Abstract
Azoxystrobin (AZ) is an aryloxy pyrimidine fungicide extensively applied in the agriculture field all over the world. There is a little information about the ecotoxicity of AZ fungicide on the freshwater snail Lymnaea luteola (L. luteola). The present study investigated the toxic effect of AZ fungicide on L. luteola by using various measures. We determined the mean LC50 value―96 h of AZ fungicide (0.79 mg/L) for L. luteola, in a static system. Based on this value, three sublethal concentrations, viz., I (0.079 mg/L), II (~ 0.40 mg/L), and III (~ 0.53 mg/L), were determined. The snails were exposed to these three concentrations of AZ fungicide for 96 h, and hemolymph and digestive glands were collected after 24 and 96 h for assessment of oxidative stress, apoptosis, and histological and genotoxic changes. The induction of intracellular reactive oxygen species (ROS) and apoptosis in hemocyte cells was increased in a dose- and time-dependent manner. It was observed that lipid peroxide (LPO) and glutathione S transferase (GST) were increased, and glutathione and superoxide dismutase decreased in digestive glands. A similar trend was observed for the DNA damage as measured in terms of the percentage of tail DNA and olive tail moment in digestive gland cells. This study showed the collective use of oxidative stress, histological, and genotoxicity parameters in in vivo laboratory studies using snails that are useful for screening the toxic potential of environmental contaminants such as AZ fungicide.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural and manufacturing industries are increasing the use of various types of pesticides and fungicides all over the world. The hazardous effect of fungicides on the aquatic ecosystem is an increasing problem worldwide. Fungicides and pesticides are hazardous to aquatic organisms due to bioaccumulation in tissues and induce toxicity to aquatic organisms. The surplus use of fungicides and pesticides was considered as pollutants. The fungicides have been less studied than herbicides and insecticides (Schäfer et al. 2011). The pesticides and fungicides pollution is caused especially by anthropogenic activities such as manufacturing, agriculture, and sewage in developing countries (AL-Ahmadi 2019). After ingesting pollutants by organisms, they are not excreted and bioaccumulates in tissues or organs.
Due to the increase of genotoxins and mutagens in the aquatic ecosystems, the improvement of useful parameters for revealing of mutagenic and genotoxic effects in aquatic organisms had increased significance (Ali et al. 2008; Hyashi et al. 1998). On the other hand, the alkaline single-cell gel electrophoresis is achieving importance over the other tests because its advantages include sensitivity for detecting very little quantity of DNA damage and the short time needed to complete a study. The AZ fungicide induced genotoxicity and oxidative stress in zebrafish (Han et al. 2016). The fungicides and pesticides are released into the aquatic environment through agricultural sources. The tissue damage occurred by the unevenness between the generation and elimination of reactive oxygen species (Lei et al. 2017).
The lymnaeids organisms are spread all over the world (Godan 1983). Freshwater snails serve an important role in the aquatic ecosystem. The freshwater snail L. luteola referred to as a significant common pond snail of East Asian countries. The freshwater L. luteola was easily cultured in the aquatic laboratory at room temperature for 70 days (Taha 1993). So, in this study, L. luteola was selected to get significant scientific information, investing in a better understanding of toxic effects of AZ fungicides on aquatic organisms. Although the studies of acute toxic effects of pesticides on human and aquatic organisms have been done earlier (Kim et al. 2017), the information regarding the genotoxic potential of AZ fungicide in aquatic organisms is scanty, especially the data about the acute genotoxic effect of AZ fungicide in the snail. Therefore, the present study investigates the genotoxic effect of AZ fungicide using oxidative stress and alkaline single-cell gel electrophoresis (SCGE) techniques in the snail.
Materials and methods
Chemicals
Snail saline buffer (5 mM HEPES, 3.7 M NaOH, 36 mM NaCl, 2 mM KCl, 2 mM MgCl2, 4 mM CaCl2, pH 7.8), EDTA salt, DMSO, ethidium bromide was procured from M/s. Sigma (St. Louis, MO, USA). All other chemicals and AZ fungicides are purchased from local markets.
