Qaraesou, Qhalepayan, Galougah, Esmailsay, Khozeini channel, and Miankaleh center. Furthermore, human biomonitoring was performed based on the protocol specified by the Medical Ethical Committee of Iran in collaboration with the staff of the health center (Ethical Committee of Golestan University of Medical Sciences (IR.GOUMS.REC.2019.126). Ethical principles and privacy were fully observed, and a written consent was received from the participants during the completion of questionnaires. Blood, hair, fingernail, and toenail samples were taken from 16 Turkmen pregnant women who lived in two sites including Chapaqli and Qaraesou.
Sample preparation for heavy metal analysis
Water and sediment sample preparations were performed according to the modified EPA digestion method (EPA 3005a and EPA 3050B, respectively). First, suspended particulate matters were removed from water samples using a Whatman filter paper no.1 (0.45 mm). A 10-mL HNO3 (65.0%) was added to 100 ml of the water sample and then heated on a hot plate at 90 to 95 °C until the volume reached 15–20 ml. Then, samples were moved into a 100-ml volumetric flask and sieved through Whatman glass filter paper (pore size 110 mm) prior to the analysis. Sediment samples were dried in an oven at 105 °C for 24 h. Afterwards, 0.5 g of each sample was digested by the combination of nitric acid (69%), hydrochloric acid (37%), and hydrogen peroxide (30%) in a ratio of 1:1:2 with continuous heating. After dilution, samples were passed through Whatman No. 41 filter paper.
Purchased fish were transferred to the laboratory using a cold flask. In the lab, the type of species was identified, and then fish were dissected to collect liver, muscle, and skin tissues for heavy metal analysis. Following the methodology, 1 g of samples was put into microwave digestion tubes, and 5-ml HNO3 (65%) and 1-ml H2O2 were added to each tube (US EPA-3052, 1996). Thermal decomposition was used to determine the concentration of total mercury (T-Hg) in fish tissues using the US Environmental Protection Agency recommendation (EPA 7473, 1998).
For human biomonitoring, Turkmen pregnant women’s sampling was performed in two villages, Chapaqli and Qaraesou (Turkaman harbor, Golestan Province, Iran). Hair strands from the occipital area of the scalp and fingernail and toenail samples were collected with a pair of sterile stainless-steel scissors. All collected hair samples were placed in plastic bags before starting the main analysis. In terms of blood samples, a volume of 5 mL of peripheral blood was captured using nonmetal needles and test tubes based on the specific protocols. For analysis, all specimens were transferred to the laboratory in containers labeled with the subject, identification number, and date. Human hair (3 g) and nail (1 g) were kept in the oven at 110 ℃ for 1 h and 550 ℃ for 4 h, respectively, and finally maintained in a desiccator pending system. The produced ash was digested with 10 ml of 6:1 mixture of concentrated nitric acid and perchloric acid, and the mixture was heated to be perfectly vaporized for obtaining a water transparent solution. Each digested sample was moved into a 100-mL volumetric flask and reached the specified volume with distilled water (Abdulrahman et al. 2012). 4 mL of HNO3 (69%) and 2 mL of H2O2 (35% w/v) were then used to digest blood samples in the microwave digestion system in 200 °C for 10 min (Alrobaian and Arida 2019). Finally, the analysis of metals (Cr, Co, Cu, As, Hg, and Pb) in surface water, sediments, fish, and human tissues was performed using ICP-MS (HP-4500) equipped with an Asx-520 autosampler.
Quality assurance and quality control
For the analytical process, quality control and assurance protocols related to tissue-based (muscle) standard reference material (SRM 2976) released by the national institute of standards and technology (NIST, Luxembourg, Belgium) were applied triplicate in analyzing all samples, standards, and blanks. The recovery rate was recorded at 93.8 to 102.8% that ensured the reliability and consistency of the measurement. To examine the replicability of detected concentrations, 10% of the samples were analyzed thrice, and relative standard deviation (RSD) was between 0.05 and 2.5% (Sahebi et al. 2018).
Human participation and questionnaire
The participants were selected from the Turkmen pregnant women who lived in two villages (Chapaqli and Qaraesou) near the wetland. Pregnant women that were householders in the age of pregnancy from 16 to 45 years old and resided more than 2 years in the studied villages were included in the study. In addition, those women whose husbands worked as a fisherman in the wetland were included. For the exclusion criteria, participants who did not live as a permanent resident in the study area and individuals with colored hairs and nails were not considered in this study. For the questionnaire, a range of inquiries considering the age, height, weight, name of consumed fish, type of fish cooking, number of fish meals per month and week, time of pregnancy, number of children, and pregnancy were designed. In addition, questionnaires were filled in person, followed by asking the questions and sampling (SI-Fig. 1).
Human health risk assessment
Target hazard quotients (THQ)
Target hazard quotient recommended by the US Environmental Protection Agency was used to calculate the noncarcinogenic risk of studied heavy metals according to Eq. 1 (EPA 2004; IRIS 2010). THQ values more and lower than 1 indicate the potential hazards and no risk of heavy metals to fish consumers, respectively. The Total target hazard quotient (TTHQ) is obtained from the cumulative risk of all metals based on Eq. 2 (Chien et al. 2002).
where THQ is the target hazard quotient, EF is the frequency of exposure (365 days/year), ED is the total exposure time (70 years), IR is the daily consumption rate of fish (33.15 g/day based on Iran Fisheries Organization 2018), C is the concentration of metal in the muscle tissue of consumed fish (mg/kg wet weight), BW is the average of adults body weight (70 kg), AT is the average exposure days (365 days/year × 70 years assumed 2550 days), and RfD is the oral reference dose (mg/kg day−1) for Cr, Co, Cu, As, Hg, and Pb as 0.003, 0.03, 0.04, 0.0003, 0.0001, and 0.0035 mg/kg BW day−1, respectively (USEPA 2011, 2012, 2019; Finley et al. 2012).
Determination of estimated daily intake
The estimated daily intake (EDI) was calculated based on the metal and food concentration and the body weight. Two hypotheses were considered to estimate heavy metals risks caused by fish consumption. First, cooking has no effect on metal concentration (Tengku et al. 2020), and the oral dose is equal to the absorbed dose of the toxic metal (USEPA 1989). EDI was calculated based on the model proposed by USEPA using Eq. 3 (Ikem and Egiebor 2005; Christophoridis et al. 2019).
where EDI is the estimated daily intake of metals through fish consumption (mg/kg BW day−1), C is the concentration of metal in the muscle tissue of the consumed fish (mg/kg ww), IRD is the (intake rate) daily consumption rate of fish, 33.15 g/day (as per Iran Fisheries Organization, 1398), and Bw is the average of adults’ body weight (70 kg).
Carcinogenic risk
According to the EPA standards, the life expectancy or number of years that a person is exposed to dangerous pollutants is equal to 70, which is used in the calculations of carcinogenic and noncarcinogenic risks (Jeszka-Skowron et al. 2017). To evaluate the carcinogenic effects of heavy metals, Eq. 4 was used based on the EPA recommended model. In this equation, CR is carcinogenic risk, and CSF shows carcinogenic slope factor for the toxic substance (ATSDR 2010; Feed 2013; Wei et al. 2020). Carcinogenic risk index between 10–6 (risk of carcinogenic during human life 1 in 1,000.000) and 10–4 (risk of carcinogenic during human life 1 in 10,000) indicated a range of potential risks predicting the probability of cancer. Therefore, CR values less than 10–6 did not show the carcinogenicity of heavy metals (Demirezen and Aksoy 2006; Liu et al. 2006; USEPA 2015). Relevant calculations were performed based on the standard assumptions proposed by USEPA, and CSF values for Cr, As, and Pb were equal to 0.5, 1.5, and 0.0085 mg/kg/day, respectively (Demirezen and Aksoy 2006).
Statistical analysis
Statistical test analysis was carried out using R software. In addition, descriptive statistics of heavy metal concentrations in water, sediment, fish, and human samples were determined. Independent sample T test, one-way analysis of variance (ANOVA), and Pearson test were used to evaluate the significant differences in metal concentrations and potential correlations in the studied factors. Principal component analysis (PCA) was performed, and eigenvalues more than 1 were considered as major contributing factors in the study. Moreover, components with the highest variance were selected to compare the possible correlations with the respective factors. Major components, including PC1, PC2, and PC3, were considered to compare the contributing factors and find the origin of the studied heavy metals in terms of anthropogenic and natural activities.
