Abstract
p-Nitrophenol (PNP) is the main end product of organophosphorus insecticides and a derivative of diesel exhaust particles. In addition to its unfavorable impact on reproductive functions in both genders, it also has various harmful physiological effects including lung cancer and allergic rhinitis. The identification of the cellular readout that functions in metabolic pathway perpetuation is still far from clear. This research aimed to study the impact of chronic PNP exposure on the health condition of the liver in Japanese quails. Quails were exposed to different concentrations of PNP as follows: 0.0 (control), 0.01mg (PNP/0.01), 0.1mg (PNP/0.1), and 1mg (PNP/1) per kg of body weight for 2.5 months through oral administration. Liver and plasma samples were collected at 1.5, 2, and 2.5 months post-treatment for biochemical, histopathology, and immunohistochemistry assessment. The plasma aspartate aminotransferase (AST) level was assessed enzymatically. The livers were collected for histopathology, glycogen accumulation, proliferating cell nuclear antigen (PCNA), and apoptosis assessment. Our results revealed an irregularity in body weight due to the long-term exposure of PNP with a significant reduction in liver weight. PNP treatment caused histopathological alterations in the hepatic tissues which increased in severity by the long-term exposure. The low dose led to mild degeneration with lymphocytic infiltration, while the moderate dose has a congestion effect with some necrosis; meanwhile severe hepatocyte degeneration and RBCs hemolysis were noticed due to high dose of PNP. Glycogen accumulation increased in hepatocytes by prolonged exposure to p-Nitrophenol with the highest intensity in the group treated by the high dose. Moderate and high doses of PNP resulted in a significant increase in apoptosis and hepatocytes’ proliferation at the different time points after treatment. This increase is markedly notable and maximized at 2.5 months post-treatment. The damage occurred in a time-dependent manner. These changes reflected on the plasma hepatic enzyme AST that was clearly increased at 2.5 months of exposure. Therefore, it could be concluded that PNP has profound toxic effects on the liver in cellular level. Taking into consideration the time and dose factors, both have a synergistic effect on the accumulation of glycogen, apoptosis, and cellular proliferation, highlighting the power of cellular investigation which will potentially open the door for earlier medical intervention to counteract this toxicity. Collectively, PNP could have critical hurtful effects on the health of human beings, wild animals as well as livestock.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorus compounds (OPs) are extensively used insecticides in the agriculture and veterinary fields (Ahmed et al. 2015b). One of the major metabolites of the organophosphorus pesticides is the p-Nitrophenol (PNP) (Abu-Qare et al. 2001; Ahmed et al. 2015a) which is beneficially used in the pharmaceutical industry and manufacture of some dyes and fungicides (Registry) 1992). On the other hand, the extensive and illegal usage of OPs insecticides leads to the PNP bioaccumulation (Abu-Qare et al. 2001; Uzunhisarcikli et al. 2007) which is a persistent organic pollutant (Banik et al. 2008; Zhang et al. 2010) in addition to its biodegradation resistance property (Call et al. 1980). p-Nitrophenol (PNP) results in disturbances of the ecosystem and appearance of the toxicity signs on different animals, fish, and birds (Kuang et al. 2020a; Zhang et al. 2015). 4-Nitrophenol (4-NP) has been also detected in the human urine samples, and so it has been classified as a priority toxic pollutant. The birds suffer from PNP toxicity through feeding on the sprayed seeds and plants and drinking of the PNP contaminated water (Ahmed et al. 2015a). Furthermore, the residues of these OPs in animal products pose a serious threat to the health of human being through their mutagenic potential and toxicity (Sodhi et al. 2006). A plethora of previous research on PNP toxicity mainly focused on the reproductive system (Li et al. 2006; Li et al. 2009) as the PNP is considered to be one of the endocrine-disrupting compounds affecting the signaling pathways of steroidal hormones (Zhang et al. 2013). Along with its anti-androgenic and estrogenic activities, it can disturb the hypothalamus-pituitary-gonadal axis (Zhang et al. 2013). Although ample shreds of evidence are clarifying the massive crosstalk between the endocrine and other organs (Ottinger et al. 2009), there is seldom research about PNP toxic effect on the other body systems. This dynamic link gives the potential to disrupt the development and function of a myriad of organs (Ahmed et al. 2015b; Ottinger et al. 2009) such as the liver. p-Nitrophenol (PNP) is mainly metabolized in the liver, before its excretion from the body, through glucuronidation and sulfation via microsomal hepatic enzymes such as CYP 450 enzymes (Machida et al. 1982; Mizuma et al. 1983). So, it is very important to study the toxic effect of PNP in the liver where it is detoxified. Previous studies showed that PNP induced the damage of hepatic parenchyma with sever vacuolation (Kuang et al. 2020b; Lam et al. 2013), and also it can interfere with the CYP 450 with modulation of heme oxygenase HO-1 expression (Ahmed et al. 2015b). However, the mechanistic studies and deep explanation of the hepatotoxic effect of PNP are still limited and under investigation.
