Abstract
Blackwater events are frequently reported over the world and become a serious environmental problem. However, the mechanisms of blackwater occurrence are not fully understood yet. This study simulated the process of blackwater with the combined pollution in an orthogonal experiment, which had 4 factors (TOC, TP, NH4+-N, and NO3−-N) and 4 levels (None, Low, Middle, and High). Results showed that the process of water condition changes was divided into two parts, which were “exogenous” and “algae-derived” blackwater, and the influence of four different pollutants on the occurrence of the blackwater was ranked as follows: TOC > TP > NO3−-N > NH4+-N. With the increase of organic matter addition, the anaerobic condition in water was prolonged and the concentration of Fe2+ had a significant increase. In addition, under the None phosphorus condition, the descent rates of DO and COD in the water were reduced, and the algae bloom was obviously deferred. Moreover, the addition of organic matter or phosphorus changed the microbial community structure and led to different water processes. Particularly, only on the condition of the high content of TOC and phosphorus, the diversity of sulfate-reducing bacteria (e.g., Pseudomonas, Paludibacter, and Bacteroides) increased significantly, which accounted for 51.4%, causing the significant production of S2− in the water. Water’s lack of phosphorus showed a low rate of decomposition of organic matter, which might be the result of a considerable increase in the abundance of aerobic Trichococcus and Malikia. This study shows that organic matter and phosphorus have synergistic effect on blackwater occurrence. In the treatment of blackwater, the exogenous pollutant control should reduce the discharge of organic pollutants, and endogenous control should focus on phosphorus abatement and reduce nitrogen control.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Blackwater is a serious phenomenon of water pollution, mainly caused by severe organic pollution or nitrogen and phosphorus nutrition pollution in water, resulting in the frequent microbial activity, the rapid consumption of dissolved oxygen, and the generation of malodorous gas and black matter in the water. When blackwater occurs, the water ecology will be seriously damaged, and human water sources will be polluted, leading to the death of fish and causing serious economic losses (Freuze et al. 2004; Zhang et al. 2016). In the last few decades, lakes, urban rivers, and some coastal areas in many countries around the world such as Lower Mystic Lake in the USA (Duval and Ludlam 2001), Taihu Lake in China (Zhang et al. 2010), the Ganges River in India (Hamzeh et al. 2014), and Florida’s coastal waters (Hu et al. 2003) have suffered from the blackwater. Therefore, the process, the causes, and key pollution factors of the blackwater under the combined pollutants have become the key problems to solve the blackwater.
At present, exogenous pollutants (e.g., untreated industrial wastewater and non-point source pollution) and endogenous pollutants (e.g., sediment release pollutants, algae decomposition) are mainly considered the sources of pollutants that cause blackwater (Rixen et al. 2008; Peter et al. 2009; Sharp 2010; Feng et al. 2014; Hua and Marsuki 2014; Mccoy et al. 2015). Organic pollutants and excessive nutrients (e.g., N and P) in the water body are considered to be important factors that cause the blackwater (Rixen et al. 2008; Feng et al. 2014). Yu et al. (2016) found that the occurrence of blackwater accompanied by low DO for 25 days and high concentration of nutrients (nearly 40 mg/L for TN, 9 mg/L for TP, and 35 mg/L for NH4+-N). Besides, the studies found that the algae bloom caused by the high content of N and P nutrients is also the main reason for the water body to turn black and odorous (He et al. 2009; Duan et al. 2016; Chen et al. 2019). Moreover, high concentrations of NO3−-N and TN are usually regarded as dangerous signs to produces odorous gas in water (Chen et al. 2010).
In addition, a wide range of microorganisms decompose the organic matter in the water, obtain electrons, and consume the dissolved oxygen of water, resulting in an anaerobic state in the water for a long time. The anaerobic state would obviously improve the growth of anaerobic microorganisms (e.g., sulfate-reducing bacteria and iron-reducing bacteria), which are considered to be “the backstage manipulator” for blackwater events. Sulfate-reducing bacteria (SRB) such as Novosphingobium, Acinetobacter were explicitly dominant genera in a black-odor urban river (Cai et al. 2019), and there is a significant positive correlation that exists between SRB population density and the degree of blackness (Feng et al. 2014). Xu et al (2014) found that the abundance of Actinobacteria in sediment would release odorous substances such as geosmin and 2-methylisoborneol. FeRB can reduce Fe(III) to Fe(II) for producing energy in an anaerobic state (Lovley et al. 1996; Gounou et al. 2010). The microbial community structures are the vital part to show the water condition, and the different pollution situations have different impacts on the microbial community of the water body. While many studies focused on the microbial community distribution in the actual blackwater and analyzed the microorganisms in a specific river (Cai et al. 2019; Wu et al. 2019), few studies paid attention to the change of the microbial community during the process from pure water to blackwater and the comparative analysis between different pollution conditions. Therefore, it is necessary to study the connection between microorganisms with blackwater process under different pollution conditions.
Moreover, although many studies had focused on the effects of some typical factors such as organic matter content, Fe, S concentration, and algae content on the occurrence of the blackwater (Chen et al. 2010; Lu et al. 2013; Shen et al. 2014; Wang et al. 2014; Duan et al. 2016; Yu et al. 2016), there are relatively few studies on the blackwater caused by the combined pollution from the perspective of the organic matter (C), nitrogen (N), and phosphorus (P) pollutants. Under different forms and contents of these pollutants, the process of blackwater and the impact of each pollutant on blackwater may be different. Sharp (2010) found that the organic substance alone will cause the water hypoxia but will not cause blackwater. Moreover, a large number of freshwater and marine ecosystems suffered from an algae bloom, which is considered a precondition for the occurrence of algae-derived black bloom, for a long time with high concentrations of N and P in the water body, and have not evolved into blackwater (Smith et al. 2006; Smith and Schindler 2009). Therefore, there are also some questions that need to be resolved, for example: (1) Which kind of pollutant plays the key role in the blackwater? (2) How elements of N and P, which are the most important factors for the algae bloom, contribute to the blackwater? (3) What is the difference between NO3−-N and NH4+-N on blackwater? (4) What is the connection between microorganisms with blackwater process under different pollution conditions?
