Abstract
Imidacloprid as a widely used neonicotinoid insecticide can cause harmful pesticide residue inevitably. Metal-organic frameworks (MOFs) were innovatively composited to improve the light absorption and degradation performance of Bi2WO6 semiconductor, which expanded the photodegradation application in solving environmental problems. Based on the synergistic effect of Bi2WO6 and NH2-MIL-88B(Fe), a Bi2WO6/NH2-MIL-88B(Fe) (BNM) heterojunction photocatalyst with high-performance of photocatalytic degradation activities was successfully synthesized. The optimized BNM catalyst had a good degradation rate under visible light, which was mainly caused by generation of the active ·OH. Transient photocurrent response and electrochemical impedance tests verified that 1:2 BNM exhibits a highest charge separation and a lowest carrier recombination rate which were favorable to the photocatalytic activity. Cycle experiments show that the composite photocatalyst had good reusability and stability which were very important for potential industry applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Imidacloprid (IMC) is the most widely used pesticide as a neonicotinoid insecticide, and it is often used to protect economic crops such as vegetables, tea trees and fruit trees. However, residual pesticides may form harmful intermediate products under the influence of the environment, which will pose a threat to humans and the environment (Satiroff et al. 2020). Photocatalytic degradation was always effective to reduce the residue of IMC in food and the environment. The pioneering photocatalyst was TiO2, which opened a door to convert solar energy into valuable chemical energy (Rahimi et al. 2016). Subsequently, ZnO, CeO2, ZnS, CdS, g-C3N4, and other have emerged in various fields of photocatalysis. However, the shortcomings of the above semiconductor materials, such as their prone to photocorrosion, rapid recombination of electron-hole, and slow catalytic kinetics on the surface, hindered their applications in eliminating the environmental contamination caused by IMC. Therefore, new photocatalysts with high photocatalytic activity and photostability are urgently needed.
Among many metal oxide photocatalysts, Bi-based metal oxide with low toxicity and high reserves have been successfully developed in recent years, such as Bi2O3 (Zhang et al. 2014), Bi2WO6 (Fu et al. 2005, Karbasi et al. 2019, Karbasi et al. 2020), BiVO4 (Kohtani et al. 2005), Bi4V2O11 (Chen et al. 2013), BiPO4 (Lv et al. 2014), Bi2O2CO3 (Madhusudan et al. 2012), BiFeO3 (Humayun et al. 2016), BiOX (Zhang et al. 2012) (X = Cl, Br, I), BiNbO4 (Bakiro et al. 2020), and other semiconductors. As one of the simplest Aurivillius oxides, bismuth tungstate (Bi2WO6) has a suitable band gap, high photoactivity and stability, non-toxicity, and low cost, which has attracted many attentions (Dona-Rodriguez and Pulido Melian 2021). The perovskite crystals of Bi2WO6 have a layered structure, which facilitates the migration of the light carrier and thus enhances its photocatalytic performance. However, the pure Bi2WO6 material is also a low-efficiency semiconductor, owing to its poor visible light response, low e−-h+ migration efficiency and high recombination efficiency; thus, many strategies have been adopted to boost the photocatalytic performance of Bi2WO6 semiconductor materials, such as morphology modification, surface modification and other photocatalyst composites. Currently, Bi2WO6 is often combined with g-C3N4 (Al-Namshah and Mohamed 2021), CdS (Zhang et al. 2021), CuS (Mao et al. 2021), rGO (Zhao et al. 2021), and Co3O4 (Sun et al. 2021) to synthesize composite catalysts to improve catalytic performance. However, these composites still did not solve all the upper challenges, thus the metal-organic framework (MOF)-based photocatalysts come into our sight due to their high photocatalytic performance and good chemical stability (Mirhosseini et al. 2021).
In recent years, MOFs, as a kind of porous materials with applications in many areas (Chen et al. 2021b, Xu et al. 2021), also have been extensively studied in the treatment of small organic pollutants (Zhang et al. 2019). By combining the advantages of metal oxide semiconductors (MOS) and MOFs, MOS/MOF composites have become a kind of universal photocatalyst (Gautam et al. 2020). Moreover, due to the synergistic effect between the components, the MOS/MOFs composite photocatalysts usually show a higher catalytic activity than that of the alone material. Thus, few composite materials of Bi2WO6/MOFs (UiO-66, MIL-53, MIL-125 and MIL-100) photocatalysts were reported for the degradation of Rhodamine B (Sha et al. 2014, Tu et al. 2020, Xu et al. 2020) and tetracycline (He et al. 2021) recently. However, only four MOFs and only two degradation targets were investigated, more efforts are needed for this kind of photocatalyst.