In this vein, this study aims to evaluate the probable hepatic toxicity associated with PNP exposure and the possible affected pathways through assessing liver function enzyme, histopathological changes, glycogen accumulation, liver cell proliferation, and rate of apoptosis. To perform our target, the Japanese quail (Coturnix japonica) was used as an experimental and biological model due to its fast development (Sedqyar et al. 2008), and it was accepted as a model to assess the acute and chronic toxic effects of pesticides as well as other chemicals (OECD 1993; US-EPA 1996). Moreover, quails are vulnerable to OPs and their metabolic toxicity as it has been intensively used in different formulations such as fumigant and contact formulas in veterinary farms (Kwong 2002). Additionally, the quail and chicken are characterized by their low catalytic activity due to the low level of CYP 450 enzymes in their livers (Abou-Donia and Nomeir 1986; Hansen et al. 2011). To sum up, both the unregulated accumulation of PNP and its moderate level of clearance potentiation in quail made the quail an interesting domesticated economic bird for studying the harmful effects of PNP (Ball and Balthazart 2010).
Materials and methods
Chemicals
The p-Nitrophenol (PNP) crystal (4-Nitrophenol; CAS No. 100027 with purity >99.9% and molecular weight at 139.11 g/mol) was used in the study (Kasei Kogyo Co. Ltd, Japan). ApopTag peroxidase detection kit and DAB Substrate Kit were purchased from Sigma-Aldrich Co. Louis St., MO, USA. PCNA (proliferating cell nuclear antigen) kit was purchased from Invitrogen, CA, USA. Xylene and paraformaldehyde were purchased from Sigma-Aldrich Co. Louis St., MO, USA. Phosphate-buffered saline (PBS) was purchased from Lonza, USA, and ethanol from Fisher Scientific, UK. Other chemicals were of analytical grade and commercially available.
Birds’ management
Twenty-eight day-old male Japanese quails (80–90g) were used to perform our study. These birds were treated humanely following the established guidelines and principles of the Faculty of Veterinary Medicine, Suez Canal University, Egypt, which is approved by the ethics committee for using laboratory animals’ experiments (protocol # 2019-056). To alleviate the suffering, the used birds in this experiment were housed in a comfortable environment in metal cages with conditions of 24 ± 2 °C temperature, 50 ± 10% humidity, and 0500–1900 h lights on (Moregaonkar et al. 2011). Birds were provided with food and water ad libitum following the quail nutrition conditions reviewed by Shim and colleagues (Shim and Vohra 1984).
Experimental design
Male Japanese quails were divided into four random groups, each one consists of n=25–26. The four groups subscribed as follows: control group (quails were daily administered PBS orally for 2.5 month), PNP/0.01 group (daily administered low dose of PNP at concentration of 0.01mg/kg of body weight), PNP/0.1 group (daily administered mid-dose of PNP at concentration 0.1mg/kg of body weight), and PNP/1 group (daily administered high dose of PNP at concentration 1mg/kg of body weight). p-Nitrophenol (PNP) was dissolved in 0.01 M PBS pH 7.2 and offered orally to quail using a plastic stomach tube daily for 2.5 months. These doses were selected relying on the pharmacokinetics of p-Nitrophenol (PNP) (Abu-Qare et al. 2000; Machida et al. 1982) and from previous studies (Li et al. 2006; Zhang et al. 2013).
Samples collection
Quails were weighed individually every 15 days. At different time intervals post-treatment (1.5, 2, and 2.5 months), seven quails from each treated group were collected and euthanized by decapitation. Blood samples were collected in heparinized plastic tubes for the biochemical test. The blood samples were centrifuged for 15 min at 1700×g at 4 °C for plasma separation. The liver was rapidly dissected out, washed using saline solution, weighed, and fixed for histopathology and immunohistochemistry examination.
Aspartate aminotransferase biochemical test
The separated plasma samples were stored at –20°C for aspartate aminotransferase (AST) measurement with enzymatic methods (Huang et al. 2006; Rej and Shaw 1984) by using DRI-CHEM 7000 veterinary chemical analyzer.
Tissue preparation for histopathology and immunohistochemistry
The liver was fixed in 4% PFA pH 7.4 (paraformaldehyde in PBS) for 24 h. Fixed tissues were dehydrated through passaging through a consequent series of ethanol with graded concentrations from 70 to 100%. The completely dehydrated tissues were clarified in three changes of xylene and then embedded in paraffin. The paraffinized tissues were sectioned at 4μm thickness. Slides were deparaffinized using xylene, rehydrated again, and washed twice with distilled water.
Histopathological examination
Liver sections were stained using hematoxylin and eosin (H&E) stain to evaluate the histopathological alteration caused by different doses of PNP (Bancroft and Gamble 2008). Sections were examined using light microscopy.
Glycogen accumulation detection
For additional pathological alteration detection, the periodic acid-Schiff (PAS) staining was applied to detect the glycogen accumulation with hematoxylin counterstaining. PAS staining intensity was scored depending on the percentage of positively stained cells, using a 4-point scales as 0 (0–15%), 1 (16–25%), 2 (26–50%), or 3 (76–100%) (Lu et al. 2013). Moreover, and to confirm our results and measurement of glycogen content, we quantified the glycogen content using the open-source ImageJ software to determine the area fraction of pink color in the image. To achieve that, we referred to Karami et al. (2016) as random pictures were equally measured from different replicates from each treatment. The staining area values were produced after using the same lower and an upper threshold of 0–145, respectively, for each picture.
