Abstract
Understanding the evolution process of hydrogeochemistry and groundwater quality is essential for water supply and health in the southwestern Ordos Basin, where groundwater is a vital source for drinking. This study systematically illustrates the hydrogeochemical characteristics and evolution mechanism based on the groundwater samples (n = 67) collected from Loess area by integrating multivariate statistical methods and hydrogeochemical methods. Furthermore, the entropy water quality index (EWQI) and water quality indices combined with spatial analysis were employed to evaluate the suitability of groundwater for drinking and irrigation purposes and analyze the spatial variation of water quality. The hierarchical cluster analysis and principal component analysis classified groundwater dataset into four clusters and four components which were examined using a Piper diagram and Gibbs diagram, representing different hydrogeochemical characteristics and controlling factors. Based on results, the groundwater chemistry was characterized by representative water types: freshwater (cluster 1, cluster 2), low salinity (half of cluster 3), high salinity (half of cluster 3, cluster 4), and the main controlling factors of hydrogeochemistry revealed by Gibbs diagram were evaporation crystallization (cluster 3, cluster 4) and water–rock interactions (cluster 1, cluster 2). Moreover, the Gaillardet diagram, chloro-alkaline indices, binary diagram, and saturation index further comprehensively illustrate that the silicate and evaporite weathering, ion exchange, dissolution of halite, gypsum, and anhydrite are responsible for hydrogeochemical process. Based on EWQI and ArcGIS, the groundwater quality is categorized as excellent (47.0%), good (31.8%), medium (4.5%), poor (6.1%), and extremely poor (10.6%) types, and the quality in the south of the study area is better than north. Additionally, the USSL diagram shows that most of samples belong to C3S1 (high-salinity hazard and low-sodium hazard) and C2S1 (medium-salinity hazard and low-sodium hazard), and Wilcox diagram shows that 77.2% of samples are suitable for irrigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater plays a significant role in domestic water supply and agricultural irrigation in arid and semi-arid regions due to the limitations of precipitation and surface water (Chen et al. 2019; Li and Gao 2019; Rao et al. 2020; Singh et al. 2020). Hence, understanding the quality of groundwater is of great significance for ensuring the safety of drinking water and human health (Li et al. 2017). Groundwater quality is largely affected by natural conditions (lithology, groundwater velocity, water–rock interactions, quality of recharge water, influence from other aquifers), anthropogenic activities (agriculture, industry, overexploitation of groundwater, leaching of pollutants in soil), and atmospheric precipitation input (Jiang et al. 2009; Li et al. 2017; Peng et al. 2018; Re et al. 2014; Zereg et al. 2018). A comprehensive understanding of groundwater geochemistry plays a critical role in identifying the changes in water quality, protecting groundwater resources, and evaluating the suitability for drinking and irrigation (Arumugam and Elangovan 2009; Cloutier et al. 2008; Fendorf et al. 2010; Yang et al. 2020). Moreover, the hydrogeochemical characteristics of groundwater largely determine the suitability of groundwater for drinking and irrigation (Belkhiri et al. 2010; Kumar et al. 2016).
The multivariate statistical methods (principal component analysis (PCA), hierarchical cluster analysis (HCA), and correlation analysis (CA)) combined with hydrogeochemical analysis have greatly promoted the understanding of the formation and evolution of water chemistry characteristics (Cloutier et al. 2008; Cortes et al. 2016; Heydarirad et al. 2019; Patil et al. 2020). Generally, statistical analysis of the groundwater geochemical data is performed first, and then the hydrogeochemical analysis method is used to identify the hydrogeochemical characteristics and the evolution process controlling groundwater chemistry (Yang et al. 2020). Hierarchical cluster analysis is an effective tool to divide water samples into different clusters based on groundwater chemistry data, representing different hydrochemical facies or water chemical types (Belkhiri et al. 2010). However, a principal component analysis divides various hydrochemical parameters into several principal components according to the relationship between different variables, which represent the factors that control groundwater chemistry (Cloutier et al. 2008; Cortes et al. 2016; Jehan et al. 2020a). The integration of principal component analysis, hierarchical cluster analysis, and correlation analysis is an effective tool in identifying groundwater hydrochemical characteristics and hydrogeochemical evolution processes and promotes the thorough understanding of groundwater quality (Ayed et al. 2017; Monjerezi et al. 2011; Noshadi and Ghafourian 2016; Yang et al. 2015).
The water quality index (WQI), proposed by (Horton 1965), is an effective tool for assessing the overall water quality (Jehan et al. 2020b) and the suitability for drinking based on hydrochemical data and water quality standards. Many previous studies have utilized the WQI method to evaluate groundwater quality in different regions of the world (Adimalla and Qian 2019; Simões et al. 2008; Jianhua et al. 2011; Logeshkumaran et al. 2015; Varol and Davraz 2015; Vasanthavigar et al. 2010). However, in the process of calculating the WQI, the assignment of hydrochemical parameters weights is usually subjective, which will obscure part of the important information of water quality (Amiri et al. 2014). In order to objectively reflect the weight of different hydrochemical parameters in water quality evaluation, the entropy weight method (Shannon 1948) is used to determine the weight of each variables, namely the entropy weight water quality index (EWQI) method. The EWQI has been widely used to evaluate the quality of groundwater for drinking (Adimalla et al. 2018; Gao et al. 2020; Karunanidhi et al. 2021; Wu et al. 2018), which is usually combined with ArcGIS to more intuitively reflect the spatial distribution characteristics and differences of water quality categories (He and Wu 2019; Hossain and Patra 2020; Karunanidhi et al. 2020; Shahid et al. 2014).
The study area is located in the southwest of Ordos Basin, which is a typical loess plateau gully area with fragmented and complex terrain. The study area belongs to the semi-arid climate, which is characterized by low precipitation and large evaporation, leading to water shortages and becoming a crucial issue restricting the development of the region. Therefore, groundwater, especially quaternary phreatic groundwater, plays a vital role in domestic drinking and agricultural irrigation. However, insufficient research and attention on the hydrochemical characteristics and the water quality evaluation of the quaternary phreatic groundwater for drinking and irrigation limits our understanding of the current status of groundwater quality and water resources management in this region. Consequently, there is an increasing significance to identify the hydrochemical characteristics and evolution process of groundwater and evaluate the suitability for drinking and irrigation purposes.
This study utilizes multivariate statistical methods (CA, PCA, HCA) and hydrogeochemical analysis methods (Piper trilinear diagram, Gibbs diagram, binary diagram, saturation index, and chloro-alkaline indices) to analyze the hydrochemical characteristics and identify the hydrogeochemical evolution process of groundwater. Furthermore, the EWQI method was used to evaluate the suitability of groundwater quality for drinking, then the ArcGIS software was employed to establish the evaluation zones. In addition, multiple water quality assessment indicators for irrigation (SAR, %Na, RSC, KR, MH, PI, USSL diagram, and Wilcox diagram) are adopted to evaluate the suitability of groundwater for irrigation in the study area.
Study area
Description of the study area
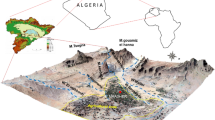
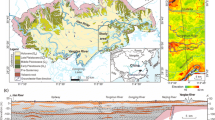
The study area (E: 106° 21′ 23″–108° 42′ 33″, N: 35° 14′ 31″–37° 9′ 31″) is located in the southwest of the Ordos Basin (Fig. 1), which belongs to a typical loess area. The terrain slopes from the northeast and west to the southeast, with an altitude of 885–2089 m. The study area is located in the gully area of the Loess Plateau in the middle reaches of the Yellow River. The terrain is high on the east, north, and west and low on the middle and southern sides, which forms a non-closed basin with an opening in the southwest. The rainfall gradually decreases from south to north, and the rainfall is mostly concentrated in July to September, accounting for about 60% of the annual precipitation. The study area has crisscross gullies and dendritic water systems, which belong to the Jinghe water system. The main rivers are Puhe River and Malianhe River. In addition, there are two larger secondary tributaries of Huanjiang River and Rouyuanhe River.