Previously, we found out that NH2-MIL-88B(Fe) was an effective adsorbent and Fenton-like catalyst for degradation of IMC by the active substance of ·OH (Chen et al. 2021a). Thus, NH2-MIL-88B(Fe) was chosen to composite with Bi2WO6 to produce a photocatalyst with high performance. Herein, a serials of composite materials Bi2WO6/NH2-MIL-88B(Fe) (BNM) heterojunction with different molar ratios were hydrothermally synthesized. The visible photodegradation performances of BNM heterojunction to IMC were studied. The introduction of NH2-MIL-88B(Fe) may enhance the adsorption capacity, accelerate the transfer of e--h+, and even broaden the range of light absorption. In the present study, the relationship between the structure and the photocatalytic activity of the photocatalyst was investigated, and the free radical capture results verified that ·OH was the main active substances in this system.
Experimental section
Synthesis
NH2-MIL-88B (Fe) was synthesized by hydrothermal method as reported (Chen et al. 2021a). Firstly, FeCl3·6H2O (2.0 mmol, 0.54 g) and NH2-BDC (2.0 mmol, 0.36 g) were added into DMF (42.0 mL), and then the mixture was stirred at room temperature for 10 min. Subsequently, the mixture was transferred to a 100 mL Teflon reactor and heated at 383 K for 12.0 h. After the reactor was cooled to room temperature, the suspension was centrifuged at a centrifugal force of 5000g for 5.0 min to obtain a brown product, which was washed 3 times with DMF and EtOH, respectively. Finally, the product was dried in a vacuum oven at 343 K for 12.0 h.
The preparation process of Bi2WO6/NH2-MIL-88B(Fe) samples was as following reports with some modifications (Hu et al. 2019, Zhang et al. 2013). Disperse 1.0 mmol (0.33 g) of Na2WO4·2H2O and 0.5 mmol (0.16 g) of NH2-MIL-88B(Fe) in 20.0 mL of ethylene glycol to form solution A. Then, the solution B formed by 2.0 mmol (0.97 g) Bi(NO3)3·5H2O and 25.0 mL of 2.0 mol/L HNO3 was added drop-wisely to solution A. After mixing evenly, the pH of the suspension was adjusted to 4.0, and the suspension was stirred at room temperature for six hours. Then, the suspension was transferred to a 100 mL Teflon reactor and heated at 393 K for 12.0 h. The resulting powder was washed with distilled water and ethanol. Finally, after drying at 333 K for 12.0 h, the product Bi2WO6/NH2-MIL-88B(Fe) (designated as 1:0.5-BNM) was obtained. By changing the molar amount of NH2-MIL-88B(Fe) added, composite photocatalysts of 1:0.3 BNM, 1:0.5 BNM, 1:1 BNM, 1:2 BNM and 1:3 BNM can be synthesized respectively.
Characterization
The high-resolution thermal field emission scanning electron microscope was recorded by the Quanta 400 FEG instrument (FEI Company, USA). The X-ray powder diffraction patterns were recorded by a BRUKER AXS D8-Advance X-ray instrument, and the test conditions were the scanning range of 5°–45° and the scanning speed of 8°/min. Fourier infrared spectroscopy analysis were recorded by a Nicolet FT-IR 5700 spectrometer. The surface areas of the catalyst were measured by the American Mike TriStart II 3020 automatic multi-station specific surface area and porosity analyzer. Ultraviolet-visible diffuse reflectance spectroscopy was a full-wavelength scan performed by Shimadzu UV-3600 plus of Japan. The transient photocurrent response was based on the traditional three-electrode system, the electrolyte was 0.5M Na2SO4 solution, the time was 200 s, and the applied bias voltage was 0.7V vs. saturated silver/silver chloride electrode. The electrochemical impedance was measured by Shanghai Chenhua CHI-660C equipment, using a standard three-electrode device.
Photodegradation experiments
Before irradiating with visible light, perform the adsorption process in the dark. After a certain amount of photocatalyst was added into IMC solution (50.0 mL), the dark adsorption process lasts for 30.0 minutes. Then Zhongke Microenergy CME-Xe300F model xenon lamp (λ> 400 nm, China) was utilized to irradiate photocatalytic process. The catalyst was removed through a 0.22-μm syringe filter. A high performance liquid chromatography (Waters 2695, USA) was used to quantitatively detect the IMC at a wavelength of 270 nm (Chen et al. 2021a). The changes in concentrations were represented by C/C0, where C0 and C were the original concentration and residual concentration of IMC, respectively.