To reveal the formation mechanism of the blackwater under combined pollutions and solve these above questions, this study set up an L16 (44) orthogonal experiment with 4 factors (TOC, TP, NH4+-N, and NO3−-N) and 4 levels (None, Low, Middle, and High) on natural condition. Moreover, the whole experiment last for 24 days and was divided into two parts, “exogenous” and “algae-derived” blackwater. The general water index was analyzed in the period of the whole experiment, and the change of microbial community structures at the initial and ultimate time of “exogenous blackwater” was characterized. The result is helpful to have a new insight into the main factors of blackwater and provides information for control and inhibition of blackwater occurrence.
Materials and methods
Reagents and materials
Glucose anhydrous (C8H16O8), potassium dihydrogen phosphate (KH2PO4), ammonium chloride (NH4Cl), and potassium nitrate (KNO3) were of analytical grade and purchased from Chengdu Kelong Chemical Reagent Company (Sichuan, China). Deionized water was applied throughout the experiment.
The sediment was collected from the Pi river (N 104° 4′ 33″, E 30° 46′ 12″, Sichuan Province of China, detail in Fig.S1) in March 2019. Pi river flows through the downtown of Chengdu and is one of the typical black and odor rivers in Chengdu. Store the collected sediment in the laboratory under dark conditions, and homogenize it before the experiment. The chemical properties of the initial sediment used in this study are presented in Table S1.
Design of the experiment
This study used orthogonal experiments L16 (44) to study the influence of various nutrients in the water on the blackwater process and the interaction between the various influencing factors. The experiment was designed with 4 factors and 4 levels (Table S2). The values of each level were set by referring to actual measured values of typical blackwater bodies in China (Table S3 for details). A total of 16 experimental groups were obtained (Table S4 for details). To facilitate the analysis of the water quality change during the black and odor process, the experimental groups 1~16 were divided into None-Organic Carbon (NC), Low-Organic Carbon (LC), Middle-Organic Carbon (MC), High-Organic Carbon (HC), None-Phosphorus (NP), Low-Phosphorus (LP), Middle-Phosphorus(MP), and High-Phosphorus (HP) according to the concentration of the initially added TOC and TP.
The size of each experimental device was 40 × 30 × 30 cm. A small amount of sediment (50 g) was added to the experiment device to provide microbial communities. Meanwhile, 20 L of purified water for each device were enriched with glucose, dihydrogen phosphate, ammonium chloride, and potassium nitrate, establishing different concentrations for TOC, TP, NH4+-N, and NO3−-N. To simulate the real process of blackwater occurrence, all the experiment devices were placed outdoors, without any human interventions and were exposed to the real natural environment, including air, sunlight, rain, and evaporation. In this experiment, 50 mL of the water sample is taken for 17 times, a total of about 1 L of the water sample is artificially consumed with the little sampling loss during sampling.
The experiment began in April 2019, and was conducted for 24 consecutive days. Water samples were gathered every day in the first 13 days, and were measured every 2 days from 13th day to 24th day. Measurement indicators of water samples included dissolved oxygen (DO), oxidation-reduction potential (ORP), pH, COD, TP, Fe (II), S2-, transparency, and chlorophyll-a (Chl-a). At the initial and 10th day of the experiment, 16s DNA high-throughput sequencing was performed on the microbes in the typical experimental groups.
Analysis of samples
The water samples were filtered through 0.45-μm Millipore filters before chemical analysis. The chemical analysis methods of the experimental water sample all used the Chinese national standard methods. The detail of the measure methods and the apparatus used in the experiment shows in the Supplementary material (Table S5).
Twenty-five millimeter syringe filters (6794-2502, Whatman, UK) and 0.22-micron microporous membranes (Shanghai Bandao Industrial Co., Ltd., China) were used to filter 200 mL of the experimental water sample for high-throughput sequencing. Store the filter in a 15-mL sterile centrifuge tube (430790, Corning, USA) for microbial measurement. High-throughput sequencing was conducted with the Illumina MiSeq system. The DNA extraction and PCR procedures were followed by previous method and the detail showed in Supplementary material (Cai et al. 2019).
Statistical analysis
Index method of water quality comprehensive pollution
To evaluate the degree of blackwater of each sample in the experiment, Comprehensive Water Quality Pollution Index (WQI) (Zhao et al. 2012; Matta et al. 2020) was used to evaluate the water quality of 16 groups of experiments, and the evaluation results were orthogonally analyzed to obtain the main factors of blackwater. The evaluation results were represented by the water quality in the whole experiment of each group. The dimensionless method of different indicators and the WQI value calculation of all samples are shown in the Supplementary material.
Orthogonal experimental analysis method
Using these above evaluation results as the test indicators for this orthogonal experiment, we performed orthogonal analysis on 16 sets of experimental data, including the visual analysis (range analysis) and the analysis of variance (ANOVA). As a result, the primary and secondary relationship of the impact of various factors on water quality were obtained.
Results
The result of orthogonal analysis
The index P of each experimental group was calculated by the water quality comprehensive pollution index method (see Supplementary material for detail). The calculation results of each group are shown in Table S3.
The visual analysis of the orthogonal experiment results (Table 1) showed that value of the range (Rj) followed the order of TOC > TP > NO3−-N > NH4+-N, and the higher the concentration of TOC, TP, and NH4+-N, the worse the water quality conditions during the experiment. However, it was showed that the higher the addition concentration of NO3−-N, the better the water quality conditions.
As shown in Table 2, the variance analysis of the orthogonal experiment showed that the P-value of factor TOC was less than 0.01, the P-value of TP was in the range of 0.01~0.05, and that of NO3−-N was in the range from 0.05 to 0.1. Moreover, there was no significant between NH4+-N and the water quality, where P-value of NH4+-N was greater than 0.1.
Analysis of water quality change process
According to the phenomenon in the experiment process and the change process of the water quality index, the whole experiment process was divided into two parts: Part 1 (P1), the exogenous blackwater which is caused by exogenous pollution, and Part 2 (P2), the algae-derived blackwater which is caused by algae residue pollution.