Results and discussion
Heavy metal concentration in water and sediment
Results showed that the mean concentrations of metals in the surface water were 44.56 ± 5.90, 10.00 ± 0.17, 69.03 ± 13.37, 8.79 ± 1.16, 6.52 ± 0.91, and 1.71 ± 0.15 µg /L for Cr, Co, Cu, As, Hg, and Pb, respectively (Table 1). In addition, findings revealed that Cu and Cr did show the highest mean concentrations, whereas Pb had the lowest mean concentration among the studied heavy metals. The mean concentrations of metals in the study area were ordered as Cu > Cr > Co > As > Hg > Pb. In line with the results, a similar study was reported high concentrations of Cu in the water due to anthropogenic activities (Gimeno-García et al. 1996; Akindele et al. 2020). The highest concentration of Cu, Co, and As was observed in Chapaqli, Hg and Pb in Esmailsay, and Cr in Qhalepayan station, and the lowest concentration of metals was observed in Galougah. To support this, Abadi et al. (2018) did a similar research, and they observed higher levels of Hg and Pb and lower levels of Co, Cu, and As in Southern Caspian Sea coasts than those obtained in this study. In our study, concentrations of Co, Cu, Pb, and As in water were higher than that of those obtained by other researches (Humbatov et al. 2015; Fan et al. 2020; Pandiyan et al. 2021). However, only the concentration of Hg was higher than the standard limit legislated by WHO and USEPA, and Cr was in line with the standards of WHO (SI-Table 1).
The mean concentrations of metals in the sediment were 3.2 ± 0.16, 1.2 ± 0.07, 3.6 ± 0.66, 4.3 ± 0.46, 113 ± 70.54, and 8 ± 0.60 µg/g for Cr, Co, Cu, As, Hg, and Pb, respectively (Table 1). Unlike water media, Hg and Pb had the highest concentration in sediment samples, whereas Co revealed the lowest concentration. Heavy metals in sedimentary samples ordered as Hg > Pb > As > Cu > Cr > Co. The highest concentration of Co, Cr, and Pb was observed in the Miankaleh center, whereas the highest concentration of Cu, As, and Hg was perceived in Ashourade station. Lahijani et al. (2018) and Agah et al. (2011) reported lower concentrations of Cr, As, Cu, and Pb in the sediment of the Southern Caspian Sea than we did in this study. Cr, Cu, and Pb concentrations observed in this study were lower than the values obtained in Yangtze River (Fan et al. 2020); however, a higher concentration of these metals was observed in Point Calimere Wildlife Sanctuary (Pandiyan et al. 2021). Only Hg concentration was higher than the permissible standards set by Canadian sediment quality guidelines for the protection of aquatic life and National Oceanic and Atmospheric Administration; therefore, it was approximately 160 times higher than PEL and ERM and 560 times higher than TEL and ERL values (SI-Table 1). Agricultural lands with overuse of fertilizers and pesticides by farmers have led to the accumulation of Pb and Hg in aquatic sediments (Hashmi et al. 2013; Pandiyan et al. 2021). A comparison between heavy metals concentration in this study with other similar studies is presented in SI-Table 1. Similar results were obtained in the study of water and sediment samples of the Point Calimere Wildlife Sanctuary by Pandiyan et al. (2021), and water samples showed the highest concentration of Cr was, and the highest concentration of Hg and Pb was observed in sediment samples.
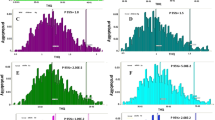
In order to accurately identify and interpret the source of heavy metals, PCA and correlation analysis were used in the present study. A correlation matrix map of six heavy metals in water and sediments is demonstrated in Fig. 2A and B. Relationships among metals in the surface water demonstrated significant positive correlations between Co and Cu (r = 0.9, P < 0.001), Cu and As (r = 0.68, P < 0.001), and Co and Pb (r = 0.47, P < 0.03). In addition, correlations between metals in sediment samples displayed significant positive correlations between Co and Cr (r = 0.85, P < 0.002), Cr and Pb (r = 0.68, P = 0.03), and Cu and As (r = 0.66, P = 0.03). The correlation of heavy metals has been investigated in many studies in water and sediment samples (Islam et al. 2020; Muhammad and Ahmad 2020).
PCA analysis showed that in water samples with eigenvalues > 1, Co, As, and Cu were in the same group and did correlate with station 8. Pb and Hg were also in the same group and were correlated with station 5. Cr did not form any clusters with other metals but was associated with station 1 (Fig. 2C and D). The first (Co, As, and Cu), second (Pb and Hg), and third component constituted 45%, 26%, and 19% of the total variance. PCA for water samples showed that three components built 90% of the total variance. The main source of metals in PC1 could be municipal wastewater which permeated into the wetland without treatment. The source of Pb and Hg in the PC2 may be originated from human activities. Many studies have reported Pb and Hg as nominated elements for an atmospheric deposition because of a strong distribution caused by human-based activities (Chaturvedi et al. 2018; Chen et al. 2019; Lin et al. 2013; Xiao et al. 2017; Yamaguchi et al. 2003). Moreover, agricultural drainage can play a pivotal role in entering such toxic metals into aquatic environments (Xiao et al. 2017; Chaturvedi et al. 2018; Chen et al. 2019). In our study, PC3, which contained only Cr, could be attributed to natural and geogenic sources as the natural origin of Cr has been observed in many environmental biomonitoring studies (Micó et al. 2006; Xu et al. 2014; Deng et al. 2020; Lin et al. 2020; Tian et al. 2020). In sediment, Cr, Co, and Pb were classified in a separate group, and Cu and As were categorized as another respective group (Fig. 2C and D). PC1 (Cr, Co, and Pb) and PC2 (Cu and As) demonstrated 46% and 32% of the total variance, respectively. Heavy metals in the surface water are the main source of toxic metals deposited and accumulated in the sediment, where the presence of such deleterious metals relies upon many biogeochemical processes (Schertzinger et al. 2018). Therefore, determining the source of the metal in the sediment is more complex than in water. Metals in PC2 were probably generated by human activities, especially municipal wastewater, while the origin of metals in PC1 could be due to agricultural and transportation activities. In the present study, Pearson correlation of Cr with Co and Pb as well as As and Cu in sediment and significant correlation of Cu with Co and As in water samples were consistent with PCA results, indicating the common source of each heavy metal group.
