Abstract
Ag2CO3/AgBr/graphene oxide (Ag2CO3/AgBr/GO) ternary composites with different percentages of GO were fabricated by a facile co-precipitation strategy. The composites were characterized in the aspect of phase composition, light absorption performance, and micromorphology etc. The activity of the composites was studied by photocatalytic degradation of colored organic dye (rhodamine B, RhB) and colorless organics (phenol) under the shine of visible light. The optimized Ag2CO3/AgBr/GO-7.5 composites revealed the most excellent photocatalytic activity, which exhibited an apparent reaction rate constant exceeding that of pristine AgBr and Ag2CO3 by a factor of 107 and 5.63, respectively. The outstanding performance can be attributed to the effective separation of electrons and holes as well as the strong light absorption ability resulting from the Ag2CO3/AgBr/GO heterostructure. Moreover, it was verified that h+ and •O2− were two major active substances responsible for the decomposition of organic pollutants according to the free radical-trapping experiments. Besides, a probable reaction mechanism referring to the charge transfer and separation in the composites was proposed and discussed in detail.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With people’s raising awareness of energy shortage and environmental pollution, the pace of developing solar energy is getting faster all over the world. Semiconductor-based photocatalysts with potential application for purification of organic pollutants have drawn wide attention (Shi et al. 2019; Mao et al. 2019; García-Muñoz et al. 2019; Doan et al. 2019; Puttaswamy et al. 2013). Among various sorts of photocatalytic materials, TiO2 has become one of the most promising green photocatalysts for environmental protection owing to its advantages such as good oxidation ability, photostability, environmental friendliness, and low cost (Wang et al. 2012; Guo et al. 2019; Zheng et al. 2014; Chen et al. 2020; Lv et al. 2017). However, this traditional photocatalyst can only respond to ultraviolet light (accounting for ca. 4% of the solar spectrum) owing to its large band gap (3.2 eV). The practical application of TiO2 photocatalysts is greatly restricted since it cannot make full use of the visible part of the sunlight. Hence, the exploration of photocatalysts with high visible-light-response activity for making efficient use of solar energy has become a promising research trend. Over the years, a variety of compounds such as sulfide, Bi-based and Ag-based semiconductor materials have proved to be excellent photocatalysts that are sensitive to visible light, which exhibited high activity for decomposition of various dyes. Cadmium sulfide (CdS) (Cai et al. 2019; Cheng et al. 2018), bismuth vanadate (BiVO4) (Jiang et al. 2019; Nguyen and Hong 2019), silver phosphate (Ag3PO4) (Liu et al. 2019a, 2019b), and silver carbonate (Ag2CO3) (Xu et al. 2015; Fang et al. 2016; Chen et al. 2017) are typical representatives of the above semiconductor photocatalysts. Ag2CO3 is such a novel photocatalytic material with a bandgap of 2.46 eV. It has shown high visible-light-responsive activity for decomposing a variety of dye solutions including methylene blue (MB), rhodamine B (RhB), and methyl orange (MO) (Yang et al. 2016; Dai et al. 2012; Jo et al. 2018). Nonetheless, the above shown Ag-based photocatalyst always suffers from photocorrosion. For instance, when the Ag2CO3 catalyst gets excited by visible light and starts to produce electrons and holes simultaneously. For one thing, the electrons would recombine with holes due to the relatively narrow energy gap of Ag2CO3. For another, the Ag2CO3 is slightly soluble which makes easy reduction of Ag+ to metallic Ag by photoelectrons and thus resulting in the decrease of the photocatalytic property and stability (Wang et al. 2017).
Up to now, variety of methods has been exploited to overcome the deactivation issue of Ag2CO3. Coupling Ag2CO3 with other compound semiconductors is expected to be an effective solution for improving its activity and stability (Yuan et al. 2017; Yin et al. 2015). Yuan et al. fabricated Ag2CO3/Ag/WO3 composites which exhibited increased photocatalytic activity compared with that of singular Ag2CO3 rods ascribing to the formation of a Z-scheme system that effectively separated the photogenerated charges (Yuan et al. 2017). Yin and co-workers synthesized AgBr/Ag2CO3 which had higher catalytic performance compared with pure Ag2CO3 resulting from the intensified light capture capability and higher charge separation efficiency of AgBr/Ag2CO3 hybrid structure (Yin et al. 2015). From then on, various binary heterojunction composites relating to AgBr/Ag2CO3 have been prepared and the mechanisms for the improved activity were investigated (Wang et al. 2016b; Mehraj et al. 2014; Xie et al. 2016; Asadollahi et al. 2017). Besides, during the past few years, one atomic-layer two-dimensional carbon material like graphene oxide (GO) has aroused wide concern because of its unique sp2 hybrid carbon network with remarkable characters such as high specific surface area, superior carrier mobility, favorable heat conductivity, and good chemical stability. The surface of GO contains many functional groups such as hydroxyl and carboxyl groups. Hence, it can be served as a desirable support material for deposition of photocatalysts. It has been demonstrated that GO sheets can increase the light absorption and promote the electron transfer, and strengthen the adsorption of organic contaminants (Song et al. 2014; Dong et al. 2014; Li et al. 2018; Xia et al. 2017;Wu et al. 2015).