Geology and hydrogeology
The study area is an integral part of the Ordos Basin and is a large syncline sedimentary basin composed of Paleozoic and Mesozoic with an axis near north to south. The stratigraphic lithology and lithofacies in the study area are relatively complex, mainly including Quaternary alluvial deposits, Quaternary loess, Neogene mudstone, Cretaceous Jingchuan Formation mudstone and sandstone, Cretaceous Luohandong Formation sandstone, and Cretaceous Huanhe Formation mudstone and sandstone from top to bottom (Fig. S1). This study focuses on the quaternary strata, including aeolian loess and river alluvial deposits. According to lithological characteristics and geological age, the quaternary strata can be divided into the following: (1) Pleistocene aeolian loess layer, which is composed of Lower Pleistocene Wucheng Formation, Middle Pleistocene Lishi Formation, and Upper Pleistocene Malan Formation. (2) Holocene alluvial–diluvial strata, which is mainly distributed in the valley terraces, large gullies, and piedmont alluvial fans of major rivers.
According to the water-bearing rock layer and the type of medium, the study area can be divided into karst groundwater system, clastic rock groundwater system, and Quaternary loose layer groundwater system. This study takes the Quaternary loose rock groundwater system as the target aquifer. The overlying Quaternary loose rock groundwater distribution is relatively independent, which is mainly divided into three sub-systems of loess hilly phreatic aquifer, loess plateau phreatic aquifer, and river valley alluvial–proluvial phreatic aquifer. The phreatic aquifers of the loess hills are distributed in the loess hilly areas. The aquifer medium is mainly Lishi loess (Q2), the overlying Malan loess (Q3) is permeable and does not contain water, and the underlying Wucheng loess (Q1) has a high content of clay particles and is relatively water-resistant. However, the phreatic aquifers in the loess plateau are mainly distributed in the 19 loess plateau areas south of Qingcheng (Fig. S2). Each plateau is a relatively independent hydrogeological unit, with the similar hydrogeological conditions. Atmospheric precipitation is the main source of replenishment for the loess phreatic water in the basin. The runoff of the loess phreatic aquifer is mainly affected by topography, and the direction of runoff is changeable, mainly from high terrain to low terrain.
Data and methods
Sampling and test
A total of 67 groundwater samples were collected from motor-pumped wells in the study area in October 2020, as shown in Fig. 1. Most of the groundwater wells in the study area are used for drinking and irrigation, and the groundwater samples are taken from Quaternary phreatic aquifers. All wells must be pumped for at least 15 min before sampling. The groundwater samples are taken into polyethylene bottles after the temperature and pH have stabilized. The portable instruments are used for on-site measurement of temperature, pH, and conductivity (EC). The water samples used to test other general hydrochemical indicators (Na+, K+, Ca2+, Mg2+, HCO3−, SO42−, Cl−, TDS, NO3−, NH4+, F−, and Fe) were put into polyethylene bottles, sealed and stored, and transported to the laboratory for testing. The detection of parameters is recommended by the water quality—determination of 32 elements—inductively coupled plasma optical emission spectrometry (Ministry of Environmental Protection of the People’s Republic of China 2015), standard examination methods for drinking water—water analysis quality control (Ministry of Health of the People’s Republic of China 2006).
Data pre-processing
The test results of hydrochemical parameters inevitably appear as censored data (the measured concentration less than detection limits). In order to utilize the data as much as possible for analysis, the commonly used methods for processing censored data are either to exclude from hydrochemical analysis or to replace it with a specific data less than the detection limit (Barescut et al. 2011; Cloutier et al. 2008; Croghan and Egeghy 2003; Güler and Thyne 2004). This study uses 75% of the detection limit to replace the censored data (VanTrump Jr and Miesch 1977; Yang et al. 2020).
Moreover, in order to facilitate groundwater chemistry analysis, the concentration data (mg/L) of all parameters are converted into milligram equivalent concentration (meq/L). Quality control was carried out by calculating the percent charge balance errors (CBE) to verify the reliability of the chemical analysis results. The formula for calculating CBE is as follows:
where all units of chemical data are meq/L.
The data with the CBE < ± 10% (Karunanidhi et al. 2020) were used in this study, while the groundwater samples with the CBE over 10% were excluded from the all data. In this study, the number of the datasets used for subsequent analysis is 66 based on the CBE.
Statistical analysis
Correlation analysis
Correlation analysis is a statistical method to test the degree of intimacy between two variables. In this study, Pearson correlation analysis was employed to discriminate the correlation between different water chemistry parameters. A statistical difference is considered at a 5% significant level (p < 0.05).
Hierarchical cluster analysis
Hierarchical cluster is a clustering method that classifies different datasets according to their similarity, which is determined by calculating the distance between the data points of each category and all the data points. In this study, the Euclidean distance is used to calculate the distance between different types of data points, which is calculated as follows:
Then, Ward’s linkage method (Ward 1963) is utilized to assess the distances between different clusters using the variance analysis (Yang et al. 2020), and the final result is presented in the form of a dendrogram. Moreover, the number of clusters is determined according to the phenon line.
Principal component analysis
Principal component analysis (PCA) is a widely used data dimensionality reduction method. PCA can retain most of the information while reducing the dimensionality of the data (Boonkaewwan et al. 2021). In this study, PCA was employed to verify whether the hierarchical cluster analysis is reasonable.
Hydrochemical analysis
Geochemical modeling
The hydrogeochemical model describes the chemical reactions that occur in the groundwater system and the state of ions in groundwater and minerals. The geochemical model can simulate various equilibrium reactions between groundwater and minerals using the chemical thermodynamic (Charlton and Parkhurst 2011; Karunanidhi et al. 2021). PHREEQC is a geochemical reaction model (Parkhurst and Appelo 1999), which is widely used to simulate the chemical equilibrium between groundwater and minerals by calculating the saturation indices (SI) in this study.
Groundwater quality evaluation
Groundwater quality evaluation for drinking
The entropy weighted water quality index (EWQI) is an improved method based on water quality index (WQI) (Amiri et al. 2014; Singh et al. 2019), which is characterized by the information entropy used to determine the weight of groundwater hydrochemical parameters. EWQI is widely used to quantitatively evaluate groundwater quality for drinking, which can be obtained through the following steps (Gorgij et al. 2017; Rao et al. 2020; Su et al. 2018):
-
1.
According to the groundwater hydrochemistry data, the eigenvalue matrix, X, which is composed of “m” groundwater samples and “n” hydrochemistry parameters. X is shown as follows:
-
2.
Due to the difference in the dimensions of the hydrochemistry parameters, in order to facilitate the analysis of different parameters, the data is normalized using Eq. (4) and matrix X is converted into a standard-grade matrix Y, which is shown in Eq. (5).
-
3.
The ratio of the index value of the jth parameter at the ith sample and the information entropy of the parameter j (ej) were calculated by Eqs. (6) and (7), which is as follows:
-
4.
The entropy weight of the parameter j (wj) was calculated by the Eq. (8).
Then, the quality rating scale of the parameter j (qj) was calculated by Eq. (9).
where Cj, CpH represents the concentration (mg/L) and pH of parameter j, respectively. Sj represents the limitations of parameter j based on the standards for drinking water quality of China.
-
5.
Finally, EWQI is calculated by following Eq. (10):
According to the classification criteria of EWQI values (Amiri et al. 2014; Gorgij et al. 2017; Islam et al. 2017), groundwater quality is divided into five categories, which are excellent, good, medium, poor, and extremely poor, respectively, as shown in Table 4.