Heavy metal concentration in fish species
Concentrations of Cr, Co, Cu, As, Hg, and Pb in the liver, muscle, and skin of C. carpio and L. auratus are presented in Table 2. Heavy metal concentration in the liver, muscle, and skin of C. carpio is ordered as Cu > Pb > Hg > Cr > As > Co, Pb > Cu > Hg > Cr > As > Co and Cu > Pb > Hg > As > Cr > Co, whereas L. auratus showed an order of Cu > Pb > As > Hg > Cr > Co, Cu > Pb > As > Hg > Cr > Co and Pb > Cu > As > Cr > Hg > Co, respectively. In both fish, Cu and Pb had the highest concentrations while Co revealed the lowest concentration in liver, muscle, and skin. High levels of Cu and Pb in different species of fish were observed in the Cauvery delta region, India, by Dhanakumar et al. (2015). Muscle of studied fish showed no significant differences among heavy metals detected in this study. The concentration of Hg and Pb showed no significant differences in the liver and skin of fish species, while Cr, Co, Cu, and As in the liver and Cu in the skin were significantly different (P < 0.05). The liver of C. carpio and L. auratus was the most accumulator tissue to absorb Hg, Pb, Cu, and Co; however, metal concentrations in L. auratus liver were more than C. carpio liver except for Hg. Cr, Hg, and Pb were the highest detected metals in C. carpio muscle while L. auratus did show Co, Cu, and As as the most accumulated metals in the muscle. In addition, the skin of C. carpio demonstrated higher levels of metals than L. auratus skin except for Pb. Compared to other tissues, liver, as a detoxification organ, can largely accumulate heavy metals due to its metallothionein protein content and metabolic activity (Kargın and Çoğun 1999; Çoğun et al. 2005; Nabavi et al. 2012). To support our findings, higher concentrations of heavy metals in the liver have been reported in many studies (Yılmaz et al. 2007; Keshavarzi et al. 2018; Aytekin et al. 2019). Overall, the muscle of studied fish showed a lower concentration of heavy metals than the liver samples. To support this, although muscle is the main edible part of the fish body, it is not an organ contributing to the metabolism and detoxification process (Esmaeilbeigi et al., 2021; Yılmaz et al. 2007; Rajeshkumar and Li 2018; Aytekin et al. 2019). Maurya et al. (2019) claimed that heavy metal concentration in seven fish species inhabited in the River Ganga basin is ordered as liver > gill > muscle. The results of the ANOVA test in the C. carpio showed that there was a significant difference between the concentration of Cr and As in the liver and skin. In addition, a significant difference was observed between liver and muscle in relation to Co and As. L. auratus demonstrated significant difference between Co, Cu, As, and Hg content of the liver and skin (Fig. 2E and F). Rajeshkumar et al. (2018) showed an order of Pb > Cu > Cr > Cd for the concentration of heavy metals in the liver of C. carpio, which was agreed with our findings. As presented in SI-Table 2, the mean values of Pb, Hg, As, and Cu exceeded the recommended limits of FAO 1983, WHO, and EU in the muscle tissue of C. carpio and L. auratus. Although, the mean concentration of Cu in the muscle of C. carpio and L. auratus was higher than the suggested limits by WHO and EU, its value in C. carpio was relatively close to FAO limits. Moreover, while Cr concentration in the muscle of C. carpio and L. auratus exceeded the maximum limit recommended by EU, its value was below the upper limits given by WHO. The heavy metal levels measured in the muscles of two fish species in this study were compared with literature (SI-Table 2). Literature have reported that metal contents of fish muscles vary depending on where and which species were caught and the type of habitat and species play an essential role in the accumulation of heavy metals in fish.
Human health risks posed by heavy metals in fish species
EDI, THQ, and CR indices were used to assess the potential human health risks of accumulated heavy metals in the muscle of studied fish (Sharafi et al. 2019; Abd-Elghany et al. 2020). The results relating to THQ values assessed based on the heavy metals concentration and fish species are portrayed in SI 1. THQ values for C. carpio and L. auratus species orderly showed Hg > As > Pb > Cr > Cu > Co and Hg > As > Cr > Pb > Cu > Co, respectively (THQ < 1 for all metals). This indicated that people did not face significant noncarcinogenic health risks through the consumption of heavy metals contaminated fish. The lowest THQ was calculated for Co at 2.6E-06 and 4.1E-06 for C. carpio and L. auratus, respectively. THQ also showed the highest values for Hg at 4E-02 and 2.1E-02 in consuming C. carpio and L. auratus, respectively, with no significant noncarcinogenic health risks. Although the studied fish in our study were safe for human consumption, exposing to a range of heavy metals can cause joint effects of toxic materials in the body (Loaiza et al., 2018). Therefore, TTHQ was calculated to find out the joint effects of various heavy metals on the human body. Our results demonstrated that the combined impact of all heavy metals was still lower than the acceptable limit of 1 for TTHQ, with the greatest risk for C. carpio compared to L. auratus (SI-Fig. 2).
EDI of Pb, Cd, Cr, As, and Hg through consumption of C. carpio and L. auratus is summarized in Table 3. EDI values were compared with the recommended international standards for heavy metals, including Joint FAO and WHO Expert Committee on Food Additive (JECFA, 1982, 1989, 2000), World Health Organization (WHO 1996, 2006), and National Research Council (NRC 1989). EDI demonstrated values less than the permissible limits, indicating that there was no risk, caused by studied heavy metals, to the human health. Our findings depicted that the lowest value of EDI was evaluated for Co through consumption of C. carpio and L. auratus at 7.71E-05 and 8.29E-05, respectively. In addition, the highest EDI was calculated for Cu in L. auratus at 1.06E-02 and Pb in C. carpio at 5.38E-03. As heavy metal exposure can cause a diverse range of cancers in the body’s organs and disorders in the nervous system, CR index was assessed using CFS values for Cr, As, and Pb as carcinogenic metals in this study (Kazemi et al., 2022). CR showed the highest values for As and Cr in fish species, which exceeded the intolerable range and represented a higher risk of cancer in fish consumers. CR values assessed based on heavy metals in both C. carpio and L. auratus ordered as As > Cr > Pb, respectively.
Biological monitoring of heavy metals in Turkmen pregnant women’s
Heavy metal concentration in Turkmen pregnant women’s blood, fingernail, toenail, and hair, considering their place of residence, fishermen’s families, age, and number of dental amalgam fillings is represented in SI-Table 3. The results showed that Cr, Co, Cu, As, and Hg contents were detected in the fingernail samples and the highest Pb content was observed in the toenail, while blood samples demonstrated the lowest concentration of heavy metals. Cu did reveal the highest concentration among the studied metals in the blood, fingernail, toenail, and hair, whereas Co was detected in the lowest concentration in all organs.
The mean concentration of Cr, Co, Cu, As, Hg, and Pb metals in human blood was 0.11, 0.01, 2.53, 0.25, 1.05, and 1.184 mg/l, respectively. In terms of the sampling locations, in general, the mean concentration of metals in Chapaqli was higher than Qaraesou except for Cr. The mean concentration of heavy metals in blood samples was lower in non-fishermen’s families than fishermen’s families except for Cr, and the mean concentration of Pb in fisherman families was significantly higher than non-fisherman families. Fish is generally consumed in fishermen’s families more than non-fishermen’s families; therefore, it is highly possible that Hg accumulates in the body of these people. Prokopowicz et al. (2014) claimed that regular fish consumption about 2–6 and more than 6 meals a month, increased the mercury content of blood by almost 2 and 3 times, respectively. In another study, researchers outlined that even small amounts of fish consumption can lead to Hg accumulation in the blood (Jedrychowski et al. 2006; Ashrap et al. 2020). Moreover, literature have reported that the increase of fish consumption during pregnancy period could elevate the Hg content of mother’s and cord blood (Davidson et al. 2004; Jeong et al. 2017).
According to the outcomes, Hg concentration in all blood samples demonstrated higher values and exceeded the standard limit recommended by USEPA (5.8 μg/L) (Council 2000) and WHO (10 μg/L) (UNIDO 2003). In our study, the Hg content of the blood of pregnant women with dental amalgam filling showed low concentration, although some studies have reported the effect of dental amalgam on the blood mercury accumulation (Mortada et al. 2002; Prokopowicz et al. 2014). Based on the findings, Hg and Pb concentrations in blood samples were much higher than the Korean female population (Lee et al. (2012), healthy women in their 50 s in an urban area of Poland (Prokopowicz et al. 2014), nonsmokers volunteers in Saudi Arabia (Alrobaian and Arida 2019), and 40–60 years old women in Belgium (Fierens et al. 2016).
The mean concentration of Cr, Co, Cu, As, Hg, and Pb metals in hair samples was 1.89, 0.09, 8.87, 1.11, 2.35, and 2.40 µg/g, respectively. Factors, including place of residence, fishermen’s families, dental amalgam, and age did have no significant effect on the concentration of heavy metals in hair. To support our findings, Okati and Esmaili-sari (2018) outlined that the concentration of As and Hg in fishermen’s families was higher than non-fishermen’s families. In our study, Hg in water and sediment bioaccumulated in studied fish species and consequently the hair of pregnant women through fish consumption. A significant positive correlation between the amount of fish consumption and Hg concentration in hair samples around Ayapel swamp, Colombia, was reported by Gracia et al. (2010). Many studies have indicated that fish is the main source of Hg exposure in communities consuming fish frequently (McDowell et al. 2004; Al-Saleh 2020). As recommended by USEPA reference dose (1 μg/g in hair) (USEPA 2005a, b) and WHO “normal” level (2.0 μg/g in hair), when mercury content of hair samples exceeds standard values, the assessment of potential human health risks is necessary. In our study,75% of the analyzed hair samples showed Hg concentrations higher than the limits suggested by USEPA and WHO. Okati and Esmaili-sari, 2018, outlined that the increase of age had no influence on the metal content of hair which is in line with our outcomes.