Collection of test specimens
The test specimens (L. luteola) are brought from freshwater ponds and transferred to the laboratory aquarium. The specimens are kept at 22 ± 4 °C in a laboratory aquarium for 10 days before starting the experiment and fed daily leaves of the Marsilia sp. plant. We have selected snail with a mean size of 19 mm (range of shell length 18–22 mm) and a wet weight of 437 mg (average range, 310–460 mg).
Measurement of test water parameters
The quality of aquarium water, i.e., temperature, pH, total conductivity, dissolved oxygen, and total hardness, is evaluated by standard methods (APHA, AWWA, WPCF 2005).
Evaluation of LC50 value
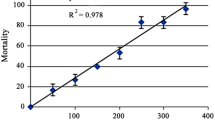
The median lethality of AZ fungicide was evaluated according to APHA, AWWA, WPCF (2005) standard methods. In this experiment, AZ fungicide (1 mg/mL) was dissolved in water. Mortality of snail in different concentrations of AZ fungicide was determined at 24 and 96 h after exposure. The photoperiod was controlled to simulate the natural day:light cycle (12 h:12 h). The snail death is calculated at 24 and 96 h after exposure with AZ fungicide. The experiment is performed in duplicates and repeated three times to confirm the results. The median lethal concentration is calculated by using the computer software probit analysis. The percentage mortality of snail is observed to be 3% and 100% at 0.1 mg/L and 4 mg/L concentration of AZ fungicide, respectively.
The LC50 96 h value of AZ fungicide is determined as 0.79 mg/L for L. luteola following the probit analysis method as described by Finney (1971) (Fig. 1).
Experimental design
The L. luteola was placed in an aquarium (10 L) in the aquatic laboratory at room temperature on a 12 h day/night cycle for 10 days. Based on LC50 96 h value, we have determined three exposure concentration such as sublethal I (1/10 of LC50 = 0.079 mg/L), sublethal II (1/2 of LC50 = (~ 0.40 mg/L), and sublethal III (2/3 of LC50 = (~ 0.53 mg/L). The feeding was stopped 24 h before exposure. Snails were kept in transparent polystyrene beakers of 2 L test water (Thomas Scientific NJ 08085 USA) for 24 and 96 h. The experiments were done in the nonrenewal system. Three replicates of 12 snails were made up at each of concentrations and control. The snails were split into four groups of twelve snails each as described below.
-
Group I: Control, snails were maintained in tap water for 24 and 96 h.
-
Group II: AZ fungicide (at sublethal I) was exposed to snails for 24 and 96 h.
-
Group III: AZ fungicide (at sublethal II) was exposed to snails for 24 and 96 h.
-
Group IV: AZ fungicide (at sublethal III) was exposed to snails for 24 and 96 h.
After exposure, digestive glands and hemocyte cells of snail were collected at intervals of 24 and 96 h from five snails per duration. The snails were maintained in tap water was considered as a negative control.
Production of reactive oxygen species (ROS)
To find out intracellular generation of ROS in L. luteola after treatment of sublethal I, II, and III concentration of AZ fungicide, we have collected hemolymph (290 μL) by slow pinching of foot sole from two snails. The hemolymph was centrifuge at 3000 rpm for 5 min to concentrate hemocytes cells of snails. The hemocytes cells seeded in 96-well black plates and incubated with H2DCF-DA (2.0 μM, 5 μL) for 40 min. After incubation, the fluorescence of dichlorofluorescein was evaluated by using a fluorescence microplate reader (Spectra MAX Gemini EM, Molecular Devices) at 480 nm (excitation wavelength) and 530 nm (emission wavelength).
For analysis of intracellular ROS generation in hemocytes cells due to fungicides, we have set an experiment in a four-well chambered slide (1 × 103 hemocyte cells/well) with H2DCF-DA (2.0 μM, 5 μL), and intracellular ROS generation was observed as green fluorescence by using a fluorescent microscope (Olympus CKX41; Olympus: Center Valley, PA, USA).