Groundwater quality evaluation for irrigation
The primary goal of evaluating the suitability of water for irrigation is to understand the salinity levels of the water, which affect the soil structure and the crops yield. The following criteria are used to identify the characteristics of water used for irrigation:
Sodium adsorption ratio
Sodium adsorption ratio (SAR) is one of the significant indicators for evaluating irrigation water quality (Ghouili et al. 2018; Srinivasamoorthy et al. 2014), which was used to indicate the degree of sodium hazard (alkali hazard) caused by irrigation water to soil or plants. It can be expressed by the ratio of Na+ concentration to the square root of the sum of Ca2+ and Mg2+ concentration, and the formula is as follows (Karanth 1987):
Soluble sodium percentage (%Na)
Soluble sodium percentage is used to evaluate the degree of sodium damage (Kumar et al. 2016), which is determined by the ratio of Na+ to (Na+ + K+ + Ca2+ + Mg2+). The calculation formula is as follows:
Residual sodium carbonate (RSC)
The suitability of groundwater for irrigation is affected by the total amount of carbonate and bicarbonate exceeding the total amount of calcium and magnesium in the water (Ghouili et al. 2018; Srinivasamoorthy et al. 2014).
Kelly’s ratio
Kelly’s ratio (KR) evaluates the suitability of water for irrigation by examining the balance between sodium ions, calcium ions, and magnesium ions. KR greater than 1 represents the excess Na+, indicating the unsuitability of water for irrigation. KR is calculated by the following formula (Kelly 1940):
Magnesium hazard (MH)
The physical properties of the soil will be affected due to the excessive absorption of magnesium. Therefore, the magnesium hazard, one of the important criteria, was used to assess the suitability of irrigation water (Szabolcs 1964). The MH is defined as the ratio of magnesium concentration to divalent cations (Ca2+ and Mg2+), which is calculated by the following formula:
Permeability index
The permeability of soil is affected by the sodium, magnesium, calcium, and bicarbonate in water (Saha et al. 2019). The permeability index (PI), proposed by Doneen (1964), provides a division scheme for irrigation water quality based on the ratio of sodium and bicarbonate to all cations, indicating the suitability of water used for irrigation. PI is calculated by the following formula:
where all units of the ions are in meq/L.
Results and discussion
Hydrogeochemical characteristics
Statistical analysis of hydrochemistry
Hierarchical cluster analysis
The ward’s linkage method and Euclidean distance are employed in HCA, which divides all groundwater samples into four clusters according to the hydrogeochemical characteristic. The dendrogram is obtained as shown in Fig. 2, representing that the four clusters are composed of 25, 30, 8, and 3 samples, respectively. Each cluster has similar hydrochemistry characteristics, but there are differences between each cluster.
The spatial distribution (Fig. S3) of the clustering results and TDS shows that cluster 3 and cluster 4 samples are located in areas where TDS is greater than 1000 mg/L (brackish water to saline water), while most of cluster 1 and cluster 2 samples are located in areas where TDS is less than 1000 mg/L (fresh water). From a spatial perspective, on the whole, clusters 3 and cluster 4 samples are located in the northwest of the study area, representing the groundwater characteristics of brackish water to saline water, while most of clusters 1 and cluster 2 samples are located in the southern and eastern of the study area, indicating that the groundwater is dominated by fresh water.
The box–whisker plot was used to identify the characteristics and differences of various hydrochemical parameters in different clusters. Figure 3 shows that the concentration of Na+, K+, Ca2+, Mg2+, SO42−, Cl−, NO3−, TH, and TDS represent the increasing trend from the cluster 1 to cluster 4. However, the pH value shows a decreasing trend. The average value of pH of the four clusters range from 7.46 (cluster 4) to 7.93 (cluster 1), indicating the weakly alkaline conditions in the groundwater. The weak alkalinity of groundwater may be caused by the reaction of groundwater with silicate minerals (potassium feldspar, albite); in addition, the evaporation and cation exchange (calcium and magnesium ions in groundwater and sodium ions in clay minerals) may have strengthened the weak alkalinity conditions (Dehbandi et al. 2019). The average values of the TDS of the four clusters are 685.4 mg/L, 690.1 mg/L, 3430.1 mg/L, and 7646.7 mg/L, respectively. According to the groundwater classification standard based on TDS (Fetter 1994). Cluster 1 and cluster 2 can be classified as the fresh water (TDS < 1000 mg/L) except for the TDS value of several samples (Q3, Q16, Q34, Q42, Q62, Q63, Q64) slightly greater than 1000 mg/L. Half of the samples in cluster 3 belong to brackish water (1000 mg/L < TDS < 3000 mg/L), while the other half of cluster 3 and all cluster 4 belong to saline water (> 3000 mg/L). Moreover, cluster 4 (Q25, Q65, Q67) has the highest concentration of Cl− and NO3− (Fig. 4), which may be caused by the human activities such as agricultural fertilization (Li and Gao 2019). Therefore, the cluster 4 can be identified as a human activity factor.
Correlation analysis
The correlation analysis reflects the interdependence between different hydrochemical parameters and was used to identify the similar sources of major ions with good correlation (Pant et al. 2018; Yin et al. 2021). As shown in Table 1, there is a strong positive relationship between Na+ and Cl− with the correlation coefficient of 0.78, indicating that the possibility of dissolution of halite. Na+ is significantly correlated with SO42− (correlation coefficient is 0.958), implying that the contribution of the dissolution of Glauber’s salt (Liu et al. 2015), which can be explained by the reaction (R3). Moreover, Ca2+ and SO42− show an excellent correlation with the correlation coefficient of 0.841, suggesting that the gypsum may be responsible for the hydrogeochemistry of groundwater as shown in reaction (R4). Mg2+ and SO42− also show the positive relationship with the correlation coefficient of 0.645, implying the dissolution of sulfur-bearing minerals.
Principal component analysis
Principal component analysis (PCA) extracted four principal components (PCs) with eigenvalues greater than 1 as shown in Table 2 and Fig. S4, and the cumulative explained variance accounted for 83.78%, indicating that PCA significantly reduced the dimensionality of the original complex hydrochemical datasets. Table 2 shows that there is a positive or negative relationship between most parameters and PCs, although some parameters have no significant correlation with the PCs. PC1 explains 55.71% of the total variance and shows a significant correlated relationship with Ca2+, Mg2+, Na+, K+, SO42−, Cl−, TDS, and TH, implying the factor controlling the increase of the salinity in groundwater (Kim et al. 2020). However, PC1, PC2, and PC3 explain 11.65%, 8.83%, and 7.59% of the total variance, respectively. PC3 and PC4 are positively correlated with F− and NH4+, while PC2 is negatively correlated with Fe. It can be preliminarily inferred that PC2 and PC4 may be controlled by anthropogenic activity factors.
It can be seen from Table 2 that the total variance explained by PC3 and PC4 is smaller than that of PC1 and PC2, which represents less importance. Therefore, PC1 and PC2 are used to represent the results of PCA to examine the rationality of using HCA for classification. Figure S4 shows the scores of all groundwater samples based on the HCA classification on the two principal components PC1 and PC2. Furthermore, four-ellipsis spatial cloud map of the four clusters is plotted. The four clusters are reasonably distinguished despite the marginal overlapping, which indicates that the classification of HCA is reasonable (Fig. S4).
Hydrogeochemical facies
The piper trilinear diagram, a graphical representation of groundwater chemistry data, is proposed by Piper (1944) and is widely used to understand the groundwater evolution (Subba Rao 2017). The piper diagram separates the anions and cations of the water samples and represents the dominant anions and cations in two triangles and then maps the combination of anions and cations in a diamond for identifying various hydrogeochemical types and hydrogeochemical facies of groundwater.
Figure 4 illustrates the hydrogeochemical facies and groundwater types. The triangle diagram of cations shows that the sodium is the leading cations in most groundwater samples, nearly half of groundwater samples are dominated by the cations of mixed type, while only few samples have cations dominated by calcium. On the anion plot, most of groundwater samples fall in the lower left corner, indicating that the bicarbonate plays a leading role in groundwater, and some of samples belong to mixed type. Moreover, only few samples are dominated by chloride, which may be due to the effect of halite dissolution and human activities. However, the overall hydrogeochemical characteristics of all samples are the sodium-dominated type in cation and bicarbonate-dominated type, respectively.