The mean content of Cr, Co, Cu, As, Hg, and Pb in fingernail samples were 17.07, 1.29, 36.46, 3.5, 6.05, and 6.63 μg/g, respectively. The mean toenail concentrations of Cr, Co, Cu, As, Hg, and Pb were 9.33, 0.29, 29.04, 2.98, 3.47, and 16.58 μg/g, respectively. The mean concentration of all metals in fingernail samples was higher than toenails except for Pb, and a significant difference was observed between the concentration of Cr, Co, Hg, and Pb metals in toenails and fingernails. In line with our results, Abdulrahman et al. (2012) reported notable differences in metal concentrations in the fingernail and toenail in Maiduguri Metropolis, Borno State, Nigeria. Numerous studies have investigated higher concentration of metals in the toenail than in the fingernail (Abdulrahman et al. 2012; Dessie et al. 2020). In addition, a higher concentration of Cr, Cu, and Pb in the fingernail has been reported in many studies (Przybylowicz et al. 2012). Compared to hair samples, nail samples showed higher heavy metals accumulation, which might be related to the combination of metals with hair keratin through binding to sulfhydryl groups in follicular protein. In this case, detergents, such as soaps, shampoos, hair pomades, lotions, hair bleaches, and dyes can wash away heavy metals from the shaft bulk (Takagi et al. 1986; Abdulrahman et al. 2012; Koseoglu et al. 2017). The mean concentration of Cr, Co, Cu, and Pb in fingernail samples were 9.4, 37.9, 4.8, and 6.6 times and in the toenail were 5.3, 6.2, 5.5, and 14.4 times higher than the healthy women in Poland, respectively (Przybylowicz et al. 2012).
The results of the ANOVA test, concerning the heavy metal contents in human organs, indicated that there was no significant difference between blood and hair, while a statistically significant difference was observed between hair and toenail (for Cr, Co, Cu, As, and Hg) and between hair and fingernail (for Cr, Cu, As, Hg, and Pb). In addition, Cr, Co, Hg, and Pb in the toenail and fingernail were significantly different compared to Cr, Co, As, and Pb in the toenail and hair (Fig. 3A). The correlation of heavy metals in different samples demonstrated that there was a positive correlation between Cr, Co, Cu, and As in blood and Cr, Co, and Cu in hair samples. In addition, positive correlations between Co and As, as well as Hg and Pb, were determined in fingernail and toenail samples, respectively (Fig. 3A, B, C, and D).
Regarding the strengths emerged to this study, it is worthy to say that this study is the first one concerned Turkmen pregnant women as unstudied ethnic minorities in the Miankaleh Peninsula district where a comprehensive biomonitoring study has not been yet conducted to examine the potential health risks of heavy metals to human. In terms of the limitations, the number of participants was relatively low which was due to low frequency of pregnant women in the study area, husband’s restrictions, and unwillingness of the pregnant women to cooperate. Moreover, the bioaccessibility of heavy metals was not considered in this study because of the limitations in human digestion and gastrointestinal studies. Bioaccessibility is defined as the quantity of a compound that is released from its matrix in the gastrointestinal tract, becoming available for absorption (e.g., enters the bloodstream). This term includes digestive transformations of foods into material ready for assimilation, the absorption/assimilation into intestinal epithelium cells, as well as the presystemic, intestinal, and hepatic metabolism.
Conclusion
The present study investigated the concentration of heavy metals (Cr, Co, Cu, As, Hg, and Pb) in the water and sediment of Miankaleh Peninsula, Southeastern Caspian Sea, Iran. In addition, heavy metal concentrations in the liver, muscle, and skin of C. carpio and L. auratus were evaluated to examine the potential carcinogenic and noncarcinogenic effects on human health. Besides, human biomonitoring was conducted using biological biomarkers, including blood, hair, fingernail, and toenail of Turkmen pregnant women who lived in the district of the wetland as fishermen’s families. Findings showed that Cu, Cr, Hg, and Pb had higher concentrations at 93.35, 80.91, 7.40, and 7.40 µg/l, respectively. Hg, in all samples, including water, sediment, fish species, and blood and hair of humans exceeded the permissible limits recommended by international organizations. In addition, mercury concentration in the fish liver and fingernail samples was in the highest levels, whereas fish muscle and human blood samples showed the lowest values. According to PCA, Hg, and Pb originated from anthropogenic activities, while Cr did show geogenic or natural origin in the study area. Turkmen people inhabited in the Northeastern Iran are ethnic minority, and to date, no study has been conducted on the biomonitoring of such residents, especially their pregnant women in fishermen’s families who lived near the Miankaleh Peninsula. Risk assessment analysis demonstrated that Hg, compared to other heavy metals, accumulated in the muscle of C. carpio and L. auratus caused the highest THQ for fish consumers, and edible tissue of C. carpio showed the highest TTHQ than L. auratus. CR index showed an order of As > Cr > Pb as carcinogenic metals; however, only As and Cr values exceeded the permissible limits representing the risk of cancer for consumers. Taken together, from the human biomonitoring perspective, this study highlighted that the Miankaleh Wetland contaminated with heavy metals in water, sediments and organisms, and fish species can act as a vector to bring such toxic metals to Turkmen pregnant women in fishermen’s families. Therefore, efforts should be made to warn such people for the deleterious impact of contaminated fish to mitigate the risk of diseases in both women and their unborn babies.
References
Abadi M, Zamani A, Parizanganeh A, Khosravi Y, Badiee H (2018) Heavy metals and arsenic content in water along the southern Caspian coasts in Iran. Environ Sci Pollut Res 25(24):23725–23735. https://doi.org/10.1007/s11356-018-2455-7
Abd-Elghany SM, Zaher HA, Elgazzar MM, Sallam KI (2020) Effect of boiling and grilling on some heavy metal residues in crabs and shrimps from the Mediterranean Coast at Damietta region with their probabilistic health risk assessment. J Food Compos Anal 93:103606. https://doi.org/10.1016/j.jfca.2020.103606
Abdulrahman F, Akan J, Chellube Z, Waziri M (2012) Levels of heavy metals in human hair and nail samples from Maiduguri Metropolis, Borno State, Nigeria. World Environ 2(4):81–89. https://doi.org/10.5923/j.env.20120204.05
Agah, H., M. Hashtroodi and W. Baeyens (2011). "Trace metals analysis in the sediments of the Southern Caspian Sea." Journal of the Persian Gulf 2(6): 1–12. http://jpg.inio.ac.ir
Ahmed MK, Baki MA, Kundu GK, Islam MS, Islam MM, Hossain MM (2016) Human health risks from heavy metals in fish of Buriganga river, Bangladesh. Springerplus 5(1):1–12. https://doi.org/10.1186/s40064-016-3357-0
Akindele EO, Omisakin OD, Oni OA, Aliu OO, Omoniyi GE, Akinpelu OT (2020) Heavy metal toxicity in the water column and benthic sediments of a degraded tropical stream. Ecotoxicol Environ Saf 190:110153. https://doi.org/10.1016/j.ecoenv.2019.110153
Al-Saleh I (2020) Reference values for heavy metals in the urine and blood of Saudi women derived from two human biomonitoring studies. Int J Hyg Environ Health 225:113473. https://doi.org/10.1016/j.ijheh.2020.113473
Alrobaian M, Arida H (2019) Assessment of heavy and toxic metals in the blood and hair of Saudi Arabia smokers using modern analytical techniques. International Journal of Analytical Chemistry 2019. https://doi.org/10.1155/2019/7125210
Angerer J, Aylward LL, Hays SM, Heinzow B, Wilhelm M (2011) Human biomonitoring assessment values: approaches and data requirements. Int J Hyg Environ Health 214(5):348–360. https://doi.org/10.1016/j.ijheh.2011.06.002
Angerer J, Ewers U, Wilhelm M (2007) Human biomonitoring: state of the art. Int J Hyg Environ Health 210(3–4):201–228. https://doi.org/10.1016/j.ijheh.2007.01.024