Apoptosis assay and cell proliferation
Apoptosis biomarker was detected using in situ ApopTag peroxidase detection assay depending on DNA modification that utilizing the terminal deoxynucleotidyl transferase (TdT) in labeling the apoptotic cells. Briefly, freshly diluted proteinase K (20μg/ml) was applied for 15 min at room temperature on pre-prepared sections in a copulin jar. The specimens were washed twice with distilled water for 2 min each. The endogenous peroxidase activity was quenched using 3.0% H2O2. The specimen was rinsed twice with PBS for 5 min each time. Immediately apply the equilibration buffer directly on the specimen and incubate them for at least 10 s at room temperature. Gently tap off excess liquid and carefully pipette onto the section working strength TDT enzyme. The slides were incubated in a humidified chamber at 37°C for 1 h. The specimen was put in a copulin jar containing working strength stop/wash buffer. The specimens were washed in 3 changes of PBS for 1 min each. Anti-digoxigenin conjugate was applied to the slide, incubated in a humidified chamber for 30 min at room temperature, and washed in 4 changes of PBS for 2 min each. The antibody binding was visualized through DAB Substrate Kit utilizing the diaminobenzidine as a chromogen substrate. Look at the slide under the microscope to determine the optimal staining time for color development. The normal rabbit IgG antibody was used as a negative control to examine the specificity of the antibody (Crosby et al. 2016). The positively stained cells were quantified in twenty and ten randomly selected fields (using magnification power ×200) for apoptosis and PCNA assays, respectively. The average number of positive cells per each slide was determined.
For cell proliferation evaluation, the previously prepared sections were treated with 3.0% H2O2 in a copulin jar to quench the endogenous peroxidase activity. Then, they were heated in (0.01 M) citrate buffer at 120 °C for 20 min to retrieve antigen. The sections were cooled and washed three times with 0.01 M PBS, pH 7.2, and then subjected to blocking buffer to prevent nonspecific binding of the primary antibody using rabbit normal serum in 0.01 M PBS for 30 min at room temperature. Finally, they were stained using a PCNA (proliferating cell nuclear antigen) kit following the manufacturer’s instructions (Kayano et al. 1992). Briefly, the sections were incubated overnight in a humidified chamber at 4°C with polyclonal first antibodies which were diluted in 0.01 M PBS at ratio a 1:500. Then the sections were treated with 0.25% biotinylated second antibody for 60 min at room temperature. After that, they were washed three times with PBS. Subsequently, they were incubated with 2% (v/v) avidin-biotin complex in PBS pH 7.4 for 30 min at room temperature. Afterward, the horseradish peroxidase was added for 30 min. The bindings of the antibodies were visualized using diaminobenzidine as a chromogen substrate. Tissue sections were counterstained with hematoxylin, dehydrated, cleared, mounted, and covered with coverslips. The specificity of the antibody was examined using normal rabbit IgG instead of the first antibody.
Statistical analysis
The statistical assessment was performed using the GraphPad Prism software (version 9, San Diego, USA) based on one-way ANOVA and Tukey’s test. All data are presented as means ± SEM, and the probability value of P<0.05 was estimated as a significant level. Histopathological data were presented as means of the number of positive cells in different quail’s liver samples (n=7) of the different groups ± SEM.
Results
Effect of p-Nitrophenol on body weight and relative liver weights (%)
Administration of PNP for 2.5 months did not lead to significant changes in body weight (P>0.05). Nevertheless, it caused irregularity in body weight as shown in Table 1. The liver weight at 1.5 months showed significant decrease in both PNP/0.01 (1.76 ± 0.07, P<0.05) and PNP/1.0 groups (1.67 ± 0.02, P<0.01) compared to the control group (2.04 ± 0.08). On the other hand, the PNP/0.1 group after 2.5 months showed an abrupt significant (P<0.05) increase (3.35 ± 0.27) (Table 2) in comparison to the control birds (2.61 ± 0.14).
Effect of p-Nitrophenol on plasma aspartate aminotransferase
Plasma aspartate aminotransferase (AST) activity was used mainly as a marker of liver injury in addition to other organs’ inflammation. Administration of PNP for 2.5 months led to an increase of plasma AST at 1.5 and 2 months and became noticeably clear at 2.5 months in all treated groups versus the control group as shown in Table 3. This increase was very high at 2.5 months in the mid-dose 503.0 U/l and the high dose 350.0 U/l versus 204 U/l in the control group.
Effect of p-Nitrophenol on liver histology
Histopathology at 1.5 months
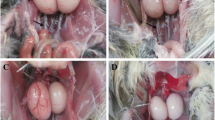
In general, liver morphology and architecture were normal in the control group at all collections time (Fig. 1. A1, A2, A3). Regarding the lowest dose at 1.5-month collection, PNP treatment induces focal lymphocytic infiltration (Fig. 1. B1). In the PNP/0.1group, the PNP caused multifocal areas of necrosis surrounded by lymphocytes aggregation (Fig. 1. C1). In the PNP/1-treated group, multiple areas of blood vessels congestion as well as focal lymphocytic infiltration with mild hepatocytes degeneration were seen clearly (Fig. 1. D1).
Histopathology of liver sections of the control and the p-Nitrophenol-treated Japanese quail after 1.5, 2, and 2.5 months post-treatment. In the control group, the liver cell shows normal cell architecture without any structural abnormality. In the PNP/0.01-, PNP/0.1-, and PNP/1-treated groups, the liver shows lymphocytic infiltration (black arrow), degeneration of hepatocytes (green arrow), and congestion of blood vessels (blue arrow). Scale bars = 100 μm at 1.5 month and =50 μm at 2 and 2.5 months (Li, lymphocyte infiltration, nec, necrosis; cong, congestion; and pig, brownish pigmentation)
Histopathology at 2 months
After 2 months of the PNP exposure, the PNP/0.01-treated group showed focal lymphocytic infiltration and mild degeneration of hepatocytes (Fig. 1. B2). In the PNP/0.1-treated group, severe congestion of portal blood vessels with a mild biliary cirrhosis and leukocytic infiltration were recorded (Fig. 1. C2). At PNP/1-treated group, we found severe congestion of blood vessels, lymphocytic aggregation, with moderate degeneration, and necrosis of hepatocytes especially around blood vessels (Fig. 1. D2).
