Abstract
Premna odorata Blanco (Lamiaceae) is an ethnomedicinal plant, where some reports claimed their anti-inflammatory, cytotoxic, and antituberculosis effects, without investigating its role on the brain. Therefore, forty mature male rats were equally divided into 4 groups; the 1st was kept as control. Rats in groups 2 and 4 were orally given P. odorata extract daily at a dose of 500 mg/kg B.W., while those in groups 3 and 4 were daily administrated aluminum chloride “AlCl3” (70 mg/kg B.W.). The treatments extended for 30 successive days. At the end of the experimental period, brain samples were collected for biochemical assay of glutathione reductase (GSH), catalase, malondialdehyde (MDA), and acetylcholinesterase activity (AChE). Besides, monoamines (norepinephrine, dopamine, serotonin), amino acids (glutamine, serine, arginine, taurine and gamma-aminobutyric acid (GABA)), neurotransmitters, DNA damage, cyclooxygenase-2 (COX-2), and tumor necrosis factor (TNF)-α genes were estimated. Moreover, brain samples were obtained for histopathological investigation. Aluminum toxicity resulted in a decline of GSH concentration, elevation of MDA, and AChE activity. Except for GABA which exhibited a significant decrease, there was a marked increase in the measured amino acid and monoamine neurotransmitters. Also, an increase in mRNA expressions of TNF-α and COX-2 was detected. It was noticed that Premna odorata extract reduced the oxidative stress and counteracted the augmentations in AChE caused by AlCl3. Marked improvements in most measured neurotransmitters with downregulation of pro-inflammatory gene expression were recorded in P. odorata + AlCl3 group. Premna odorata restores the altered histopathological feature induced by AlCl3. In conclusion, the present findings clarify that P. odorata extract could be important in improving and treatment of neurodegenerative disorders as it was able to reduce oxidative stress, DNA damage, biochemical alterations, and histopathological changes in rats exposed to AlCl3 toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neurotoxicity of aluminum (Al) was recognized a long time ago. Exposure to aluminum most frequently occurs through food additives, cooking utensils, deodorants, and aerospace (Singh and Goel 2015). Aluminum is considered a major environmental factor that can cause neurodegenerative diseases (Wei et al. 2017). After aluminum absorption, it accumulates in almost every organ particularly nerve and in glial cells. It gains access to the brain through transferrin receptors expressed in the blood-brain barrier (Yokel et al. 1999). In the brain, it causes degeneration of neuronal cells increasing the risk of neurodegenerative disorders. There are multiple mechanisms involved in aluminum-induced neurotoxicity; the major ones are the initiations of oxidative stress, inflammatory reactions (Kumar et al. 2009), and alteration of signaling processes mediated by neurotransmitters (Dalla Torre et al. 2019). Most of the drugs used for the treatment of neurological diseases have limited effectiveness and side effects (Hussien et al. 2018). The close link among oxidative stress, neurodegenerative disorders, and metals suggests a new possible therapeutic strategy for the use of free radical scavengers and metal chelators. In this context, the use of natural medicinal herbs and natural extracts as new disease-modifying therapy is required to block the neurodegenerative process.
Genus Premna is represented by many species of plants such as Premna integrifolia, Premna corymbosa, and Premna odorata that are cultivated in tropical and subtropical areas. Premna species have gained much attention and are used in a wide variety of traditional medicine applications because of their antioxidant (Elmaidomy et al. 2020), immunomodulatory, anti-inflammatory, antitumor, anti-diabetic, and anti-atherosclerotic activities (Hien et al. 2019; Kabra et al. 2015). The Premna odorata (P. odorata) plant contains a variety of chemical compounds such as iridoid glycosides and rhamnopyranoses (Elmaidomy et al. 2017). Iridoid glycoside compound has been reported to have neuroprotective and nerve growth factor–potentiating activities (Hang et al. 2008). However, few studies have investigated the medicinal effects of P. odorata. Therefore, the present study was performed to characterize the neuroprotective effect of P. odorata following oral supplementation against a potent neurotoxicant aluminum chloride.
Material and methods
Preparation of Premna odorata extract
Premna odorata leaves were obtained from the Department of Pharmacognosy, Faculty of Pharmacy, Beni-Suef University, Egypt. The plant leaves (2 kg) were collected and air-dried in a shaded area for 1 month. After drying, the leaves were finely powdered using Laboratory Small Jet Mill Machinery, Mill Jet, Mill Classifier (ALPA) (1-1000 V, ISO9001, CE, Shandong, China). The finely powdered plant was extracted by maceration without agitation using 70% ethanol (3 L, 2×, 7 days each) at room temperature and concentrated under vacuum at 45 °C using a rotary evaporator (BuchiRotavapor R-300, Cole-Parmer, Vernon Hills, IL, USA) to afford 150 g crude extract which was kept at 4 °C for biological investigations.
Chemicals
Aluminum chloride and all reagents used for biochemical estimation were procured from Sigma-Aldrich (St Louis, MO, USA). RNeasy mini kit (Qiagen, Germany) was used. Ethanol (EtOH) was purchased from El-Nasr Company for Pharmaceuticals and Chemicals, Egypt.
Animals and experimental design
Forty mature male Wistar rats (110–150 g) were used for this study. After acclimatization, rats were equally divided into four groups; the 1st was the control group and orally given 0.5 ml saline via stomach tube. In the 2nd group (Premna odorata group), each rat was orally given 0.5 ml of P. odorata extract via stomach tube at a dose rate of 500 mg/kg. In the 3rd group (AlCl3 group), each rat was given 70 mg/kg B.W. of AlCl3 orally, while in the 4th group (Premna odorata + AlCl3 group), each rat administrated P. odorata extract and AlCl3 at the same previous doses. All treatments were done daily for 30 days. The dose and route of AlCl3 administration were based on a previous study of Kadhem and Enaya (2018). This study was performed in accordance with the Institutional Animal Care and Use Committee of Beni-Suef University (BSU-IACUC) ethical guidelines. Rats were housed in a clean stainless steel cage under controlled temperature (23 ± 5 °C), relative humidity (50 ± 5%), and 12-h light/dark cycle. Food and water were offered ad libitum.