Biochemical assay
Sample preparation
The digestive gland of each snail from each group was weighed and homogenized with 10 volumes (w/v) in an ice-cold saline solution using a Polytron homogenizer for 5 min. The homogenate was centrifuged at 13000 rpm for 20 min at 4 °C. The supernatant was used as a source for lipid peroxidation (LPO), glutathione (GSH), super oxide dismutase (SOD), glutathione-S-transferase (GST), as well as protein content.
Lipid peroxidation levels (LPO)
LPO was determined by the estimation of MDA formation by measuring thiobarbituric acid reactive products in the digestive gland of snail according to Ali et al. (2014) method. The tissue homogenate (250 μL) was added with 1 mL trichloroacetic acid (15% (w/v) in 25 mM HCl and 750 μL thiobarbituric acid (0.37% (w/v) in 25 mM HCl). Then samples were heated in a water bath at 95 °C for 20 min. The samples were cooled at room temperature and centrifuged at 10000 rpm for 10 min. The optical density of samples was read at 535 nm using a spectrophotometer.
Glutathione content
The GSH level is measured using Rao et al. (1996) method. One milliliter reaction mixture was prepared by adding 100 μL tissue homogenate, 600 μL phosphate buffer (100 mM, pH 7.5), 100 μL EDTA (1 mM), 100 μL NADPH (0.02 mM), and 100 μL oxidized glutathione (0.5 mM). It was incubated for 30 min at room temperature. The concentration of GSH was expressed as U/mg protein.
Superoxide dismutase (SOD)
The level of SOD was evaluated according to Ali et al. (2014) method. Superoxide radicals react with hydroxylamine, as a result NO2- ion is produced. The NO2- ion is catalyzed by hypoxanthine and xanthine oxidase. NO2- ion was determined at 550 nm by using a color reagent. The quantity of superoxide dismutase required inhibiting the rate of nitrite ion generation by 50% was defined as one unit of superoxide dismutase activity.
Glutathione-S-transferase
GST activity was evaluated by measuring the variation in absorbance due to the presence of glutathione dinitrobenzene complex as a product of the reaction between GSH and1-chloro-2, 4 dinitrobenzenes (CDNB) (Habig et al. 1974).
The reaction mixture consist 100 μL tissue homogenate, 600 μL phosphate buffer (0.1 M), 100 μL GSH (10 mM), 100 μL EDTA (60 mM), and 100 μL CDNB (10 mM) in a cuvette. The alteration of absorbance was observed at 340 nm every 30 s for 6 min. The unit of GST is defined as the amount of the enzyme that catalyzes the production of 1 μ mole of CDNB per min.
Annexin V-FITC/PI staining
After exposure to AZ fungicide, the hemolymph (150 μL/snail) was collected and mixed in snail saline buffer (50 μL) and suspended in 300 μL binding buffer. Then, propidium iodide (10 μL) and Annexin V-FITC (5 μL) were added in cell suspension and incubated for 30 min in dark at room temperature. After incubation, the stained cells were examined by FACS and detected as apoptotic (Annexin V-bound FITC) and necrotic (DNA-bound propidium iodide) hemocyte cells. Data was analyzed by FACS Diva 6.1.2 software.
Determination of DNA strand breakage
DNA damage in snail after exposure to AZ fungicide was determined using SCGE techniques (Ali et al. 2008). The digestive glands (20 mg/snail) was collected from each group. The viability of digestive gland cells was determined using the Trypan blue exclusion test method (Ali et al. 2008). Two slides were made from each concentration, and a total of 100 cells were analyzed randomly from each using an image analysis system (Komet-5.0, Kinetic Imaging, Liverpool UK) attached to a fluorescent microscope (DMLB, Leica, Germany) equipped with appropriate filters. DNA damage was expressed as percent tail DNA (i.e., % tail DNA = 100 % head DNA) as observed by the software.