Asante, K. (2015). Assessment of heavy metal contamination in water, sediment and fish from the Jimi Reservoir, Obuasi. http://ir.knust.edu.gh/
Ashrap P, Watkins DJ, Mukherjee B, Boss J, Richards MJ, Rosario Z, Vélez-Vega CM, Alshawabkeh A, Cordero JF, Meeker JD (2020) Predictors of urinary and blood metal (loid) concentrations among pregnant women in northern Puerto Rico. Environ Res 183:109178. https://doi.org/10.1016/j.envres.2020.109178
Atique Ullah AKM, Akter M, Musarrat M, Quraishi SB (2019) Evaluation of possible human health risk of heavy metals from the consumption of two marine fish species Tenualosa ilisha and Dorosoma cepedianum. Biol Trace Elem Res 191(2):485–494. https://doi.org/10.1007/s12011-018-1616-3
ATSDR (2010). (Agency for toxic substance and disease registry) Public health assessment and health consultation. Washington:, CENEX supply and marketing, Incorporated, Quicy, Grant County, https://www.atsdr.cdc.gov
Aytekin T, Kargın D, Çoğun HY, Temiz Ö, Varkal HS, Kargın F (2019) Accumulation and health risk assessment of heavy metals in tissues of the shrimp and fish species from the Yumurtalik coast of Iskenderun Gulf, Turkey. Heliyon 5(8):e02131. https://doi.org/10.1016/j.ecoenv.2018.04.035
Basim Y, Khoshnood Z (2016) Target hazard quotient evaluation of cadmium and lead in fish from Caspian Sea. Toxicol Ind Health 32(2):215–220. https://doi.org/10.1177/0748233713498451
Bianchi, M., F. Chopin, T. Farmer, N. Franz, C. Fuentevilla, L. Garibaldi, N. Grainger, F. Jara, I. Karunasagar and A. Laurenti (2014). "FAO: the state of world fisheries and aquaculture." Rome, Italy: Food and Agriculture Organization of the United Nations. https://scholar.google.com/
Canada, H. (2010). "Report on human biomonitoring of environmental chemicals in Canada-results of the Canadian Health Measures Survey Cycle 1 (2007–2009). https://scholar.google.com/
Canada, H. (2013). Second report on human biomonitoring of environmental chemicals in Canada, Health Canada Ottawa, Ontario, Canada. https://scholar.google.com
Canada, H. (2017). "Fourth report on human biomonitoring of environmental chemicals in Canada." https://scholar.google.com
Chaturvedi A, Bhattacharjee S, Singh AK, Kumar V (2018) A new approach for indexing groundwater heavy metal pollution. Ecol Ind 87:323–331. https://doi.org/10.1016/j.ecolind.2017.12.052
Chen L, Liang S, Liu M, Yi Y, Mi Z, Zhang Y, Li Y, Qi J, Meng J, Tang X (2019) Trans-provincial health impacts of atmospheric mercury emissions in China. Nat Commun 10(1):1–12. https://doi.org/10.1038/s41467-019-09080-6
Cherrie JW, Semple S, Christopher Y, Saleem A, Hughson GW, Philips A (2006) How important is inadvertent ingestion of hazardous substances at work? Ann Occup Hyg 50(7):693–704. https://doi.org/10.1093/annhyg/mel035
Chien L-C, Hung T-C, Choang K-Y, Yeh C-Y, Meng P-J, Shieh M-J, Han B-C (2002) Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Sci Total Environ 285(1–3):177–185. https://doi.org/10.1016/S0048-9697(01)00916-0
Christensen JM (1995) Human exposure to toxic metals: factors influencing interpretation of biomonitoring results. Sci Total Environ 166(1–3):89–135. https://doi.org/10.1016/0048-9697(95)04478-J
Christophoridis, C., A. Kosma, E. Evgenakis, A. Bourliva and K. Fytianos (2019). "Determination of heavy metals and health risk assessment of cheese products consumed in Greece." Journal of Food Composition and Analysis 82: 103238. Determination of heavy metals and health risk assessment of cheese products consumed in Greece https://doi.org/10.1016/j.jfca.2019.103238
Clewell HJ, Tan YM, Campbell JL, Andersen ME (2008) Quantitative interpretation of human biomonitoring data. Toxicol Appl Pharmacol 231(1):122–133. https://doi.org/10.1016/j.taap.2008.04.021
Coffie, J. (2015). Assessment of physicochemical properties and heavy metals in water, sediment and fish (oreochromis niloticus) from the Upper Volta basin (stratum vii), Ghana. http:F//hdl.handle.net/123456789/7167
Çoğun, H., T. Yüzereroğlu, F. Kargin and Ö. Firat (2005). "Seasonal variation and tissue distribution of heavy metals in shrimp and fish species from the yumurtalik coast of iskenderun gulf, mediterranean." Bulletin of Environmental Contamination & Toxicology 75(4). https://web.b.ebscohost.com/
Council NR (2000) Toxicological effects of methylmercury. National Academy Press, Washington, DC
Dadar M, Adel M, Ferrante M, Nasrollahzadeh Saravi H, Copat C, Oliveri Conti G (2016) Potential risk assessment of trace metals accumulation in food, water and edible tissue of rainbow trout (Oncorhynchus mykiss) farmed in Haraz River, northern Iran. Toxin Reviews 35(3–4):141–146. https://doi.org/10.1080/15569543.2016.1217023
Davidson PW, Myers GJ, Weiss B (2004) Mercury exposure and child development outcomes. Pediatrics 113(Supplement 3):1023–1029. https://doi.org/10.1542/peds.113.4.S1.1023
Demirezen, D. and A. Aksoy (2006). "Heavy metal levels in vegetables in Turkey are within safe limits for Cu, Zn, Ni and exceeded for Cd and Pb." Journal of food quality 29(3): 252–265.doi/epdf/https://doi.org/10.1111/j.1745-4557.2006.00072
Deng M, Yang X, Dai X, Zhang Q, Malik A, Sadeghpour A (2020) Heavy metal pollution risk assessments and their transportation in sediment and overlay water for the typical Chinese reservoirs. Ecol Ind 112:106166. https://doi.org/10.1016/j.ecolind.2020.106166
Dessie BK, Melaku S, Robele Gari S, Ayele BT, Desta AF, Mihret A (2020) Evaluation of toxic elements in nails of tannery workers in Addis Ababa, Ethiopia. Microchem J 159:105589. https://doi.org/10.1016/j.microc.2020.105589
Dhanakumar S, Solaraj G, Mohanraj R (2015) Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region, India. Ecotoxicol Environ Saf 113:145–151. https://doi.org/10.1016/j.ecoenv.2014.11.032
Esmaeilbeigi M, Kalbassi MR, Seyedi J et al (2021) Intra and extracellular effects of benzo [α] pyrene on liver, gill and blood of Caspian White fish (Rutilus frissi kutum): cyto-genotoxicity and histopathology approach. Mar Pollut Bull 163:111942. https://doi.org/10.1016/j.marpolbul.2020.111942
Sahebi Z, Emtyazjoo M, Mohammad Shafiee MR et al (2018) Examination of relationship between mercury rate with zinc and copper changes in muscle tissue of Otolithes rubber in Mahshahr Port—the Persian Gulf. J Water Chem Technol 40:177–183. https://doi.org/10.3103/s1063455x18030104
Elkady AA, Sweet ST, Wade TL, Klein AG (2015) Distribution and assessment of heavy metals in the aquatic environment of Lake Manzala, Egypt. Ecol Ind 58:445–457. https://doi.org/10.1016/j.ecolind.2015.05.029
EPA (2004). Risk assessment guidance for superfund, Human Health Evaluation Manual (Part a). vol. I. https://www.epa.gov/risk/risk-assessment-guidance
Esilaba F, Moturi WN, Mokua M, Mwanyika T (2020) Human health risk assessment of trace metals in the commonly consumed fish species in Nakuru Town, Kenya. Environmental Health Insights 14:1178630220917128. https://doi.org/10.1177/1178630220917128
Eykelbosh A, Werry K, Kosatsky T (2018) Leveraging the Canadian health measures survey for environmental health research. Environ Int 119:536–543. https://doi.org/10.1016/j.envint.2018.07.011
Ezemonye LI, Adebayo PO, Enuneku AA, Tongo I, Ogbomida E (2019) Potential health risk consequences of heavy metal concentrations in surface water, shrimp (Macrobrachium macrobrachion) and fish (Brycinus longipinnis) from Benin River, Nigeria. Toxicol Rep 6:1–9. https://doi.org/10.1016/j.toxrep.2018.11.010
Fallah AA, Saei-Dehkordi SS, Nematollahi A, Jafari T (2011) Comparative study of heavy metal and trace element accumulation in edible tissues of farmed and wild rainbow trout (Oncorhynchus mykiss) using ICP-OES technique. Microchem J 98(2):275–279. https://doi.org/10.1016/j.microc.2011.02.007
Fan H, Chen S, Li Z, Liu P, Xu C, Yang X (2020) Assessment of heavy metals in water, sediment and shellfish organisms in typical areas of the Yangtze River Estuary, China. Mar Pollut Bull 151:110864. https://doi.org/10.1016/j.marpolbul.2019.110864
FAO (1983). FAO (1983) Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fisheries Circular. 764. FAO, Rome, 102p. Available from: http://www.fao.org/fi/oldsite/eims_search/1_ dett.asp,calling=simple_s_result&lang=fr&pub_id=65155.