Brain samples
At the end of the experiment, rats were sacrificed by cervical dislocation and their brains were removed, washed with saline, and divided into four sections. The first section was used for the preparation of brain homogenates. The brain was homogenized in 10% w/v phosphate buffer (pH 7.4) and then centrifuged at 10000 xg for 15 min at 4 °C. The obtained supernatant was used for the biochemical estimations. The second section was used for DNA extraction, while the third section was used for RNA extraction to analyze cyclooxygenase-2 (COX-2) and tumor necrosis factor (TNF)-α gene expression. The previously mentioned three sections were stored at – 80 °C, while the fourth one was placed in 10% paraformaldehyde for histopathology examination.
Biochemical estimation
Assessment of antioxidant/oxidant parameters
Reduced glutathione (GSH) concentration was determined according to the method of Van Doorn et al. (1978). This method is based upon the development of stable yellow color when DTNB is added to sulfhydryl compounds. Catalase (CAT) activity was estimated according to Aebi (1984). The decomposition of H2O2 which was catalyzed by CAT can be followed by ultraviolet spectroscopy at 240 nm. Malondialdehyde (MDA) concentration was measured following the method of Uchiyama and Mihara (1978). It depends on that MDA, formed from the breakdown of polyunsaturated fatty acids, reacts with the thiobarbituric acid in acidic medium to give a pink color that can be measured spectrophotometrically at 535 nm and 520 nm.
Assessment of acetylcholine esterase (AChE) activity
Acetylcholine esterase activity was measured by the colorimetric method of Ellman et al. (1961) modified by Gorun et al. (1978). Brain sample was prewarmed (37 °C) for 5 min and incubated with acetylthiocholine solution for 30 min. Enzyme activity was stopped by the addition of DTNB reagent in ethanol. Absorbance of the 5-thio-2-nitrobenzoate chromophore formed from the reaction of thiocholine with DTNB was measured at 412 nm. The principle of this method is based on measurement of the thiocholine production rate as a result of acetylthiocholine hydrolysis.
Determination of monoamines neurotransmitters
Norepinephrine (NE), dopamine (DA), and serotonin (5-HT) were determined according to Ciarlone (1978). The principle of the method is the measurement of the fluorescent product that results from the reaction with alkaline sulfite and iodine solution in case of NE and DA and reaction with orthophthalaldehyde solution in case of 5-HT.
Assessment of amino acids neurotransmitters
Glutamine (Gln), serine (Ser), arginine (Agr), taurine (Tau), and γ-aminobutyric acid (GABA) were analyzed according to Cui et al. (2017). Determination of amino acids was conducted by high-performance liquid chromatography (HPLC) analysis with an Agilent 1200 series HPLC apparatus (USA). An ultrasphere C18 phase column at wavelength 254 nm with UV detector was used.
Determination of brain protein concentration
The protein content of the supernatants, resulting from the centrifugation of brain homogenate, was determined following the method of Lowry et al. (1951) using Folin’s phenol reagent. It depends on the formation of blue color that resulted from the reduction of the phosphomolybdic-phosphotungstic reagent by the copper-treated protein in alkaline medium at room temperature.
DNA laddering assay
DNA laddering assay was conducted according to Ibrahim and Ibrahem (2020). Briefly, The brain tissues were homogenized in lysis buffer (10 mM Tris-HCl, pH 7.4, 10 mM EDTA, 0.5% Triton X-100) then centrifuged at 13800 xg for 10 min. The pellets containing total intact DNA (P) and the supernatants containing smaller fragments of DNA (S). Both the S and P fractions were treated with 0.5 ml of 10% trichloroacetic acid (TCA) and were left overnight at 4 °C. A 80 μl of 5% TCA was added and incubated at 90 °C for 15 min. Freshly prepared 1 ml diphenylamine reagent, 1.5 g of diphenylamine dissolved in 100 ml acetic acid, 1.5 ml of sulfuric acid, and 0.50 ml of acetaldehyde (16 mg/ml) were added in each sample, tubes were allowed to stand overnight at room temperature, and OD was recorded at 600 nm. DNA laddering percentage was calculated as:
Agarose gel electrophoresis of the fragmented DNA
The agarose gel electrophoresis was done for the DNA fragments extracted from the supernatant portion using the DNeasy kit (Qiagen) electrophoresed in 1.5% agarose gel for 90 min at 5 V/cm and visualized with ethidium bromide.
Quantitative real-time PCR for cyclooxygenase-2 (COX-2) and TNF-α genes
Total RNA was isolated from the brain tissue using RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. First-strand cDNA was generated by reverse transcription of 10 μg RNA samples. The primer set used for COX-2 (Khalaf et al. 2017) are forward primer 5'AAGCCTCGTCCAGATGCTA-3' and reverse primer, 5'ATGGTGGCTGTCTTGGTAGG3', and those of the TNF-α were forward primer 5'ATGGGCTCCCTCTCATCAGT-3' and reverse primer 5'CAAGGGCTCTTGATGGCAGA-3'. Real-time PCR was done using a real-time PCR system (Applied Biosystems, USA) which was run for 40 cycles of denaturation at 95 °C for 45 s, annealing at 60 °C for both genes for 30 s and extension at 72 °C for 30 s.. The GAPDH gene was amplified in the same reaction to serve as the internal control (HelmyAbdou et al. 2019). Each assay was repeated twice, and the values were used to calculate the gene/GAPDH ratio, with a value of 1.0 used as the control (calibrator) (Ibrahim et al. 2020). The normalized expression ratio was calculated using the Mxprosoftware (Khalaf et al. 2019).
Histopathology examination of brain
By using a rotary microtome, paraffin blocks of the brain were sectioned into 4–5-μm-thick sections, and then these sections were stained using hematoxylin/eosin (H&E) staining procedure as described by Banchroft et al. (1996).
Statistical analysis
The data of this study were analyzed with one-way ANOVA followed by Tukey post-hoc test, performed by for GraphPad Prism v5.0 (GraphPad Software, Inc.) Windows Software. Values were expressed as the mean ± standard error, and differences were considered to indicate a statistically significant at P ≤ 0.05.