Histopathology
Snails were exposed to each concentration (sublethal I, II, III) of AZ fungicide for 24 and 96 h. After breaking the shell, the digestive glands were collected and fixed in a fixative (10% formalin) solution. The dehydration was processed in using 70%, 80%, 90%, 95%, and 100% grades of ethanol. After dehydration, the samples were dipped two times in xylene for 5 min. The cleared tissues were permeated in melted paraffin wax, embedded, and prepared block. The tissue block was cut in the microsection (4 μm) and was stained using histologic stains as described by Bancroft and Stevens (1986). Stained sections of the digestive gland of each snail of control and exposed group were seen, and images were captured using an optical microscope (Olympus, Tokyo, Japan) for detecting alterations in architecture, digestive tubules, hemolymphatic sinuses, and hemocytes and necrosis.
Statistical analysis
The results were analyzed using a one-way analysis of variance (ANOVA). A p value of less than 0.05 and 0.01 was considered statistically slightly and highly significant, respectively. At least three independent experiments were carried out in duplicates for each exposure. Data were expressed as mean ± SE.
Result
Properties of experimental water
The pH of water (from 6.78 to 7.50), temperature (from 22.9 to 24.5 °C), and dissolved oxygen (DO) 6.56–8.06 mg/L were found during the experiment. The total hardness of test water was found 160.4 to 182 μg/mL as CaCO3. The Cl- level and conductivity of test water were 46.2 to 54.0 μg/mL and 246 to 295 μM/cm, respectively.
Mortality and behavioral response of test specimens
After exposure to AZ fungicide (0.1, 0.3, 0.6, 0.8, 2, and 4 mg/L), the freshwater snails died 3%, 9.1%, 21.4 %, 53 %, 69 %, and 100 % for 96 h, respectively (Fig. 1). The median lethal (LC50, 96 h value) with 95 % confidence limits for 96 h of AZ fungicide exposure was 0.79 mg/mL. The change in the behavior of L. luteola was observed in different exposure periods at 24 and 96 h. Behavioral changes occurred due to intoxication, and it compared with control snails. The results were a loss of chemoreception so that the snails were no longer attracted the test water reservoirs. Snails became restless, calm down to the bottom of reservoirs, and died without showing any movement and feeding activities at higher concentration exposure of AZ fungicide. The color of the foot becomes discolored, leading to snail death. On the other hand, snails without exposure to AZ fungicide and with exposure to lower concentration, snails became attached to the underside of the water surface or attached to the wall of the test water reservoir and showed more activity by laying many eggs mass during the experimental periods.
Oxidative stress
After the treatment of AZ fungicide to the snail, the reactive oxygen species (ROS) is produced in hemocyte cells of snail (Fig. 2 a, b). This intracellular ROS generation is concentration and time-dependent (Fig. 2 a, b). The maximum generation of ROS was found at sublethal III exposure, and it was increased 162% than the control for 96 h in the snail.
Intracellular ROS generation after exposure to azoxystrobin fungicide (a). The fluorescence image hemocyte cells treated with sublethal II and III for 24 and 96 h. b % ROS production due to azoxystrobin fungicide in hemocyte cells. Each value represents the mean ± SE of three experiments. *p < 0.05, **p < 0.01 vs. control
After the treatment of AZ fungicide, the level of MDA is significantly increased in the digestive gland tissue of snail compared with control (Fig. 3a). The alterations of the MDA level demonstrate the LPO of digestive gland cells. The change in MDA in the digestive gland tissue of snail is concentration and time-dependent manner (Fig. 3a).
After exposure to AZ fungicide, GSH level is significantly reduced as a dose- and time-dependent manner (Fig. 3b). More reduction of glutathione is found at sublethal III exposure for 96-h duration (Fig. 3b).
SOD is increased at the 0.4 mg/L concentration of AZ fungicide exposure but is markedly decreased at the 0.53 mg/L concentration (Fig 3c). GST activity increases at all concentrations, except when exposed for 24 h to the 0.4 mg/L concentration (Fig 3d).
Apoptosis
Apoptotic and necrotic hemocyte cells were determined using FACS after dyeing with Annexin V-FITC and PI. The obtained histogram revealed that control snail has healthy hemocyte cells (99.59%), but the apoptotic hemocyte cells are found 7.03% at sublethal I, 12.07% at sublethal II, and 16.06% at sublethal III in exposed snails (Fig. 4).