Given the characteristics of anions and cations, the overall hydrogeochemical characteristics can be expressed as the groundwater chemistry types. As shown by the central diamond in Fig. 4, the groundwater is characterized by the dominance of the alkaline earth elements (Ca + Mg) over the alkalis (Na + K) and the nearly balance of the weak acids (HCO3) and the strong acids (Cl + SO4). The diamond diagram shows that 37.9% of samples fall into the zone 1, which represents the Ca–HCO3 type. In comparison, one-third of the water samples fall into zone 3 (mixed type) and are divided into Ca–Mg–Cl type (24.2%) and Ca–Na–HCO3 type (9.1%). In particular, cluster 4 samples (Q25, Q65, Q67) are located in zone 4 and belong to Ca–Cl type. Moreover, 16.7% of samples fall into zone 2, representing the Na–Cl type. Only four samples fall into the zone 5 and are subclassified as Na–HCO3 type.
Controlling factors of hydrochemistry characteristics
Hydrochemical evolution process
Gibbs diagram (Gibbs 1970) is an effective tool in identifying the factors that control the hydrochemical characteristics of groundwater and is widely used to understand the formation process of hydrogeochemistry (Marandi and Shand 2018; Nazzal et al. 2014). The Gibbs diagram is represented by the plot of TDS versus (Cl−/Cl− + HCO3−) and TDS versus (Na+/Na+ + Ca2+) and divides the factors controlling the groundwater chemical processes into three aspects, which are evaporation crystallization, rock weathering, and precipitation, respectively. Figure 5 shows that all the cluster 3 and cluster 4 samples fall into evaporation crystallization dominance zone, indicating the possibility of evaporation and dissolution of evaporites, such as halite, gypsum, and mirabilite (Luo et al. 2018). Most of cluster 1 and cluster 2 samples fall within the rock weathering zone with the low TDS (< 1000 mg/L), suggesting that the water–rock interactions are the main hydrogeochemical formation mechanism in areas where cluster 1 and cluster 2 are located. Moreover, some of the cluster 1 and cluster 2 samples are located in evaporation dominance zone, which indicates that the possibility of evaporation or the existence of ancient brackish water (Li et al. 2018).
In order to further decipher the major weathering/dissolution mechanisms of the water–rock interactions processes, bivariate diagrams are used to explain which lithological minerals (carbonate, silicate, evaporite) are dissolved (Li et al. 2018; Luo et al. 2018). The sodium normalized ratios represented by Mg/Na, HCO3/Na, and Ca/Na were calculated. The relationships between (Mg/Na) and (Ca/Na), (HCO3/Na), and (Ca/Na) are plotted in the Gaillardet diagram (Gaillardet et al. 1999).
The Gaillardet diagram (Fig. 6) shows that most samples fall near the carbonate–silicate dissolution line and silicates weathering zone, suggesting that the dissolution of silicate plays a dominant role in groundwater chemistry. The albite, a common silicate, has the following weathering process (Ghouili et al. 2018):
Moreover, some samples fall on the transition zone of silicate weathering to carbonate weathering and the transition zone from silicates weathering to evaporites weathering, which indicates that the carbonates and the evaporites may be responsible for groundwater chemistry.
Water–rock interactions
Ion exchange
Chloro-alkaline indices (CAI)
Ion exchange is an essential process in water–rock interactions and plays a significant role in groundwater chemistry. In different hydrogeochemical conditions, an aquifer is composed of different minerals, resulting in the unique controlling factors of groundwater hydrochemistry. To better understand the ion exchange process that occurs between groundwater and its host environment during the process of residence or travel (Marghade et al. 2012), the chloro-alkaline indices (CAI-I and CAI-II) proposed by Schoeller (1977) have been widely used to identify the cation exchange process between Na, K and Ca, Mg. The formulas for calculating CAI are as follows:
where the units of all ions are meq/L.
The Na+ in groundwater is replaced by the Ca2+ or Mg2+ absorbed on the aquifer medium, indicating the reverse ion exchange (Zaidi et al. 2015). Correspondingly, the CAI-I and CAI-II are all greater than 0, which resulted in the increase of Ca2+ or Mg2+ concentration in groundwater. On the contrary, negative values of CAI-I and CAI-II imply that the Ca2+ or Mg2+ in groundwater replaced by the Na+ absorbed on the aquifer medium, suggesting the ion exchange may cause the increase of Na+ in groundwater. The ion exchange processes are as follows:
-
Ion exchange: Ca2+(Mg2+) + 2Na − X → 2Na+ + Ca(Mg) − X2
-
Reverse ion exchange: 2Na+ + Ca(Mg) − X2 → Ca2+(Mg2+) + 2Na − X
The relationship of CAI-I and CAI-II were plotted to discriminate the ion exchange process, as shown in Fig. 7a. It can be seen that 89.4% of the samples fall on the bottom left of plot, indicating the ion exchange, which leads to the enrichment of Na+ in groundwater. Moreover, 10.6% of samples that falls on the upper right represents the reverse ion exchange.
In order to further identify the ion exchange between groundwater and aquifer materials, the relationship between (Na+–Cl−) and (Ca2+ + Mg2+–HCO3−–SO42−) was established to examine the ion exchange of sodium and calcium. If there is a cation exchange, the slope of the fitted line representing the above relationship should be close to − 1 (Fisher and Mullican III 1997). In this study, the slope of the fitted line of (Na+–Cl−)/(Ca2+ + Mg2+–HCO3−–SO42−) is − 1.297, and 81.8% of the groundwater samples fall on the zone of ion exchange (Fig. 7b), which further confirmed the existence of cation exchange.
Ion ratio
The stoichiometric relationship between different ions can be used to identify the source of the main ions in groundwater (Liu et al. 2018). The relationship between Na+ and Cl− would be located on the 1:1 line if the halite is the only source of the Na+ and Cl−. Figure 7c shows that some of the cluster 1, cluster 2, and cluster 3 samples are near the 1:1 line, indicating the dissolution of the halite is responsible for the groundwater chemistry. However, most of cluster 1, cluster 2, and cluster 3 samples are above the 1:1 line, representing the excess Na+, which may be the contribution of the ion exchange and the silicate weathering (Zhu et al. 2011). The sodium normalized bivariate plots (Fig. 6) indicates that there may be silicate weathering. Moreover, the ion exchange may be the source of excess Na+, which may be responsible for the less Ca2+ over SO42− (Fig. 7d). However, cluster 4 samples are below the 1:1 line, and the chlorine excess may be caused by human activities such as agricultural fertilization (Deshmukh 2013).
The ratio of Ca/SO4 would be 1:1 if there is only the dissolution of gypsum in the groundwater. Most of samples are below the 1:1 line, indicating the dominance of SO42− over Ca2+. Meanwhile, the relationship between Mg2+ and SO42− exhibits the same characteristics (Fig. 7e), that is, excess SO42−. On the one hand, the dissolution of gypsum plays a major role in groundwater chemistry, which can be explained by the negative saturation indices of gypsum. In addition, the bicarbonate-bearing rock is oversaturated, calcite and dolomite will not dissolve to produce Ca2+ and Mg2+, and the Ca2+ forming the dissolution of gypsum tends to precipitate calcite and dolomite, which further reduces the concentration of Ca2+ and Mg2+.
The ratio close to 1 between (Ca2+ + Mg2+) and (HCO3− + SO42−) indicates that the dissolution of bicarbonate-bearing rocks (calcite and dolomite) and sulfate minerals (gypsum) are responsible for groundwater chemistry (Edmunds et al. 2002). Figure 7f represents that most samples are below 1:1 line of (Ca2+ + Mg2+) versus (HCO3− + SO42−) scatter plot with the deficient Ca2+ + Mg2+ over HCO3− + SO42−, indicating that the dissolution of silicate weathering and ion exchange (exchange of Ca2+ and Mg2+ in groundwater with Na+ on clay minerals) may be responsible for groundwater chemistry (Han et al. 2013). However, few samples fall above the 1:1 line, suggesting the possibility of the reverse ion exchange, which can be explained by the (Na+–Cl−) versus (Ca2+ + Mg2+–HCO3−–SO42−) scatter plot (Fig. 7b).