Variations in the dissolved oxygen and chlorophyll-a
The low concentration of dissolved oxygen in the water body and the outbreak of algae had always been the important characteristics of the blackwater. In the P1 of the experiment (from the 1st to 10th day), it was found from Fig. 1a that when the water body lacked P nutrients (NP), the higher the concentration of added organic carbon (OC), the lower the dissolved oxygen level of the water body. Due to the decomposition and utilization of organic matter by microorganisms, the dissolved oxygen concentration of the water body was decreased. Moreover, the dissolved oxygen of MC + HP and HC + HP in the water had been in an anoxic and anaerobic state (DO <2 mg/L) from the 6th to the 12th day.
From Fig. 1b–d, it was found that the DO concentration of all samples (excluding the NC group) decreased rapidly after the initial day of the experiment, reaching an anaerobic state on 2nd day (DO less than 0.5 mg/L), and the higher the OC concentration was, the longer period the water stayed in the anaerobic state (HC > MC > LC). However, regardless of the P concentration in the water, the dissolved oxygen in the NC groups had been maintained at a high level. In addition, compared with NP groups and others, under the same OC concentration, NP groups had a higher DO concentration, and the decrease rate of DO was slow.
With the rapid DO enrichment of the water body (Fig. 1) and the rapid increase of the chlorophyll-a concentration of the water body (Fig. 2), the experiment entered the second stage, P2 (from the 10th to 24th day). The concentration of DO and chlorophyll-a in the water began to increase rapidly on the 10th day, especially DO concentration of the LP, MP, and HP groups all exceeded 18 mg/L on the 11th day. By the 15th day, the chlorophyll-a concentration of LP, MP, and HP groups reached its peak (Fig. 2b–d), and the peak chlorophyll-a concentrations in LC + LP, MC + MP, HC + LP, HC + MP, and HC + HP were 80.02, 108.2, 100.7, 61.11, and 85.1 μg/L, respectively. After the 15th day, due to the death of algae, chlorophyll-a and DO concentrations showed a decreasing trend.
However, the chlorophyll-a concentration in Fig. 2a exhibited a consistently increasing trend in the whole experiment, compared with Fig. 2b–d, in which the highest concentration was only 20.22 μg/L at the end of the experiment.
Variations in Fe2+ and S2- concentration
Fe2+ and S2− are the most important factors for the black matter and odorous gas. When the water is under anaerobic conditions, the reduced Fe2+ and S2− can produce black FeS and volatile sulfides with malodorous odor, such as hydrogen sulfide gas (Duval and Ludlam 2001; Lu et al. 2013; Shen et al. 2014; Wang et al. 2014).
As shown in Fig. 3, the Fe2+ concentration for all samples exhibited an increasing trend at the beginning of the experiment. The peak value of Fe2+ concentration in HC+NP reached 0.65 mg/L on the 9th day, and the values in HC+LP, HC+MP, and HC+HP were 0.65 mg/L on 3rd day, 0.95 mg/L on 4th day, and 0.67 mg/L on 3rd day, respectively. What’s more, there was a strong correlation between the added OC concentration and the peak of Fe2+ concentration (R2 = 0.806), while the correlation coefficient between added TP concentration and the peak of Fe2+ concentration was only 0.101 (shown in Table S6). From the 11th to the 13th days, the Fe2+ concentration in LP, MP, and HP groups had a small peak again, which might due to the release of Fe2+ from algae residues.
It could be found from Fig. 4 that under HC conditions, only the S2− concentration of the MP and HP water groups increased significantly, and the sulfide concentration was low under low P conditions. Moreover, the S2− concentration remained at low value from the initial to the 5th day and began to increase significantly after the 6th day. On the 10th day, the S2− concentration of HP reached the peak value, 1.19 mg/L. After the 10th day, the S2− concentration began to decrease, and the sulfide was completely oxidized on the 15th day. Besides, the remaining sulfide concentration of each other group was at a low value (Table S7) during the entire experimental period. Only when the water body was polluted by organic matter and P at the same time, the S2− content would increase significantly (shown in Fig. S2).
Variations in TP and COD concentration
It was found that the TP concentration in Fig. S3 A had been at a low value (TP <0.25 mg/L), and there was a small fluctuation during the whole experiment. This was due to the release of a small amount of bottom mud added in the water. In Fig. S3 (B–D), the TP concentration in the water body changed similarly during the experiment, showing a gradually decreasing trend overall. From the initial time to the 4th day, the TP concentration decreased rapidly. From the 4th day to the 7th day, the LP group fluctuated greatly, while the TP concentration of the MP and HP groups remained stable. From the 7th day to the 15th day, the concentration of TP decreased rapidly. After the 15th day, TP was at a stable stage, and some experimental groups had a slow downward trend.
As showed in Fig S4, the COD concentration for all samples decreased rapidly from the initial day of the experiment. The COD concentration of the MC-NP and HC-NP groups reached the lowest value on the 19th day, while the HC group and the MC group in Fig. S4 (B–D) reached the lowest value on the 12th day and 10th day, in which the decreasing rate was significantly increased. The results showed that the decreasing rate of COD concentration (K) on HC+NP was 19.79 mg·L−1·d−1, while the K on HC+LP, HC+MP, and HC+HP were 36.08, 40.73, and 38.67 mg·L−1·d−1, respectively.
When the water entered the algal-derived black odor (P2), the COD concentration of MC and HC groups in Fig. S4 (B–D) increased slightly and leveled off after the 19th day. This might be due to the continuous death and regeneration of algae after an algae outbreak, which could lead to a slight increase in the amount of organic matter in the water.
Analysis of microbial changes in water
To further explore the mechanism of adding different pollutants to the blackwater, the initial water sample (Control) and the NC + NP, NC + HP, HC + NP, and HC + HP four groups of water samples in 10th days were analyzed by high-throughput sequencing to determine water body microbial flora abundance, diversity and flora structure.
Analysis of microbial diversity
Table 3 showed the microbial sequence number, species abundance, and diversity in the control group and the 10th day. The number of effective sequences ranged from 44750 to 74044, the number of OUT was between 357 and 912, and the coverage of the sample reached 100%, indicating that the results of this microorganism sequencing could truly express the diversity of microorganisms in the sediment.