Results and discussion
Characterizations
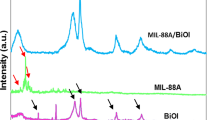
The powder XRD patterns of composite photocatalysts such as pure NH2-MIL-88B(Fe), pure Bi2WO6, and different molar ratio BNM are shown in the range of 5° < 2θ < 80° (2θ) in Fig. 1a. The diffraction peaks of pure Bi2WO6 at 28.3°, 32.8°, 47.1°, 55.9°, 58.6°, 68.8°, 76.1°, 78.4° can be attributed to the crystal faces (1 3 1), (2 0 0), (2 0 2), (3 3 1), (2 6 2), (0 8 3), (1 9 3), (2 0 4) of the orthorhombic phase (Bi2WO6, PDF#39-0256) (Zhao et al. 2018), indicating the high crystalline quality of Bi2WO6. The BNM composite photocatalysts with different molar ratios all showed different levels of characteristic absorption peaks of Bi2WO6 and NH2-MIL-88B(Fe), which could verify the successful composites synthesis. In order to determine certain chemical vibration bands in the composite material, the FT-IR spectra of the pure Bi2WO6, NH2-MIL-88B(Fe) and BNM composite photocatalyst with different molar ratio are shown in Fig. 1b. The absorption peaks of Bi2WO6 at 443 cm−1, 587 cm−1, and 817 cm−1 can be ascribed to the vibrations of the Bi–O bond, the absorption peak at 730 cm-1 can be attributed to the W–O bond (Zhang 2017), and the absorption band in the range of 3415-3435 cm−1 is related to the vibrations of O–H bond (Aljerf 2018). As the ratio of NH2-MIL-88B(Fe) increases, its characteristic absorption peaks increase, indicating that NH2-MIL-88B(Fe) has been successfully integrated into the BNM composite photocatalyst. SEM image shows the aggregated morphology of the obtained samples (Fig. S1, supporting information). Pure Bi2WO6 has several disordered nanosheets, and the 1:2 BNM composite exhibits a large volume morphology with many nanosheets. EDS and elemental diagrams showed that Bi2WO6 contained C, N, O, W and Bi elements, and C, N, O, W, Fe, and Bi elements were present in the 1:2 BNM complex.
X-ray photoelectron spectroscopy (XPS) analysis of NH2-MIL-88B(Fe), Bi2WO6, and 1:2 BNM were carried out to study the surface chemical compositions of the photocatalyst. In the entire spectra of XPS (Fig. S2), Bi, W, Fe, and C elements can be observed in 1:2 BNM. The signal peak of C 1s may be caused by CO2 in the air (Wan et al. 2017). In Fig. 2a–d, the high-resolution Bi 4f spectrum of Bi2WO6 semiconductor shows two peaks at 165.1 and 159.8 eV, corresponding to Bi 4f5/2 and Bi 4f7/2, respectively. The peaks at 38.3 and 36.1 eV can be ascribed to W 4f5/2 and W 4f7/2 respectively. The peak at 530.7 eV may correspond to O1s. The binding energy of 1:2 BNM Bi 4f, W 4f and O 1s is almost the same as that of pure Bi2WO6. In the Fe 2p spectrum, the Fe 2p1/2 and Fe 2p3/2 characteristic absorption peaks of 1:2 BNM are similar to those of NH2-MIL-88B(Fe). The above characterization results indicate that the 1:2 BNM heterojunction is successfully synthesized.
The surface areas and corresponding pore size distributions of the pure Bi2WO6, NH2-MIL-88B(Fe) and 1:2 BNM composite photocatalyst materials were analyzed by N2 adsorption-desorption analysis method. As shown in Fig. 3, isotherms of all samples are type IV and showed obvious mesoporous characteristics with H3 type hysteresis loop, Bi2WO6 has the largest SBET of 70.556 m2 g−1 (Table 1), and 1:2 BNM material has a larger SBET than that of NH2-MIL-88B(Fe).
Catalytic degradation of IMC
Effect of different molar ratios of BNM
In order to screen out the photocatalyst with the best degradation effect, the visible photocatalytic activity to IMC of different catalysts were investigated. As shown in Fig. 4, IMC did not self-degrade under visible light, and all catalysts could promote the degradation of IMC, and the 1:2 BNM composite showed the best activity among them. As the mass of NH2-MIL-88B (Fe) increases, the degradation efficiency first increases and then decreases. When the molar ratio of Bi2WO6 to NH2-MIL-88B(Fe) was 1:3, most of the IMC was adsorbed, and the photocatalytic ability was significantly inhibited. Therefore, 1:2 BNM was chose for subsequent experiments.