The previously published works mainly focused on the construction of binary heterojunction composites such as AgBr/Ag2CO3 (Yin et al. 2015; Wang et al. 2016b; Mehraj et al. 2014; Xie et al. 2016; Asadollahi et al. 2017), Ag2O/Ag2CO3 (Jo et al. 2018), g-C3N4/Ag2CO3 (Shi et al. 2015; Xu et al. 2014), Ag2CO3/TiO2 (Feng et al. 2014), and rGO/Ag2CO3 (Dai et al. 2014; Song et al. 2016; Xu et al. 2014). However, little concern has been aroused to the synthesis of ternary semiconductor composites (Ren et al. 2015; Wang et al. 2016a). According to what we know about the existing literature, the research on the Ag2CO3/AgBr/graphene oxide (Ag2CO3/AgBr/GO) ternary composites has never been reported. In this work, Ag2CO3/AgBr/GO composites containing varying quantities of GO were prepared by means of one-step co-precipitation. The as-prepared ternary composites displayed an improved photocatalytic activity compared with that of Ag2CO3/AgBr, Ag2CO3/GO, and AgBr/GO binary composites towards decomposition of rhodamine B (RhB) and phenol under visible-light illumination. A feasible charge transfer mechanism for the ternary composites with improved performance has been investigated and proposed.
Experimental
Synthesis of Ag2CO3/AgBr/GO composites
Analytical reagents were used in the whole synthesis process. The graphene oxide (GO) was purchased from the Suzhou Tanfeng Graphene Technology Co., Ltd. Typically, silver nitrate (6 mmol) was dissolved into deionized water (40 mL) under magnetic stirring in dark condition. Then, a certain volume of GO aqueous solution (1 × 10−6 g/mL) was instilled into the AgNO3 solution under ultrasonic vibration for 20 min. Simultaneously, 1 mmol of cetyltrimethylammonium bromide (CTAB) and 3 mmol of ammonium bicarbonate (NH4HCO3) were completely dissolved together in deionized water (40 mL) under magnetic agitation. Afterwards, the CO32−- and Br−-contained solutions were continuously dropped to the AgNO3/GO solution along with ceaseless agitation in dark condition. And then yellow precipitates were obtained, followed by filtration and several times of washing with ethanol and distilled water. Finally, the wet powder was put in an oven to dry for 12 h at 60 °C. The dried powders prepared with different volume of GO solution (5, 7.5, 10 mL) were denoted as Ag2CO3/AgBr/GO-5, Ag2CO3/AgBr/GO-7.5, and Ag2CO3/AgBr/GO-10, respectively. For comparison, the pure Ag2CO3, pure AgBr, and Ag2CO3/AgBr samples were also prepared in the same way without adding GO.
Characterization
The X-ray diffraction (XRD) patterns referring to crystalline structures of the samples were obtained on a D8-Advance X-ray powder diffractometer equipped with Cu Kα radiation. The chemical construction of the sample can be further acquired on a Raman spectrometer (LabRAM HR800) using Ar+ laser (632.8 nm) as excitation source. The chemical states and molecular structures analysis were conducted by an X-ray photoelectron spectrometer (XPS, ESCALAB 250Xi) with Al Kα source. The observation of surface morphology of the samples was taken on a field emission scanning electron microscopy (FESEM, SU8010). The element composition and their distribution on the surface of the samples were then analyzed by an X-ray fluorescence (iXRF) spectrometer assembled on SEM. The light absorption capability of the samples was acquired by UV-visible diffuse reflectance spectra (DRS) on a UV-vis spectrophotometer using BaSO4 as reference.
Measurement of photocatalytic activity
To test the photocatalytic activities of the samples, rhodamine B (RhB) and phenol (typical organic contaminant) were selected as degradation targets. A xenon arc lamp (350 W, λ > 420 nm) was served as the illuminating source. Specifically, the catalyst powder (100 mg) was mixed with RhB aqueous solution (50 mL, 1 × 10−5 M) to form a suspension, which was stirred constantly in a darkroom until the adsorption and desorption of RhB on the surface of the photocatalysts get balanced. At fixed time intervals, the RhB was separated from the powders by centrifugation and analyzed by the UV-vis spectrophotometer. The concentration change of RhB in the degradation conforms to the pseudo-first-order kinetics, and the apparent reaction rate constant k can be computed by k = − ln(Ct/C0)/t, where C0 and Ct represent the initial RhB concentration as well as that in the time of t, respectively. Meanwhile, the photodegradation of colorless phenol (50 mL, 2 × 10−4 M) was also carried out under the same conditions. The total organic carbon (TOC) experiment was carried out using a Vario TOC analyzer (Elementar Corporation, Germany). The removal rate can be calculated by R = (TOC0 − TOCt)/TOC0 × 100%, where TOC0 and TOCt represent the TOC value of initial RhB and TOC value of RhB after photocatalytic degradation at the time of t, respectively.