Chao index and ACE index are commonly used in ecology to estimate the number of species. From the above table, it could be found that the Chao and ACE indexes between the experimental groups were 380.1~986.1 and 395.3~1034.5, respectively, indicating that the microbial community abundances among the groups are quite different. Among them, Chao and ACE in NC + NP group and control group were between 939.1 and 1034.5 and Chao and ACE index of NC + HP and HC + NP were between 677.5 and 742.8, while Chao and ACE in HC + HP group were at the lowest value, 380.1 and 395.3, respectively. It showed that after 10 days of the experiment, the abundance of microbial communities lacking organic matter and phosphorus-containing compounds changed little compared with that at the beginning of the experiment, and the increase of organic matter or phosphorus-containing compounds in the water body would reduce the abundance of the microbial community. However, the sample with higher values of phosphorus-containing compounds had lower microbial community abundance.
Shannon index and Simpson index are commonly used to reflect the diversity of microbial species. The larger the Shannon index, the higher the community diversity, while the larger the Simpson index, the lower the community diversity. It was found from the table above that, compared with control, the Shannon index of each experimental group decreased, while the Simpson index increased and the diversity of the microbial community decreased. Among them, the Shannon and Simpson indexes of the NC + NP group changed little, and the diversity of the microbial community lacking organic matter and phosphorus-containing compounds changed little, while the addition of organic matter or phosphorus-containing compounds reduced the diversity of the microbial community.
Analysis of microorganism community structure in genera level
Figure 5 showed the relative abundance of microorganisms in water samples of control, NC + NP, NC + HP, HC + NP, and HC + HP on the 10th day. The main bacteria in the control were Curvibacter (7.8%), Novosphingobium (6.1%), Pseudarcicella (5.6%), Bacteriovorax (5.3%), Flavobacterium (4.2%), Pseudomonas (4.2%), Herbaspirillum (3.4%), Methylophilus (3.4%), and Brevundimonas (2.3%).
Compared with the control group, the abundance of Brevundimonas or Pseudomonas in each group of the experimental group increased. The abundance of Brevundimonas in NC + NP group increased from 2.3 to 7.9%, the abundance of Pseudomonas increased from 4.2 to 6.1%, the abundance of Pseudomonas in NC + HP group increased from 4.2 to 6.1%, the abundance of Brevundimonas in HC + HP group increased from 2.3 to 3.6%, and the abundance of Pseudomonas in the HC + HP group increased from 4.2 to 11.5%. Pseudomonas belongs to the genus Heterotrophic Denitrifying Bacteria under Proteobacteria. The study had shown that Pseudomonas and Brevundimonas can stimulate the growth of algae by reducing the photosynthetic oxygen tension (Park et al. 2008).
The comparison between the control group and the NC + NP group found that the main genus species of the NC + NP was similar to the control after 10 days of the experiment, indicating that with the absence of OC and P in water, the microbial environment remained unchanged.
From the comparison between the control group and the NC + HP group, it was found that after adding high concentrations of phosphorus-containing compounds to the water, Flavobacterium, Curvibacter, Novosphingobium, Sediminibacterium, and other fungi disappeared, while Malikia (22.5%), Anaerocella (9.9%), Paludibacter (7.2%), etc. became the dominant genus of NC + HP. Malikia is strictly aerobic chemoorganotrophic, always occurring in activated sludge and river water (Leifson 1962; Spring et al. 2005). Under carbon-limited conditions, Malikia can degrade polyhydroxyalkanoates granules, and accumulate polyphosphate granules. This capacity for storage makes Malikia a potentially important player in the enhanced biological phosphorus removal (EBPR) process used in some wastewater treatment plants (Spring et al. 2005).
From the comparison between the control group and the HC + NP group, it was found that after adding high concentrations of organic matter to the water body, Pseudomonas, Novosphingobium, Sediminibacterium, and other bacteria disappeared. Flavobacterium increased from 4.2 to 12.8%, and new major genera such as Trichococcus (39.7%) and Malikia (8.4%) were newly produced. Trichococcus is the microorganism using dissolved organic to form activated sludge, which includes the filamentous type (Scheff et al. 1984), and cause poor settling properties of the activated sludge, so-called “bulking sludge” (Eikelboom 1975). Moreover, in a nutrient-rich condition with Malikia, filamentous cells of up to 50 mm length may occur (Spring et al. 2005).
From the comparison between the control group and the HC + HP group, it was found that after adding high concentrations of phosphorus compounds and organics, the Pseudomonas genus increased from 4.2 to 11.5%, and new major genera such as Paludibacter (35.9%), Clostridium sensu stricto (11.6%), Psychrosinus (6.7%), and Bacteroides (4.1%) were newly produced. Among them, Clostridium sensu stricto (11.6%) and Psychrosinus were the genus of anaerobic bacteria. In the action of high organic matter and phosphorus-containing compounds, the water body was in an anaerobic state for a long time, and the abundance of anaerobic bacteria in the water body increased. Pseudomonas, Paludibacter, and Bacteroides were common sulfate-reducing bacteria (Cai et al. 2019), which together accounted for 51.4% of the abundance of HC + HP bacteria. They were able to reduce SO42− in water to S2−, and might be an important cause of blackwater in the HC + NP group.
Discussion
The water body changed from the exogenous blackwater to algae-derived blackwater
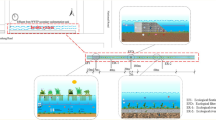
From the variations of dissolved oxygen and chlorophyll-a concentration (Fig. 1 and Fig. 2), it was found that the change in water quality during the experiment was divided into two parts, namely, “the exogenous blackwater” (P1) and “the algae-derived blackwater” (P2).
A schematic diagram, highlighting the blackwater processes occurring after a serious exogenous pollution, including the exogenous blackwater and the algae-derived blackwater, is shown in Fig. 6. In the process of exogenous blackwater (Fig. 6a–b), the added organic matter would be quickly consumed (Fig. S4), and dissolved oxygen would rapidly fall into an anaerobic state (Fig. 1). Under long-term anoxic and anaerobic conditions, the number of anaerobic organisms began to rise rapidly (Fig. 5). Some microorganisms (such as Pseudomonas, Paludibacter, and Bacteroides) used SO42−, Fe3+, and other high-valence elements in the water body as electron acceptors, reducing them to low-valence S2− and Fe2+ to generate odorous gases such as H2S and black substances such as FeS.