In order to further reflect the controlling effect of silicate minerals weathering on groundwater chemistry, the relationship between Na+ + K+ and TZ+ is used to indicate the weathering process of silicate minerals (Subramani et al. 2010). Figure 7g shows that most of samples are located near the Na + K = 0.5 × TZ line, indicating that the silicate weathering is involved in the geochemical process. However, some of samples are deviated from the that line, which may be the cause of ion exchange.
The formation mechanism of high salinity in groundwater
The groundwater is characterized by high salinity, which may be caused by the dissolution of minerals. Analysis of the correlation between salinity and Ca2+, Mg2+, Na+, Cl−, and SO42− shows that high salinity may come from the dissolution of halite, gypsum, anhydrite, sulfate, and sulfur-containing minerals (Liu et al. 2018).
Figure 8 shows that most of cluster 1 and cluster 2 samples have a high ratio Na+/Cl−, Ca2+/Cl−, Mg2+/Cl−, and Ca2+/SO42−, while they have a low salinity (< 2000 mg/L), suggesting that the mineral weathering (silicate weathering (Fig. 8a, carbonate weathering (Fig. 8d), evaporite dissolution (Fig. 8b), or cation exchange may be responsible for the groundwater chemistry. However, cluster 3 and cluster 4 samples are featured by the high salinity, indicating the possibility of evapotranspiration, halite dissolution (Fig. 8a), or gypsum dissolution (Fig. 8d) in groundwater belongs to cluster 3 and cluster 4.
Mineral dissolution processes
Saturation indices
In order to further examine the contribution of the mineral dissolution process to the groundwater hydrochemistry, the saturation indices (SI) can be used to indicate the saturation status of minerals in groundwater, when the solubility of the minerals in groundwater reaches the limit, and the ions in groundwater precipitate and reattach to the minerals. Moreover, SI can identify whether dissolution or precipitation has occurred, which was calculated by the following formula:
where IAP represents the value of ionic activity product, Ks represents the solubility product of different minerals. When SI is greater than 0, equal to 0 and less than 0, it denotes the oversaturation, equilibrium, and unsaturated state of minerals in groundwater, respectively (Boudot et al. 1996). The unsaturated state means that the minerals still have the potential to release ions into groundwater.
The typical minerals are selected to calculate the SI of various minerals based on the hydrogeochemical characteristics. Figure 9a shows that the SI of calcite and dolomite of all samples are greater than zero, suggesting that groundwater are oversaturated and has a trend of precipitation in term of calcite and dolomite. However, the SI of gypsum, anhydrite, and fluorite are less than zero, indicating that groundwater are unsaturated. In particular, the SI of halite is far below the zero (− 4.06~− 8.6), indicating that groundwater has the capacity to dissolve halite. The dissolution processes of various minerals are as follows (García et al. 2001):
Figure 9c–f illustrates the relationship of SI (Gypsum) versus (Ca2+ + SO42−), SI (halite) versus (Na+ + Cl−), SI (fluorite) versus (Ca2+ + F−), and SI (anhydrite) versus (Ca2+ + SO42−). The SI of gypsum, halite, and anhydrite show an increasing trend toward zero from cluster 1 to cluster 4, indicating a tendency toward a saturation state. Moreover, groundwater of cluster 1 and cluster 2 has the significant potential to dissolve gypsum, halite, fluorite, and anhydrite, which cause the hydrogeochemical characteristic.
Evaluation of groundwater quality for drinking
The EWQI method was used to evaluate the groundwater quality and the suitability of quaternary phreatic groundwater for drinking. According to the limits of various indicators in the standards for drinking water quality of China, the value of EWQI of all groundwater samples were calculated using Eqs. (3)–(10). Furthermore, the groundwater quality can be divided into five categories (excellent, good, medium, poor, extremely poor) based on the classification criteria of the EWQI values (Table 3), corresponding to the five ranks (I, II, III, IV, V) of the Quality Standard for Groundwater of China (Table S1, QSGC 2015). Table 4 shows the overall groundwater quality and classification results, and the EWQI values of all samples range from 16.30 to 549.81. The critical limit of EWQI is specified as 150; 6.1% and 10.6% of samples are classified as poor water and extremely poor water, suggesting that these samples are unsuitable for drinking purpose. Moreover, 47.0% and 31.8% of samples are classified as excellent water and good water, representing that these samples are suitable for drinking purpose. Meanwhile, only 4.5% of samples are classified as medium water, indicating the possibility of being used for drinking after additional treatment.
The results of groundwater quality categories based on the HCA show that out of 16 and 6 groundwater samples of cluster 1 are categorized as excellent water and good water, representing that the groundwater is suitable for drinking. Only 1 groundwater sample is categorized as poor water and is unsuitable for drinking, while 2 samples are categorized as medium water and can be used to drinking and agricultural purposes with the additional treatment. Moreover, all cluster 2 samples are classified as excellent water and are suitable for drinking. However, out of 7 samples of cluster 3 are categorized as poor or extremely water and are unsuitable for drinking, while only 1 sample is classified as medium water. In addition, all cluster 4 samples are categorized as extremely poor water and are unsuitable for drinking purpose.
In order to understand the spatial distribution characteristics of groundwater quality, the inverse distance weighting method in ArcGIS is used to interpolate EWQI, and the spatial distribution of EWQI is obtained. Then, the spatial distribution map of different hydrochemical parameters were plotted to further explain the spatial relationship between hydrogeochemistry and groundwater quality. Figure 10 shows that the spatial distribution characteristics of groundwater quality is characterized by high value of EWQI in the northwest and low value of EWQI in the east and south, indicating that the overall groundwater quality in the northwest of the study area is poor and not suitable for drinking, while the groundwater quality in the south of the study area is good and suitable for drinking purpose on the whole. According to the analysis of the hydrochemistry characteristics and evolution process of groundwater in the previous sections, it may be caused by the effects of cation exchange, evaporation, and precipitation. In addition, it is also related to the lithofacies paleogeographic conditions of the study area (Ding et al. 2021; Hou et al. 2008).
According to the classification limit in the Quality Standard for Groundwater of China (Table S1, QSGC 2015), the Na+, Cl−, SO42−, NO3−, TDS, and TH are classified as five ranks (I, II, III, IV, V). Figure 10 illustrates the relationship of the spatial distribution characteristics between the above six parameters and EWQI values. It can be seen that the samples with the rank 4 and rank 5 groundwater quality are located at the area where EWQI values are greater than the critical limit (150), indicating a good correlation between groundwater hydrochemical characteristics and groundwater quality based on EWQI.
Evaluation of groundwater quality for irrigation
Irrigation water quality parameters
The sodium adsorption ratio (SAR), soluble sodium percentage (%Na), residual sodium carbonate (RSC), Kelly’s ratio (KR), magnesium hazard (MH), and permeability index (PI) were calculated by Eqs. (11)–(16), as shown in Table 4. The RSC in the study area ranges from − 108.61 to 6.70 with the average of − 6.39. It can be seen that most of samples belong to safe category (77.27%), while some samples belong to the category of doubtful (10.61%) and unsuitable (12.12%). The KR values range from 0.10 to 6.75 with the average of 1.15, and most of samples are classified as suitable (68.18%), while the category of marginal suitable and unsuitable account for 19.7 and 12.12%. However, for the Mg hazard indicator, most of samples belong to harmful category (75.76%), while the rest of samples (24.24%) belong to good category. In addition, according to the value of PI, the water quality can be divided into three categories, which are class I (PI > 75%), class II (25% < PI < 75%), and class III (PI < 25%), respectively. The results (Table 4) show that 78.79% of groundwater samples belong to class I category, while 21.21% of samples belong to class II category. Therefore, the evaluation results based on the PI show that all groundwater samples are suitable for irrigation.