Results and discussion
Texture and component analysis
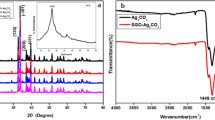
Figure 1 displays the X-ray diffraction patterns of the as-prepared Ag2CO3/AgBr/GO ternary composites with various GO content as well as pure Ag2CO3 and AgBr. The pure Ag2CO3 exhibited evident diffraction peaks at 2θ = 18.55°, 20.55°, 32.66°, 33.67°, 37.13°, and 39.67° (labeled with black diamond), which correspond to the (020), (110), (− 101), (− 130), (200), and (031) lattice planes of Ag2CO3 crystal in the form of monoclinic (JCPDS No. 26-0339) (Wang et al. 2017). For the pure AgBr, diffraction peaks at 30.94°, 44.33°, 55.02°, 64.47°, and 73.22° (labeled with black club suit) were indexed to (200), (220), (222), (400), and (420) crystal planes relating to the cubic structure of AgBr (JCPDS No. 06-0438) (Li et al. 2015). The hybrid photocatalysts exhibited both Ag2CO3 and AgBr phases, indicating the successful formation of Ag2CO3/AgBr composites. Nonetheless, there were no characteristic peaks corresponding to the GO discerned in the complex owing to its low content and weak peak intensity and the broke regular-stacking of GO by the loading of Ag2CO3/AgBr composites.
The existence of GO can be ascertained by the Raman spectroscopy (Fig. 2). Three peaks at around 704, 1068, and 1387 cm−1 were related to the characteristic Raman spectrum of Ag2CO3. After adding GO into the Ag2CO3/AgBr/GO composites, the characteristic peaks of Ag2CO3 significantly decreased, possibly because of the GO wrapping on the surface of Ag2CO3/AgBr. Meanwhile, two broad peaks at 1349 and 1590 cm−1 corresponded to the graphitized structure of GO (Xiang et al. 2011), which suggested the presence of GO in the Ag2CO3/AgBr/GO composites. The surface elemental components and chemical status of the as-prepared Ag2CO3/AgBr/GO-7.5 composites were further identified by XPS and high-resolution spectra of Ag 3d, C 1s, and O 1s. Four elements containing Ag, C, O, and Br can be found in the composites, as shown in the full survey spectrum (Fig. 3a). The Ag 3d spectrum (Fig. 3b) consists of two peaks at 367.2 and 373.2 eV, which correspond to the Ag 3d5/2 and Ag 3d3/2 binding energies of both Ag2CO3 and AgBr (Wang et al. 2017). In the case of C 1s spectrum (Fig. 3c), three fitted peaks locating at 288.2, 286.0, and 284.3 eV can be assigned to the carboxyl (O–C=O), epoxy/hydroxyl (C–O), and graphitic sp2 carbon (C=C–C) originated from graphene oxide, respectively (Dubin et al. 2010; Yang et al. 2014; Song et al. 2014). The spectral peak of O 1s (Fig. 3d) appears at 530.7 eV attributing to the lattice oxygen in Ag2CO3.
SEM analysis
The surface features of the as-prepared Ag2CO3, AgBr, Ag2CO3/AgBr and Ag2CO3/AgBr/GO-7.5 composites are shown in Fig. 4. The pure Ag2CO3 powders showed irregular polyhedral structures with length of 2–6 μm (Fig. 4a and b). The pristine AgBr powder was composed of near-spherical particles with smooth surface, the diameter of which is between 0.5 and 2 μm (Fig. 4c and d) (Lou et al. 2011). In the case of Ag2CO3/AgBr composites, both size of the Ag2CO3 and AgBr dramatically became smaller compared with that of before coupling (Fig. 4e and f). The diameter of the near-spherical AgBr particles reduced to about 0.25–0.6 μm. The Ag2CO3 particles exhibited polyhedral rod-like morphology with length of 2–5 μm. The AgBr particles are clustered around the Ag2CO3 particles. For the Ag2CO3/AgBr/GO-7.5 ternary composites, there were few changes in the sizes and morphologies when compared with that of Ag2CO3/AgBr composites, as shown in Fig. 4g and h. Besides, quantities of AgBr particles aggregated and attached on the surface of the Ag2CO3 rods, indicating a heterostructure of Ag2CO3 and AgBr was formed. The intimate contact between Ag2CO3 and AgBr can be attributed to the bonding force between Ag2CO3/AgBr and GO formed in the synthesis procedure. Thus, the photoexcited electrons can be easily separated from holes through this heterostructure, which was conductive to the improvement of the photocatalytic activity.