This blackwater caused by metal sulfides and volatile sulfides (e.g., FeS, MnS, and H2S) was common in rivers and urban rivers (Duval and Ludlam 2001; Hua and Marsuki 2014). The wastewater generated by human activities (such as industry, agriculture, and living) was directly discharged into rivers or urban rivers, resulting in local enrichment of large amounts of organic pollutants in the rivers. Within a few days, the water quickly became black and odor.
The distinguishing factors between “exogenous” and “algae-derived” blackwater are the consumption of organic matter in the water body and the change of Chl-a content. On the 10th day of the experiment, the COD content of the experimental group with P added dropped to the lowest point and the Chl-a content began to rise, and the exogenous blackwater process ended and entered the algae-derived blackwater.
When the water entered the algae-derived black odor (Fig. 6b–c), the organic matter in the water was exhausted, and the dissolved oxygen concentration in the water began to rise (Fig. 1 and Fig. S4). Accordingly, algae contained in the sediment began to use the remaining N and P substances in the water body. The amounts of algae increased exponentially, and the concentration of chlorophyll-a in the water body increased rapidly (Fig. 2), resulting in a serious algal bloom. At this time, with the death of algae, abundant organic matter and nutrients were released from the algae residues. In this way, the water might return to anoxic or anaerobic conditions, causing a vicious blackwater cycle.
This algae-derived blackwater, caused by an algal bloom, often occurred in lakes and some urban rivers (Pucciarelli et al. 2008; Zhang et al. 2010). Due to the slow water flow rate and poor water exchange capacity of lakes, nutrients such as nitrogen and phosphorus in the water were concentrated in the sediment. When the temperature rises, the nitrogen and phosphorus nutrients in the sediment were released, and the algae in the lake began to multiply. A large number of algae released high concentrations of organic matter and nutrients when they die, resulting in the blackwater in the water (He et al. 2009; Wang et al. 2014; Yu et al. 2016).
Organic matter is the main cause of the blackwater in the water
Organic matter factors were considered the main cause of blackwater. Excessive concentration of organic matter would cause the concentration of dissolved oxygen in the water to stay at a low level (Rixen et al. 2010; Feng et al. 2014; Cai et al. 2019). According to the results of the orthogonal analysis, it indicated that TOC was the most important factor affecting the water quality during the blackwater, followed by TP, and finally NO3−-N and NH4+-N. Moreover, the concentration of organic matter in the water body also has an important influence on the Fe2+ and S2− concentration. As shown in Fig 3, the higher concentration of added organic matter, the higher the Fe2+ concentration produced by the reduction in the water. In the blackwater, organic matter acted as an electron donor during decomposition, while high-valence metals and sulfur acted as electron acceptors (Ghattas et al. 2017), and metal ions and sulfur-containing compounds in water were reduced, resulting in black suspended substances (metal sulfides).
Besides, it was found through experiments that the sulfide concentration of the HC+MP and HC+HP increased significantly only. According to the energy theory, in the anaerobic state, microorganisms would preferentially use ferric salts with higher redox potential, and then used sulfates with lower potential (Ghattas et al. 2017). As shown in Fig. S5(A), Fe was reduced in the water body from the initial to the 4th day, while the sulfide concentration of the water body began to increase after the 6th day. In the black zone (BZ) period, the water of HC + HP kept an anaerobic condition and generated FeS black substance. However, in the BZ period of MC + MP (Fig.S5 B), the dissolved oxygen in the water body had been restored from the 6th to 11th day, and the low concentration of Fe2+ and S2− would not blacken the water. Therefore, the excessively high concentration of organic matter in the water was the deep reason for the generation of reduced sulfur and the most important factor that caused the blackwater.
Moreover, different components of organic matter exhibited different bioavailability, which may cause different effects on the formation of blackwater. Sulfur-containing protein (e.g., methionine and cysteine) can provide available sulfur for SRB, and produce the volatile organic sulfur compounds (VOSCs) (Landaud et al. 2008). However, the presence of glucose might stimulate the degradation of methionine, an important precursor of the VOSCs, while postponing the occurrence of the blackwater and inhibiting the production of VOSCs (Lu et al. 2013). Kiene and Hines (1995) also found that high concentrations of glucose would enhance CO2 or CH4 production and inhibit the thiol methylation potential and ultimately DMS formation. Starch is also one of the common organic pollutants in the water bodies. Rusch et al. (1998) carried out the field experiment in coastal sandy land by adding starch, and found that blackwater occurred in a large area and caused the sulfide concentration to be high for a long time.
Also, some studies had found that when black odor occurs, chromophoric dissolved organic matter (CDOM) in the water body was significantly higher than that of ordinary water bodies, which made the water body became low scattering and high absorption, resulting in the significantly lower reflectivity of the blackwater water body than surrounding normal water bodies (Duan et al. 2014, 2016). In the eutrophic water, the organic matter formed from the algae residues, which lead to the reduction of the backscattering of the particles and the increase of the absorption of the short visible wavelength, would provide favorable conditions for the blackwater (Inamdar et al. 2012).
Phosphorus limitation suppresses the occurrence of blackwater in the water
Phosphorus was an essential element of life, and the concentration of phosphorus in the water body affected the structure and function of the water ecosystem. When phosphorus stress or phosphorus limitation occurred in the water body, it will lead to fundamental changes in the structure and function of the ecosystem (Karl 2014; Guan et al. 2018).
According to the variations of DO, Chl-a, Fe2+, and COD concentration through the whole experiment, there was an obvious delay of blackwater occurrence under the None-Phosphorus condition. Phosphorus limitation would affect the microbial community structure. Under the High-C and None-P condition, Trichococcus and Malikia became the dominant genera, which could form filamentous cells and might reduce the rate of degradation of organic matter and the rate of decrease of oxygen in the water (Eikelboom 1975; Scheff et al. 1984).