Sodium adsorption ratio and USSL diagram
The SAR evaluates the sodium hazard of excessive sodium in the water to the soil. The water with higher SAR is even more unsuitable for irrigation. Long-term use of high SAR water for irrigation will destroy the structure of soil and affect the permeability of soil, resulting in soil compaction (Salifu et al. 2017), thereby affecting crop yields. The water quality grades used for irrigation are divided into four categories according to various range of SAR. The evaluation results of irrigation water quality using SAR indicator show that all groundwater samples are excellent and good for irrigation, which accounted for 89.39 and 10.61%, respectively. Therefore, the sodium hazards are low and groundwater can be used for irrigation of most crops. Furthermore, the US salinity laboratory’s diagram (USSL diagram) is widely used to comprehensively evaluate the quality of irrigation water based on SAR (representing the sodium hazard) and EC (representing the salinity hazard). The USSL diagram is classified into 16 zones according to different classification standards of SAR and EC. Most samples fall on the zone of C3S1 (high-salinity hazard and low-sodium hazard) and C2S1 (medium-salinity hazard and low-sodium hazard), as shown in Fig. 11a, indicating that the necessity of drainage or harm to the soil and the suitability to all plants but drainage should be good, respectively (Madhav et al. 2018).
Percent sodium and Wilcox diagram
Long-term use of groundwater containing high concentration of sodium will diminish the soil permeability and reduce the crop yield (Marghade et al. 2021). The %Na values range from 8.88 to 86.97 with the average value of 45.69 (Table 4). Water quality classification based on %Na shows that 78.79% of groundwater samples are suitable for irrigation. Furthermore, the classification scheme based on %Na and EC, proposed by Wilcox (1948), was used to categorize groundwater quality for irrigation. The Wilcox diagram is divided into five zones, representing excellent to good, good to permissible, permissible to doubtful, doubtful to unsuitable, and unsuitable, respectively. Figure 11b shows that 15.2% of groundwater samples belong to unsuitable category, while the “doubtful to unsuitable” category accounts for 7.6%, indicating that these two categories are unsuitable for irrigation due to the low agricultural yield irrigated by these types of water (Ramesh and Elango 2012).
Conclusions
In this paper, multivariate statistical methods and hydrogeochemical methods are employed to identify the hydrochemistry characteristics and controlling mechanisms of the phreatic groundwater in the study area. Furthermore, using the EWQI method and multiple characteristic indicators to evaluate the suitability of groundwater for drinking and irrigation purposes, the conclusions are as follows:
-
1.
The hierarchical cluster analysis divides 66 groundwater samples into four clusters, and the principle component analysis is used to further examine the rationality of classification. Cluster 1 and cluster 2 are dominated by fresh water (TDS < 1000 mg/L), cluster 3 and cluster 4 are dominated by brackish water (1000–3000 mg/L)–salinity water (> 3000 mg/L). Furthermore, the Piper diagram determines the main hydrochemical facies: Ca–HCO3 type (cluster 1 and cluster 2), mixed type (mainly cluster 1 and cluster 2), Na–Cl type (mainly cluster 2 and cluster 3) and Ca–Cl type (cluster 4).
-
2.
The Gibbs diagram identifies the main controlling factors, which are evaporation crystallization (cluster 3 and cluster 4) and water–rock interactions (cluster 1 and cluster 2). Moreover, the saturation index combined with chloro-alkaline indices (CAI-I, CAI-II) indicates that silicate and evaporite weathering, dissolution of minerals (halite, gypsum, and anhydrite), and cation exchange are responsible for groundwater hydrochemistry. In addition, human activities may contribute to groundwater chemistry characteristics.
-
3.
The entropy water quality index method is used to evaluate the suitability of groundwater for drinking purpose and combined with ArcGIS to analyze the spatial distribution characteristics. The results reveal that groundwater quality is categorized as the excellent (47.0%), good (31.8%), medium (4.5%), poor (6.1%), and extremely poor (10.6%) water types, respectively. Therefore, 16.7% of samples are unsuitable for drinking. On the whole, the water quality in the southern part of the study area is better than that in the northwest.
-
4.
Multiple characteristic indicators are used to evaluate the suitability of groundwater for irrigation purpose. The USSL diagram shows that most of samples belong to C3S1 (high-salinity hazard and low-sodium hazard) and C2S1 (medium-salinity hazard and low-sodium hazard), indicating that the necessity of drainage or harm to the soil and the suitability to all plants but drainage should be good. Moreover, the Wilcox diagram shows that 22.8% of groundwater samples are doubtful or unsuitable for irrigation due to the high salinity of groundwater. Therefore, it is necessary to strengthen the continuous monitoring of groundwater quality and pay more attention to the variations of groundwater quality in the future for sustainable development and human health.
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
Adimalla N, Qian H (2019) Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, South India. Ecotoxicol Environ Saf 176:153–161
Adimalla N, Li P, Venkatayogi S (2018) Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environ Process 5(2):363–383
Amiri V, Rezaei M, Sohrabi N (2014) Groundwater quality assessment using entropy weighted water quality index (EWQI) in Lenjanat, Iran. Environ Earth Sci 72(9):3479–3490
Arumugam K, Elangovan K (2009) Hydrochemical characteristics and groundwater quality assessment in Tirupur Region, Coimbatore District, Tamil Nadu, India. Environ Geol 58(7):1509–1520
Ayed B, Jmal I, Sahal S, Mokadem N, Saidi S, Boughariou E, Bouri S (2017) Hydrochemical characterization of groundwater using multivariate statistical analysis: the Maritime Djeffara shallow aquifer (Southeastern Tunisia). Environ Earth Sci 76(24):1–22
Barescut J, Lariviere D, Stocki T, Wood M, Beresford N, Copplestone D (2011) Limit of detection values in data analysis: do they matter? Radioprotection 46(6):S85–S90
Belkhiri L, Boudoukha A, Mouni L, Baouz T (2010) Application of multivariate statistical methods and inverse geochemical modeling for characterization of groundwater-a case study: Ain Azel plain (Algeria). Geoderma 159(3-4):390–398
Boonkaewwan S, Sonthiphand P, Chotpantarat S (2021) Mechanisms of arsenic contamination associated with hydrochemical characteristics in coastal alluvial aquifers using multivariate statistical technique and hydrogeochemical modeling: a case study in Rayong province, eastern Thailand. Environ Geochem Health 43(1):537–566
Boudot J-P, Maitat O, Merlet D, Rouiller J (1996) Occurrence of non-monomeric species of aluminium in undersaturated soil and surface waters: consequences for the determination of mineral saturation indices. J Hydrol 177(1-2):47–63
Charlton SR, Parkhurst DL (2011) Modules based on the geochemical model PHREEQC for use in scripting and programming languages. Comput Geosci 37(10):1653–1663
Chen J, Huang Q, Lin Y, Fang Y, Qian H, Liu R, Ma H (2019) Hydrogeochemical characteristics and quality assessment of groundwater in an irrigated region, Northwest China. Water 11(1):96
Cloutier V, Lefebvre R, Therrien R, Savard MM (2008) Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J Hydrol 353(3-4):294–313
Cortes J, Muñoz L, Gonzalez C, Niño J, Polo A, Suspes A, Siachoque S, Hernández A, Trujillo H (2016) Hydrogeochemistry of the formation waters in the San Francisco field, UMV basin, Colombia–a multivariate statistical approach. J Hydrol 539:113–124
Croghan C, Egeghy PP (2003) Methods of dealing with values below the limit of detection using SAS. Southern SAS User Group, pp 22–24