Effect of the reaction parameters
As shown in Fig. 5a, the effects of the initial concentrations of IMC (2–25 mg/L) on the degradation were investigated. The degradation ratio decreased with the increase of initial concentration. As the reaction concentration increases, Fig. 5b shows a linear relationship between -In(C/C0) and the irradiation time t; thus, the first-order kinetic model could be applied to this system. The degradation rate constants K of 5.0, 10.0, 15.0, 20.0, and 25.0 mg/L IMC were 0.00479 min−1, 0.00548 min−1, 0.00321 min−1, 0.00158 min−1, and 0.00168 min−1, respectively. Therefore, an initial concentration of 10.0 mg/L of IMC was chose for subsequent tests.
IMC (10.0 mg/L) was used to study the effect of the photocatalyst 1:2 BNM dose on the photocatalytic activity, and the results are shown in Fig. 5c. However, when the dosage increased to 0.50 g/L, the degradation ratio decreased. Excessive use of 1:2 BNM should be avoided, because the high concentration of the photocatalyst would lead to a decrease in light transmittance, which would cause a decrease of the degradation efficiency to IMC pollutants. Therefore, 0.40 g/L 1:2 BNM composite photocatalyst was selected in the follow-up experiment. Performing first-order kinetic fitting, where the 1:2 BNM degradation IMC fitting rate constants k of 0.1, 0.2, 0.3, 0.40, and 0.50 g/L were 0.00395 min−1, 0.00207 min−1, 0.00242 min−1, 0.0054 min−1, and 0.00124 min−1 (Fig. 5d), it is obvious that the degradation rate of 0.40 g/L 1:2 BNM was the fastest one.
The pH of the reaction system has a great influence on the adsorption and photocatalytic reactions. Here, we adjusted the pH value of the initial IMC solution by using 1.0 M HCl or NaOH solution. The effects of pH of the initial solution from 3.0 to 11.0 on the photocatalytic degradation of IMC were studied (Fig. 5e). The fitting rate constants k were 0.00196 min−1, 0.00337 min−1, 0.00471 min−1, 0.00425 min−1, 0.0054 min−1, and 0.00185 min−1 (Fig. 5f). Obviously, when pH value was 9.0, this system had the fastest degradation rate.
Evaluation of photocatalytic properties
The UV-Vis DRS absorption spectra of the sample are shown in Fig. 6a. It can be clearly observed that compared with pure Bi2WO6, the light absorption range of composite 1:2 BNM is further expanded to the visible light region. However, the light absorption capacity of NH2-MIL-88B(Fe) is similar to that of 1:2 BNM, which is not sufficient to show that the BNM heterojunction significantly improves the photocatalytic activity.
The conversion characteristic curve of the photocatalyst is used to detect whether the catalyst is sensitive to light. As can be seen from the Fig. 6b, in the traditional three-electrode system with the electrolyte of 0.50 M Na2SO4 aqueous solution, the current of 1:2 BNM catalyst is very low in the dark state, but the current rises quickly when turning on the light. It can be seen that the catalyst is very sensitive to light, and the photosensitivity of the sample will not be reduced when the catalyst is switched on and off repeatedly.
Electrochemical impedance spectroscopy (EIS) measurement is used to study the properties of catalyst charge transfer and recombination. The arc diameter represents the charge transfer resistance (Rct). The smaller the arc diameter, the lower the Rct. Figure 6c shows that 1:2 BNM has more effective photogenerated electron-hole separation and faster interface charge transfer than Bi2WO6. According to the above results, it can be concluded that the combination of NH2-MIL-88B(Fe) and Bi2WO6 is an effective method to promote the photocatalytic efficiency. The main reason for the enhanced activity of 1:2 BNM is the effective charge separation and transfer ability.