Results
Plant extract content
Figure S1 shows that the chemical profiling of the of P. odorata leaves secondary metabolites, using LC–HRESIMS, chromatographic, and spectroscopic techniques, resulted in the characterization of a variety of constituents, including iridoids (premnoside A and 6- O-α- L-(2”-O-trans-caffoyl) rhamnopyranosylcatalpol, premnoside C, D, H and 6- O-α- L-(2”-O-trans-methoxycinnamoyl) rhamnopyranosylcatalpol, premnoside G, and 6- O-α- L-(4”-O-trans-feruolyl) rhamnopyranosylcatalpol, premnoside E-F, premnaodoroside A-D and premnoside D, premcoryoside, flavones (vitexin, acacetin, diosmetin, luteolin, and apigenin), phenylethanoid verbascoside, rhamnopyranoside esters (1-O-trans-p-coumaroyl- 2-O-trans-caffeoyl-α-L-rhamnopyranose,1-O-trans-p-coumaroyl-2-O-trans-caffeoyl-α-Lrhamnopyranose), and sterols, triterpenes, fatty acids (ducosterol, stigmasterol, β-sitosterol, linolenic acid, β-amyrin, arjunolic acid) (Elmaidomy et al. 2017).
Oxidant/antioxidant markers
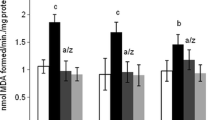
Table 1 shows that treatment with the P. odorata extract had no statistical differences in GSH, CAT, and MDA values as compared with the control. The administration of AlCl3 significantly lowered GSH concentration and elevated MDA concentration as compared with control and P. odorata group values. AlCl3 treatment did not significantly lower the activity of CAT enzyme comparing with the control group. Combination of both Premna odorata and AlCl3 induced significant elevation of GSH concentration and reduction in MDA concentration as compared with AlCl3 group.
Acetylcholine esterase activity
A significant elevation in the AChE activity was observed in AlCl3-treated group when compared with either control or P. odorata groups. The AChE activity of rats treated with P. odorata extract had no significant effect in comparison with the control group. Administration of P. odorata extract with AlCl3 was able to significantly inhibit the AChE activity when compared with AlCl3-treated group (Table 1).
Neurotransmitters markers
Results of monoamine neurotransmitters are shown in Table 2. Premna odorata extract has no adverse effect on NE, DA, and 5-HT concentrations as indicated by the insignificant difference of their concentrations in P. odorata group and control group. Aluminum chloride treatment induced a significant increase of NE, DA, and 5-HT concentrations when compared with control and P. odorata groups. The brain of rats co-administered P. odorata extract with AlCl3 showed a significant decrease in NE concentration as compared with AlCl3-treated group, but its concentration remained higher than the control. Exposure to P. odorata and AlCl3 caused a significant decrease of the DN content in the brain when compared with AlCl3-treated group without showing any statistical difference from those of the control. Serotonin concentrations in AlCl3+ P. odorata group showed insignificant changes as compared with the control and AlCl3 groups.
A total of five amino acids were evaluated in the brain homogenate (Table 3). The concentrations of the measured amino acids in P. odorata extract group did not reveal any significant change from the control group. It appears that the concentrations of glutamine (Gln), serine (Ser), agrinine (Agr), and taurine (Tau) were significantly increased, while the concentration of γ-aminobutyric acid (GABA) was decreased in AlCl3-treated group when compared with control and P. odorata groups (Table 3). Treatment with P. odorata + AlCl3 was able to significantly lower the concentration of Gln as compared with AlCl3-treated group, but its concentration was still significantly higher than the control value. Concentrations of Ser and Agr amino acids were significantly decreased in P. odorata + AlCl3 group as compared with AlCl3-treated group. On the other hand, administration of P. odorata with AlCl3 exhibited no effect on GABA concentration as it remained significantly lower when compared with control and P. odorata groups.
DNA damage
As shown in Fig. 1, the highest DNA fragmentation percentage was detected in the group treated by AlCl3 (68%) which is significantly different from the control (20%) and Premna odorata–treated group (22%) and the AlCl3 + P. odorata group (33%). The same results were detected by the electrophoretic mobility of the DNA fragments on the agarose gel.
a Electrophoretic pattern of the isolated DNA fragments on 2% agarose gel. M, 100 bp DNA ladder; lane 1, control group; lane 2, aluminium chloride group; lane 3, P. odorata group; and lane 4, AlCl3 + P. odorata group. b The DNA laddering percentage. The dissimilar superscript letters (significantly differing at P < 0.05) (n = 5): asignificantly different from control; bsignificantly different from the P. odorata group; and csignificantly different from the AlCl3 group
Cyclooxygenase-2 and TNF-α genes
Aluminum chloride–treated group showed a significant upregulation in the mRNA levels of both genes in the brain compared with that in the control group. Premna odorata treatment downregulated the mRNA expression level of both TNF-α and COX-2 compared with the intoxicated group (Fig. 2).
The relative expression level of a TNF-α and b COX-2 in different experimental groups (n = 5). The dissimilar superscript letters (significantly differing at P < 0.05): asignificantly different from control; bsignificantly different from the P. odorata group; and csignificantly different from the AlCl3 group
Histopathological observation
The brain histopathological picture is illustrated in Fig. 3. The cerebral cortex of control rats showed normal histological structure (Fig. 3a). No histopathological changes were observed in P. odorata–treated group (Fig. 3b). In AlCl3-treated group, the histopathologic changes were represented by marked vascular congestion and edema of pia matter layer with hypercellularity and neuronal degeneration. Also, there was an increased number of apoptotic neural cells with pyknotic dark-stained nuclei and cytoplasmic vacuolation (Fig. 3c–e). In the hippocampus of AlCl3-treated group, the pyramidal layer in C3 region showed decreased thickness with increased apoptotic pyramidal cells and cytoplasmic vacuolation (Fig. 3f). The morphological alterations induced by AlCl3 were reduced in the cerebral cortex of P. odorata + AlCl3-treated group. However, there was a few number of apoptotic neurons and minimal vascular congestion (Fig. 3g).