UV-vis diffuse reflectance spectra analysis
Figure 5 shows the UV-vis optical absorption performances of as-prepared samples. The primary absorption edge of pure AgBr appeared at around 470 nm while the pure Ag2CO3 displayed a strong absorption of visible light with wavelength larger than 420 nm. The Ag2CO3/AgBr/GO composites with different contents of GO exhibited both absorption property of Ag2CO3 and AgBr. Besides, the optical absorption intensity of the Ag2CO3/AgBr/GO composites increased continuously as the amount of GO increased. Furthermore, all the Ag2CO3/AgBr/GO composites showed a wide absorption peak from 470 to 600 nm compared with pure AgBr and Ag2CO3. This is caused by the Ag nanoparticles that generate the surface plasmon response (SPR) effect (Wang et al. 2013), which is beneficial to capture solar energy and enhance performance.
Photocatalytic activity
The photodegradation experiments of the Ag2CO3, AgBr, Ag2CO3/AgBr, and Ag2CO3/AgBr/GO-x (x = 5, 7.5, 10) composites were carried out in a floating reaction system exposed to visible light. The contrast experiment verified that only about 2.8% variation of the RhB concentration was detected under visible light illumination for 50 min without the addition of the photocatalyst (Fig. 6a). It is evident from Fig. 6a that all the Ag2CO3/AgBr/GO composites as well as Ag2CO3/AgBr composites represented higher activity than that of the pure Ag2CO3 and AgBr photocatalysts. It took about 46 min for pure Ag2CO3 to completely degrade RhB solution, whereas only 12 min was needed for Ag2CO3/AgBr/GO-7.5 ternary composite sample. To quantitatively evaluate the catalytic performance of different samples, the apparent reaction rate constants (k) were worked out. It is clear that the k value increased first and then decreased as the GO content increased (Fig. 6b). The Ag2CO3/AgBr/GO-7.5 composites reached a highest k value of 0.321 min−1, which was 107 and 5.63 times that of pure AgBr and Ag2CO3. The total organic carbon (TOC) removal of RhB over the Ag2CO3/AgBr/GO-7.5 composites at different time intervals is illustrated in Fig. 6c. It was found that 45.83% of TOC was mineralized after irradiation for 12 min, indicating that the RhB can be photo-oxidized to small molecules and further mineralized to CO2 by the Ag2CO3/AgBr/GO-7.5 composites under visible light irradiation.
Usually, dyes are the most frequently used model pollutants for evaluating the photocatalytic activity. However, some dye molecules could be activated by visible light and the relating chromophoric group can be destroyed, resulting in the decolorization of the dyes in the absence of photocatalysts. Hence, phenol with hardly visible light absorption characteristics was available as a colorless model contaminant to better reflect the real contribution of the Ag2CO3/AgBr/GO-7.5 composites irradiated with visible light. Figure 7 shows that the peak intensity of phenol gradually decreased with prolonged illumination time and almost vanished after 45 min. Further observation indicates that the space between each peak gradually reduced with increasing time, illustrating the deactivation of Ag2CO3/AgBr/GO-7.5 composites during the prolonged illumination. It is interesting to note that the quondam polyhedral rod-like Ag2CO3 had been corroded into porous structure with rough surface (Fig. 8a). The correlative XRD pattern of Ag2CO3/AgBr/GO-7.5 after photocatalytic reaction exhibited three new diffraction peaks at 38.10, 64.38, and 77.60° (Fig. 8b) besides the peaks of Ag2CO3 and AgBr, which corresponded to the (111), (220), and (311) crystal planes of silver (Wang et al. 2018), respectively. These metallic Ag particles originated from the reduction of Ag+ ions by capturing electrons (Wang et al. 2017). Meanwhile, the photocatalyst powder had turned color from original yellow to black, demonstrating the decomposition of Ag2CO3/AgBr/GO-7.5 composites.
Possible mechanism for enhanced performance
In the course of the photocatalytic oxidation, the decomposition of organic contaminants is closely related to three reactive species including photogenerated holes (h+), superoxide radicals (•O2−), and hydroxyl radicals (•OH). Hence, radical-trapping experiments were carried out to identify the main active substances participating in the photodegradation of RhB. Benzoquinone (BQ, 0.1 mM), disodium ethylenediamine tetraacetate (EDTA-2Na, 1 mM), and isopropanol (IPA, 1 mM) were adopted as scavengers for •O2−, h+, and •OH, respectively. The diagram for the degradation percentage of RhB by the Ag2CO3/AgBr/GO-7.5 composites with or without scavengers exposed to visible light for 12 min is illustrated in Fig. 9. It is clear that about 98% of RhB was photodegraded by the Ag2CO3/AgBr/GO-7.5 without scavenger. The photodegradation of RhB was almost impervious to IPA while dramatically suppressed by EDTA-2Na and BQ. This result demonstrates that the h+ and •O2− are the two primary oxidizing substances responsible for the photodegradation of RhB (Yin et al. 2015).