Histopathology at 2.5 months
At 2.5-month collection, the low dose showed congestion of portal blood vessels with peri-lobular cirrhosis and mild degeneration of hepatocytes (Fig. 1. B3). The mid-dose showed severe congestion of blood vessels, degeneration of hepatocytes with some necrotic cells, and severe peri-lobular cirrhosis (Fig. 1.C3). Furthermore, in the high dose (PNP/1 mg/kg), severe congestion of blood vessels in portal areas with hemolysis of RBCS, presence of golden-brown pigmentation of hemosiderosis, and severe degeneration of hepatocytes was seen clearly (Fig. 1. D3).
Liver glycogen accumulation
Investigating the difference between the glycogen metabolic profile in the control and PNP groups (Fig. 2), we noticed a slight non-significant increase in glycogen accumulation at all doses at 1.5 months. Furthermore, the glycogen accumulation significantly (P<0.01) increased at 2 months post-PNP administration without showing dose-dependent manner. This increment becomes highly significant at 2.5 months post-exposure at PNP/0.01, PNP/0.1, and PNP/1 groups. In general, the accumulation increased with time in “a time-dependent manner” as shown in Fig. 2.
Periodic acid-Schiff staining analysis of liver sections of both the control and the p-Nitrophenol-treated quails with different doses (PNP/0.01, PNP/0.1, and PNP/1mg/kg) at different time points (1.5, 2, 2.5 months). Accumulated glycogen appeared in purple color (a). Scale bars = 100 μm. b PAS stain was scored depending on the intensity of the positively stained cells that ranged from 0 (0–15%), 1 (16–25%), 2 (26–50%), or 3 (76–100%). c Quantitative periodic acid-Schiff (PAS) staining analysis of quail’s liver treated with PNP at various time intervals depending on the area fraction %
Liver apoptosis and cell proliferation
Japanese quails that were treated with PNP for 2.5 months had significantly over-expression of apoptotic cells than the control birds as shown in Fig. 3. At 1.5 months, the apoptotic cells still within the control level but with time the apoptotic cells increased significantly (P<0.05) at 2.5 months in the PNP/0.1- and PNP/1-treated groups. Cell proliferation gradually increased in the birds orally administered 0.01 mg/kg of the PNP (PNP/0.01), as indicated by PCNA staining. However, birds that were exposed to 0.1 and 1.0 mg/kg showed the strongest PCNA staining at 1.5 and 2.5 months (P<0.01) followed by 2 months (P<0.05) as shown in Fig. 4. Taken together, data suggest that chronic exposure to PNP, taking into consideration the time and dose factors, has a synergistic effect on the accumulation of glycogen, apoptosis, and cellular proliferation.
Representative apoptotic stained liver section from the control and p-Nitrophenol-treated quails with different doses (PNP/0.01, PNP/0.1, and PNP/1 mg/kg) at different time points (1.5 and 2.5 months). Positive apoptotic cells show brown nuclei, scale bars = 20 μm (a). Quantitative analysis of apoptotic cells in the liver of male quail after 1.5 and 2.5 months. The positive cells were counted in 20 fields for each sample (b)
PCNA stained liver section from the control and the p-Nitrophenol-treated quails with different doses (PNP/0.01, PNP/0.1, and PNP/1 mg/kg) at different time points (1.5, 2, 2.5 months). Positive cells have brown nuclei, scale bars = 50 μm (a). The number of positive stained cells in different treated groups at different time points (b)
Discussion
In recent years, environmental pollution has emerged as one of the main reasons implicated in health problems such as liver (Ahmed et al. 2015b) and reproductive impairment (Li et al. 2006; Li et al. 2009; Taneda et al. 2004), lung cancer (Ichinose et al. 1997), and bronchial asthma-like disease (Sagai et al. 1993). Extensive use of p-Nitrophenol and its low degradation rate caused several toxicological effects on human beings that is the reason for listing the PNP and its derivatives as a top pollutant with limitation of their concentration in natural water to no more than 10 μg/l as stated by the United State Environmental Protection Agency (US EPA 1987; WWAP. 2019). Despite our previous findings that p-Nitrophenol might cause hepatotoxicity by disturbing the cytochromes P450 and oxidative stress enzymes (Ahmed et al. 2015b), the toxicological effect and the mechanism of PNP to induce liver toxicity is still unclear. This prompted us for more investigation about the PNP effect on the liver by expanding the study with precise details to explore other hepatic enzymes and cellular proliferation as well as apoptosis. Here we treated groups of Japanese quails with different doses of the PNP aiming to examine its hepatotoxicity along with different times of exposure. Administration of PNP with different concentrations for 28 male Japanese quail did not show an obvious alteration in the body weight during the 2.5 months of exposure in agreement with the previously reported data showing that PNP treatment had no change in body weight of the immature rats (Li et al. 2006). However, the relative liver weights (%) were significantly changed in a dose-independent manner. This is consistent with a previous study on one of the nitrophenol compound in the DEP (3-methyl-4-nitrophenol, PNMC) which caused a significant reduction of testis weight and did not show a dose dependency in treated rats (Yue et al. 2011). The liver is a vital organ for metabolism and detoxification of numerous toxicants (Timbrell 2020). Mainly, the liver injury or inflammation manifested through the morphological changes which are usually accompanied by biochemical and histopathological alterations indicating the hepatotoxicity. In this study and confirming our previous observations (Ahmed et al. 2015b), upon exposure to PNP, the parenchymal structure of hepatocytes was significantly damaged, and we noted that liver injury was manifested by hepatocyte vacuolation, dilatation, and congestion. Deep investigations showed that the degeneration level increased with higher concentration of PNP along with the increasing of exposure time. The effect of low dose started by lymphocytic infiltration and by time caused congestion and mild degeneration; meanwhile the exposure to moderate dose led to lymphocytic aggregation after 1.5 months and mild biliary cirrhosis along with congestion after 2.5 months of treatment. On the other hand, the high dose severely affected the liver within short duration causing congestion with some necrosis which is maximized by prolonged exposure time leading to hemolysis and severe hepatocyte degeneration. Chen et al. reported the same effect of exposure duration and dose concentration (Chen et al. 2016). Our results suggested that PNP even at low concentrations with long-term exposure can lead to liver function damage. Kuang and his colleague reported that exposure of marine fish to low PNP dose resulted in liver congestion, slight parenchyma disorganization, as well as pyknotic nuclei. And at high PNP concentrations, they noted significant liver vacuolation, parenchyma disorganization, with irregular cell arrangement, pyknotic nucleus, and loss of original architecture (Kuang et al. 2020a). These histopathological alterations are a kind of defense and stress response against external pollutants proving the chronic harmful effect of the PNP.
