Abstract
Denitrification plays an important role in nitrogen (N) removal in freshwater ecosystems. Aquatic plants might have an impact on the sediment denitrification of water body, especially in macrophytes-dominated lake; however, there were different opinions about it. Our hypothesis was that the sediment denitrification rates differ significantly in different vegetation zones and seasons because of direct and indirect effect of the aquatic plants. Therefore, we studied sediment denitrification in Dongping Lake, a typical macrophytes-dominated lake located in the north of China. The acetylene inhibition technique was used to quantify the sediment denitrification rates (DRs) in the Phragmites communis (P. communis) zone, aquaculture zone, Potamogeton crispus (P. crispus) zone and mixed vegetation zone in July (summer), October (autumn), December (winter) of 2015 and March (spring) of 2016. The results showed that the average DRs were significantly higher in the P. communis zone (69.0 ± 91.6 μmol N m−2 h−1) than the mixed vegetation zone (8.70 ± 5.44 μmol N m−2 h−1), and the average DRs represented significant seasonal difference as in the order of winter (74.5 ± 88.3 μmol N m−2 h−1) > autumn (15.7 ± 18.6 μmol N m−2 h−1) ≈ summer (10.7 ± 5.90 μmol N m−2 h−1) > spring (3.85 ± 1.29 μmol N m−2 h−1). The DRs generally decreased with the increasing of depth; however, significant increase of DRs with depth were found in certain seasons at the vegetated zones except the non-vegetated zone (the aquaculture zone) indicating the possible rhizosphere effect of aquatic plants on denitrification. The higher DRs and cycling rates of nitrate in the P. communis zone might be related to the larger biomass and oxygen transporting capacity of P. communis than those of the other aquatic plants. Winter peaks of DRs might be attributed to the higher NO3− load and the absence of the plant uptake. The high cycling rates of nitrate in Dongping Lake indicated an enhanced internal N cycling by aquatic plants. Sediment denitrification could remove about 537.7 t N every year, which was about 26.5% of annual TN loading in Dongping Lake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic activities were responsible for 210 Tg N of reactive nitrogen to terrestrial and marine ecosystem, which was about half of global nitrogen fixation (Fowler et al. 2013), and this resulted in one-third of the world’s population affected by nitrogen pollution (WWAP 2017). In the past 40 years, the nitrogen concentrations of lakes in China have increased dramatically, and the nitrogen concentrations of some lakes even have increased 32 times (Guan et al. 2011). High nitrogen loading can lead to water eutrophication, oxygen deficit, and water quality deterioration (Guan et al. 2011), so nitrogen pollution in aquatic ecosystem becomes a research hotspot (Camargo and Álvaro 2006; Shibata et al. 2015).
Nitrate is the main form of nitrogen in water body and has strong mobility and bioavailability, so its transportation and transformation have always been the focus. Nitrate in the aquatic ecosystem can be mainly removed by two ways. One way is to be assimilated by phytoplankton and other aquatic plants or microbes in water body and become an integral part of the organisms (O’brien et al. 2012). The other is to be transformed to N2 or N2O through denitrification and then transported into air (Burgin and Hamilton 2007; Scott et al. 2008; Beaulieu et al. 2011; Yin et al. 2015; Deng et al. 2019). Denitrification is the primary mechanism of nitrogen retention, followed by uptake of aquatic plants (Saunders and Kalff 2001). Furthermore, compared with the assimilation of nitrogen, denitrification is the more thorough way to remove nitrogen because its product, i.e., N2 or N2O, cannot be assimilated by most living creatures and can be discharged into atmosphere. Therefore, denitrification is of greatly important ecological significance to reduce the nitrogen load and remediate nitrogen pollution in water body.