Azoxystrobin-induced apoptosis in hemocyte cells of L. luteola. Histogram of flow cytometric analysis of Annexin V-FTIC/PI stained hemocyte cells. a Control. b Sublethal I. c Sublethal II. d Sublethal III. e Percentage of apoptotic and necrotic cells. Data represent mean ± SE three experiments. *p 0.05, **p 0.01 compared with sublethal I
DNA fragmentation
Figure 5 shows the DNA damage as % tail DNA and olive tail moment in the control and AZ fungicide exposed snails. Some cells were damaged after 24 h at 0.076 mg/L of the fungicide, as Fig. 5 shows that damage at this time and concentration was not significantly different from the controls, but after 24 h at 0.4 mg/L, there was a significant increase in damage above the controls. Maximum fragmentation of DNA was found at AZ fungicide (sublethal III) at 96 h exposure (Fig. 5 a, b, c). Figure 5 also shows that at all the sublethal concentrations, there is evidence of more damage over the longer exposure times, and this is very apparent at the 0.4 mg/L concentration, so that one might expect that over longer exposure times than 96 h even 0.4 mg/L would cause severe damage and might cause > 50% mortality. We have observed a reduction of GSH, and induction of DNA damage is mutually co-related (Fig. 6a, b).
Histopathological studies
Digestive glands of control snails show normal digestive tubules separated by intertubular connective tissue containing hemolymphatic sinuses and hemocytes (Fig. 7 a). There are various types of digestive cells, excretory cells, and calcium cells (Fig. 7a). We have observed some tubules with atrophy, hemocyte infiltration after the treatment of AZ fungicide (Fig. 7b). The basement of tubules was ruptured, and digestive gland cells accumulated dark granules, and it seem to lead breakdown into membrane-bound vesicles. The cytoplasm of most calcium cells was replaced by large vacuoles containing darkly stained granules. Calcium cells contain an increased deposition of calcified dark granules with vacuolated cytoplasm and karyolysis nuclei (Fig. 7c).
Microphotographs. a Digestive gland of Lymnea leuteola (control) for 96 hrs demonstrating normal histological architecture, H & E. 100 X. b Ultra structure of digestive gland of Lymnea leuteola exposed to Subletthal III for 24 hrs, H & E. 100 X. c Ultra structure of digestive gland of Lymnea leuteola exposed to Subletthal III for 96 hrs, H & E. 100 X
Discussion
Investigation of this experiment indicated that AZ fungicide is toxic to freshwater snail, and it can be used as molluscicide chemicals for the snail. The effect of AZ fungicide used as molluscicides differs according to whether it comes in contact with the skin or is eaten or absorbed through the intestine. Thomas (1948) reported that the toxicity of chemicals depends upon the route of exposure; for example, the rate of mortality of slug Deroceros reticulatum was more when metaldehyde is absorbed to the skin, but the rate of death was less when it consumed orally in Helix aspersa. The finding of this experiment indicated that AZ fungicide increased DNA damage and oxidative stress in concentration and duration-dependent basis. We have found that the 96 h LC50 values of AZ fungicide with 95% confidence limits were 0.79 mg/mL. The snails showed behavior change due to adjusting to the new environment. This is a sensitive factor of an organism’s response to stress including water pollutants. The change in the behavior of freshwater snail demonstrates the deterioration of water quality. In this finding, the behavioral change in snails due to fungicide was an agreement with the finding of Tripathi et al. (2013) in snail Lymnaea acuminate toward photo and chemoattractants.