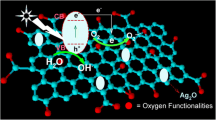
The above experimental and analytical results have demonstrated that the hybrid structure of Ag2CO3/AgBr with GO performs valuable function for the enhanced performance of composites, which can be explained by the mechanism as illustrated in Fig. 10. Both Ag2CO3 and AgBr were synchronously excited to produce pairs of electrons and holes when exposed to visible light. In the light of previous reports, the Ag2CO3 possesses higher valence band (VB, EVB = 2.75 eV) and conduction band (CB, ECB = 0.29 eV) than that of AgBr (EVB: 2.55 eV, ECB: 0.07 eV) (Yin et al. 2015). Hence, the photogenerated holes in the VB of Ag2CO3 (more positive) would migrate to the VB of AgBr, while the electrons in the CB of AgBr (less positive) tend to migrate down to the CB of Ag2CO3 (more positive). Thus, the separation of electrons from holes can be effectively promoted. The transfer process of electrons from AgBr to Ag2CO3 can also be confirmed by the SEM image of Ag2CO3/AgBr/GO-7.5 composites after photocatalytic reaction, as depicted in Fig. 8a. The structure of AgBr particles deposited on the surface of Ag2CO3 had been well reserved, while the polyhedral rod-like Ag2CO3 had been corroded into porous structure with rough surface due to the partial decomposition of Ag2CO3. The partly dissolved Ag+ ions had been reduced into metallic Ag particles by the accumulated electrons transferred from AgBr and photogenerated electrons from itself.
For one thing, the RhB or phenol can be oxidized into H2O and CO2 directly by the holes in the AgBr rather than by •OH radicals with E0 (•OH/H2O) of 2.27 eV. Furthermore, it has been reported that the E0 (O2/•O2−) potential (− 0.046 eV vs SHE) is more negative than the ECB potential of Ag2CO3 (0.29 eV) or AgBr (0.07 eV) (Yao and Liu 2014; Mehraj et al. 2014). As a result, no •O2− reactive species can be produced by the electrons in the conduction band of Ag2CO3 or AgBr. Therefore, the existing •O2− radicals were generated by the reaction between the dissolved O2 and electrons over the GO which accepted electrons from the CB of Ag2CO3 or AgBr owing to its excellent electron transport property. The charge transfer through the heterostructure between GO and Ag2CO3 (or AgBr) effectively inhibited the combination of electrons with holes. Meanwhile, the •O2− radicals with powerful oxidative ability can effectively decompose the RhB or phenol. As a consequence, the activity of the Ag2CO3/AgBr/GO composite photocatalyst was significantly improved.
Conclusions
In summary, the Ag2CO3/AgBr/GO ternary composites with varying content of GO were successfully synthesized through a facile one-step co-precipitation procedure. The as-prepared hybrid photocatalysts exhibited improved activity than single Ag2CO3 and AgBr for photodecomposition of RhB and phenol irradiated with visible light. Especially, the Ag2CO3/AgBr/GO-7.5 composites displayed the highest photocatalytic activity mainly attributing to the effective separation of electrons from holes bought by the multi-heterostructure. Besides, the light absorption of the Ag2CO3/AgBr/GO composites was conspicuously enhanced with the aid of GO. We believe that this ternary composites photocatalyst may be a prospective material that actually gets practical application for decontamination of organic wastewater.
References
Asadollahi A, Sohrabnezhad S, Ansari R (2017) Enhancement of photocatalytic activity and stability of Ag2CO3 by formation of AgBr/Ag2CO3 heterojunction in mordenite zeolite. Adv Powder Technol 28:304–313. https://doi.org/10.1016/j.apt.2016.10.004
Cai FY, Zhang YQ, Wang JT, Zhou JR, Cao HL, Liu J (2019) Mixed phase nano-CdS supported on activated biomass carbon as efficient visible light-driven photocatalysts. Environ Sci Pollut Res 26:31055–31061. https://doi.org/10.1007/s11356-019-06267-8
Chen F, Wu YD, Ning GQ, Ren JB, Zhang ZY, Zheng CC, Zhong YG, Hu Y (2017) Facile preparation of ternary Ag2CO3/Ag/PANI composite nanorods with enhanced photoactivity and stability. J Mater Sci 52:4521–4531. https://doi.org/10.1007/s10853-016-0697-7