What’s more, under the phosphorus stress or phosphorus limitation, the production of Fe2+ and S2− in the water body was significantly inhibited (Figs. 3 and 4). As shown in Fig. 3, the concentration of Fe2+ on HC+NP reached a peak at 9th day, while those on HC+LP, HC+MP, and HC+HP were 3rd day, 4th day, and 3rd day, respectively. Yang et al. (2015) found that the concentration of organic matter and TP in water was closely related to the diversity of sulfate-reducing bacteria communities. Pseudomonas, Paludibacter, and Bacteroides, the typical sulfate-reducing bacteria, which together accounted for 51.4% of the abundance of HC + HP bacteria, might be the reason for blackwater on HC+HP. Therefore, controlling the phosphorus concentration in the water could effectively control and inhibit the occurrence of exogenous blackwater.
Besides, phosphorus was also the main factor restricting the growth of algae in the water. During the experiment of algae-derived black odor, it was found from Fig. 2a that chlorophyll-a was always at a low value in the NP group throughout the experiment, and the growth of algae in the water body was suppressed. A study of 227 lakes in Northwest Ontario, Canada, found that as long as there is enough phosphorus in the water body, the water body would remain eutrophic (Schindler et al. 2008). However, after controlling the concentration of phosphorus, the chlorophyll-a of archipelago estuary decreased from 30 to 16 μg/L (Karl 2014). When the input of phosphorus was restricted, the microorganisms could not obtain phosphorus through other channels, and the number of algae in the water would decline rapidly. When the input of nitrogen was restricted, the water body would stimulate the growth of nitrogen-fixing bacteria and had little effect on the number of algae in the water body (Wang and Wang 2009). Studies indicated that some species of non-heterocellular filamentous cyanobacteria and unicellular cyanobacteria could contribute considerable fixed N even under aerobic conditions in marine and coastal ocean (Zehr et al. 2001; Newell et al. 2016). Therefore, phosphorus was the main factor to adjust the algae in the water (Wang et al. 2008; Xu et al. 2010). For lakes with algal-derived blackwater, priority should be given to limiting the TP concentration of the water and controlling the phosphorus concentration of the water, which could slow down and inhibit the occurrence of algal-derived blackwater.
Conclusion
This paper studies the changes of water quality and the process of blackwater under the cooperation of different content and pollutants (organic matter, phosphorus-containing, and nitrogen-containing). The conclusions from the results are the following:
-
(1)
The influence of four different pollutants on the occurrence of the blackwater was ranked as follows: organic matter > phosphorus pollutants > nitrate-nitrogen pollutants > ammonium nitrogen pollutants (TOC > TP > NO3−-N > NH4+-N).
-
(2)
The high concentration of organic matter in the water, causing the longer anaerobic condition and a higher concentration of Fe2+, was the deep reason for the generation of reduced sulfur, and the most important factor that caused the blackwater.
-
(3)
The addition of organic matter or phosphorus-containing compounds may reduce the diversity of the microbial community, while the abundance of sulfate-reducing bacteria, such as Pseudomonas, Paludibacter, and Bacteroides, will increase, which accounted for 51.4% on HC + HP.
-
(4)
Phosphorus stress or phosphorus limitation had a significant delay for the occurrence of the blackwater by reducing the rate of DO, Chl-a, and Fe2+ concentration variation. Moreover, it can significantly reduce the rate of decomposition of organic matter in water, which may due to the poor settling properties of Trichococcus and Malikia.
-
(5)
In the prevention and control of exogenous pollution, more attention should be paid to the control of organic matter discharge, and the TOC content should be controlled below 100 mg/L, which can effectively reduce the time of being anaerobic water. For endogenous pollution, it is helpful to reduce nitrogen control and focus on phosphorus abatement. Controlling the phosphorus content below 1mg/L can effectively control the outbreak of algae and delay the occurrence of black odor.
Data availability
Some data generated or analysed during this study are included in this published article and its supplementary information files. The other raw or processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
Cai W, Li Y, Shen Y, Wang C, Wang P, Wang L, Niu L, Zhang W (2019) Vertical distribution and assemblages of microbial communities and their potential effects on sulfur metabolism in a black-odor urban river. J Environ Manag 235:368–376. https://doi.org/10.1016/j.jenvman.2019.01.078
Chen J, Xie P, Ma Z, Niu Y, Tao M, Deng X, Wang Q (2010) A systematic study on spatial and seasonal patterns of eight taste and odor compounds with relation to various biotic and abiotic parameters in Gonghu Bay of Lake Taihu, China. Sci Total Environ 409:314–325. https://doi.org/10.1016/j.scitotenv.2010.10.010
Chen C, Shi X, Yang Z, Fan F, Li Y (2019) An integrated method for controlling the offensive odor and suspended matter originating from algae-induced black blooms. Chemosphere 221:526–532. https://doi.org/10.1016/j.chemosphere.2019.01.072
Duan H, Ma R, Loiselle SA, Shen Q, Yin H, Zhang Y (2014) Optical characterization of black water blooms in eutrophic waters. Sci Total Environ 482–483:174–183. https://doi.org/10.1016/j.scitotenv.2014.02.113