a A photomicrograph of a section in the cerebral cortex of control group showing a delicate layer of pia matter (arrowhead) with normal appearance of six layers of cerebral cortex molecular layer (I), outer granular layer (II), outer pyramidal layer (III), inner granular layer (IV), inner pyramidal layer (V), and polymorphic layer (VI). These layers show the normal neurons with rounded vesicular nuclei with prominent nucleoli and eosinophilic neuropil (red arrow) and neuroglial cells with scantly cytoplasm (black arrow) × 100. b A photomicrograph of the cerebral cortex of P. odorata group showing no histopathological alteration × 100. c A photomicrograph of the cerebral cortex of AlCl3 group showing marked congestion and edema of pia matter layer with disarrangement of its six layers with hypercellularity and neuronal degeneration × 100. d A photomicrograph of the cerebral cortex of AlCl3 group showing marked vascular congestion with extravasation RBCs (black arrow) and increased number of apoptotic neural cells with pyknotic dark-stained nuclei and cytoplasmic vacuolation (red arrows) × 200. e A photomicrograph of the cerebral cortex of AlCl3 group showing diffuse distribution of apoptotic neural cells with pyknotic dark-stained nuclei and cytoplasmic vacuolation (red arrows) × 400. f A photomicrograph of the hippocampus AlCl3 group showing decreased thickness of pyramidal layer in C3 region in the hippocampus showing increased apoptotic pyramidal cells with pyknotic dark-stained nuclei and cytoplasmic vacuolation (red arrows) with dilated congested blood vessels with perivascular space (black arrows ) × 200. g A photomicrograph of a section in the cerebral cortex of the P. odorata + AlCl3 group showing slightly normal neurons showing rounded vesicular nuclei with prominent nucleoli (black arrows) and a few number of apoptotic neurons with neuropil vacuolation (edema) (red arrows) with minimal vascular congestion (arrow head ) × 100
Discussion
The brain is considered a sensible organ to oxidative damage as it contains a low concentration of antioxidant enzymes and abundant polyunsaturated fatty acids (Nehru and Anand 2005). The neurotoxic effect of aluminum (Al) is well proved and confirmed in vivo, in cell culture, and in vitro (Azib et al. 2019). Aluminum is a pro-oxidant metal which can induce the generation of reactive oxygen species (ROS) (Tian et al. 2017). Aluminum superoxide semi-reduced radical ion is formed by the combination of superoxide radical anion and Al3+, causing oxidative stress damage which further induces the death of neurons (Wei et al. 2017).
Previous studies of Zhao et al. (2019), Alzahrani et al. (2020), and Marzouk et al. (2020) clarified that concentration of GSH was significantly declined after aluminum toxicity, a finding which comes in agreement with the results of the current study. Also, in the present study, the activity of CAT did not change due to AlCl3 toxicity, a result which follows those of Sánchez-Iglesias et al. (2009) and Liaquat et al. (2019). Both CAT and GSH are antioxidants which are1 responsible for the reduction of the peroxides that are produced from the reaction catalyzed by SOD. Therefore, the consumption of GSH might be sufficient to meet the brain needs (Musalmah et al. 2013). Moreover, in rat brain, the catalase mRNA is present in a high level in the cytoplasm of neuronal cells with heterogeneous distribution pattern along with different subsets of neuron in the brain of rat (Schad et al. 2003). Thus, CAT content may not be depleted rapidly, and its activity declined under severe stress. Meanwhile, the concentration of MDA, which is a marker of lipid peroxidation, was significantly elevated by AlCl3. The neuron cell membrane is a target for lipid peroxidation due to its high concentration of polyunsaturated fatty acids. The most important mechanism by which aluminum induces neurotoxicity is peroxidation of brain membrane, as aluminum can aggravate iron peroxidative action and thus initiate lipid peroxidation (Nehru and Anand 2005). Furthermore, aluminum has a high affinity to phosphate groups, so it can directly bind to the membrane, causing rearrangement of membrane lipids thus facilitating the iron attack. Aluminum potentiates the ability of iron to induce oxidation of nucleic acids, proteins, and lipid peroxidation that prompts neuronal lesions, leading to inflammation, neurochemical changes, neuro-histopathological, and oxidative events (Azib et al. 2019).
On the other hand, treatment with P. odorata restored GSH and reduced the MDA concentration. The abilities of P. odorata iridoids flavones, phenylethanoid, and rhamnopyranoside esters in the protection against oxidative stress and lipid peroxidation are well-known (Peng et al. 2015; Liang et al. 2016; Wei et al. 2019).
To the best of our knowledge, the current investigation is the first to identify the neuroprotective prosperities of P. odorata extract. Two studies have evaluated the hypnotic and the neuropharmacological effects of Premna species on animal models. Devi et al. (2003) evaluated the effects of the methanol extract of P. tomentosa leaves as a central nervous system (CNS) depressant using potentiation of phenobarbitone-induced hypnotic and locomotor activities on rats. At doses of 400 and 500 mg/kg orally, the extract decreased the locomotor activity and moderately increased the sleeping time that were comparable with CNS depressant, chlorpromazine (10 mg/kg, i.p.), yet significantly different to CNS stimulant, ephedrine hydrochloride (10 mg/kg, i.p). A recent study also evaluated the effect of P. integrifolia bark on locomotor activity of the rats in the open field and hole-cross tests (Khatun et al. 2014). The findings suggested that P. integrifolia significantly affected locomotor activity of the rats at the doses of 250 and 500 mg/kg, orally on both methods, therefore, might act as CNS depressant.
In the present study, AChE activity is enhanced by AlCl3 treatment. Acetylcholinesterase is the key enzyme responsible for the degradation of acetylcholine neurotransmitter. Thus, increased AChE activity will lead to deficiency of acetylcholine concentration in the brain causing cognitive and memory impairments (Auti and Kulkarni 2019). Aluminum can cause an increase of AChE activity through interaction between cation (Al+3) and anionic sites of AChE enzyme leading to alteration of the cholinergic projection function and activation of AChE enzyme (Rather et al. 2018; Auti and Kulkarni 2019; Marzouk et al. 2020). Conversely, inhibition of AChE activity caused by Al was reported by other authors (Lakshmi et al. 2014; Taïr et al. 2016; Abdelghany et al. 2019). The dual effect of Al on AChE activity may be related to the metal exposure duration (Kumar 1999), dose, and route of administration (Abdelghany et al. 2019). Another possible explanation for the different effects of Al on the brain AChE activity may be related to the different membrane compositions and the presence of different AChE molecular forms (Kaizer et al. 2008).