Chen LQ, Tian LJ, Xie JY, Zhang CJ, Chen JN, Wang Y, Qin L, Lv KL, Deng KJ (2020) One-step solid state synthesis of facet-dependent contact TiO2 hollow nanocubes and reduced graphene oxide hybrids with 3D/2D heterojunctions for enhanced visible photocatalytic activity. Appl Surf Sci 504:144353. https://doi.org/10.1016/j.apsusc.2019.144353
Cheng L, Xiang QJ, Liao YL, Zhang HW (2018) CdS-based photocatalysts. Energy Environ Sci 11:1362–1391. https://doi.org/10.1039/c7ee03640j
Dai GP, Yu JG, Liu G (2012) A new approach for photocorrosion inhibition of Ag2CO3 photocatalyst with highly visible-light-responsive reactivity. J Phys Chem C 116:15519–15524. https://doi.org/10.1021/jp305669f
Dai GP, Liu SQ, Liang Y, Liu K (2014) Fabrication of a nano-sized Ag2CO3/reduced graphene oxide photocatalyst with enhanced visible-light photocatalytic activity and stability. RSC Adv 4:34226–34231. https://doi.org/10.1039/c4ra04792c
Doan VD, Luc VS, Nguyen TLH, Nguyen TD, Nguyen TD (2019) Utilizing waste corn-cob in biosynthesis of noble metallic nanoparticles for antibacterial effect and catalytic degradation of contaminant. Environ Sci Pollut Res 27:1–15. https://doi.org/10.1007/s11356-019-07320-2
Dong C, Wu KL, Wei XW, Li XZ, Liu L, Ding TH, Wang J, Ye Y (2014) Synthesis of graphene oxide-Ag2CO3 composites with improved photoactivity and anti-photocorrosion. CrystEngComm 16:730–736. https://doi.org/10.1039/c3ce41755g
Dubin S, Scott G, Wang K, Tung VC, Cha K, Hall AS, Farrar J, Varshneya R, Yang Y, Kaner RB (2010) A one-step, solvothermal reduction method for producing reduced graphene oxide dispersions in organic solvents. ACS Nano 4:3845–3852. https://doi.org/10.1021/nn100511a
Fang SS, Ding CY, Liang Q, Li ZY, Xu S, Peng YY, Lu DY (2016) In-situ precipitation synthesis of novel BiOCl/Ag2CO3 hybrids with highly efficient visible-light-driven photocatalytic activity. J Alloys Comp 684:230–236. https://doi.org/10.1016/j.jallcom.2016.05.168
Feng CX, Li GG, Ren PH, Wang Y, Huang XS, Li DL (2014) Effect of photo-corrosion of Ag2CO3 on visible light photocatalytic activity of two kinds of Ag2CO3/TiO2 prepared from different precursors. Appl Catal B 158–159:224–232. https://doi.org/10.1016/j.apcatb.2014.04.020
García-Muñoz P, Zussblatt NP, Pliego G, Zazo JA, Fresno F, Chmelka BF, Casas JA (2019) Evaluation of photoassisted treatments for norfloxacin removal in water using mesoporous Fe2O3-TiO2 materials. J Environ Manage 238:243–250. https://doi.org/10.1016/j.jenvman.2019.02.109
Guo H, Jiang N, Wang HJ, Shang KF, Lu N, Li J, Wu Y (2019) Enhanced catalytic performance of graphene-TiO2 nanocomposites for synergetic degradation of fluoroquinolone antibiotic in pulsed discharge plasma system. Appl Catal B 248:552–566. https://doi.org/10.1016/j.apcatb.2019.01.052
Jiang LD, Chen D, Qin LS, Liang JH, Sun XG, Huang YX (2019) Enhanced photocatalytic activity of hydrogenated BiVO4 with rich surface-oxygen-vacancies for remarkable degradation of tetracycline hydrochloride. J Alloys Compd 783:10–18. https://doi.org/10.1016/j.jallcom.2018.12.290
Jo WK, Kumar S, Yadav P, Tonda S (2018) In situ phase transformation synthesis of unique Janus Ag2O/Ag2CO3 heterojunction photocatalyst with improved photocatalytic properties. Appl Surf Sci 445:555–562. https://doi.org/10.1016/j.apsusc.2018.03.194
Li JJ, Xie YL, Zhong YJ, Hu Y (2015) Facile synthesis of Z-scheme Ag2CO3/Ag/AgBr ternary heterostructured nanorods with improved photostability and photoactivity. J Mater Chem A 3:5474–5481. https://doi.org/10.1039/c4ta06075j
Li YH, Wu XF, Ho WK, Lv KL, Li Q, Li M, Lee SC (2018) Graphene-induced formation of visible-light-responsive SnO2-Zn2SnO4 Z-scheme photocatalyst with surface vacancy for the enhanced photoreactivity towards NO and acetone oxidation. Chem Eng J 336:200–210. https://doi.org/10.1016/j.cej.2017.11.045
Liu Y, Wang WG, Si MZ, Yu YF, Zhang HY (2019a) (Yb3+, Er3+) co-doped TiO2/Ag3PO4 hybrid photocatalyst with enhanced activity for photodegradation of phenol. Appl Surf Sci 463:159–168. https://doi.org/10.1016/j.apsusc.2018.08.188