Another indicator of hepatotoxicity is the enzymatic level in the circulating plasma including the metabolic enzymes such as cytochrome (CYP) system. Previous studies showed that fluctuations in the mRNA expression level of some genes belong to metabolism as CYP 1B1, 1A4, and 1A5 which interpreted as a way to overcome the toxicity effect (Ahmed et al. 2015b). Here, we mainly focused on the enzymes reflecting the degree of liver disease and toxicity such as aminotransferase enzymes. These catalytic enzymes convert the amino acids into 2-oxo acids through transferring of amino groups such as aspartate amino transferase (AST) and alanine amino transferase (ALT) (Esani 2014). AST is highly expressed in the liver and other tissues including skeletal muscle and cardiac tissue with small expression in the pancreas, kidney, and erythrocytes; meanwhile the predominant expression of ALT is in the hepatic tissue (Kunutsor et al. 2013). Upregulation of the aminotransferases activities in the plasma indicate the loss of functional integrity of cell membranes in the liver cells and cellular leakage with cytoplasmic release of the enzymes. This is depending on the fact that metabolic enzymes are normally allocated in the cytoplasm and they are released into the circulation in case of cellular damage (David and Zafar 1990). ALT expression is higher in the acute inflammatory conditions; meanwhile the chronic hepatitis cases is accompanied by higher activity level of serum AST as in case of cirrhosis and alcoholic hepatitis (David and Zafar 1990; Esani 2014). In accordance with the histopathological findings, our results revealed an elevation in the AST level which increased proportionally with the concentration and duration of exposure to the p-Nitrophenol. These findings of AST increase suggested the chronic inflammatory effect of PNP long-term exposure which can reflect the necrosis and hepatic cellular degeneration as well as releasing the mitochondrial AST (David and Zafar 1990). This is in accordance with the study reported that deltamethrin (synthetic pyrethroid insecticide and acaricide) led to an elevation in the serum level of liver function enzymes (AST and ALT) in rats (Abdel-Daim et al. 2013). Also, challenged broilers with infectious bursal disease (IBD) virus at 28 days old showed an increase in liver AST to 390 ± 27 U l−1 (Maroufyan et al. 2010) that could be part of an adaptive response by the liver to minimize cell damage. During the PNP treatment, dramatic changes occurred in the liver, and the body tries to overcome this stress condition. Due to its persistent stress and toxic condition, p-Nitrophenol toxicity is involved in a global disturbance of gene expressions leading to loss of the liver cellular adaptive resistance mechanism and loss of the liver cell control. Hence, the implication of this study is important from both pharmacological and environmental perspectives (Taneda et al. 2004). In the same line of these stress-related liver changes, we noticed that p-Nitrophenol treatment induced glycogen accumulation, cell proliferation, and apoptosis. In the current study, PAS staining showed an increase in the glycogen accumulation with the PNP-treated birds with especial attention to the increase by dose and time of exposure as it showed the highest at 2.5 months with the high dose. Glycogen accumulation at different levels occurred in inflammation and proliferative stage as noticed in human patients and zebrafish with liver diseases in addition to the reported increase of cell proliferation and apoptosis (Lu et al. 2013). Accumulation of hepatocellular glycogen has been reported to be induced by several toxicants and by-products such as dichloroacetate (DCA) which causes hepatocellular carcinoma (Lingohr et al. 2002). Also, glycogen deposition in liver cells results in glycogen hepatopathy in which the imbalance of glycogen biosynthesis lead to hepatocyte dysfunction and leakage of the enzymes out of the hepatocytes (Alenazy et al. 2020). Besides, serious hepatotoxicity has been reported from the anabolic steroids dietary supplements such as corticosteroids through its promoting action of glycogen accumulation (Kafrouni et al. 2007). These corticosteroids increased with PNP treatments (Ahmed et al. 2015b) which may elucidate the glycogen accumulation effect. These evidences supported that hepatotoxic effect of PNP might be through its glycogen accumulation effect and this open the sight to explore the mechanism through which PNP causes the glycogen accumulation which need deep molecular studies investigating the molecular pathway of glycogen biosynthesis.