Aquatic plants are an indispensable part of the lacustrine ecosystem, especially in the macrophytic lakes. There are different opinions on the effect of aquatic plants on nitrogen transformation. Liu et al. (2018) found that denitrification was controlled by physicochemical properties of water body, such as pH, Eh, temperature, concentration of nitrogen, etc. instead of the types of aquatic plants. Palacin-Lizarbe et al. (2020) found that nitrate concentration and temperature provided the most explanatory power for the actual denitrification rates in sediments of several lake types and habitats by using the multiple linear regression models. However, Zhang et al. (2017) found that aquatic plants could change physicochemical properties of water body and then affect denitrification indirectly. Eriksson and Weisner (1999) found that submersed vegetation (Potamogeton pectinatus) might greatly enhance the transformation of NH4+ to N2 by the sequential action of nitrification and denitrification in NH4+-rich freshwater ecosystem. Nizzoli et al. (2014) also found that P. pectinatus would modify the N dynamics by enhancing N removal via assimilation and denitrification. Veraart et al. (2011) studied the effect of floating vegetation and submerged vegetation on denitrification rates using microcosm, and found that the oxygen production by photosynthesis of plants inhibited denitrification in the top layer of the sediment. Besides, aquatic plants can transport oxygen to the root zone and increase the oxygen supply to the root zone microbes (Sand-Jensen et al. 1982; Mi et al. 2008), which can stimulate the activities of the microbes and enhance the denitrification (Choi et al. 2009; Vila-Costa et al. 2016; Palacin-Lizarbe et al. 2020). Aquatic plants create an ideal environment for denitrification by increasing the supply of potentially limiting organic carbon and nitrate to denitrifying bacteria (Saunders and Kalff 2001). Moreover, when aquatic plants die and decay, they can release carbon and nitrogen which is to affect the denitrification rates (Bastviken et al. 2005; Li et al. 2014).
Dongping Lake is a typical macrophytes lake in the north of China. As a water collection center and a retention reservoir, the main role of Dongping Lake is to regulate and store floods of the Yellow River and the Dawen River. In addition, Dongping Lake serves important roles in the East Route of the South-to-North Water Diversion Project of China and water transmission from the west to the east of Shandong Province. One of the key potential water quality concerns in Dongping Lake is eutrophication (Yang et al. 2017), and NO3− as a non-carcinogen has health risk on human body. Although the non-carcinogenic risk values for NO3− were lower than the standard recommended by US EPA (Zhang et al. 2011), external nitrogen retention will increase the risk of eutrophication and the health risk of NO3−. We hypothesized that the sediment denitrification rates differ significantly in different vegetation zones and seasons because of direct and indirect effect of the aquatic plants. Taking the Dongping Lake as the study area, the objectives of this research are to (1) investigate the sediment denitrification rates in different aquatic vegetation zones and seasons, (2) identify the main factors that impact the denitrification rates, (3) compare the N cycling rates in different aquatic vegetation zones, and (4) calculate the capability of sediment to eliminate external nitrogen loading into the drinking water reservoir and examine the role of denitrification in controlling the nitrogen concentrations in the water body of different aquatic vegetation to make a suggestion for vegetation management.
Materials and methods
Study site
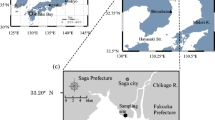
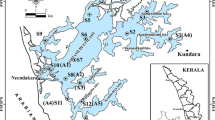
Dongping Lake (35° 30′~36° 20′ N, 116° 00′~116° 30′ E) is located in Dongping County, southwest of Shandong Province, China (Fig. 1). With an area of 134 km2 retaining water all year round, Dongping Lake is the second largest freshwater lake in Shandong Province. The lake is a flat basin and its multi-annual mean water depth is 2~4 m (Yang et al. 2017). The area of Dongping Lake experiences a warm and semi-humid continental monsoon climate and has four distinct seasons, and the average annual precipitation and temperature were 601 mm and 13.3 °C respectively; and the average monthly water temperature varies between 4 °C (January) and 30 °C (August) (Compiling Team for Annals of Dongping County Shandong Province 2006). The average annual evapotranspiration water demand of Dongping Lake is 7.7 × 107 m3 (Wang et al. 2014). Dongping Lake was at mesotrophic or eutrophic level (Yang et al. 2017) and the water quality was at moderate pollution level with TN as the predominant pollutant factor (He et al. 2010; Shi et al. 2011). Recharge to Dongping Lake relies mainly on surface runoff via the Dawen River which is about 10.6 × 108 m3 a−1 and the East Route of the South-to-North Water Diversion Project (Yang et al. 2017). The water in the lake flows northward through the Xiaoqing River and the East Route of the South-to-North Water Diversion Project, finally entering the water-receiving area. In addition to the upstream water input, precipitation is the other main external source of nitrogen to the water body.