Liu Y, Wang WG, Si MZ, Zhang HY (2019b) Carbon Cloth-supported MoS2/Ag2S/Ag3PO4 composite with high photocatalytic activity and recyclability. ChemCatChem 11:1017–1025. https://doi.org/10.1002/cctc.201801771
Lou ZZ, Huang BB, Qin XY, Yang XY, Wang ZY, Zheng ZK, Cheng HF, Wang P, Dai Y (2011) One-step synthesis of AgBr microcrystals with different morphologies by ILs-assisted hydrothermal method. CrystEngComm 13:1789–1793. https://doi.org/10.1039/c0ce00856g
Lv KL, Fang S, Si LL, Xia Y, Ho WK, Li M (2017) Fabrication of TiO2 nanorod assembly grafted rGO (rGO@TiO2-NR) hybridized flake-like photocatalyst. Appl Surf Sci 391:218–227. https://doi.org/10.1016/j.apsusc.2016.03.195
Mao J, Li PW, Wang JM, Wang HT, Zhang Q, Zhang LX, Li H, Zhang W, Peng TY (2019) Insights into photocatalytic inactivation mechanism of the hypertoxic site in aflatoxin B1 over clew-like WO3 decorated with CdS nanoparticles. Appl Catal B 248:477–486. https://doi.org/10.1016/j.apcatb.2019.01.057
Mehraj O, Mir NA, Pirzada BM, Sabir S, Muneer M (2014) In-situ anion exchange synthesis of AgBr/Ag2CO3 hybrids with enhanced visible light photocatalytic activity and improved stability. J Mol Catal A: Chem 395:16–24. https://doi.org/10.1016/j.molcata.2014.07.027
Nguyen DT, Hong SS (2019) Synthesis of needle-like BiVO4 with improved photocatalytic activity under visible light irradiation. J Nanosci Nanotechnol 19:7696–7701. https://doi.org/10.1166/jnn.2019.16731
Puttaswamy M, Malahalli VK, Tadashi I, Kenji T, Kazuyoshi U, Mineo S (2013) Hydrothermal synthesis of meso/macroporous BiVO4 hierarchical particles and their photocatalytic degradation properties under visible light irradiation. Environ Sci Pollut Res 20:6638–6645. https://doi.org/10.1007/s11356-013-1694-x
Ren HT, Jia SY, Wu SH, Zhang TH, Han X (2015) Phase transformation synthesis of novel Ag2O/Ag2CO3/g-C3N4 composite with enhanced photocatalytic activity. Mater Lett 142:15–18. https://doi.org/10.1016/j.matlet.2014.11.082
Shi L, Liang L, Wang FG, Liu MS, Sun JM (2015) Enhanced visible-light photocatalytic activity and stability over g-C3N4/Ag2CO3 composites. J Mater Sci 50:1718–1727. https://doi.org/10.1007/s10853-014-8733-y
Shi E, Xu ZL, Wang WJ, Xu Y, Zhang YH, Yang XZ, Liu Q, Zeng T, Song S, Jiang YZ, Li LXY, Sharma VK (2019) Ag2S-doped core-shell nanostructures of Fe3O4@Ag3PO4 ultrathin film: Major role of hole in rapid degradation of pollutants under visible light irradiation. Chem Eng J 366:123–132. https://doi.org/10.1016/j.cej.2019.02.018
Song YX, Zhu JX, Xu H, Wang C, Xu YG, Ji HY, Wang K, Zhang Q, Li HM (2014) Synthesis, characterization and visible-light photocatalytic performance of Ag2CO3 modified by graphene-oxide. J Alloys Compd 592:258–265. https://doi.org/10.1016/j.jallcom.2013.12.228
Song SQ, Cheng B, Wu NS, Meng AY, Cao SW, Yu JG (2016) Structure effect of graphene on the photocatalytic performance of plasmonic Ag/Ag2CO3-rGO for photocatalytic elimination of pollutants. Appl Catal B 181:71–78. https://doi.org/10.1016/j.apcatb.2015.07.034
Wang WG, Yu JG, Xiang QJ, Cheng B (2012) Enhanced photocatalytic activity of hierarchical macro/mesoporous TiO2-graphene composites for photodegradation of acetone in air. Appl Catal B 119–120:109–116. https://doi.org/10.1016/j.apcatb.2012.02.035
Wang WS, Du H, Wang RX, Wen T, Xu AW (2013) Heterostructured Ag3PO4/AgBr/Ag plasmonic photocatalyst with enhanced photocatalytic activity and stability under visible light. Nanoscale 5:3315–3321. https://doi.org/10.1039/c3nr00191a
Wang HQ, Li JZ, Huo PW, Yan YS, Guan QF (2016a) Preparation of Ag2O/Ag2CO3/MWNTs composite photocatalysts for enhancement of ciprofloxacin degradation. Appl Surf Sci 366:1–8. https://doi.org/10.1016/j.apsusc.2015.12.229
Wang PF, Wu TF, Ao YH, Wang C, Hou J, Qian J, Li Y (2016b) One-pot synthesis of AgBr/Ag2CO3 heterojunctions with enhanced visible-light photocatalytic activity. Mater Lett 163:258–261. https://doi.org/10.1016/j.matlet.2015.10.050
Wang WG, Liu Y, Zhan HY, Qian YN, Guo ZC (2017) Re-investigation on reduced graphene oxide/Ag2CO3 composite photocatalyst: an insight into the double-edged sword role of RGO. Appl Surf Sci 396:102–109. https://doi.org/10.1016/j.apsusc.2016.11.030