Co-treatment of AlCl3 with P. odorata significantly suppressed the activity of AChE. These results may be attributed to the polyphenolic content of P. odorata that inhibited the activity of AChE (Tungmunnithum et al. 2018)
Monoaminergic neurotransmitters (NE, DA, and 5-HT) participate in many physiological activities including emotion, learning, and memory (Wu et al. 2016). Aluminum may disrupt serotonergic neurotransmission through deviated levels of neurotransmitters in the brain (Abu-Taweel et al. 2012). Dopamine, an inhibitory transmitter, is secreted from the neurons that originate in the substantia nigra. In the current study, NE and DA concentrations showed a significant increase after Al administration. The increase in NE has been linked to the oxidative stress caused by Al which activates the NE synthetic pathway, leading to an increase in the biosynthesis of NE (Hassan et al. 2015; Kinawy 2019). Serova et al. (1999) stated that under stress condition induced by Al metal toxicity, locus coeruleus neurons stimulated and released more NE through increase the activity of tyrosine hydroxylase (TH) and TH mRNA within these neurons. Kandeil et al. (2019) attributed the increase in dopamine, after neurotoxicity by titanium oxide nanoparticles, to neural damage. Also, Pereira et al. (2011) reported a perturbation in the neurotransmission systems that resulted in inhibition of dopamine degradation and increase the neurotransmitters synthesis due to sodium fluoride neurotoxicity. Conversely, Bhalla et al. (2010), Mohamed and Abd El-Moneim (2017), and Singla and Dhawan (2017) reported a significant decrease in DA concentration after Al exposure. Serotonin is involved in sleep, eating, neuroendocrine function, and behavior. Increased serotonin concentration was reported by Kumar (2002) in different brain regions at 4 and 14 days after AlCl3 administration. The author mentioned that the accumulation of serotonin may be due to decreased breakdown. Furthermore, Laabbar et al. (2014) clarified the effect of AlCl3 on increased brain serotonin content as Al either potentiates serotonin synthesis or decreases its clearance. The current results about serotonin concentration disagree with some others reports that indicated a decline in brain serotonin concentration after exposure to aluminum (Bhalla et al. 2010; Mohamed and Abd El-Moneim 2017; Said and Abd Rabo 2017). This controversy data could be related to the duration of aluminum exposure, used dose, or route of administration (Laabbar et al. 2014).
Amino acid neurotransmitters are divided into excitatory and inhibitory according to their effects on the postsynaptic neuron. Their dynamic balance in the brain is important to sustain functions of the nervous system. It has been demonstrated herein that Al produced a significant increase in Gln, Ser, Tau, and Agr concentrations accompanied by a decrease in GABA. Glutamine is an amino acid produced from glutamate via the glutamine synthetase pathway. Although it has no neurotransmitter action, it is the main precursor of the inhibitory neurotransmitter GABA and the main precursor of the excitatory neurotransmitter glutamate (Strużyńska and Sulkowski 2004). Aluminum induces neurotoxicity through exacerbation of oxidative stress; interfering with synthesis and storage of neurotransmitters, triggering their release from the presynaptic terminal, interacting with receptors on the postsynaptic cell and inactivation of neurotransmitters (Gonçalves and Silva 2007). The increase in glutamine concentration could be attributed to Al potentiating the glutamine-synthetase activity by increasing its mRNA and decreasing the glutaminase activity (Ibraheem et al. 2011). The aforesaid authors observed an elevation in the excitatory amino acid neurotransmitters (glutamic acid, glutamine, aspartic acid, serine, glycine, and histidine) and the inhibitory amino acid GABA and Tau. They claimed the decrease in GABA concentration due to Al increases GABA catabolism through enzymatic activity. Another interpretation could be the selective loss of GABAergic neurons in response to Al exposure (Gonçalves and Silva 2007).
Administration of P. odorata in combination with Al ameliorated the imbalance in the neurotransmitters induced by Al through counteracting the oxidative stress, probably by scavenging the species that initiate peroxidation, quenching singlet oxygen, chelating metals, breaking free radical chain reactions, and reducing the concentration of O2, thereby reducing the neuronal damage; these effects may be implied due to the phenolic contents specially verbascoside that has powerful antioxidant effect (Elmaidomy et al. 2020).
Tumor necrosis factor-α, one of the neuroinflammatory factors, is a cell-signaling protein involved in an inflammatory response, which acts as a trigger for other cytokines. It is produced mainly by activated macrophages and to a lesser extent by many other cell types such as neurons (Martínez-Cué and Rueda 2020). The dysregulation of TNF expression has been implicated in a variety of diseases (Swardfager et al. 2010). Tumor necrosis factor-α can result in nerve cell degeneration and death through reducing the early phagocytic clearance and immune surveillance of microglia cells (Ouyang et al. 2017). Cyclooxygenase-2 is an enzyme involved in the production of inflammatory mediator prostaglandins and their metabolites. Oxidative stress, excitotoxic insults, as well as inflammatory mediators such as TNF-α can enhance the expression of COX-2 in neurons (Rather et al. 2018). Aluminum exacerbates ROS production leading to genotoxicity and DNA damage in brain cells. In the current study, AlCl3 induced neuro-inflammation through mediating gene expression of TNF-α, COX-2. Zaky et al. (2017) reported that AlCl3 affected the regulation of different genes that mediate cellular arrest and DNA function. Exposure to Al significantly induces neurotoxicity through enhancing the expression of COX-2 NF-kB, COX-2, interleukin (IL)-1β, IL-4, IL-6,TNF-α and inducible nitric oxide synthase in brain cells (Justin-Thenmozhi et al. 2018; Rather et al. 2018; Dhivya Bharathi et al. 2019).
The current study displayed that the administration of P. odorata exerts a neuroprotective effect as revealed by DNA laddering assay. A significant reduction in the DNA laddering percentage was detected in the P. odorata +AlCl3 group as compared with the AlCl3 group. The P. odorata extract suppresses the elevation of TNF-α and COX-2 expression levels which may indicate the inhibition of the pro-inflammatory mediators and ultimately provide neuroprotection.