Dehbandi R, Abbasnejad A, Karimi Z, Herath I, Bundschuh J (2019) Hydrogeochemical controls on arsenic mobility in an arid inland basin, Southeast of Iran: the role of alkaline conditions and salt water intrusion. Environ Pollut 249:910–922
Deshmukh KK (2013) Impact of human activities on the quality of groundwater from Sangamner area, Ahmednagar District, Maharashtra, India. Int Res J Environ Sci 2(8):66–74
Ding L, Yang Q, Yang Y, Ma H, Martin JD (2021) Potential risk assessment of groundwater to address the agricultural and domestic challenges in Ordos Basin. Environ Geochem Health 43(2):717–732
Doneen LD (1964) Notes on Water Quality in Agriculture, Water Science and Engineering
Edmunds W, Carrillo-Rivera J, Cardona A (2002) Geochemical evolution of groundwater beneath Mexico City. J Hydrol 258(1-4):1–24
Fendorf S, Michael HA, van Geen A (2010) Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science 328(5982):1123–1127
Fetter CW (1994) Applied hydrogeology. Englewood Cliffs, Prentice Hall
Fisher RS, Mullican WF III (1997) Hydrochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the northern Chihuahuan Desert, Trans-Pecos, Texas, USA. Hydrogeol J 5(2):4–16
Gaillardet J, Dupré B, Louvat P, Allegre C (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159(1-4):3–30
Gao Y, Qian H, Ren W, Wang H, Liu F, Yang F (2020) Hydrogeochemical characterization and quality assessment of groundwater based on integrated-weight water quality index in a concentrated urban area. J Clean Prod 260:121006
García GM, Del Hidalgo MV, Blesa MA (2001) Geochemistry of groundwater in the alluvial plain of Tucuman Province, Argentina. Hydrogeol J 9(6):597–610
Ghouili N, Hamzaoui-Azaza F, Zammouri M, Zaghrarni MF, Horriche FJ, de Melo MTC (2018) Groundwater quality assessment of the Takelsa phreatic aquifer (Northeastern Tunisia) using geochemical and statistical methods: implications for aquifer management and end-users. Environ Sci Pollut Res 25(36):36306–36327
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090
Gorgij AD, Kisi O, Moghaddam AA, Taghipour A (2017) Groundwater quality ranking for drinking purposes, using the entropy method and the spatial autocorrelation index. Environ Earth Sci 76(7):269
Güler C, Thyne GD (2004) Delineation of hydrochemical facies distribution in a regional groundwater system by means of fuzzy c-means clustering. Water Resour Res 40(12):455–474
Han Y, Wang G, Cravotta CA III, Hu W, Bian Y, Zhang Z, Liu Y (2013) Hydrogeochemical evolution of Ordovician limestone groundwater in Yanzhou, North China. Hydrol Process 27(16):2247–2257
He S, Wu J (2019) Relationships of groundwater quality and associated health risks with land use/land cover patterns: a case study in a loess area, northwest China. Hum Ecol Risk Assess 25(1-2):354–373
Heydarirad L, Mosaferi M, Pourakbar M, Esmailzadeh N, Maleki S (2019) Groundwater salinity and quality assessment using multivariate statistical and hydrogeochemical analysis along the Urmia Lake coastal in Azarshahr plain, North West of Iran. Environ Earth Sci 78(24):1–16
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37(3):300–306
Hossain M, Patra PK (2020) Water pollution index–a new integrated approach to rank water quality. Ecol Indic 117:106668
Hou GC, Liang YP, Su XS, Zhao ZH, Tao ZP, Yin LH, Yang YC, Wang XY (2008) Groundwater systems and resources in the Ordos Basin, China. Acta Geol Sin 85(5):1061–1069
Islam ARMT, Ahmed N, Bodrud-Doza M, Chu R (2017) Characterizing groundwater quality ranks for drinking purposes in Sylhet district, Bangladesh, using entropy method, spatial autocorrelation index, and geostatistics. Environ Sci Pollut Res 24(34):26350–26374
Jehan S, Khattak SA, Muhammad S, Ali L, Rashid A, Hussain ML (2020a) Human health risks by potentially toxic metals in drinking water along the Hattar Industrial Estate, Pakistan. Environ Sci Pollut Res 27(3):2677–2690
Jehan S, Ullah I, Khan S, Muhammad S, Khattak SA, Khan T (2020b) Evaluation of the Swat River, Northern Pakistan, water quality using multivariate statistical techniques and water quality index (WQI) model. Environ Sci Pollut Res 27(31):38545–38558
Jiang Y, Wu Y, Groves C, Yuan D, Kambesis P (2009) Natural and anthropogenic factors affecting the groundwater quality in the Nandong karst underground river system in Yunan, China. J Contam Hydrol 109(1-4):49–61
Jianhua W, Peiyue L, Hui Q (2011) Groundwater quality in Jingyuan County, a semi-humid area in Northwest China. E-J Chem 8(2):787–793
Karanth KR (1987) Ground water assessment: development and management. Tata McGraw-Hill Education, New York
Karunanidhi D, Aravinthasamy P, Deepali M, Subramani T, Bellows BC, Li P (2021) Groundwater quality evolution based on geochemical modeling and aptness testing for ingestion using entropy water quality and total hazard indexes in an urban-industrial area (Tiruppur) of Southern India. Environ Sci Pollut Res 28(15):18523–18538
Karunanidhi D, Aravinthasamy P, Deepali M, Subramani T, Roy PD (2020) The effects of geochemical processes on groundwater chemistry and the health risks associated with fluoride intake in a semi-arid region of South India. RSC Adv 10(8):4840–4859
Kelly WP (1940) Permissible composition and concentration of irrigated waters. Proc ASCF 66:607
Kim KH, Yun ST, Yu S, Choi BY, Kim MJ, Lee KJ (2020) Geochemical pattern recognitions of deep thermal groundwater in South Korea using self-organizing map: identified pathways of geochemical reaction and mixing. J Hydrol 589:125202
Kumar VS, Amarender B, Dhakate R, Sankaran S, Kumar KR (2016) Assessment of groundwater quality for drinking and irrigation use in shallow hard rock aquifer of Pudunagaram, Palakkad District Kerala. Appl Water Sci 6(2):149–167
Li C, Gao X (2019) Assessment of groundwater quality at Yuncheng Basin: denotation for the water management in China. Groundwater 57(3):492–503
Li P, Tian R, Xue C, Wu J (2017) Progress, opportunities, and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ Sci Pollut Res 24(15):13224–13234
Li D, Gao X, Wang Y, Luo W (2018) Diverse mechanisms drive fluoride enrichment in groundwater in two neighboring sites in northern China. Environ Pollut 237:430–441
Liu F, Song X, Yang L, Han D, Zhang Y, Ma Y, Bu H (2015) The role of anthropogenic and natural factors in shaping the geochemical evolution of groundwater in the Subei Lake basin, Ordos energy base, Northwestern China. Sci Total Environ 538:327–340
Liu Y, Jin M, Wang J (2018) Insights into groundwater salinization from hydrogeochemical and isotopic evidence in an arid inland basin. Hydrol Process 32(20):3108–3127
Logeshkumaran A, Magesh N, Godson PS, Chandrasekar N (2015) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci 5(4):335–343
Luo W, Gao X, Zhang X (2018) Geochemical processes controlling the groundwater chemistry and fluoride contamination in the Yuncheng Basin, China-an area with complex hydrogeochemical conditions. PLoS One 13(7):e0199082
Madhav S, Ahamad A, Kumar A, Kushawaha J, Singh P, Mishra P (2018) Geochemical assessment of groundwater quality for its suitability for drinking and irrigation purpose in rural areas of Sant Ravidas Nagar (Bhadohi), Uttar Pradesh. Geol Ecol Landsc 2(2):127–136
Marandi A, Shand P (2018) Groundwater chemistry and the Gibbs diagram. Appl Geochem 97:209–212
Marghade D, Malpe D, Zade A (2012) Major ion chemistry of shallow groundwater of a fast growing city of Central India. Environ Monit Assess 184(4):2405–2418
Marghade D, Malpe DB, Duraisamy K, Patil PD, Li P (2021) Hydrogeochemical evaluation, suitability, and health risk assessment of groundwater in the watershed of Godavari basin, Maharashtra, Central India. Environ Sci Pollut Res 28(15):18471–18494
Monjerezi M, Vogt RD, Aagaard P, Saka JD (2011) Hydro-geochemical processes in an area with saline groundwater in lower Shire River valley, Malawi: an integrated application of hierarchical cluster and principal component analyses. Appl Geochem 26(8):1399–1413
Nazzal Y, Ahmed I, Al-Arifi NS, Ghrefat H, Zaidi FK, El-Waheidi MM, Batayneh A, Zumlot T (2014) A pragmatic approach to study the groundwater quality suitability for domestic and agricultural usage, Saq aquifer, northwest of Saudi Arabia. Environ Monit Assess 186(8):4655–4667