Duan H, Loiselle SA, Li Z, Shen Q, du Y, Ma R (2016) A new insight into black blooms: Synergies between optical and chemical factors. Estuar Coast Shelf Sci 175:118–125. https://doi.org/10.1016/j.ecss.2016.03.029
Duval B, Ludlam SD (2001) The black water chemocline of meromictic Lower Mystic Lake, Massachusetts, U.S.A. Int Rev Hydrobiol 86:165–181. https://doi.org/10.1002/1522-2632(200104)86:2<165::AID-IROH165>3.0.CO;2-Y
Eikelboom DH (1975) Filamentous organisms observed in activated sludge. Water Res 9:365–388. https://doi.org/10.1016/0043-1354(75)90182-7
Feng Z, Fan C, Huang W, Ding S (2014) Microorganisms and typical organic matter responsible for lacustrine “black bloom”. Sci Total Environ 470–471:1–8. https://doi.org/10.1016/j.scitotenv.2013.09.022
Freuze I, Brosillon S, Herman D, Laplanche A, Démocrate C, Cavard J (2004) Odorous products of the chlorination of phenylalanine in water: formation, evolution, and quantification. Environ Sci Technol 38:4134–4139. https://doi.org/10.1021/es035021i
Ghattas AK, Fischer F, Wick A, Ternes TA (2017) Anaerobic biodegradation of (emerging) organic contaminants in the aquatic environment. Water Res 116:268–295. https://doi.org/10.1016/j.watres.2017.02.001
Gounou C, Bousserrhine N, Varrault G, Mouchel JM (2010) Influence of the iron-reducing bacteria on the release of heavy metals in anaerobic river sediment. Water Air Soil Pollut 212:123–139. https://doi.org/10.1007/s11270-010-0327-y
Guan DX, Wang X, Xu H, Chen L, Li P, Ma LQ (2018) Temporal and spatial distribution of Microcystis biomass and genotype in bloom areas of Lake Taihu. Chemosphere 209:730–738. https://doi.org/10.1016/j.chemosphere.2018.06.141
Hamzeh M, Ouddane B, Daye M, Halwani J (2014) Trace metal mobilization from surficial sediments of the Seine River estuary. Water Air Soil Pollut 225. https://doi.org/10.1007/s11270-014-1878-0
He X, Ren L, Lin Y, Tian X, Zhang P, Li X, Han G, Huang F (2009) Dynamics of water-extractable phosphorus during the degradation of Microcystis aeruginosa by four bacteria species. Ecol Eng 35:570–575. https://doi.org/10.1016/j.ecoleng.2008.05.010
Hu C, Hackett KE, Callahan MK et al (2003) The 2002 ocean color anomaly in the Florida Bight: a cause of local coral reef decline? Geophys Res Lett 30:1–4. https://doi.org/10.1029/2002GL016479
Hua AK, Marsuki MZ (2014) Public perception towards environmental awareness case study: Malacca River. Int J Acad Res Environ Geogr 1:53–61. https://doi.org/10.6007/IJAREG/v1-i2/1504
Inamdar S, Finger N, Singh S, Mitchell M, Levia D, Bais H, Scott D, McHale P (2012) Dissolved organic matter (DOM) concentration and quality in a forested mid-Atlantic watershed, USA. Biogeochemistry 108:55–76. https://doi.org/10.1007/s10533-011-9572-4
Karl DM (2014) Microbially mediated transformations of phosphorus in the sea: new views of an old cycle. Annu Rev Mar Sci 6:279–337. https://doi.org/10.1146/annurev-marine-010213-135046
Kiene RP, Hines ME (1995) Microbial formation of dimethyl sulfide in anoxic Sphagnum peat. Appl Environ Microbiol 61:2720–2726. https://doi.org/10.1128/aem.61.7.2720-2726.1995
Landaud S, Helinck S, Bonnarme P (2008) Formation of volatile sulfur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl Microbiol Biotechnol 77:1191–1205. https://doi.org/10.1007/s00253-007-1288-y
Leifson E (1962) Pseudomonas spinosa n. sp. Int J Syst Evol Microbiol 12:89–92. https://doi.org/10.1099/0096266X-12-3-89
Lovley DR, Coates JD, Blunt-Harris EL, Phillips EJP, Woodward JC (1996) Humic substances as electron acceptors for microbial respiration. Nature 382:445–448. https://doi.org/10.1038/382445a0
Lu X, Fan C, He W, Deng J, Yin H (2013) Sulfur-containing amino acid methionine as the precursor of volatile organic sulfur compounds in algea-induced black bloom. J Environ Sci (China) 25:33–43. https://doi.org/10.1016/S1001-0742(12)60019-9
Matta G, Kumar A, Nayak A, Kumar P, Kumar A, Tiwari AK (2020) Water quality and planktonic composition of river henwal (india) using comprehensive pollution index and biotic-indices. Trans Indian Natl Acad Eng 5:541–553. https://doi.org/10.1007/s41403-020-00094-x
Mccoy N, Chao B, Gang DD (2015) Nonpoint source pollution. Water Environ Res 87:1576–1594. https://doi.org/10.2175/106143015x14338845156263
Newell SE, McCarthy MJ, Gardner WS, Fulweiler RW (2016) Sediment nitrogen fixation: a call for re-evaluating coastal N budgets. Estuar Coasts 39:1626–1638. https://doi.org/10.1007/s12237-016-0116-y
Park Y, Je KW, Lee K, Jung SE, Choi TJ (2008) Growth promotion of Chlorella ellipsoidea by co-inoculation with Brevundimonas sp. isolated from the microalga. Hydrobiologia 598:219–228. https://doi.org/10.1007/s10750-007-9152-8
Peter A, Köster O, Schildknecht A, von Gunten U (2009) Occurrence of dissolved and particle-bound taste and odor compounds in Swiss lake waters. Water Res 43:2191–2200. https://doi.org/10.1016/j.watres.2009.02.016
Pucciarelli S, Buonanno F, Pellegrini G, Pozzi S, Ballarini P, Miceli C (2008) Biomonitoring of Lake Garda: identification of ciliate species and symbiotic algae responsible for the “black-spot” bloom during the summer of 2004. Environ Res 107:194–200. https://doi.org/10.1016/j.envres.2008.02.001
Rixen T, Baum A, Pohlmann T, Balzer W, Samiaji J, Jose C (2008) The Siak, a tropical black water river in central Sumatra on the verge of anoxia. Biogeochemistry 90:129–140. https://doi.org/10.1007/s10533-008-9239-y
Rixen T, Baum A, Sepryani H, Pohlmann T, Jose C, Samiaji J (2010) Dissolved oxygen and its response to eutrophication in a tropical black water river. J Environ Manag 91:1730–1737. https://doi.org/10.1016/j.jenvman.2010.03.009