In addition to the glycogen accumulation, the hepatic dysfunction effect of PNP is accompanied by an increase in the percentage of apoptotic cell. PNP exposure led to an elevation in the apoptotic cells as well the percentage of cells expressing the pCNA indicating the highly proliferation status of the affected livers. Zhan and his/her colleagues showed that DEP (diesel exhaust particles) (PNMC) induced cell apoptosis in male rat testis (Zhan et al. 2020). The same effects on the liver tissues were observed under exposure to tobacco smoke and ethyl alcohol (Wisniewska et al. 2013) and in the different degrees of heat stress (Li et al. 2012). Previous studies modulated the involvement of PNP in the hepatocellular apoptosis through its uncoupling oxidative phosphorylation effect which subsequently led to increase of tumor suppressor genes (Rb and P53) and reactive oxygen species (ROS). This oxidative damage stress activities the Bax and intracellular caspases which are the main pathway triggering apoptosis (Lam et al. 2013). The same effect apoptotic effect of p-Nitrophenol has been detected in chicken ovarian stroma where the nitrophenol compounds stimulate the expression of apoptotic genes (Grzegorzewska et al. 2020). In the research under investigation, no dose-dependent effects of PNP with non-monotonic effects were reported. Furthermore, the same phenomena were recorded in previous research papers (Li et al. 2006; Yue et al. 2011; Zhang et al. 2013). In conclusion, the presented findings of this research contribute to an understanding concerning liver toxicity with PNP pollutants. p-Nitrophenol has the ability to disrupt liver health conditions by affecting liver enzyme and cell apoptosis. The current results clarified that even a very small amount of PNP was able to cause severe side effects. Hence, the potential for PNP accumulation in the environment is expected to have deleterious outcomes on human beings, animals, and wildlife that cannot be neglected. Even though there is a modulation of many targets including PCNA and apoptosis rate, the exact mechanism by which this compound works cannot exactly be defined but considered to be a point toward the understanding of PNP toxicity. In addition, measurement of other biological markers such as pro-apoptotic, anti-apoptotic, and oxidative markers will shed the light in understanding the mechanism of PNP hepatotoxicity. Mechanistic detailed studies regarding the molecular pathways involved in glycogen metabolism and apoptosis are still needed to be uncovered to understand more about the nitrophenol in hepatotoxicity. So far, PNP ought to be considered an element evidently involved in the harmful impact on the liver health condition.
References
Abdel-Daim MM, Abuzead SM, Halawa SM (2013) Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One 8:e72991
Abou-Donia MB, Nomeir AA (1986) The role of pharmacokinetics and metabolism in species sensitivity to neurotoxic agents. Fundam Appl Toxicol 6:190–207
Abu-Qare AW, Abdel-Rahman AA, Kishk AM, Abou-Donia MB (2000) Placental transfer and pharmacokinetics of a single dermal dose of [14C]methyl parathion in rats. Toxicol Sci 53:5–12
Abu-Qare AW, Abdel-Rahman AA, Ahmad H, Kishk AM, Abou-Donia MB (2001) Absorption, distribution, metabolism and excretion of daily oral doses of [14C]methyl parathion in hens. Toxicol Lett 125:1–10
Ahmed E, Nagaoka K, Fayez M, Abdel-Daim MM, Samir H, Watanabe G (2015a) Suppressive effects of long-term exposure to P-nitrophenol on gonadal development, hormonal profile with disruption of tissue integrity, and activation of caspase-3 in male Japanese quail (Coturnix japonica). Environ Sci Pollut Res Int 22:10930–10942
Ahmed E, Nagaoka K, Fayez M, Samir H, Watanabe G (2015b) Long-term p-nitrophenol exposure can disturb liver metabolic cytochrome P450 genes together with aryl hydrocarbon receptor in Japanese quail. Jpn J Vet Res 63:115–127
Alenazy LA, Javed M, Elsiesy H, Raddaoui E, Al-Hamoudi WK (2020) Glycogenic hepatopathy: a rare hepatic complication of poorly controlled type 1 DM. Case Rep Med 2020:1294074
Ball GF, Balthazart J (2010) Japanese quail as a model system for studying the neuroendocrine control of reproductive and social behaviors. ILAR J 51:310–325
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Elsevier health sciences
Banik RM, Mayank, Prakash R, Upadhyay SN (2008) Microbial biosensor based on whole cell of Pseudomonas sp. for online measurement of p-Nitrophenol. Sensors Actuators B Chem 131:295–300
Call DJ, Brooke LT, Lu PY (1980) Uptake, elimination, and metabolism of three phenols by fathead minnows. Arch Environ Contam Toxicol 9:699–714
Chen J, Song M, Li Y, Zhang Y, Taya K, Li C (2016) The effect of phytosterol protects rats against 4-nitrophenol-induced liver damage. Environ Toxicol Pharmacol 41:266–271