In Fig. 6d, based on the Kubelke–Munk transformation, the band gaps (Eg) of NH2-MIL-88B (Fe) and Bi2WO6 are 2.64 and 3.37 eV, respectively. In addition, the conduction band potential and valence band potential can be calculated according to Eqs. (1) and (2), where the χ values of Bi2WO6 and NH2-MIL-88B (Fe) are 6.36 (Ju et al. 2014) and 5.86 (Na et al. 2018), respectively. Then, the potentials of the conduction band (CB) are calculated to be 0.04 eV and 0.175 eV/NHE for NH2-MIL-88B (Fe) and Bi2WO6, respectively. The potentials of valence band (VB) are 2.680 eV and 3.545 eV/NHE for NH2-MIL-88B (Fe) and Bi2WO6, respectively. All in all, as shown in Table 2, this catalyst shows an acceptable degradation ratio with low-level catalyst dosage. Although the catalyst of PANI/ZnO-CoMoO4 shown the highest degradation ratio, the catalyst dosage was high for low concentration of IMC (163.5 mg for 4.5 mg/L in 4.0 mL) (Adabavazeh et al. 2021).
where EVB is the edge potential of VB, ECB is the edge potential of CB, χ is the electronegativity, Eg is the band gap, EC is the energy of free electrons on the hydrogen scale (about 4.5 eV).
Stability and reusability of BNM
The structure of 1:2 BNM before and after IMC degradation was characterized to evaluate the stability of the catalyst under the current experimental conditions. As can be seen from Fig. 7, the FT-IR and XRD spectra of 1:2 BNM before and after IMC adsorption and degradation did not change significantly. In addition, the stability and reusability of the composite material were further studied by conducting 5 cycles of dye degradation tests on 1:2 BNM. As shown in Fig. 8, as the number of cycles increases, the activity of the photocatalyst decreases slightly. The possible reason is that some IMC molecules and degradation products may be adsorbed in the pores of the composite material. These trapped molecules will reduce the accessible surface area of the catalyst, leading to a decrease in its activity. After 5 cycles, the stability of the 1:2 BNM photocatalyst did not decrease significantly, indicating that it has good stability.
Possible mechanism of IMC degradation
Detection of active species
As shown in Fig. 9, IPA, BQ, and EDTA were used to capture ·OH, O2−, and h+, respectively. Obviously, it can be seen that IPA and EDTA have significant inhibitory effects on the photocatalytic degradation ratio of 1:2 BNM, indicating that ·OH and h+ are the main active substances. A LC-MS instrument was used to investigate the products of imidacloprid degradation as in previous report (Chen et al. 2021a). Owing to the products were similar to that of previous report, no detail results will be shown in here.
Possible photocatalytic mechanism
Based on the above results of active species, a possible photocatalytic degradation mechanism of IMC was proposed. As shown in Fig. 10, when the Bi2WO6 semiconductor and NH2-MIL-88B(Fe) are irradiated by visible light, VB electrons can be excited by light to generate e--h+, and then the electron transition from VB to the CB happen and leave a corresponding number of h+ in the VB. As the CB potential of NH2-MIL-88B(Fe) is lower than that of Bi2WO6 (0.175 eV), the electron in CB of NH2-MIL-88B(Fe) is easily transferred to CB of Bi2WO6. And the VB potential of Bi2WO6 (3.545 eV) is higher than that of NH2-MIL-88B(Fe) (2.68 eV), which help the h+ in VB of Bi2WO6 can easily transfer to VB of NH2-MIL-88B(Fe). Thus, e− aggregates in CB of Bi2WO6, while h+ aggregates in VB of NH2-MIL-88B(Fe), and achieving the charge separation. The VB potential of NH2-MIL-88B(Fe) (2.68 eV) is higher than that of OH–/·OH (2.38 eV), thus OH– can generate ·OH easily under visible light. However, the CB potential of Bi2WO6 (0.1875 eV) is higher than the reduction potential of O2/O2− (− 0.33 eV vs. NHE), thus it is difficult to generate ∙O2− from O2 under visible light. This is also consistent with the free radical quenching experiment, so based on the analysis, a reasonable photocatalytic mechanism of this composite catalyst was proposed, which is similar but not same to the reported results (He et al. 2021, Tu et al. 2020).