The current data are supported by the histopathological observation in the brain tissue of AlCl3- treated group. Aluminum possesses a cholinotoxin nature that causes apoptotic neuronal loss thereby resulting in neurodegeneration (Ghribi et al. 2001). Aluminum may induce these morphologic alterations in astrocytes and neural cells through inhibition of membrane-bound Na+, K+, Ca2+ ATPases activity (El-Rahman 2003). The presence of cytoplasmic vacuolation might be due to the ability of Al to cause an increase in glutamate-induced intracellular calcium overload with severe mitochondria swelling resulting in an apparent microvacuolation of the neuronal cytoplasm (Matyia 2000). The current results showed oxidative stress–induced lipid peroxidation which is strongly associated with neuronal cell death (Auti and Kulkarni 2019). Also, Premna odorata co-administration to AlCl3 was able to restore the altered morphology which may be due to the antioxidant potential of P. odorata.
Conclusion
Based on the current results, aluminum induces oxidative stress with an increase in AChE activity, imbalance in monoamines neurotransmitters, alteration of amino acid neurotransmitters, and apoptosis-related gene expression, which are important mechanisms of aluminum neurotoxicity. Premna odorata extract exerted significant neuroprotective effects through its antioxidants potentials, acetylcholinesterase inhibition, and anti-inflammatory effect. Moreover, P. odorata improves the degenerative lesions in the brain induced by AlCl3 administration. Therefore, this study suggests that the use of P. odorata extract could be important in improving and treatment of neurodegenerative disorders.
References
Abdelghany AK, Khalil F, Azeem NMA, El-Nahass ES, El-Kashlan AM, Emeash HH (2019) Ginseng and moringa olifera ameliorated cognitive impairments induced by aluminium chloride in albino rat. Adv Anim Vet Sci 7(7):557–565
Abu-Taweel GM, Ajarem JS, Ahmad M (2012) Neurobehavioral toxic effects of perinatal oral exposure to aluminum on the developmental motor reflexes, learning, memory and brain neurotransmitters of mice offspring. Pharmacol Biochem Behav 101:49–56
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Alzahrani YM, Sattar MA, Kamel FO, Ramadan WS, Alzahrani YA (2020) Possible combined effect of perindopril and Azilsartan in an experimental model of dementia in rats. Saudi Pharm J 28:574–581. https://doi.org/10.1016/j.jsps.2020.03.00
Auti ST, Kulkarni YA (2019) Neuroprotective effect of cardamom oil against aluminum induced neurotoxicity in rats. Front Neurol 10:399
Azib L, Debbache-Benaida N, Da Costa G, Atmani-Kilani D, Saidenea N, Ayouni K, Richard T, Atmani D (2019) Pistacialentiscus L. leaves extract and its major phenolic compounds reverse aluminium-induced neurotoxicity in mice. Ind Crop Prod 137:576–584
Banchroft JD, Stevens A, Turner D (1996) Theory and practice of histologic techniques, 4th edn. Churchil Livingstone, New York, London, San Francisco, Tokyo
Bhalla P, Garg ML, Dhawan DK (2010) Protective role of lithium during aluminium-induced neurotoxicity. Neurochem Int 56:256–262
Ciarlone AE (1978) Further modification of a fluorometric method for analyzing brain amines. Microchem J 23(1):9–12
Cui T, Qiu H-M, Huang D, Zhou Q, Fu XY, Li HY, Jiang XH (2017) Abnormal levels of seven amino neurotransmitters in depressed rat brain and determination by HPLC - FLD. Biomed Chromatogr 31:e3937
Dalla Torre G, Mujika JI, Lachowicz JI, Ramos MJ, Lopez X (2019) The interaction of aluminum with catecholamine-based neurotransmitters: can the formation of these species be considered a potential risk factor for neurodegenerative diseases? Dalton Trans 48(18):6003–6018. https://doi.org/10.1039/c8dt04216k
Devi KP, Sreepriya M, Devaki T, Balakrishna K (2003) Antinociceptive and hypnotic effects of Premna tomentosa L. (Verbenaceae) in experimental animals. Pharmacology. Biochem Behav 75:261–264
Dhivya Bharathi M, Justin-Thenmozhi A, Manivasagam T, Ahmad Rather M, Saravana Babu C, Mohamed Essa M, Guillemin GJ (2019) Amelioration of aluminum maltolate-induced inflammation and endoplasmic reticulum stress-mediated apoptosis by tannoid principles of Emblica officinalis in neuronal cellular model. Neurotox Res 35(2):318–330
Ellman GL, Courtney KD, Andres VJ, Feather-stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Elmaidomy AH, Mohyeldin MM, Ibrahim MM, Hassan HM, Amin E, Rateb ME, Hetta MH, El Sayed KA (2017) Acylated iridoids and rhamnopyranoses from Premna odorata (Lamiaceae) as novel mesenchymal–epithelial transition factor receptor inhibitors for the control of breast cancer. Phytother Res 31:1546–1556
Elmaidomy AH, Mohammed R, Owis AI, Hetta MH, AboulMagd AM, Siddique A, Abdelmohsen UR, Rateb ME, El Sayed KA, Hassan HM (2020) Triple-negative breast cancer suppressive activities, antioxidants and pharmacophore model of new acylated rhamnopyranoses from Premna odorata. RSC Adv 10:10584–10598
El-Rahman SSA (2003) Neuropathology of aluminum toxicity in rats (glutamate and GABA impairment). Pharmacol Res 47:189–194
Ghribi O, DeWitt DA, Forbes MS, Herman MM, Savory J (2001) Co-involvement of mitochondria and endoplasmic reticulum in regulation of apoptosis: changes in cytochrome c, Bcl-2 and Bax in the hippocampus of aluminum-treated rabbits. Brain Res 903:66–73. https://doi.org/10.1016/S0006-8993(01)02406-4
Gonçalves PP, Silva VS (2007) Does neurotransmission impairment accompany aluminium neurotoxicity? J Inorg Biochem 101:1291–1338