Faure S, Noisel N, Werry K, Karthikeyan S, Aylward LL, St-Amand A (2020) Evaluation of human biomonitoring data in a health risk based context: an updated analysis of population level data from the Canadian Health Measures Survey. Int J Hyg Environ Health 223(1):267–280. https://doi.org/10.1016/j.ijheh.2019.07.009
Feed, F. a. (2013). Maximum limit of heavy metals. Iranian National Standard, Institute of Standard and Industrial Research of Iran.
Fierens S, Rebolledo J, Versporten A, Brits E, Haufroid V, De Plaen P, Van Nieuwenhuyse A (2016) Human biomonitoring of heavy metals in the vicinity of non-ferrous metal plants in Ath, Belgium. Archives of Public Health 74(1):1–11. https://doi.org/10.1186/s13690-016-0154-8
Finley BL, Monnot AD, Paustenbach DJ, Gaffney SH (2012) Derivation of a chronic oral reference dose for cobalt. Regul Toxicol Pharmacol 64(3):491–503. https://doi.org/10.1016/j.yrtph.2012.08.022
Food, F. (2018). "Agriculture organization of the United Nations. 2012." Producción. Ganadería. Online https://rsis.ramsar.org/ris/36.
Fuentes-Gandara F, Herrera-Herrera C, Pinedo-Hernández J, Marrugo-Negrete J, Díez S (2018) Assessment of human health risk associated with methylmercury in the imported fish marketed in the Caribbean. Environ Res 165:324–329. https://doi.org/10.1016/j.envres.2018.05.001
Ganzleben, C., J.-P. Antignac, R. Barouki, A. Castaño, U. Fiddicke, J. Klánová, E. Lebret, N. Olea, D. Sarigiannis and G. R. Schoeters (2017). "Human biomonitoring as a tool to support chemicals regulation in the European Union." International journal of hygiene and environmental health 220(2 Pt A): 94–97. https://doi.org/10.1016/j.ijheh.2017.01.007
Garnero, P. L., M. de los Angeles Bistoni and M. Monferran (2020). "Trace element concentrations in six fish species from freshwater lentic environments and evaluation of possible health risks according to international standards of consumption." Environmental science and pollution research international. Vol. 27 Issue 22, p27598–27608. 11p.
Gimeno-García E, Andreu V, Boluda R (1996) Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environ Pollut 92(1):19–25. https://doi.org/10.1016/0269-7491(95)00090-9
Gracia L, Marrugo JL, Alvis EM (2010) Contaminación por mercurio en humanos y peces en el municipio de Ayapel, Córdoba, Colombia, 2009. Revista Facultad Nacional De Salud Pública 28(2):118–124
Griboff J, Wunderlin DA, Monferran MV (2017) Metals, As and Se determination by inductively coupled plasma-mass spectrometry (ICP-MS) in edible fish collected from three eutrophic reservoirs. Their consumption represents a risk for human health? Microchem J 130:236–244. https://doi.org/10.1016/j.microc.2016.09.013
Haines DA, Saravanabhavan G, Werry K, Khoury C (2017) An overview of human biomonitoring of environmental chemicals in the Canadian Health Measures Survey: 2007–2019. Int J Hyg Environ Health 220(2):13–28. https://doi.org/10.1016/j.ijheh.2016.08.002
Hashmi MZ, Malik RN, Shahbaz M (2013) Heavy metals in eggshells of cattle egret (Bubulcus ibis) and little egret (Egretta garzetta) from the Punjab province, Pakistan. Ecotoxicol Environ Saf 89:158–165. https://doi.org/10.1016/j.ecoenv.2012.11.029
Hays SM, Aylward LL (2009) Using biomonitoring equivalents to interpret human biomonitoring data in a public health risk context. J Appl Toxicol 29(4):275–288. https://doi.org/10.1002/jat.1410
Humbatov FY, Ahmadov MM, Balayev VS, Suleymanov BA (2015) Trace metals in water samples taken from Azerbaijan sector of Caspian Sea. J Chem 9:288–295. https://doi.org/10.17265/1934-7375/2015.04.007
Ikem A, Egiebor NO (2005) Assessment of trace elements in canned fishes (mackerel, tuna, salmon, sardines and herrings) marketed in Georgia and Alabama (United States of America). J Food Compos Anal 18(8):771–787. https://doi.org/10.1016/j.jfca.2004.11.002
Iran Fisheries Organization (1398). Statistical yearbook of Iran Fisheries Organization. Deputy of Planning and Resource Management, Planning and Budget Office, Planning and Statistics Department.
IRIS (2010). "Agency Uep (Ed.), Integrated Risk Information System." Cincinnati, OH: 4–9.
Islam MA, Das B, Quraishi SB, Khan R, Naher K, Hossain SM, Karmaker S, Latif SA, Hossen MB (2020) Heavy metal contamination and ecological risk assessment in water and sediments of the Halda river, Bangladesh: a natural fish breeding ground. Mar Pollut Bull 160:111649. https://doi.org/10.1016/j.marpolbul.2020.111649
Javed M, Usmani N (2016) Accumulation of heavy metals and human health risk assessment via the consumption of freshwater fish Mastacembelus armatus inhabiting, thermal power plant effluent loaded canal. Springerplus 5(1):776. https://doi.org/10.1186/s40064-016-2471-3
Jedrychowski W, Jankowski J, Flak E, Skarupa A, Mroz E, Sochacka-Tatara E, Lisowska-Miszczyk I, Szpanowska-Wohn A, Rauh V, Skolicki Z (2006) Effects of prenatal exposure to mercury on cognitive and psychomotor function in one-year-old infants: epidemiologic cohort study in Poland. Ann Epidemiol 16(6):439–447. https://doi.org/10.1016/j.annepidem.2005.06.059
Jeong KS, Ha E, Shin JY, Park H, Hong Y-C, Ha M, Kim S, Lee S-J, Lee KY, Kim JH (2017) Blood heavy metal concentrations in pregnant Korean women and their children up to age 5 years: Mothers’ and Children’s Environmental Health (MOCEH) birth cohort study. Sci Total Environ 605:784–791. https://doi.org/10.1016/j.scitotenv.2017.06.007
Jeszka-Skowron M, Zgoła-Grześkowiak A, Stanisz E, Waśkiewicz A (2017) Potential health benefits and quality of dried fruits: Goji fruits, cranberries and raisins. Food Chem 221:228–236. https://doi.org/10.1016/j.foodchem.2016.10.049
Kazemi A, Esmaeilbeigi M, Sahebi Z, Ansari A (2022) Health risk assessment of total chromium in the qanat as historical drinking water supplying system. Sci Total Environ 807:150795. https://doi.org/10.1016/j.scitotenv.2021.150795
Kargın F, Çoğun H (1999) Metal interactions during accumulation and elimination of zinc and cadmium in tissues of the freshwater fish Tilapia nilotica. Bull Environ Contam Toxicol 63(4):511–519
Keshavarzi B, Hassanaghaei M, Moore F, Mehr MR, Soltanian S, Lahijanzadeh AR, Sorooshian A (2018) Heavy metal contamination and health risk assessment in three commercial fish species in the Persian Gulf. Mar Pollut Bull 129(1):245–252. https://doi.org/10.1016/j.marpolbul.2018.02.032