Conclusion
In summary, a hydrothermal method was developed to prepare Bi2WO6/NH2-MIL-88B(Fe) heterostructures. The prepared composite material exhibits better adsorption affinity and photocatalytic activity than that of Bi2WO6 for the photodegradation to IMC under visible light. 1:2 BNM (the best molar ratio Bi2WO6: NH2-MIL-88B(Fe)) can remove 84.46% of IMC through co-adsorption and visible light-induced photocatalytic degradation. This is mainly because NH2-MIL-88B (Fe) can enhance charge transfer and inhibit electron-hole recombination effectively. Importantly, the cycle experiment also shows that the Bi2WO6/NH2-MIL-88B(Fe) photocatalyst has good chemical stability and reusability which are necessary for the industrial applications.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adabavazeh H, Saljooqi A, Shamspur T, Mostafavi A (2021) Synthesis of polyaniline decorated with ZnO and CoMoO4 nanoparticles for enhanced photocatalytic degradation of imidacloprid pesticide under visible light. Polyhedron 198. https://doi.org/10.1016/J.Poly.2021.115058
Al-Namshah KS, Mohamed RM (2021) Development of mesoporous Bi2WO6/g-C3N4 heterojunctions via soft- and hard-template-assisted procedures for accelerated and reinforced photocatalytic reduction of mercuric cations under vis light irradiation. Ceram Int 47:5003–5012. https://doi.org/10.1016/j.ceramint.2020.10.076
Aljerf L (2018) High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: kinetics and equilibrium study. J Environ Manage 225:120–132. https://doi.org/10.1016/j.jenvman.2018.07.048
Bakiro M, Ahmed SH, Alzamly A (2020) Cycloaddition of CO2 to propylene oxide using BiNbO4/NH2-MIL-125(Ti) composites as visible-light photocatalysts. J Environ Chem Eng 8:104461. https://doi.org/10.1016/J.Jece.2020.104461
Chen M-L, Lu T-H, Long L-L, Xu Z, Ding L, Cheng Y-H (2021) NH2-Fe-MILs for effective adsorption and Fenton-like degradation of imidacloprid: Removal performance and mechanism investigation. Environ Eng Res 27:200702. https://doi.org/10.4491/eer.2020.702
Chen ML, Ning P, Jiao Y, Xu Z, Cheng YH (2021) Extraction of antioxidant peptides from rice dreg protein hydrolysate via an angling method. Food Chem 337:128069. https://doi.org/10.1016/j.foodchem.2020.128069
Chen XF, Liu JB, Wang H, Ding YL, Sun YX, Yan H (2013) One-step approach to novel Bi4V2O11 hierarchical hollow microspheres with high visible-light-driven photocatalytic activities. J Mater Chem A 1:877–883. https://doi.org/10.1039/c2ta00312k
Dona-Rodriguez JM, Pulido Melian E (2021) Nano-photocatalytic materials: possibilities and challenges. Nanomaterials-Basel 11 https://doi.org/10.3390/nano11030688
Fu H, Pan C, Yao W, Zhu Y (2005) Visible-light-induced degradation of rhodamine B by nanosized Bi2WO6. J Phys Chem B 109:22432–22439. https://doi.org/10.1021/jp052995j
Gautam S, Agrawal H, Thakur M, Akbari A, Sharda H, Kaur R, Amini M (2020) Metal oxides and metal organic frameworks for the photocatalytic degradation: a review. J Environ Chem Eng 8. https://doi.org/10.1016/J.Jece.2020.103726
He Y, Wang D, Li X, Fu Q, Yin L, Yang Q, Chen H (2021) Photocatalytic degradation of tetracycline by metal-organic frameworks modified with Bi2WO6 nanosheet under direct sunlight. Chemosphere 284:131386. https://doi.org/10.1016/j.chemosphere.2021.131386
Hu LX, Zhang YY, Lu WC, Lu YS, Hu HM (2019) Easily recyclable photocatalyst Bi2WO6/MOF/PVDF composite film for efficient degradation of aqueous refractory organic pollutants under visible-light irradiation. J Mater Sci 54:6238–6257. https://doi.org/10.1007/s10853-018-03302-w
Humayun M, Zada A, Li ZJ, Xie MZ, Zhang XL, Qu Y, Raziq F, Jing LQ (2016) Enhanced visible-light activities of porous BiFeO3 by coupling with nanocrystalline TiO2 and mechanism. Appl Catal B-Environ 180:219–226. https://doi.org/10.1016/j.apcatb.2015.06.035
Ju P, Wang P, Li B, Fan H, Ai SY, Zhang D, Wang Y (2014) A novel calcined Bi2WO6/BiVO4 heterojunction photocatalyst with highly enhanced photocatalytic activity. Chem Eng J 236:430–437. https://doi.org/10.1016/j.cej.2013.10.001