Dongping Lake has an abundance of vegetation types including Phragmites, Potamogeton, Nymphaea, Typha, Cyperus, etc. in which Phragmites communis (P. communis) and Potamogeton crispus (P. crispus) are the dominant species of the aquatic vascular plants (Shi et al. 2011). P. communis as an emergent plant shoots and grows in spring and summer, and it withers and decays in autumn and winter; while P. crispus as a submerged plant forms shoots in the autumn and grows in the winter and spring and it begins to die and decompose in early summer, which causes the deterioration of the aquatic ecological environment of Dongping Lake (Deng et al. 2016). According to the field investigation, P. communis is distributed mainly near estuary and outlet, while P. crispus is widely distributed in Dongping Lake except in estuary and outlet. A variety of aquatic plants including P. communis, P. crispus, Nymphaea tetragona, Typha orientails Presl, and Cyperus rotundus is found in the area near the lakeshore. Due to the intense aquaculture activities, the vegetation in the water body of the aquaculture zone were destroyed resulting in the less biomass of the vegetation. Considering the vegetation types and the impact of aquaculture activities, four sampling sites were established in Dongping Lake, which were P. communis zone (site 1), aquaculture zone (site 2), P. crispus zone (site 3), and the mixed vegetation zone (site 4) respectively (Fig. 1).
Sampling
Nine surface sediment cores (5-cm depth) were collected seasonally at each site in July (summer), October (autumn), December (winter) of 2015, and March (spring) of 2016 using the Perspex tubes (35 mm inner diameter × 350 mm long). Two caps with sealing washers inside were used to cover the two ends of each tube and the tubes were stored vertically in a large plastic box filled with the in situ water. Meanwhile, triplicate samples of surface water (150 ml ca. 10 cm depth) were collected and stored in a plastic bottle under ice. Temperature, pH, and dissolved oxygen (DO) concentrations of surface water were measured in situ by calibrated portable instruments, which were pH instrument (pH 400, SPECTRUM, USA) and DO instrument (HI9147-04, Italy). All the samples were then transported back to the laboratory as soon as possible.
Water and sediment analysis
All the collected water samples were processed in the laboratory within 6 h for inorganic nitrogen and soluble ortho-phosphorus (SOP) measurement. The concentrations of ammonia (NH3), nitrate (NO3−), nitrite (NO2−), and SOP were analyzed using Nessler’s reagent spectrophotometry, UV-Spectrophotometry, N-(1-naphthyl) ethylene diamine dihydrochloride spectrophotometry, and phosphomolybdate-blue spectrophotometry, respectively (SEPA (State Environmental Protection Administration) 2002).
Sediment cores were sliced into 1 cm layer subsamples, which were weighted to determine wet weight. A portion of each wet subsample was dried at 50 °C until constant weight. Water content was calculated by the ratio of the loss of wet subsample and the weight of dried subsample. Dry bulk density (BD) was calculated for each subsample according to the wet weight, water content, and volume. About 5 g of wet sediment sample was extracted by 2 mol L−1 KCl solution for determination of extractable nitrate (NO3−) and ammonium (NH4+). Extractable NO3− and NH4+ in sediments were measured by a standard colorimetric method (Grasshof et al. 1983). Dried sediment subsamples were ground and passed through a 60 mesh (250 μm) sieve for sediment organic matter (OM) content determination by K2Cr2O7-H2SO4 oxidation method (Nelson and Sommers 1996).