Rusch A, Töpken H, Böttcher ME, Höpner T (1998) Recovery from black spots: results of a loading experiment in the Wadden sea. J Sea Res 40:205–219. https://doi.org/10.1016/S1385-1101(98)00030-6
Scheff G, Salcher O, Lingens F (1984) Trichococcus flocculiformis gen. nov. sp. nov. A new gram-positive filamentous bacterium isolated from bulking sludge. Appl Microbiol Biotechnol 19:114–119. https://doi.org/10.1007/BF00302451
Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ, Beaty KG, Lyng M, Kasian SEM (2008) Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc Natl Acad Sci U S A 105:11254–11258. https://doi.org/10.1073/pnas.0805108105
Sharp JH (2010) Estuarine oxygen dynamics: what can we learn about hypoxia from long-time records in the Delaware Estuary? Limnol Oceanogr 55:535–548. https://doi.org/10.4319/lo.2010.55.2.0535
Shen Q, Zhou Q, Shang J, Shao S, Zhang L, Fan C (2014) Beyond hypoxia: occurrence and characteristics of black blooms due to the decomposition of the submerged plant Potamogeton crispus in a shallow lake. J Environ Sci (China) 26:281–288. https://doi.org/10.1016/S1001-0742(13)60452-0
Smith VH, Schindler DW (2009) Eutrophication science: where do we go from here? Trends Ecol Evol 24:201–207. https://doi.org/10.1016/j.tree.2008.11.009
Smith VH, Joye SB, Howarth RW (2006) Eutrophication of freshwater and marine ecosystems. Limnol Oceanogr 51:351–355. https://doi.org/10.4319/lo.2006.51.1_part_2.0351
Spring S, Wagner M, Schumann P, Kämpfer P (2005) Malikia granosa gen. nov., sp. nov., a novel polyhydroxyalkanoate- and polyphosphate- accumulating bacterium isolated from activated sludge, and reclassification of Pseudomonas spinosa as Malikia spinosa comb. nov. Int J Syst Evol Microbiol 55:621–629. https://doi.org/10.1099/ijs.0.63356-0
Wang H, Wang H (2009) Mitigation of lake eutrophication: loosen nitrogen control and focus on phosphorus abatement. Prog Nat Sci 19:1445–1451. https://doi.org/10.1016/j.pnsc.2009.03.009
Wang HJ, Liang XM, Jiang PH et al (2008) TN : TTP ratio and planktivorous fish do not affect nutrient-chlorophyll relationships in shallow lakes. Freshw Biol 53:935–944. https://doi.org/10.1111/j.1365-2427.2007.01950.x
Wang G, Li X, Fang Y, Huang R (2014) Analysis on the formation condition of the algae-induced odorous black water agglomerate. Saudi J Biol Sci 21:597–604. https://doi.org/10.1016/j.sjbs.2014.07.002
Wu H, Li Y, Zhang W, Wang C, Wang P, Niu L, du J, Gao Y (2019) Bacterial community composition and function shift with the aggravation of water quality in a heavily polluted river. J Environ Manag 237:433–441. https://doi.org/10.1016/j.jenvman.2019.02.101
Xu H, Paerl HW, Qin B, Zhu G, Gaoa G (2010) Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnol Oceanogr 55:420–432. https://doi.org/10.4319/lo.2010.55.1.0420
Xu M, Zhang Q, Xia C, Zhong Y, Sun G, Guo J, Yuan T, Zhou J, He Z (2014) Elevated nitrate enriches microbial functional genes for potential bioremediation of complexly contaminated sediments. ISME J 8:1932–1944. https://doi.org/10.1038/ismej.2014.42
Yang X, Huang TL, Guo L et al (2015) Abundance and diversity of sulfate-reducing bacteria in the sediment of the Zhou Cun drinking water reservoir in Eastern China. Genet Mol Res 14:5830–5844. https://doi.org/10.4238/2015.May.29.15
Yu D, Xie P, Zeng C, Xie L, Chen J (2016) In situ enclosure experiments on the occurrence, development and decline of black bloom and the dynamics of its associated taste and odor compounds. Ecol Eng 87:246–253. https://doi.org/10.1016/j.ecoleng.2015.11.039
Zehr JP, Waterbury JB, Turner PJ, Montoya JP, Omoregie E, Steward GF, Hansen A, Karl DM (2001) Unicellular cyanobacteria fix N2 in the subtropical north Pacific Ocean. Nature 412:635–638. https://doi.org/10.1038/35088063
Zhang X, Chen C, Ding J, Hou A, Li Y, Niu ZB, Su XY, Xu YJ, Laws EA (2010) The 2007 water crisis in Wuxi, China: analysis of the origin. J Hazard Mater 182:130–135. https://doi.org/10.1016/j.jhazmat.2010.06.006
Zhang Y, Shi K, Liu J, Deng J, Qin B, Zhu G, Zhou Y (2016) Meteorological and hydrological conditions driving the formation and disappearance of black blooms, an ecological disaster phenomena of eutrophication and algal blooms. Sci Total Environ 569–570:1517–1529. https://doi.org/10.1016/j.scitotenv.2016.06.244
Zhao Y, Xia XH, Yang ZF, Wang F (2012) Assessment of water quality in Baiyangdian Lake using multivariate statistical techniques. Procedia Environ Sci 13:1213–1226. https://doi.org/10.1016/j.proenv.2012.01.115
Acknowledgements
The authors would like to acknowledge the financial support from the Major Scientific and Technological Special Program of Sichuan Province, China (2018SZDZX0027 and 2019-YF09-00081-SN).
Funding
This study was funded by Major Scientific and Technological Special Program of Sichuan Province, China (2018SZDZX0027 and 2019-YF09-00081-SN).
Author information
Authors and Affiliations
Contributions
Naiwen Li and Chao Liu contributed to the conception of the study and provided financial support.
Lanmiao Li and Linjun Wu performed the experiment.
Lanmiao Li and Lingxiao Yang contributed significantly to analysis and manuscript preparation.
Lanmiao Li performed the data analyses and wrote the manuscript.
Jun Li and Naiwen Li helped perform the analysis with constructive discussions.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 818 kb).
Rights and permissions
About this article
Cite this article
Li, L., Wu, L., Yang, L. et al. Combined impact of organic matter, phosphorus, nitrate, and ammonia nitrogen on the process of blackwater. Environ Sci Pollut Res 28, 32831–32843 (2021). https://doi.org/10.1007/s11356-021-13050-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13050-1