Crosby K, Simendinger J, Grange C, Ferrante M, Bernier T, Standen C (2014) Immunohistochemistry protocol for paraffin‐embedded tissue sections advertisement. Cell Signal Technol 86:e50641–e50646. Retrieved from https://www.jove.com/t/5064/immunohistochemistry-protocol-for-paraffin-embedded-tissue-sections
David HV, Zafar I (1990): Aminotransferases. In: rd, Walker HK, Hall WD, Hurst JW (eds) Clinical methods: the history, physical, and laboratory examinations. Boston
Esani M (2014) The physiological sources of, clinical significance of, and laboratory-testing methods for determining enzyme levels. Lab Med 45:e16–e18
Grzegorzewska AK, Hrabia A, Kowalik K, Katarzynska-Banasik D, Kozubek A, Sechman A (2020) In vitro effects of PNP and PNMC on apoptosis and proliferation in the hen ovarian stroma and prehierarchal follicles. Acta Histochem 122:151463
Hansen WM, D GJ, Sandra MC (2011) Enzymatic activity in turkey, duck, quail and chicken liver microsomes against four human cytochrome P450 prototypesubstrates and aflatoxin B. Xenobiotics 1.IPCS, I.P.o.C.S.Organophosphate Pesticides
Huang X-J, Choi Y-K, Im H-S, Yarimaga O, Yoon E, Kim H-S (2006) Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors 6:756–782
Ichinose T, Yajima Y, Nagashima M, Takenoshita S, Nagamachi Y, Sagai M (1997) Lung carcinogenesis and formation of 8-hydroxy-deoxyguanosine in mice by diesel exhaust particles. Carcinogenesis 18:185–192
Kafrouni MI, Anders RA, Verma S (2007) Hepatotoxicity associated with dietary supplements containing anabolic steroids. Clin Gastroenterol Hepatol 5:809–812
Karami A, Romano N, Hamzah H, Simpson SL, Yap CK (2016) Acute phenanthrene toxicity to juvenile diploid and triploid African catfish (Clarias gariepinus): molecular, biochemical, and histopathological alterations. Environ Pollut 212:155–165
Kayano K, Yasunaga M, Kubota M, Takenaka K, Mori K, Yamashita A, Kubo Y, Sakaida I, Okita K, Sanuki K (1992) Detection of proliferating hepatocytes by immunohistochemical staining for proliferating cell nuclear antigen (PCNA) in patients with acute hepatic failure. Liver 12:132–136
Kuang S, Le Q, Hu J, Wang Y, Yu N, Cao X, Zhang M, Sun Y, Gu W, Yang Y (2020a) Effects of p-nitrophenol on enzyme activity, histology, and gene expression in Larimichthys crocea. Comp Biochem Physiol C Toxicol Pharmacol 228:108638
Kuang S, Le Q, Hu J, Wang Y, Yu N, Cao X, Zhang M, Sun Y, Gu W, Yang Y, Zhang Y, Li Y, Liu H, Yan X (2020b) Effects of p-nitrophenol on enzyme activity, histology, and gene expression in Larimichthys crocea. Comp Biochem Physiol C Toxicol Pharmacol 228:108638
Kunutsor S, Apekey T, Walley J (2013) Liver aminotransferases and risk of incident type 2 diabetes: a systematic review and meta-analysis. Am J Epidemiol 178:159–171
Kwong TC (2002) Organophosphate pesticides: biochemistry and clinical toxicology. Ther Drug Monit 24:144–149
Lam SH, Ung CY, Hlaing MM, Hu J, Li ZH, Mathavan S, Gong Z (2013) Molecular insights into 4-nitrophenol-induced hepatotoxicity in zebrafish: transcriptomic, histological and targeted gene expression analyses. Biochim Biophys Acta 1830:4778–4789
Li C, Taneda S, Suzuki AK, Furuta C, Watanabe G, Taya K (2006) Estrogenic and anti-androgenic activities of 4-nitrophenol in diesel exhaust particles. Toxicol Appl Pharmacol 217:1–6
Li X, Li C, Suzuki AK, Taneda S, Watanabe G, Taya K (2009) 4-Nitrophenol isolated from diesel exhaust particles disrupts regulation of reproductive hormones in immature male rats. Endocrine 36:98–102
Li SQ, Li RF, Xi SM, Hu S, Jia ZQ, Li SP, Wen XL, Song YK, Li S, Li SP, Wei FB, Chen XL (2012) Systematical analysis of impacts of heat stress on the proliferation, apoptosis and metabolism of mouse hepatocyte. J Physiol Sci 62:29–43
Lingohr MK, Bull RJ, Kato-Weinstein J, Thrall BD (2002) Dichloroacetate stimulates glycogen accumulation in primary hepatocytes through an insulin-independent mechanism. Toxicol Sci 68:508–515
Lu JW, Yang WY, Lin YM, Jin SL, Yuh CH (2013) Hepatitis B virus X antigen and aflatoxin B1 synergistically cause hepatitis, steatosis and liver hyperplasia in transgenic zebrafish. Acta Histochem 115:728–739
Machida M, Morita Y, Hayashi M, Awazu S (1982) Pharmacokinetic evidence for the occurrence of extrahepatic conjugative metabolism of p-nitrophenol in rats. Biochem Pharmacol 31:787–791
Maroufyan E, Kasim A, Hashemi SR, Loh TC, Bejo MH (2010) Change in growth performance and liver function enzymes of broiler chickens challenged with infectious bursal disease virus to dietary supplementation of methionine and threonine. Am J Anim Vet Sci 5:20–26
Mizuma T, Hayashi M, Awazu S (1983) p-Nitrophenol sulfation in rat liver cytosol: multiple forms and substrate inhibition of aryl sulfotransferase. Aust J Pharm 6:851–858
Moregaonkar S, Somkuwar A, Pathan M (2011): quail laboratory model for toxicological studies, National seminar on Toxicopathology, IAVP, West Zone, BVC, Mumbai
OECD (1993): (Organization for Economic Cooperation and Development): OECD guidelines for the testing of chemicals. Section2 – Effect on Biotic Systems: Test Guideline 206: Avian Reproduction Test. Paris, France: OCD