Denitrification rate measurement and calculation
The measurement of the sediment denitrification rates was carried out as soon as the samples were sent to the laboratory. The acetylene inhibition technique is a relatively easy and rapid technique and has been one of the most frequently used methods for measuring denitrification in aquatic sediments. However, a potential drawback is that incomplete inhibition of N2O reduction can occur or nitrification can be partially inhibited, which would underestimate the rates particularly in nitrate poor systems (Seitzinger et al. 1993). Nevertheless, this drawback is not essential in eutrophic lakes like Dongping Lake; the acetylene inhibition method was applied to measure denitrification in this study. About 6 g of each fresh sediment subsample was put into a 125 ml narrow mouth bottle. Each bottle was sealed with a rubber stopper and purged with 99.999% N2 for 20 min to create anoxic environments by two needles which were inserted into bottle through rubber stopper, and then 20 ml gas sample was collected as initial sample, and then one needle was withdrawn. Fifteen milliliters of water which had been aerated with 99.999% N2 for 30 min and 14 ml C2H2 were injected into the bottle through the remaining needle, and then the needle was withdrawn too. At the same time, three blank bottles were also treated in the same way and measured as the background. Next, all the bottles were incubated at the field temperature in the dark for 2 h (28 °C in summer, 13 °C in autumn, 4 °C in winter, and 19 °C in spring). At the end of incubation, 20 ml gas sample from each bottle was collected using a syringe and then injected into a gas chromatograph (GC) (Agilent 7890A, USA) equipped with an electron capture detector (ECD) for N2O analysis. A 95:5 mixture of Ar/CH4 was used as the carrier gas and the reference gas offered by National Institute of Metrology and National Research Center for Certified Reference Materials of China was analyzed for calibration. And the measurement precision was less than 1% relative standard deviation (six consecutive measurement) at approximately ambient concentrations (345.5 ppbv N2O standard). N2O concentrations in laboratory ambient air and in ultrahigh purity nitrogen gas (in case of any contamination) used in the incubations were also measured.
Denitrification rate was calculated by change of N2O concentrations in the headspace of the bottle during the incubation, and the formula is as follows:
where DRate is the denitrification rate in sediment (ng N g−1 h−1), C1m and C2m are the N2O concentration in N2 and water in the bottle at the end of incubation (ng N·L−1) respectively, C2m is calculated as the product of α and C1v, α is the solubility coefficient of in water (Weiss and Price 1980; Wang et al. 2015), C1v is the N2O concentration in the bottle at the end of incubation (ppbv), C3m is the N2O concentration in N2 in the headspace of the bottle at the beginning of incubation (ng N L−1), V1 and V2 are the volume of N2 and water in the bottle respectively (L), \( {V}_{{\mathrm{C}}_2{\mathrm{H}}_2} \) is the volume of C2H2 injected into the bottle (L), W is the dry weight of the sediment sample in the bottle (g), and T is the incubation time (h).
Total denitrification rate of the 5-cm depth sediment per m2 (\( {D}_{\mathrm{Rate}/{m}^2} \)) was calculated according to the bulk density of the sediment sample, and the formula is as follows:
where \( {D}_{\mathrm{Rate}/{\mathrm{m}}^2} \) is the total denitrification rate in sediment per m2 (μmol N m−2 h−1), DRate,i is the denitrification rate in sediment at i-cm depth (ng N g−1 h−1), and BDi is the dry bulk density of the sediment at i-cm depth (g cm−3).
Quantifying of internal cycling rate and residence time of nitrate
The denitrification was affected by the diffusion flux of NO3− from the water column to the sediment (Liu et al. 2014); therefore, the denitrification rates could be used to represent the removal of nitrate from the water column and to quantify the internal cycling rate of nitrate if only denitrification was considered in the nitrogen removal process. Residence time in hydrodynamics was also used as a reference (Monsen et al. 2002), which was defined as the inverse of its cycling rate (Wu et al. 2019). Specifically, the cycling rate (CR) and residence time could be expressed as:
where DR and NO3− represented the denitrification rate and nitrate concentration in sediment. This equation excludes the influence of other nitrogen removal processes like plant uptake, dissimilatory nitrate reduction to ammonium (DNRA), and anaerobic ammonium oxidation (Anammox) which lead to underestimation of CR.