Kortei NK, Heymann ME, Essuman EK, Kpodo FM, Akonor PT, Lokpo SY, Boadi NO, Ayim-Akonor M, Tettey C (2020) Health risk assessment and levels of toxic metals in fishes (Oreochromis noliticus and Clarias anguillaris) from Ankobrah and Pra basins: impact of illegal mining activities on food safety. Toxicol Rep 7:360–369. https://doi.org/10.1016/j.toxrep.2020.02.011
Koseoglu E, Koseoglu R, Kendirci M, Saraymen R, Saraymen B (2017) Trace metal concentrations in hair and nails from Alzheimer’s disease patients: relations with clinical severity. J Trace Elem Med Biol 39:124–128. https://doi.org/10.1016/j.jtemb.2016.09.002
Kris-Etherton PM, Harris WS, Appel LJ (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106(21):2747–2757. https://doi.org/10.1161/01.CIR.0000038493.65177.94
LaKind JS, Aylward LL, Brunk C, DiZio S, Dourson M, Goldstein DA, Kilpatrick ME, Krewski D, Bartels MJ, Barton HA (2008) Guidelines for the communication of biomonitoring equivalents: report from the biomonitoring equivalents expert workshop. Regul Toxicol Pharmacol 51(3):S16–S26. https://doi.org/10.1016/j.yrtph.2008.05.007
Lee JW, Lee CK, Moon CS, Choi IJ, Lee KJ, Yi S-M, Jang B-K, B. jun Yoon, D. S. Kim and D. Peak, (2012) Korea national survey for environmental pollutants in the human body 2008: heavy metals in the blood or urine of the Korean population. Int J Hyg Environ Health 215(4):449–457. https://doi.org/10.1016/j.ijheh.2012.01.002
Lemos VA, G. d. S. Novaes, A. L. de Carvalho, E. M. Gama and A. G. Santos, (2009) Determination of copper in biological samples by flame atomic absorption spectrometry after precipitation with Me-BTAP. Environ Monit Assess 148(1–4):245–253. https://doi.org/10.1007/s10661-008-0155-3
Lin L, Li C, Yang W, Zhao L, Liu M, Li Q, Crittenden JC (2020) Spatial variations and periodic changes in heavy metals in surface water and sediments of the Three Gorges Reservoir, China. Chemosphere 240:124837. https://doi.org/10.1016/j.chemosphere.2019.124837
Liu C-W, Liang C-P, Huang FM, Hsueh Y-M (2006) Assessing the human health risks from exposure of inorganic arsenic through oyster (Crassostrea gigas) consumption in Taiwan. Sci Total Environ 361(1–3):57–66. https://doi.org/10.1016/j.scitotenv.2005.06.005
Loaiza I, De Troch M, De Boeck G (2018) Potential health risks via consumption of six edible shellfish species collected from Piura-Peru. Ecotoxicol Environ Saf 159:249–260. https://doi.org/10.1016/j.ecoenv.2018.05.005
Louro H, Heinälä M, Bessems J, Buekers J, Vermeire T, Woutersen M, van Engelen J, Borges T, Rousselle C, Ougier E, Alvito P, Martins C, Assunção R, Silva MJ, Pronk A, Schaddelee-Scholten B, Del Carmen Gonzalez M, de Alba M, Castaño A, Viegas S, Humar-Juric T, Kononenko L, Lampen A, Vinggaard AM, Schoeters G, Kolossa-Gehring M, Santonen T (2019) Human biomonitoring in health risk assessment in Europe: Current practices and recommendations for the future. Int J Hyg Environ Health 222(5):727–737. https://doi.org/10.1016/j.ijheh.2019.05.009
Marrugo-Negrete JL, Ruiz-Guzmán JA, Díez S (2013) Relationship between mercury Levels in hair and fish consumption in a population living near a hydroelectric tropical dam. Biol Trace Elem Res 151(2):187–194. https://doi.org/10.1007/s12011-012-9561-z
Maurya PK, Malik D, Yadav KK, Kumar A, Kumar S, Kamyab H (2019) Bioaccumulation and potential sources of heavy metal contamination in fish species in River Ganga basin: possible human health risks evaluation. Toxicol Rep 6:472–481. https://doi.org/10.1016/j.toxrep.2019.05.012
McDowell MA, Dillon CF, Osterloh J, Bolger PM, Pellizzari E, Fernando R, de Oca RM, Schober SE, Sinks T, Jones RL (2004) Hair mercury levels in US children and women of childbearing age: reference range data from NHANES 1999–2000. Environ Health Perspect 112(11):1165–1171. https://doi.org/10.1289/ehp.7046
Micó C, Recatalá L, Peris M, Sánchez J (2006) Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 65(5):863–872. https://doi.org/10.1016/j.chemosphere.2006.03.016
Mortada WI, Sobh MA, El-Defrawy MM, Farahat SE (2002) Reference intervals of cadmium, lead, and mercury in blood, urine, hair, and nails among residents in Mansoura city, Nile delta, Egypt. Environ Res 90(2):104–110. https://doi.org/10.1006/enrs.2002.4396
Muhammad S, Ahmad K (2020) Heavy metal contamination in water and fish of the Hunza River and its tributaries in Gilgit–Baltistan: evaluation of potential risks and provenance. Environ Technol Innov 20:101159. https://doi.org/10.1016/j.eti.2020.101159
Nabavi SF, Nabavi SM, Latifi AM, Eslami S, Ebrahimzadeh MA (2012) Determination of trace elements level of pikeperch collected from the Caspian Sea. Bull Environ Contam Toxicol 88(3):401–405. https://doi.org/10.1007/s00128-011-0513-7
Ogbomida ET, Nakayama SM, Bortey-Sam N, Oroszlany B, Tongo I, Enuneku AA, Ozekeke O, Ainerua MO, Fasipe IP, Ezemonye LI (2018) Accumulation patterns and risk assessment of metals and metalloid in muscle and offal of free-range chickens, cattle and goat in Benin City, Nigeria. Ecotoxicol Environ Saf 151:98–108. https://doi.org/10.1016/j.ecoenv.2017.12.069
Okati N, Esmaili-sari A (2018) Determination of mercury daily intake and hair-to-blood mercury concentration ratio in people resident of the coast of the Persian Gulf, Iran. Arch Environ Contam Toxicol 74(1):140–153. https://doi.org/10.1007/s00244-017-0456-z
Pandiyan J, Mahboob S, Govindarajan M, Al-Ghanim KA, Ahmed Z, Al-Mulhm N, Jagadheesan R, Krishnappa K (2021) An assessment of level of heavy metals pollution in the water, sediment and aquatic organisms: a perspective of tackling environmental threats for food security. Saudi Journal of Biological Sciences 28(2):1218–1225. https://doi.org/10.1016/j.sjbs.2020.11.072
Prokopowicz, A., N. Pawlas, P. Ochota, M. Szuła, A. Sobczak and K. Pawlas (2014). "Blood levels of lead, cadmium, and mercury in Healthy Women in their 50s in an urban area of Poland: a pilot study." Polish Journal of Environmental Studies 23(1).
Przybylowicz A, Chesy P, Herman M, Parczewski A, Walas S, Piekoszewski W (2012) Examination of distribution of trace elements in hair, fingernails and toenails as alternative biological materials. Application of chemometric methods. Cent Eur J Chem 10(5):1590–1599. https://doi.org/10.2478/s11532-012-0089-z
Rajeshkumar S, Li X (2018) Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol Rep 5:288–295. https://doi.org/10.1016/j.toxrep.2018.01.007
Ramsar (1975). from https://rsis.ramsar.org/ris/36.