Noshadi M, Ghafourian A (2016) Groundwater quality analysis using multivariate statistical techniques (case study: Fars Province, Iran). Environ Monit Assess 188(7):1–13
Pant RR, Zhang F, Rehman FU, Wang G, Ye M, Zeng C, Tang H (2018) Spatiotemporal variations of hydrogeochemistry and its controlling factors in the Gandaki River Basin, Central Himalaya Nepal. Sci Total Environ 622:770–782
Parkhurst DL, Appelo C (1999) User’s guide to PHREEQC (version 2): a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Water-Resour Investig Rep 99(4259):312
Patil VBB, Pinto SM, Govindaraju T, Hebbalu VS, Bhat V, Kannanur LN (2020) Multivariate statistics and water quality index (WQI) approach for geochemical assessment of groundwater quality-a case study of Kanavi Halla Sub-Basin, Belagavi, India. Environ Geochem Health 42(9):2667–2684
Peng C, He JT, Wang ML, Zhang ZG, Wang L (2018) Identifying and assessing human activity impacts on groundwater quality through hydrogeochemical anomalies and NO3−, NH4+, and COD contamination: a case study of the Liujiang River Basin, Hebei Province, PR China. Environ Sci Pollut Res 25(4):3539–3556
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans Am Geophys Union 25(6):914–928
QSGC (2015) Quality Standard for Groundwater of China. Ministry of Land and Resources of the People's Republic of China
Ramesh K, Elango L (2012) Groundwater quality and its suitability for domestic and agricultural use in Tondiar River basin, Tamil Nadu, India. Environ Monit Assess 184(6):3887–3899
Rao NS, Sunitha B, Adimalla N, Chaudhary M (2020) Quality criteria for groundwater use from a rural part of Wanaparthy District, Telangana State, India, through ionic spatial distribution (ISD), entropy water quality index (EWQI) and principal component analysis (PCA). Environ Geochem Health 42(2):579–599
Re V, Sacchi E, Mas-Pla J, Menció A, El Amrani N (2014) Identifying the effects of human pressure on groundwater quality to support water management strategies in coastal regions: a multi-tracer and statistical approach (Bou-Areg region, Morocco). Sci Total Environ 500:211–223
Saha S, Reza AHMS, Roy MK (2019) Hydrochemical evaluation of groundwater quality of the Tista floodplain, Rangpur, Bangladesh. Appl Water Sci 9(8):1–12
Salifu M, Aidoo F, Hayford MS, Adomako D, Asare E (2017) Evaluating the suitability of groundwater for irrigational purposes in some selected districts of the Upper West region of Ghana. Appl Water Sci 7(2):653–662
Schoeller H (1977) Geochemistry of groundwater. Groundwater studies, an international guide for research and practice. UNESCO, Paris, pp 1–18
Shahid SU, Iqbal J, Hasnain G (2014) Groundwater quality assessment and its correlation with gastroenteritis using GIS: a case study of Rawal Town, Rawalpindi, Pakistan. Environ Monit Assess 186(11):7525–7537
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27(3):379–423
Simões FS, Moriera AB, Bisinoti MC, Gimenez SMN, Yabe MJS (2008) Water quality index as a simple indicator of aquaculture effects on aquatic bodies. Ecol Indic 8(5):476–484
Singh KR, Dutta R, Kalamdhad AS, Kumar B (2019) Information entropy as a tool in surface water quality assessment. Environ Earth Sci 78(1):15
Singh G, Rishi MS, Herojeet R, Kaur L, Sharma K (2020) Evaluation of groundwater quality and human health risks from fluoride and nitrate in semi-arid region of northern India. Environ Geochem Health 42(7):1833–1862
Srinivasamoorthy K, Gopinath M, Chidambaram S, Vasanthavigar M, Sarma V (2014) Hydrochemical characterization and quality appraisal of groundwater from Pungar sub basin, Tamilnadu, India. J King Saud Univ Sci 26(1):37–52
Su H, Kang W, Xu Y, Wang J (2018) Assessing groundwater quality and health risks of nitrogen pollution in the Shenfu mining area of Shaanxi Province, northwest China. Expos Health 10(2):77–97
Subba Rao N (2017) Controlling factors of fluoride in groundwater in a part of South India. Arab J Geosci 10:524
Subramani T, Rajmohan N, Elango L (2010) Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environ Monit Assess 162(1):123–137
Szabolcs I (1964) The influence of irrigation water of high sodium carbonate content on soils. Agrokém Talajt 13(sup):237–246
VanTrump G Jr, Miesch AT (1977) The US Geological Survey RASS-STATPAC system for management and statistical reduction of geochemical data. Comput Geosci 3(3):475–488
Varol S, Davraz A (2015) Evaluation of the groundwater quality with WQI (Water Quality Index) and multivariate analysis: a case study of the Tefenni plain (Burdur/Turkey). Environ Earth Sci 73(4):1725–1744
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Ganthi RR, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2010) Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environ Monit Assess 171(1):595–609
Ward JH (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58(301):236–244
Wilcox LV (1948) The quality of water for irrigation use. United States Department of Agriculture, Economic Research Service
Wu C, Wu X, Qian C, Zhu G (2018) Hydrogeochemistry and groundwater quality assessment of high fluoride levels in the Yanchi endorheic region, Northwest China. Appl Geochem 98:404–417
Yang Q, Zhang J, Wang Y, Fang Y, Martín JD (2015) Multivariate statistical analysis of hydrochemical data for shallow ground water quality factor identification in a coastal aquifer. Pol J Environ Stud 24(2):769–776
Yang J, Ye M, Tang Z, Jiao T, Song X, Pei Y, Liu H (2020) Using cluster analysis for understanding spatial and temporal patterns and controlling factors of groundwater geochemistry in a regional aquifer. J Hydrol 583:124594
Yin Z, Luo Q, Wu J, Xu S, Wu J (2021) Identification of the long-term variations of groundwater and their governing factors based on hydrochemical and isotopic data in a river basin. J Hydrol 592:125604
Zaidi FK, Nazzal Y, Jafri MK, Naeem M, Ahmed I (2015) Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: a case study from northwestern Saudi Arabia. Environ Monit Assess 187(10):1–18
Zereg S, Boudoukha A, Benaabidate L (2018) Impacts of natural conditions and anthropogenic activities on groundwater quality in Tebessa plain, Algeria. Sustain Environ Res 28(6):340–349
Zhu B, Yang X, Rioual P, Qin X, Liu Z, Xiong H, Yu J (2011) Hydrogeochemistry of three watersheds (the Erlqis, Zhungarer and Yili) in northern Xinjiang, NW China. Appl Geochem 26(8):1535–1548
Acknowledgements
The authors would like to thank the editor and reviewers for their constructive suggestions.
Funding
The research was financially supported by the Inner Mongolia Science and Technology Major Project (2020ZD0020-1), the National Natural Science Foundation of China (41972259), the Open Project Program of the Shandong Provincial Lunan Geo-engineering Exploration Institute (LNY2020-Z01), the Natural Science Foundation of Hebei Province (E2020402087), and the National Key Research and Development Program of China (2018YFC0406404).
Author information
Authors and Affiliations
Contributions
Xiong Wu and Xiao Zhang were responsible for methodology, conceptualization, reviewing, and editing. Xiao Zhang and Zhao Rong were responsible for data processing, analysis, visualization, writing—original draft preparation, and writing—reviewing and editing. Wenping Mu was responsible for writing—reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable since the manuscript has not been involved the use of any animal or human data or tissue.
Consent to participate
All authors gave informed and consent to participate in the article.
Consent for publication
All authors consent to the publication of the article.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Xianliang Yi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1732 kb)
Rights and permissions
About this article
Cite this article
Zhang, ., Zhao, R., Wu, X. et al. Hydrogeochemistry, identification of hydrogeochemical evolution mechanisms, and assessment of groundwater quality in the southwestern Ordos Basin, China. Environ Sci Pollut Res 29, 901–921 (2022). https://doi.org/10.1007/s11356-021-15643-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15643-2