Statistical analyses
SPSS (18.0) was used for statistical analysis of the spatial and temporal difference of the denitrification rates, the correlation and multiple linear regression analysis between the denitrification rates and environmental factors, as well as the interaction effect of season, vegetation, and depth on denitrification rates at the α = 0.05 level of significance. The data was first tested for the normality distribution. If the data was normally distributed, ANOVA was used to compare the difference; and if not, nonparametric test was performed. Specifically, the non-normal distributed data was first checked for difference using Kruskal-Wallis H test and then followed by Mann-Whitney U test for pairwise comparison. Before the correlation and regression analysis, variables were log10+1 transformed to reduce the influence of extreme values. The correlation between the denitrification rates and environmental factors was tested by Pearson correlation. For regression analysis, all log-transformed variables were further standardized to z-scores to obtain regression coefficients that are proportional to the influence of each explanatory variable, such as their relative importance can be immediately evaluated. The stepwise method was used to identify the factors driving the denitrification rates. The interaction effect of season, vegetation, and depth on denitrification rates was tested by three-way ANOVA.
Results and discussion
Physicochemical properties of water and sediment in different zones of Dongping Lake
As shown in Table 1, except NH3 in the overlying water, there were no significant differences in the average concentrations of the other property index between the sites. The average concentration of NH3 in the overlying water was significantly higher (P < 0.05) in the site 4 (the mixed vegetation zone) than those in the other sites. Furthermore, the average NH3 concentrations in the overlying water all exceeded the limit (≤ 1.0 mg/L) for class III water body of the GB3838-2002 National Environmental Quality Standards for Surface Water. Significant seasonal difference was found in T, NO3−, NO2−, and SOP in the overlying water as well as NH4+ and OM in sediments. The NO3− concentrations in the overlying water were significantly higher in winter and autumn and lower in summer and spring. The average NO2− concentration was lowest in summer compared with the other seasons. Highest SOP concentration was found in spring, which was more than ten times of the average in winter. As for the sediment, the average concentration of extractable NH4+ was significantly higher in summer than in autumn and spring. The average concentration of OM in sediment was significantly lower in spring than in others seasons.
Sediment denitrification rates in different zones of Dongping Lake
The average denitrification rates in the top 5 cm sediment decreased from site 1 to site 4, which were 69.0 ± 91.6, 15.3 ± 17.2, 11.8 ± 15.5, and 8.70 ± 5.44 μmol N m−2 h−1 respectively; however, significant difference (P < 0.05) in denitrification rates was only found between site 1 (P. communis zone) and site 4 (the mixed vegetation zone). The average sediment denitrification rates in different seasons were in the order of winter (74.5 ± 88.3 μmol N m−2 h−1) > autumn (15.7 ± 18.6 μmol N m−2 h−1) > summer (10.7 ± 5.90 μmol N m−2 h−1) > spring (3.85 ± 1.29 μmol N m−2 h−1). There were significant seasonal difference (P < 0.05) in sediment denitrification rates except between autumn and summer. The variation of sediment denitrification rates by site and season were illustrated in Fig. 2. Significant seasonal and spatial differences were only found in site 2 and in autumn respectively. In aquaculture zone, the sediment denitrification rates in summer were significantly higher than in autumn and spring; while in autumn, the sediment denitrification rates in site 1 were significantly higher than in site 3.
The denitrification rates at each sediment layer in different vegetation zones were illustrated in Fig. 3. The significant decrease (P < 0.05) of sediment denitrification with increasing depth was found both in autumn and winter at site 1 and site 2, and also in spring at site 3 indicating that denitrification mainly occurred in the top layer sediment, which was similar to other water body (Liu et al. 2014; Fernandes et al. 2016; Wu et al. 2018). However, there were significant increase of sediment denitrification with depth in summer at site 1 and site 3, and in winter and spring at site 4; and the maximum of the denitrification rates occurred at the 3~5 cm sediment, which suggested the possible influence of aquatic plants compared with the non-vegetated site 2.