Kanwal M, Tariq SR, Chotana GA (2018) Photocatalytic degradation of imidacloprid by Ag-ZnO composite. Environ Sci Pollut Res Int 25:27307–27320. https://doi.org/10.1007/s11356-018-2693-8
Karbasi M, Karimzadeh F, Kiwi J, Raeissi K, Pulgarin C, Rtimi S (2019) Flower-like magnetized photocatalysts accelerating an emerging pollutant removal under indoor visible light and related phenomena. J Photochem Photobio A: Chem 378:105–113. https://doi.org/10.1016/j.jphotochem.2019.04.017
Karbasi M, Karimzadeh F, Raeissi K, Rtimi S, Kiwi J, Giannakis S, Pulgarin C (2020) Insights into the photocatalytic bacterial inactivation by flower-like Bi2WO6 under solar or visible light, Through in situ monitoring and determination of reactive oxygen species (ROS). Water 12:1099
Kohtani S, Hiro J, Yamamoto N, Kudo A, Tokumura K, Nakagaki R (2005) Adsorptive and photocatalytic properties of Ag-loaded BiVO4 on the degradation of 4-n-alkylphenols under visible light irradiation. Catal Commun 6:185–189. https://doi.org/10.1016/j.catcom.2004.12.006
Liang F, Lu M, Zhang YH, Shi Q, Shi FN (2021) Synthesis and structure of a bismuth-cobalt bimetal coordination polymer for green efficient photocatalytic degradation of organic wastes under visible light. J Mol Struct 1230. https://doi.org/10.1016/j.molstruc.2020.129636
Liang SH, Zhang DF, Pu XP, Yao XT, Han RT, Yin J, Ren XZ (2019) A novel Ag2O/g-C3N4 p-n heterojunction photocatalysts with enhanced visible and near-infrared light activity. Sep Purif Technol 210:786–797. https://doi.org/10.1016/j.seppur.2018.09.008
Lv YH, Liu YF, Zhu YY, Zhu YF (2014) Surface oxygen vacancy induced photocatalytic performance enhancement of a BiPO4 nanorod. J Mater Chem A 2:1174–1182. https://doi.org/10.1039/c3ta13841k
Madhusudan P, Yu J, Wang W, Cheng B, Liu G (2012) Facile synthesis of novel hierarchical graphene-Bi2O2CO3 composites with enhanced photocatalytic performance under visible light. Dalton Trans 41:14345–14353. https://doi.org/10.1039/c2dt31528a
Mao W, Zhang LX, Wang TY, Bai YC, Guan YT (2021) Fabrication of highly efficient Bi2WO6/CuS composite for visible-light photocatalytic removal of organic pollutants and Cr(VI) from wastewater. Front Env Sci Eng 15:52. https://doi.org/10.1007/s11783-020-1344-8
Mirhosseini H, Shamspur T, Mostafavi A, Sargazi G (2021) A novel ultrasonic reverse micelle-assisted electrospun efficient route for Eu-MOF and Eu-MOF/CA composite nanofibers: a high performance photocatalytic treatment for removal of BG pollutant. Environ Sci Pollut Res Int 28:4317–4328. https://doi.org/10.1007/s11356-020-10746-8
Na Y, Miao S, Zhou L, Wei P, Cao Y (2018) Bio-inspired model of photosystem II: supramolecular assembly of an electron mediator into an SnO2 photoanode co-sensitized by a porphyrin photosensitizer and ruthenium molecular catalyst. Sustainable Energy Fuels 2:545–548. https://doi.org/10.1039/c7se00515f
Rahimi N, Pax RA, Gray EM (2016) Review of functional titanium oxides. I: TiO2 and its modifications. Prog Solid State Chem 44:86–105. https://doi.org/10.1016/j.progsolidstchem.2016.07.002
Satiroff JA, Messer TL, Mittelstet AR, Snow DD (2020) Pesticide occurrence and persistence entering recreational lakes in watersheds of varying land uses. Environ Pollut 273:116399. https://doi.org/10.1016/j.envpol.2020.116399
Sha Z, Sun JL, Chan HSO, Jaenicke S, Wu JS (2014) Bismuth tungstate incorporated zirconium metalorganic framework composite with enhanced visible- light photocatalytic performance. Rsc Adv 4:64977–64984. https://doi.org/10.1039/c4ra13000f
Sun J, Shen CH, Guo J, Guo H, Yin YF, Xu XJ, Fei ZH, Liu ZT, Wen XJ (2021) Highly efficient activation of peroxymonosulfate by Co3O4/Bi2WO6 p-n heterojunction composites for the degradation of ciprofloxacin under visible light irradiation. J Colloid Interface Sci 588:19–30. https://doi.org/10.1016/j.jcis.2020.12.043