Wang P, Yu CD, Ding JJ, Wang XF, Yu HG (2018) Facile synthesis and improved photocatalytic performance of Ag-AgCl photocatalyst by loading basic zinc carbonate. J Alloys Compd 752:238–246. https://doi.org/10.1016/j.jallcom.2018.04.163
Wu XF, Wen LL, Lv KL, Deng KJ, Tang DG, Ye HP, Du DY, Liu SN, Li M (2015) Fabrication of ZnO/graphene flake-like photocatalyst with enhanced photoreactivity. Appl Surf Sci 358:130–136. https://doi.org/10.1016/j.apsusc.2015.08.061
Xia Y, Li Q, Lv KL, Tang DG, Li M (2017) Superiority of graphene over carbon analogs for enhanced photocatalytic H2-production activity of ZnIn2S4. Appl Catal B Environ 206:344–352. https://doi.org/10.1016/j.apcatb.2017.01.060
Xiang QJ, Yu JG, Jaroniec M (2011) Enhanced photocatalytic H2-production activity of graphene-modified titania nanosheets. Nanoscale 3:3670–3678. https://doi.org/10.1039/c1nr10610d
Xie JS, Fang C, Zou JJ, Lu HD, Tian CG, Han CL, Zhao DF (2016) In situ ultrasonic formation of AgBr/Ag2CO3 nanosheets composite with enhanced visible-driven photocatalytic performance. Mater Lett 170:62–66. https://doi.org/10.1016/j.matlet.2016.02.002
Xu H, Song YX, Song YH, Zhu JX, Zhu TT, Liu CB, Zhao DX, Zhang Q, Li HM (2014) Synthesis and characterization of g-C3N4/Ag2CO3 with enhanced visible-light photocatalytic activity for the degradation of organic pollutants. RSC Adv 4:34539–34547. https://doi.org/10.1039/c4ra03443k
Xu H, Zhu JX Zhu, Song YX, Zhu TT, Zhao WK, Song YH, Da ZL, Liu CB, Li HM (2015) Fabrication of AgX-loaded Ag2CO3 (X = Cl, I) composites and their efficient visible-light-driven photocatalytic activity. J Alloys Comp 622:347–357. https://doi.org/10.1016/j.jallcom.2014.09.148
Yang JK, Zhang XT, Li B, Liu H, Sun PP, Wang CH, Wang LL, Liu YC (2014) Photocatalytic activities of heterostructured TiO2-graphene porous microspheres prepared by ultrasonic spray pyrolysis. J Alloys Compd 584:180–184. https://doi.org/10.1016/j.jallcom.2013.08.203
Yang XF, Li R, Wang YQ, Wu KQ, Chang SF, Tang H (2016) Solvent-induced controllable synthesis of recyclable Ag2CO3 catalysts with enhanced visible light photocatalytic activity. Ceram Int 42:13411–13420. https://doi.org/10.1016/j.ceramint.2016.05.119
Yao XX, Liu XH (2014) One-pot synthesis of ternary Ag2CO3/Ag/AgCl photocatalyst in natural geothermal water with enhanced photocatalytic activity under visible light irradiation. J Hazard Mater 280:260–268. https://doi.org/10.1016/j.jhazmat.2014.07.079
Yin L, Wang Z, Lu L, Wan XK, Shi HX (2015) Universal degradation performance of a high-efficiency AgBr/Ag2CO3 photocatalyst under visible light and an insight into the reaction mechanism. New J Chem 39:4891–4900. https://doi.org/10.1039/c5nj00385g
Yuan XZ, Jiang LB, Chen XH, Leng LJ, Wang H, Wu ZB, Xiong T, Liang J, Zeng GM (2017) Highly efficient visible-light-induced photoactivity of Z-scheme Ag2CO3/Ag/WO3 photocatalysts for organic pollutant degradation. Environ Sci Nano 4:2175–2185. https://doi.org/10.1039/c7en00713b
Zheng Y, Cai JH, Lv KL, Sun J, Ye HP, Li M (2014) Hydrogen peroxide assisted rapid synthesis of TiO2 hollow microspheres with enhanced photocatalytic activity. Appl Catal B 147:789–795. https://doi.org/10.1016/j.apcatb.2013.10.011
Funding
This work was supported by the Guangzhou Science and Technology Project (grant number 201904010131); the National Natural Science Foundation of China (grant number 51302043); the National Natural Science Foundation of China-Guangdong Joint Fund (grant number U1401246); and the Science and Technology Program of Guangdong Province of China (grant numbers 2017B050504004, 2015B010135011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Sami Rtimi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Si, M., Wang, W., Guan, Q. et al. Facile fabrication of highly catalytic-active Ag2CO3/AgBr/graphene oxide ternary composites towards the photocatalytic wastewater treatment. Environ Sci Pollut Res 28, 4173–4183 (2021). https://doi.org/10.1007/s11356-020-10740-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10740-0