Gorun V, Proinov I, Baltescu V, Balaban G, Barzu O (1978) Modified Ellman procedure for assay of cholinesterases in crude enzymatic preparations. Anal Biochem 86(1):324–326
Hang NT, Ky PT, Minh CV, Cuong NX, Thao NP, Kiem PV (2008) Study on the chemical constituents of Premna integrifolia L.Natural. Product Commun 3(9):1449–1452
Hassan HA, Serage HM, Gad W (2015) Black berry juice attenuates neurological disorders and oxidative stress associated with concurrent exposure of aluminum and fluoride in male rats. Egypt J Basic Appl Sci 2:281–288
HelmyAbdou KA, Ahmed RR, Ibrahim MA, Abdel-Gawad DRI (2019) The anti-inflammatory influence of Cinnamomum burmannii against multi-walled carbon nanotube-induced liver injury in rats. Environ Sci Pollut Res Int 26(35):36063–36072
Hien TT, Mai NT, Tam BT, Hien NT, Cuong CVL, Anh HLT (2019) Glycosides isolated from the aerial parts of premna integrifolia growing in Thai Binh. Vietnam J Sci Technol 57(4):420–427. https://doi.org/10.15625/2525-2518/57/3/13076
Hussien HM, Abd-Elmegied A, Ghareeb DA, Hafez HS, Ahmed HEA, El-Moneam NA (2018) Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food Chem Toxicol 111:432–444. https://doi.org/10.1016/j.fct.2017.11.025
Ibraheem EM, Mohamed EW, Taher MA (2011) Perturbation of brain neurotransmitters by aluminum in male rats and the potential role of sage. Egypt J Exp Biol (Zool) 7(2):249–259
Ibrahim MA, Ibrahem MD (2020) Acrylamide-induced hematotoxicity, oxidative stress, and DNA damage in liver, kidney, and brain of catfish (Clarias gariepinus). Environ Toxicol 35(2):300–308
Ibrahim MA, Radwan MI, Kim HK, Han J, Warda M (2020) Evaluation of global expression of selected genes as potential candidates for internal normalizing control during transcriptome analysis in dromedary camel (camelus dromedarius). Small Rumin Res 184:106050
Justin-Thenmozhi A, Dhivya Bharathi M, Kiruthika R, Manivasagam T, Borah A, Essa MM (2018) Attenuation of aluminum chloride-induced neuroinflammation and caspase activation through the AKT/GSK-3β pathway by Hesperidin in Wistar rats. Neurotox Res 34(3):463–476
Kabra A, Kabra R, Baghel US (2015) Premna Species: A Review. J Biol Chem Chron 1(1):55–59
Kadhem WM, Enaya H (2018) Effect of lead and aluminium in the levels of dopamine and acetylcholine in the brain male rats. Res J Pharm Tech 11(5):1–3
Kaizer RR, Corrêa MC, Gris LR, da Rosa CS, Bohrer D, Morsch VM, Maria Rosa CS (2008) Effect of long-term exposure to aluminum on the acetylcholinesterase activity in the central nervous system and erythrocytes. Neurochem Res 33:2294–2301
Kandeil MA, Mohammed ET, Hashem KS, Aleya L, Abdel-Daim MM (2019) Moringa seed extract alleviates titanium oxide nanoparticles (TiO2-NPs)-induced cerebral oxidative damage, and increases cerebral mitochondrial viability. Environ Sci Pollut Res:1–16. https://doi.org/10.1007/s11356-019-05514-2
Khalaf AA, Ibrahim MA, Tohamy AF, Abd Allah AA, Zaki AR (2017) Protective effect of vitazinc on chlorsan induced oxidative stress, genotoxicity and histopathological changes in testicular tissues of male rats. Int J Pharmacol 13:22–32
Khalaf AA, Ahmed WM, Moselhy WA, Abdel-Halim BR, Ibrahim MA (2019) Protective effects of selenium and nano-selenium on bisphenol-induced reproductive toxicity in male rats. Hum Exp Toxicol 38(4):398–408
Khatun H, Majumder R, Mamun A, Alam EK, Jami SI, Alam B (2014) Preliminary pharmacological activity of the methanolic extract of Premna integrifolia barks in rats. Avicenna J Phytomed 4(3):215–224
Kinawy AA (2019) The potential roles of aluminum chloride and sodium fluoride on the neurotoxicity of the cerebral cortex, hippocampus, and hypothalamus of male rat offspring. J Basic Appl Zool 80:17
Kumar S (1999) Aluminium-induced biphasic effect. Med Hypotheses 52(6):557–559
Kumar S (2002) Aluminium-induced changes in the rat brain serotonin system. Food Chem Toxicol 40:1875–1880
Kumar A, Dogra S, Prakash A (2009) Protective effect of curcumin (Curcuma longa), against aluminium toxicity: possible behavioural and biochemical alterations in rats. Behav Brain Res 205(2):384–390
Laabbar W, Elgota A, Kissani N, Gamrani H (2014) Chronic aluminum intoxication in rat induced both serotonin changes in the dorsal raphe nucleus and alteration of glycoprotein secretion in the subcommissural organ: Immunohistochemical study. Neurosci Lett 577:72–76
Lakshmi BV, Sudhakar M, Aniska M (2014) Neuroprotective role of hydroalcoholic extract of Vitis vinifera against aluminium-induced oxidative stress in rat brain. Neurotoxicology 41:73–79
Liang J-Q, Wang L, He J-C, Hua X-D (2016) Verbascoside promotes the regeneration of tyrosine hydroxylase-immunoreactive neurons in the substantia nigra. Neural Regen Res 11:101
Liaquat L, Sadir S, Batool Z, Tabassuma S, Shahzada S, Afzal A, Haider S (2019) Acute aluminum chloride toxicity revisited: study on DNA damage and histopathological, biochemical and neurochemical alterations in rat brain. Life Sci 217:202–211
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Martínez-Cué C, Rueda N (2020) Cellular Senescence in Neurodegenerative Diseases. Front Cell Neurosci 14:16. https://doi.org/10.3389/fncel.2020.00016 eCollection 2020
Marzouk MM, Ibrahim LF, El-Hagrassi AM, Fayed DB, Elkhateeb A, Abdel-Hameed ES, Hussein SR (2020) Phenolic profiling and anti-Alzheimer’s evaluation of Eremobiumaegyptiacum. Orient Pharm Exp Med 20:233–241. https://doi.org/10.1007/s13596-019-00408-7
Matyia E (2000) Aluminum enhances glutamate-mediated neurotoxicity in organotypic cultures of rat hippocampus. Folia Neuropathol 38(2):47–53