Tu Y, Ling LQ, Li QW, Long XY, Liu N, Li ZQ (2020) Greatly enhanced photocatalytic activity over Bi2WO6 by MIL-53 (Fe) modification. Opt Mater 110. https://doi.org/10.1016/J.Optmat.2020.110500
Wan SP, Zhong Q, Ou M, Zhang SL (2017) Synthesis and characterization of direct Z-scheme Bi2MoO6/ZnIn2S4 composite photocatalyst with enhanced photocatalytic oxidation of NO under visible light. J Mater Sci 52:11453–11466. https://doi.org/10.1007/s10853-017-1283-3
Xu YF, Zhou Y, Deng YH, Xiang Y, Tan YW, Tang HQ, Zou H (2020) Synthesis of Bi2WO6@NH2-MIL-125(Ti): A S-Scheme Photocatalyst with Enhanced Visible Light Catalytic Activity. Catal Lett 150:3470–3480. https://doi.org/10.1007/s10562-020-03258-0
Xu Z, Long LL, Chen YQ, Chen ML, Cheng YH (2021) A nanozyme-linked immunosorbent assay based on metal-organic frameworks (MOFs) for sensitive detection of aflatoxin B1. Food Chem 338:128039. https://doi.org/10.1016/j.foodchem.2020.128039
Zhang JF, Hu YF, Jiang XL, Chen SF, Meng SG, Fu XL (2014) Design of a direct Z-scheme photocatalyst: Preparation and characterization of Bi2O3/g-C3N4 with high visible light activity. J Hazard Mater 280:713–722. https://doi.org/10.1016/j.jhazmat.2014.08.055
Zhang K, Liang J, Wang S, Liu J, Ren KX, Zheng X, Luo H, Peng YJ, Zou X, Bo X, Li JH, Yu XB (2012) BiOCl sub-microcrystals induced by citric acid and their high photocatalytic activities. Cryst Growth Des 12:793–803. https://doi.org/10.1021/cg201112j
Zhang XJ (2017) Synthesis and photocatalytic activity of Bi2WO6 microspheres with hierarchical structure. Ferroelectrics 514:34–42. https://doi.org/10.1080/00150193.2017.1359042
Zhang YH, Zhang N, Tang ZR, Xu YJ (2013) Identification of Bi2WO6 as a highly selective visible-light photocatalyst toward oxidation of glycerol to dihydroxyacetone in water. Chem Sci 4:1820–1824. https://doi.org/10.1039/c3sc50285f
Zhang YY, Liu Q, Yang C, Wu SC, Cheng JH (2019) Magnetic aluminum-based metal organic framework as a novel magnetic adsorbent for the effective removal of minocycline from aqueous solutions. Environ Pollut 255:113226. https://doi.org/10.1016/j.envpol.2019.113226
Zhang ZQ, Lin YZ, Liu F (2021) Preparation and photoelectric properties of Bi2WO6-CdS hybrid nanocrystals. Colloid Surface A 611:125883. https://doi.org/10.1016/j.colsurfa.2020.125883
Zhao Y, Liang X, Hu X, Fan J (2021) rGO/Bi2WO6 composite as a highly efficient and stable visible-light photocatalyst for norfloxacin degradation in aqueous environment. J Colloid Interface Sci 589:336–346. https://doi.org/10.1016/j.jcis.2021.01.016
Zhao YY, Wang YB, Liu EZ, Fan J, Hu XY (2018) Bi2WO6 nanoflowers: An efficient visible light photocatalytic activity for ceftriaxone sodium degradation. Appl Surf Sci 436:854–864. https://doi.org/10.1016/j.apsusc.2017.12.064
Funding
This research was funded by the National Natural Science Foundation of China (31601550) and Natural Science Foundation of Hunan (2019JJ50638).
Author information
Authors and Affiliations
Contributions
MLC: conceptualization, validation, writing review and editing. THL: conceptualization, data curation, formal analysis, investigation, writing-original draft. SSL: conceptualization, data curation, formal analysis, investigation. LW: conceptualization, data curation, writing-review and editing. ZX: conceptualization, validation, writing-review and editing. YHC: resources, writing-review and editing.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable in this work.
Consent to participate
Not applicable.
Consent to publish
All authors read and approved the final manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Sami Rtimi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, ML., Lu, TH., Li, SS. et al. Photocatalytic degradation of imidacloprid by optimized Bi2WO6/NH2-MIL-88B(Fe) composite under visible light. Environ Sci Pollut Res 29, 19583–19593 (2022). https://doi.org/10.1007/s11356-021-17187-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17187-x