Analysis of factors affecting the sediment denitrification rates in Dongping Lake
Denitrification, the stepwise reduction of nitrate to N2 under low-oxygen and anaerobic conditions, represents a permanent nitrogen removal from aquatic systems (Liu et al. 2014). We need to know the basic facts of denitrification, including its rates and controlling factors, if we want to enhance N removal through it. Using the correlation analysis, previous studies uncovered a group of factors that could exert influences on denitrification, including the nitrate concentration, temperature, oxygen, availability of organic matter, the presence/absence of aquatic vegetation, and so on (Wu et al. 2013; Liu et al. 2014; Li et al. 2018). These environmental factors were interrelated, in which nitrate concentration and temperature were typically identified as the main drivers of denitrification (Zhong et al. 2020; Palacin-Lizarbe et al. 2018, 2020). Nevertheless, the influence of the two main drivers could be complex and the most limiting ones could vary under different environmental conditions (Zhong et al. 2010; Rissanen et al. 2011).
Pearson correlation analysis was conducted between the sediment denitrification rates (DRs) and the physicochemical properties of the overlying water and sediment, and the results were listed in Tables 2 and 3 respectively. In general, the DRs in Dongping Lake were significantly negatively correlated with the temperature (T) and the SOP concentrations in the surface water. However, for each site, these significant negative correlations only existed between the DRs and SOP at site 2 and site 3; furthermore, for each season, no significant correlations were found (Table 2). The DRs were positively correlated with the NO3− concentrations in the surface water, but the correlations were not significant (P > 0.05). On the whole, the DRs were significantly positively correlated with the NH4+ content and OM but negatively correlated with the NO3− content. The significant positive correlations between DRs and OM were also found in the other three seasons except spring. Otherwise, these significant correlations were only found in a certain site or season or at a certain layer sediment (Table 3).
The impact of the physicochemical properties of surface water and sediment on the DRs was further analyzed by the multiple linear regression. The optimal model for surface water was DR = − 0.578 × T-0.575 × NH3-0.509 × DO-0.334 × SOP (n = 16, P = 0.000, R2 = 0.914, Adj. R2 = 0.883), while the optimal model for sediment was DR = 0.606 × OM-0.476 × NO3− (n = 80, P = 0.000, R2 = 0.388, Adj. R2 = 0.372). As all the variables were log-transformed and then scaled to z-scores, we could directly use the regression coefficients to evaluate the relative importance of the variables in the models. The influence of the properties of the surface water on DRs were decreased in the order of T > NH3 > DO>SOP, and the model could explain 88.3% of the DR variation, while for sediment, OM had more impact on the DRs than NO3−; however, this regression model could only account for 37.2% of the DRs variation.
In most of studies (Wang et al. 2018; de Klein et al. 2017), temperature was a fundamental factor affecting the denitrification rates, although the correlations were not uniform. Wu et al. (2013) found that the denitrification rates were significantly positively related to temperature in the sediments due to the increased metabolic activity that occurs at higher temperatures. However, Luo et al. (2000) found that there was not a positive temperature effect on denitrification due to the wide variation among environmental factors. Organic matter can provide essential carbon sources for the denitrification (Lin et al. 2018); therefore, positive relationship is often found between denitrification rates and organic matter or carbon contents of sediment (Bruesewitz et al. 2011; Yao et al. 2016). The significant positive correlation of the DRs with NH4+ and significant negative correlation with NO3− in sediment might indicate that nitrate from nitrification provide major N source for denitrification (Wang et al. 2017a; Wu et al. 2019); however, as both correlation coefficients were lower than 0.3 (Table 3) and moreover the sediment model could only explained less than 40% of the variation of DRs, the impact of sediment properties on DRs still needs further study.
Based on the results of correlation and regression analysis, we categorized and summarized three factors which were season, vegetation, and the depth of the sediment according to their internal links. The factor of season represented the change of the temperatures. The growth of vegetation could change the physicochemical properties of surface water and sediment including nitrate concentrations, pH, oxygen, total organic carbon, and so on (Mi et al. 2008). The sediment at different depth also had different physicochemical properties such as the nitrate concentrations, Eh, and organic matter content.