Ottinger MA, Lavoie ET, Thompson N, Bohannon M, Dean K, Quinn MJ Jr (2009) Is the gonadotropin releasing hormone system vulnerable to endocrine disruption in birds? Gen Comp Endocrinol 163:104–108
Registry AAfTSaD (1992) Toxicological profile for nitrophenols. Public Health Service, U.S. Department of Health and Human Services, Atlanta
Rej R, Shaw LM (1984) Measurement of aminotransferases: Part 1. Aspartate aminotransferase. CRC Crit Rev Clin Lab Sci 21:99–186
Sagai M, Saito H, Ichinose T, Kodama M, Mori Y (1993) Biological effects of diesel exhaust particles. I. In vitro production of superoxide and in vivo toxicity in mouse. Free Radic Biol Med 14:37–47
Sedqyar M, Weng Q, Watanabe G, Kandiel MM, Takahashi S, Suzuki AK, Taneda S, Taya K (2008) Secretion of inhibin in female Japanese quails (Coturnix japonica) from hatch to sexual maturity. J Reprod Dev 54:52–57
Shim K, Vohra P (1984) A review of the nutrition of Japanese quail. World’s Poultry Sci J 40:261–274
Sodhi S, Sharma A, Brar RS (2006) A protective effect of vitamin E and selenium in ameliorating the immunotoxicity of malathion in chicks. Vet Res Commun 30:935–942
Taneda S, Mori Y, Kamata K, Hayashi H, Furuta C, Li C, Seki K, Sakushima A, Yoshino S, Yamaki K, Watanabe G, Taya K, Suzuki AK (2004) Estrogenic and anti-androgenic activity of nitrophenols in diesel exhaust particles (DEP). Biol Pharm Bull 27:835–837
Timbrell JA (2020) The liver as a target organ for toxicity. Target Organ Toxicity, Volume I, 1
US EPA (1987) Health effects assessment for nitrophenols. United States Environmental Protection Agency, Washington
US-EPA (1996): US Environmental Protection Agency, Avian Reproduction Test: OPPTS 850·2300 ‘Public Draft’ Ecological Effects Test Guidelines. EPA 712-C-96–141. United States Environmental Protection Agency, Washington
Uzunhisarcikli M, Kalender Y, Dirican K, Kalender S, Ogutcu A, Buyukkomurcu F (2007) Acute, subacute and subchronic administration of methyl parathion-induced testicular damage in male rats and protective role of vitamins C and E. Pestic Biochem Physiol 87:115–122
Wisniewska E, Dylik A, Kulza M, Florek E, Piekoszewski W, Senczuk-Przybylowska M, Marszalek A (2013) Exposure to ethanol and tobacco smoke in relation to level of PCNA antigen expression in pancreatic and hepatic rat cells. Pharmacol Rep 65:914–926
WWAP (2019) UNESCO World Water Assessment Programme: The United Nations World Water Development Report 2019: Leaving No One Behind; UNESCO: Paris, France.
Yue Z, She R, Bao H, Li W, Wang D, Zhu J, Chang L, Yu P (2011) Exposure to 3-methyl-4-nitrophenol affects testicular morphology and induces spermatogenic cell apoptosis in immature male rats. Res Vet Sci 91:261–268
Zhan F, Zhong Y, Qin Y, Li L, Wu W, Yao M (2020) SND1 facilitates the invasion and migration of cervical cancer cells by Smurf1-mediated degradation of FOXA2. Exp Cell Res 388:111809
Zhang G, Gao Y, Zhang Y, Guo Y (2010) Fe2O3-pillared rectorite as an efficient and stable Fenton-like heterogeneous catalyst for photodegradation of organic contaminants. Environ Sci Technol 44:6384–6389
Zhang Y, Piao Y, Li Y, Song M, Tang P, Li C (2013) 4-Nitrophenol induces Leydig cells hyperplasia, which may contribute to the differential modulation of the androgen receptor and estrogen receptor-alpha and -beta expression in male rat testes. Toxicol Lett 223:228–235
Zhang Y, Song M, Rui X, Pu S, Li Y, Li C (2015) Supplemental dietary phytosterin protects against 4-nitrophenol-induced oxidative stress and apoptosis in rat testes. Toxicol Rep 2:664–676
Acknowledgements
The authors gratefully acknowledge the help of Dr. Gen Watanabe, professor and head of Physiology Department of Veterinary Medicine, Tokyo University of Agriculture and Technology, for providing us with the PNP.
Availability of data and materials
Not applicable
Author information
Authors and Affiliations
Contributions
E.A. Ahmed contribute to the design and implementation of the research, data analysis, and manuscript revision, H.E. Khaled contributed to writing and manuscript revision, and A.K. Elsayed contributed to discussion of the results, data analysis, and writing and revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
These birds were treated humanely following the established guidelines and principles of Faculty of Veterinary medicine, Suez Canal University, Egypt, which are approved by the ethics committee for using laboratory animals’ experiments.
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmed, E.A., Khaled, H.E. & Elsayed, A.K. Long-term exposure to p-Nitrophenol induces hepatotoxicity via accelerating apoptosis and glycogen accumulation in male Japanese quails. Environ Sci Pollut Res 28, 44420–44431 (2021). https://doi.org/10.1007/s11356-021-13806-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13806-9