Lipids have an important role in biology as signaling molecules (Ayala et al. 2014). Peroxidation of lipids triggered by the oxidative stress between ROS and membrane lipids is one of the well-known mechanisms of cell death (El-Beltagi and Mohamed 2013). Ayala et al. (2014) reported that ROS such as hydroxyl radical (HO•) and hydroperoxyl (HO• 2) may affect the lipids. The HO• radical is a tiny, high active, water-soluble, and chemically most reactive species of activated oxygen and short-lived molecule. The HO• can be produced from O2 in cell metabolism and under a variety of stress conditions. MDA is one of the main oxidized products of per-oxidized polyunsaturated fatty acids, and increased MDA content is a specific marker of LPO (Demir et al. 2010). MDA also forms adducts with DNA bases that are mutagenic (Vöhringer et al. 1998) and possibly carcinogenic. This study reveals that there is a significant difference between controls and exposed groups regarding the MDA level in the digestive glands. Ali et al. (2015) documented that oxidative stress is an important mechanism for ecotoxicity. Superoxide dismutase enzyme may induce the breakdown of ROS and inhibit from adverse effects of oxidative stress. The upturns in antioxidant enzyme levels were due to the high production of oxygen free radicals, which might encourage antioxidant activities to overwhelm the oxidative stress and defend the organism from damage.
In this experiment, the alkaline nature (pH > 13) of the comet test was applied because it might have intensified the expression of alkali-labile sites as single-strand breaks. Ussery (2001) reported that the denaturation and unwinding of DNA occurred at high pH values more than 12 in double-stranded DNA. Also, Kohn (1991) documented that alkali-labile sites (apurinic sites) are rapidly altered to DNA strand breaks at pH 12.6 or higher.
The formation of depurination DNA adducts and declining the N-glycosidic bond induce the development of alkali-labile sites, as a result, DNA strand breaks (Akcha et al. 2003). Thus, the free and fragmented DNA and released super-coiled DNA close the sites of cleavage. Under the electrophoresis, the fragments of DNA and free DNA move to the anode side, forming the DNA tail of the comet. Fragments of DNA incline to passage easily under the electrophoresis, whereas the released DNA is drawn DNA head. Thus, the length of the DNA tail governs the migration of damaged DNA of the cell. Kumaravel and Jha (2006) reported that tiny DNA travels the extreme distance, thus DNA tail length is detected by the size of the DNA fragments produced during the alkaline unwinding stage of the SCGE test. The percentage of DNA in the tail is a general and important parameter that evaluates the percentage of DNA that has migrated from the head. The histopathological observation reveals that AZ fungicide affected the digestive gland of snails. The secretory granules pass to the apical region of the secretory cells from where they are discharged into the lumen.
Conclusions
Thus, on the basis above finding, it can be concluded that AZ fungicide induced genotoxicity and histological change in digestive gland cells of snail by inducing oxidative stress in response to AZ fungicide.
References
Akcha F, Vincent-Hubert F, Pfhol-Leszkowicz A (2003) Potential value of the comet assay and DNA adduct measurement in dab (Limanda limanda) for assessment of in situ exposure to genotoxic compounds. Mutat Res 534:21–32
AL-Ahmadi MS (2019) Pesticides, anthropogenic activities, and the health of our environment safety. Pesticides-use and misuse and their impact in the environment. Intech Open:1–23
Ali D, Nagpure NS, Kumar S, Kumar R, Kushwaha B (2008) Genotoxicity assessment of acute exposure of chlorpyrifos to freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Chemo. 71:1823–1831
Ali D, Kumar PG, Kumar S, Ahmed M (2014) Evaluation of genotoxic and oxidative stress response to dimethoate in freshwater fish Channa punctatus (Bloch). Chem Speciat Bioavailab 26(2):111–118
Ali D, Ahmed M, Alarif S, Ali H (2015) Ecotoxicity of single-wall carbon nanotubes to freshwater snail Lymnaea luteola L.: Impacts on oxidative stress and genotoxicity. Environm.Toxic. 30(6):674–682