The three-way multiplicative model is believed to be highly useful for parsimonious descriptions of three-way interactions. It is conceptually simple, easy to program, and with some extensions and precautions, it is also applicable to (mildly) incomplete data (Eeuwijk and Kroonenberg 1998). Many articles have been published describing three factors interactions (Singh and Agrawal 2005; Nelissen et al. 2014). In this study, the three-way multiplicative model was adopted to analyze the main effect and the interaction effect of the three factors, and the results were listed in Table 4. It could be found that season, vegetation, and depth had both significant main and interaction effect (P < 0.01) on the denitrification rates in Dongping Lake.
The winter peak of denitrification rates may be attributed to the higher NO3− load in surface water (Table 1), stimulating rates of sediment denitrification (Fear et al. 2005; Liu et al. 2014) and the absence of the plant uptake, resulting in lower competence for nitrate. In spring and summer, higher DRs were measured in deeper sediment layers of vegetated zones, pointing to a higher influence of oxygen root exudation, enhancing nitrification, which promoted denitrification during the growing season of aquatic plants (Yu et al. 2012; Liu et al. 2014). Different aquatic plants have different root system development. P. communis is a typical emergent plant, and a liquid film with thickness less than 1 mm filled with oxygen could be formed around its root hairs (Hupfer and Dollan 2003; Mi et al. 2008; Xing et al. 2008), and this effect was weaker for submerged plants such as P. crispus than P. communis (Xing et al. 2008). That might be the reason for the higher DRs in P. communis zones than in the other vegetated zones (Fig. 2).Higher DRs in summer at site 2 (the aquaculture zone) might be related to the dense aquaculture activities and the absence of the plant uptake for nitrate in the area. Nevertheless, the acetylene-block technique infra-estimates the denitrification coupled to nitrification, as it not only blocks the N2O reduction but also blocks partially nitrification (Seitzinger et al. 1993) especially during the growing season of the aquatic plants when the root exudation effect is more intense. Besides, the slurry incubation approach could overestimate the in situ denitrification rates by enhancing the nitrate availability (Ambus 1993; Laverman et al. 2006); and this methodological artifact can be especially important in deeper sediment layers of the non-vegetated zone (site 2). Therefore, the method for the DR measurement needs to be improved in the future research (Laverman et al. 2006).
Internal N cycling rate and sediment N removal capacity in Dongping Lake
The cycling rates (CR) of nitrate were quantified by Eq. (3), while the residence time of nitrate was defined as the inverse of the CR and calculated by Eq. (4). As illustrated in Fig. 4, the cycling rates of nitrate were fastest in winter in all the sites, followed by autumn in sites 1, 3, and 4 but summer in site 2. Moreover, in the P. communis zone, the average residence time of nitrate was about 18.0 day, and the average cycling rate was about 20.4 to 31.4 times faster than those in the other vegetation zones where the residence time was about from 100.0 to 240.2 days.
In order to calculate the nitrogen removal capacity of sediment in Dongping Lake, it was necessary to determine the area of the different vegetation zones. Using the density segmentation and object-oriented method, Wang et al. (2017b) found the area of Dongping Lake retaining water all year round was 134.0 km2, including 10.83 km2 reed wetland. According to the notice of the People’s Government of Dongping County on the centralized renovation of the cage and seine aquaculture in 2017, the area of aquaculture zone was 13.33 km2. As the mixed vegetation zone was usually located near the lakeshore area, based on the reconnaissance and measurement of lakeshore line, the total area of the mixed vegetation zone was calculated and was about 7.06 km2. The area of P. crispus zone was the total area minus the sum of the other area and is about 102.78 km2.