Sadeghi P, Loghmani M, Frokhzad S (2020) Human health risk assessment of heavy metals via consumption of commercial marine fish (Thunnus albacares, Euthynnus affinis, and Katsuwonus pelamis) in Oman Sea. Environ Sci Pollut Res 27(13):14944–14952. https://doi.org/10.1007/s11356-020-07907-0
Saha N, Mollah M, Alam M, Rahman MS (2016) Seasonal investigation of heavy metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control 70:110–118. https://doi.org/10.1016/j.foodcont.2016.05.040
Schertzinger G, Ruchter N, Sures B (2018) Metal accumulation in sediments and amphipods downstream of combined sewer overflows. Sci Total Environ 616:1199–1207. https://doi.org/10.1016/j.scitotenv.2017.10.199
Sharafi K, Nodehi RN, Mahvi AH, Pirsaheb M, Nazmara S, Mahmoudi B, Yunesian M (2019) Bioaccessibility analysis of toxic metals in consumed rice through an in vitro human digestion model–Comparison of calculated human health risk from raw, cooked and digested rice. Food Chem 299:125126. https://doi.org/10.1016/j.foodchem.2019.125126
Takagi Y, Matsuda S, Imai S, Ohmori Y, Masuda T, Vinson J, Mehra M, Puri B, Kaniewski A (1986) Trace elements in human hair: an international comparison. Bull Environ Contam Toxicol 36(1):793–800. https://doi.org/10.1007/BF01623585
Tengku Nur Alia TKA, Hing LS, Sim SF et al (2020) Comparative study of raw and cooked farmed sea bass (Lates calcarifer) in relation to metal content and its estimated human health risk. Mar Pollut Bull 153:111009. https://doi.org/10.1016/j.marpolbul.2020.111009
Tian K, Wu Q, Liu P, Hu W, Huang B, Shi B, Zhou Y, Kwon B-O, Choi K, Ryu J, Seong Khim J, Wang T (2020) Ecological risk assessment of heavy metals in sediments and water from the coastal areas of the Bohai Sea and the Yellow Sea. Environ Int 136:105512. https://doi.org/10.1016/j.envint.2020.105512
Ullah AA, Maksud M, Khan S, Lutfa L, Quraishi SB (2017) Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol Rep 4:574–579. https://doi.org/10.1016/j.toxrep.2017.10.002
UNESCO. (1976). from https://en.unesco.org/biosphere/aspac/miankaleh.
UNIDO (2003). (United Nations Industrial Development Organization) Removal of barriers to introduction of cleaner artisanal gold mining and extraction technologies. http://www.cetem.gov.br/gmp/GMP_News/GMP_News_January_2003.pdf.
USEPA (1989) Risk assessment guidance for superfund, human health evaluation manual part A, Interim Final, I. United States Environmental Protection Agency, Washington DC
USEPA (2005). "U.S. Environmental Protection Agency, Office of Scienceand Technology, Office of Water) Water quality criterion for the protection of human health. Methyl mercury 2005. /http://www.epa.gov/waterscience/ criteria/methylmercury/document.htmlS.".
USEPA (2005). Water quality criterion for the protection of human health: methylmercury. http://www.epa.gov/waterscience/criteria/methylmercury. Accessed 29 June 2005.
USEPA (2011). "USEPA Regional Screening Level (RSL) Summary Table: November 2011."
USEPA (2012). "EPA Region III risk based concentration table." Philadelphia (PA (: United States Environmental Protection Agency, Region III.
USEPA (2015). Regional screening level (RSL) for chemical contaminant at superfound sites, U.S. Environmental Protection Agency: 176.
USEPA (2019). Regional screening levels (RSLs) - generic tables [WWW document]. Risk Assess. URL. https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables, Accessed date: 29 November 2019.
Usero J, Izquierdo C, Morillo J, Gracia I (2004) Heavy metals in fish (Solea vulgaris, Anguilla anguilla and Liza aurata) from salt marshes on the southern Atlantic coast of Spain. Environ Int 29(7):949–956. https://doi.org/10.1016/S0160-4120(03)00061-8
USNAS (2017) Using 21st century science to improve risk-related evaluations. D.C., National Academies Press, Washington
Vahter M, Berglund M, Åkesson A, Liden C (2002) Metals and women’s health. Environ Res 88(3):145–155. https://doi.org/10.1006/enrs.2002.4338
Varol M, Kaya GK, Alp A (2017) Heavy metal and arsenic concentrations in rainbow trout (Oncorhynchus mykiss) farmed in a dam reservoir on the Firat (Euphrates) river: risk-based consumption advisories. Sci Total Environ 599:1288–1296. https://doi.org/10.1016/j.scitotenv.2017.05.052
Varol M, Sünbül MR (2019) Environmental contaminants in fish species from a large dam reservoir and their potential risks to human health. Ecotoxicol Environ Saf 169:507–515. https://doi.org/10.1016/j.ecoenv.2018.11.060
Varol M, Sünbül MR (2020) Macroelements and toxic trace elements in muscle and liver of fish species from the largest three reservoirs in Turkey and human risk assessment based on the worst-case scenarios. Environ Res 109298. https://doi.org/10.1016/j.envres.2020.109298
Vrhovnik P, Arrebola JP, Serafimovski T, Dolenec T, Šmuc NR, Dolenec M, Mutch E (2013) Potentially toxic contamination of sediments, water and two animal species in Lake Kalimanci, FYR Macedonia: relevance to human health. Environ Pollut 180:92–100. https://doi.org/10.1016/j.envpol.2013.05.004
Walker, C., S. Hopkin, R. Sibly and D. Peakall (2001). "Principles of ecotoxicology. Taylor & Francis." Nueva York: 163–178.
Wei R, Wang X, Tang W, Yang Y, Gao Y, Zhong H, Yang L (2020) Bioaccumulations and potential human health risks assessment of heavy metals in ppk-expressing transgenic rice. Sci Total Environ 710:136496. https://doi.org/10.1016/j.scitotenv.2020.136496
Xiao C, Jian H, Chen L, Liu C, Gao H, Zhang C, Liang S, Li Y (2017) Toxic metal pollution in the Yellow Sea and Bohai Sea, China: distribution, controlling factors and potential risk. Mar Pollut Bull 119(1):381–389. https://doi.org/10.1016/j.marpolbul.2017.03.027
Xu L, Wang T, Ni K, Liu S, Wang P, Xie S, Meng J, Zheng X, Lu Y (2014) Ecological risk assessment of arsenic and metals in surface sediments from estuarine and coastal areas of the southern Bohai Sea, China. Hum Ecol Risk Assess Int J 20(2):388–401. https://doi.org/10.1080/10807039.2012.762281
Yılmaz F, Özdemir N, Demirak A, Tuna AL (2007) Heavy metal levels in two fish species Leuciscus cephalus and Lepomis gibbosus. Food Chem 100(2):830–835. https://doi.org/10.1016/j.foodchem.2005.09.020
Zhuang P, McBride MB, Xia H, Li N, Li Z (2009) Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci Total Environ 407(5):1551–1561. https://doi.org/10.1016/j.scitoten.2008.10.061
Acknowledgements
The authors gratefully thank the Mazandaran Department of Environment for sampling permissions in the Miankaleh Wetland, and the respective park rangers and staff are also sincerely appreciated for their assistance in the sampling process. We also appreciate the Golestan University of Medical Sciences and its respective health experts in Bandar Torkaman county for permissions and assistance in human samplings approved by IR.GOUMS.REC.2019.126 ethics code number as well.
Author information
Authors and Affiliations
Contributions
Abbas Esmaili-sari, supervision, conceptualization, data validation; Zahra Shaabani, writing—first draft, investigation, lab-based analysis, field sampling; Ali Mashinchian Moradi, data curation, graphical, visualization; Lobat Taghavi, data analyzing, software operations; Forough Farsad, revisions, editing and English corrections.
Corresponding author
Ethics declarations
Ethics approval
Human biomonitoring was performed based on the protocol specified by the Medical Ethical Committee of Iran in collaboration with the staff of the health center “Ethical Committee of Golestan University of Medical Sciences (IR.GOUMS.REC.2019.126).” Ethical principles and privacy were fully considered in this study.
Consent to participate
A written informed consent was received from the participants while completing the questionnaire. Turkmen pregnant women from two villages, Qharesou and Chapaqli, living in the northern part of Miankaleh wetland participate in this study.
Consent for publication
The authors consent to participate in this study and declare that there is no problem to publish the paper.
Competing interest
The authors declare no competing interests.
Availability of data and materials
All data and materials used in this paper are available in the reference link or DOI of the used papers.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shaabani, Z., Esmaili-sari, A., Moradi, A.M. et al. Possible health risk assessment for heavy metal concentrations in water, sediment, and fish species and Turkmen pregnant women’s biomonitoring in Miankaleh Peninsula, Iran. Environ Sci Pollut Res 29, 37187–37203 (2022). https://doi.org/10.1007/s11356-021-17894-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17894-5