Mohamed NE, Abd El-Moneim AE (2017) Ginkgo biloba extract alleviates oxidative stress and some neurotransmitters changes induced by aluminum chloride in rats. Nutrition 35:93–99
Musalmah M, Leow KS, Nursiati MT, Raja Najmi Hanis RI, Fadly Syah A, Renuka S, Siti Norsyamimi MS, Mohamad Fairuz Y, Azian AL (2013) Selective uptake of alpha-tocotrienol and improvement in oxidative status in rat brains following short- and long term intake of tocotrienol rich fraction. Malays J Nutr 19(2):251–259
Nehru B, Anand P (2005) Oxidative damage following chronic aluminium exposure in adult and pup rat brains. J Trace Elem Med Biol 19:203–208
Ouyang QQ, Zhao S, Li SD, Song C (2017) Application of chitosan, chitooligosaccharide, and their derivatives in the treatment of Alzheimer’s disease. Marine Drugs 15:322–337. https://doi.org/10.3390/md15110322
Peng XM, Gao L, Huo SX, Liu XM, Yan M (2015) The mechanism of memory enhancement of acteoside (Verbascoside) in the senescent mouse model induced by a combination of d-gal and AlCl3. Phytother Res 29:1137–1144
Pereira M, Dombrowski PA, Losso EM, Chioca LR, Da Cunha C, Andreatini R (2011) Memory impairment induced by sodium fluoride is associated with changes in brain monoamine levels. Neurotox Res 19(1):55–62
Rather MA, Thenmozhi AJ, Manivasagam T, Bharathi DM, Essa MM and Guillemin GJ (2018) Neuroprotective role of Asiatic acid in aluminium chloride induced rat model of Alzheimer’s disease. Front Biosci, Scholar, 10: 262-275
Said MM, Abd Rabo MM (2017) Neuroprotective effects of eugenol against aluminium-induced toxicity in the rat brain. Arh Hig Rada Toksikol 68:27–37
Sánchez-Iglesias S, Méndez-Álvarez E, Iglesias-González J, Muñoz-Patiño A, Sánchez-Sellero I, Labandeira-García JL, Soto-Otero R (2009) Brain oxidative stress and selective behaviour of aluminium in specific areas of rat brain: potential effects in a 6-OHDA-induced model of Parkinson’s disease. J Neurochem 109:879–888. https://doi.org/10.1111/j.1471-4159.2009.06019.x
Schad AH, Fahimi D, Völkl A, Baumgart E (2003) Expression of catalase mRNA and protein in adult rat brain: detection by nonradioactive in situ hybridization with signal amplification by catalyzed reporter deposition (ISH–CARD) and immunohistochemistry (IHC)/immunofluorescence (IF). J Histochem Cytochem 51(6):751–760
Serova LI, Nankova BB, Feng Z, Hong JS, Hutt M, Sabban EL (1999) Heightened transcription for enzymes involved in norepinephrine biosynthesis in the rat locus coeruleus by immobilization stress. Biol Psychiatry 45:853–862
Singh T, Goel RK (2015) Neuroprotective effect of Allium cepa L. in aluminium chloride induced neurotoxicity. NeuroToxicology 49:1–7
Singla N, Dhawan DK (2017) Zinc improves cognitive and neuronal dysfunction during aluminium-induced neurodegeneration. Mol Neurobiol 54:406–422
Strużyńska L, Sulkowski G (2004) Relationships between glutamine, glutamate, and GABA in nerve endings under Pb-toxicity conditions. J Inorg Biochem 98:951–958
Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, Herrmann N (2010) A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry 68(10):930–941
Taïr K, Kharoubi O, Taïr OA, Hellal N, Benyettou I, Aoues A (2016) Aluminium-induced acute neurotoxicity in rats: treatment with aqueous extract of Arthrophytum (Hammadascoparia). J Acute Dis 5(6):470–482
Tian F, Yu L, Zhai Q, Xiao Y, Shi Y, Jiang J, Liu X, Zhao J, Zhang H, Chen W (2017) The therapeutic protection of a living and dead Lactobacillus strain against aluminum-induced brain and liver injuries in C57BL/6mice. PLoS One 12(4):e0175398. https://doi.org/10.1371/journal.pone.0175398
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicine 5:93
Uchiyama M, Mihara M (1978) Determination of malonaldehydeprecursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Van Doorn R, Leijdekkers C-M, Henderson PT (1978) Synergistic effects of phorone on the hepatotoxicity of bromobenzene and paracetamol in mice. Toxicology 11:225–233
Wei Y, Liu D, Zheng Y, Li H, Hao C, Ouyang W (2017) Protective effects of kinetin against aluminum chloride and D-galactose induced cognitive impairment and oxidative damage in mouse. Brain Res Bull 134:262–272
Wei W, Lu M, Lan X, Liu N, Wang H, Du J, Sun T, Li Y, Yu J (2019) Neuroprotective effect of verbascoside on hypoxic-ischemic brain damage in neonatal rat. Neurosci Lett 711:134415
Wu LL, Yan L, Yan C, Yi P, Su JF, Wu WK (2016) Antidepressant-like effects of fractions prepared from Danzhi-xiaoyao-san decoction in rats with chronic unpredictable mild stress: effects on hypothalamic-pituitary-adrenal axis, arginine vasopressin, and neurotransmitters. Evid Based Complement Alternat Med 3:1–11
Yokel RA, Allen DD, Ackley DC (1999) The distribution of aluminum into and out of the brain. J InorgBiochem 76(2):127–132
Zaky A, Bassiouny A, Farghaly M, El-Sabaa BM (2017) A combination of resveratrol and curcumin is effective against aluminum chloride-induced Neuroinflammation in Rats. J Alzheimers Dis 60(s1):S221–S235
Zhao Y, Wang D, Bais S, Wang H (2019) Modulation of pro-inflammatory mediators by eugenol in AlCl3 induced dementia in rats. Int J Pharmacol 15:457–464
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 195 kb)
Rights and permissions
About this article
Cite this article
Ahmed, W.M.S., Helmy, N.A., Ibrahim, M.A. et al. Premna odorata extract as a protective agent on neurotoxic effect of aluminum: neurochemical, molecular, and histopathological alterations. Environ Sci Pollut Res 28, 2146–2157 (2021). https://doi.org/10.1007/s11356-020-10659-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10659-6