APHA, AWWA, WPCF (2005) Standard methods for the examination of water and wastewater, 21st edn. American Publication of Health Association, Washington, DC
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med Cell Longev 2014:360438
Bancroft JD, Stevens A (1986) Connective tissues and stains. In: Theory and Practice of Histological Techniques. Churchill-Livingstone, London, pp 122–145
Demir E, Kocaoğlu S, Kaya B (2010) Assessment of genotoxic effects of benzyl derivatives by the comet assay. Food Chem Toxicol 48(5):1239–1242
El-Beltagi HS, Mohamed HI (2013) Reactive oxygen species, lipid peroxidation and antioxidative defense mechanism. Not Bot Horti Agrobo 41(1):44–57
Finney DJ (1971) Probit analysis. Cambridge University Press, Cambridge, p 333
Godan D (1983) Pest slugs and snails. Springer-Verlag, Berlin, Heidelberg, New York vi + 445 pp
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Han Y, Liu T, Wang J, Wang J, Zhang C, Zhu L (2016) Genotoxicity and oxidative stress induced by the fungicide azoxystrobin in zebrafish (Danio rerio) livers. Pestic Biochem Physiol 133:13–19
Hyashi M, Ueda T, Uyeno K, Wada K, Kinae N, Saotome K, Tanaka N, Takai A, Sasaki YF, Asano N, Sofuni T, Ojima Y (1998) Development of genotoxicity assay systems that use aquatic organisms. Mutat Res 399:125–133
Kim KH, Kabir E, Jahan SA (2017) Exposure to pesticides and the associated human health effects. Sci Total Environ 575:525–535
Kohn KW (1991) Principles and practice of DNA filter elution. Pharmacol Ther 49:55–77
Kumaravel TS, Jha AN (2006) Reliable Comet assay measurements for detecting DNA damage induced by ionizing radiation and chemicals. Mutat Res 605:7–16
Lei W, Wang DD, Dou TY, Hou J, Feng L, Yin H, Luo Q, Sun J, Ge GB, Yang L (2017) Assessment of the inhibitory effects of pyrethroids against human carboxyl-esterases. Toxicol Appl Pharmacol 321:48–65
Rao MV, Paliyath G, Ormrod DP (1996) Ultraviolet-B-and ozone induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110:125–136
Schäfer RB, van den Brink PJ, Liess MN (2011) Impacts of pesticides on freshwater ecosystems. Eco Impacts Toxic Chem:111–137
Taha TC (1993) Effect of temperature on the growth, life cycle and fecundity of the freshwater snails Lymnaea (Radix) luteola and Lymnaea (Radix) acuminata (Mollusca: Gastropoda). Philipp J Sci 121(4):419–434
Thomas DC (1948) The use of metaldehyde against slugs. Ann Appl Biol 35:207–227
Tripathi AP, Singh VK, Singh DK (2013) Behavioral responses of the snail Lymnaea acuminata towards photo and chemo attractants: a new step in control program of Fasciolosis Anupam. Int J Zool 2013:Article ID 439276 6 pages
Ussery DW (2001) DNA denaturation. Encyclopedia of Genetics Pages 550-553.
Vöhringer ML, Becker TW, Krieger G, Jacobi H, Witte I (1998) Synergistic DNA damaging effects of malondialdehyde/Cu(II) in PM2 DNA and human fibroblasts. Toxicol Lett 94(3):159–166
Availability of data and materials
All relevant data are within the manuscript and available from the corresponding author upon request.
Funding
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no. RG-1435-076.
Author information
Authors and Affiliations
Contributions
Conceptualization: Daoud Ali
Data curation: Daoud Ali, Khalid E Ibrahim
Formal analysis: Daoud Ali, Khalid E Ibrahim
Investigation: Daoud Ali, Khalid E Ibrahim, Seik Altaf Hussain
Methodology: Daoud Ali, Khalid E Ibrahim
Project administration: Daoud Ali
Writing original draft: Daoud Ali
Writing review and editing: Daoud Ali, Seik Altaf Hussain, Mohamed M. Abdel-Daim
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable
Consent to participate
All authors were participated in this work
Consent to publish
All authors agree to publish.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, D., Ibrahim, K.E., Hussain, S.A. et al. Role of ROS generation in acute genotoxicity of azoxystrobin fungicide on freshwater snail Lymnaea luteola L. Environ Sci Pollut Res 28, 5566–5574 (2021). https://doi.org/10.1007/s11356-020-10895-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10895-w