Precipitation and inflow of rivers are the two main sources of nitrogen loading into Dongping Lake. Due to the lack of the TN concentrations in the tributary Dawen River, the TN concentrations of Dongping Lake were used to calculate the N input. From 2008 to 2013, the total nitrogen (TN) in water body of Dongping Lake was 1.98 ± 0.81 mg N L−1 (Shi et al. 2015). According to the annual volume of river water entering the lake (1.08 × 109 m3 a−1), there was 2.1 × 109 g N imported into reservoir annually. The average TN concentration in the precipitation near Dongping Lake was 1.95 mg N L−1 (Wang et al. 2006; Xu et al. 2016). Hence, based on the lake area and the average annual precipitation, the annual N input through precipitation was 2.5 × 106 g. As the quality of inflowing and outflowing water in the Dongping Lake is required to meet the standards for class III water body of the GB3838-2002 National Environmental Quality Standards for Surface Water, and the inflow and outflow amount of water was equal; therefore, the nitrogen loading by the East Route of the south-to-north water diversion project was not considered in the calculation. At the same time, the TN standard value (1 mg L−1) for Dongping Lake was used as the TN concentration of outgoing water by others rivers. Based on water balance equations, the effluent volume was 1.1 × 109 m3 a−1. Then, the nitrogen balance was calculated according to the balance principle, and the retention of nitrogen in Dongping Lake was about 2.03 × 109 g N a−1.
Based on the hypothesis that the NO3− would be quickly exhausted at the surface of sediment, average sediment denitrification rates at the four sites were used to calculate the nitrogen removal capacity, and the results were listed in Table 5. Due to the highest denitrification rates in winter (Fig. 2), the nitrogen removal capacity was largest in the winter at all the sites. As the area of P. crispus zone was about 10 times of that of P. communis zone, the P. crispus zone had the largest nitrogen removal capacity despite of the low denitrification rates. On the whole, the denitrification of the sediment in Dongping Lake could remove about 537.7 t N every year, which was about 26.5% of the annual TN loading (2.03 × 109 g N a−1). Therefore, there was still a potential increase of bioavailable nitrogen concentrations and eutrophication risk in Dongping Lake. Nevertheless, according to Table 1, the nitrogen concentrations in Dongping Lake had exceeded the TN standard value (1 mg L−1), and using the standard value for calculation might overestimate the annual TN loading, which might result in the underestimation of the nitrogen removal ratio by denitrification.
Conclusions
The average sediment denitrification rates in the P. communis zone, the aquaculture zone, the P. crispus zone, and the mixed vegetation zone of Dongping Lake were 69.0 ± 91.6, 15.3 ± 17.2, 11.8 ± 15.5, and 8.70 ± 5.44 μmol N m−2 h−1 respectively. The DRs in the P. communis zone were significantly higher than in the mixed vegetation zone. The average sediment denitrification rates in different season were significantly decreased as follows: winter (74.5 ± 88.3 μmol N m−2 h−1) > autumn (15.7 ± 18.6 μmol N m−2 h−1) ≈ summer (10.7 ± 5.90 μmol N m−2 h−1) > spring (3.85 ± 1.29 μmol N m−2 h−1). The DRs generally decreased with the increasing of depth; however, there were significant increase of DRs in the 3~5 cm sediment in summer at the P. communis zone and the P. crispus zone, and in winter and spring at the mixed vegetation zones. The DRs in Dongping Lake were jointly controlled by factors of season, vegetation, and depth of sediment. Specifically, the winter peaks of denitrification rates and cycling rates of nitrate might be related to the higher NO3− load in winter, stimulating rates of sediment denitrification and the absence of plant uptake, resulting in lower competence for nitrate, while the highest DRs and cycling rates of nitrate in the P. communis zone might be attributed to the larger biomass and oxygen transporting capacity of P. communis than of the other aquatic plants. On the whole, the denitrification of the sediment could remove about 537.7 t N every year, which was about 26.5% of the annual TN loading (2.03 × 109 g N a−1) in Dongping Lake. Considering the high sediment denitrification rates and nitrate cycling rates in the P. communis zone, we suggest that P. communis (reeds) should be the preferred plant for eutrophication prevention and ecological remediation in Dongping Lake. Results of this study might underestimate the effect of different aquatic plants and the total nitrogen removal capability of the sediment. Further investigation is needed to probe the influence mechanism of plants on sediment denitrification.
References
Ambus P (1993) Control of denitrification enzyme activity in a streamside soil. FEMS Microbiol Ecol 102:225–234
Bastviken SK, Eriksson PG, Premrov A, Tonderski K (2005) Potential denitrification in wetland sediments with different plant species detritus. Ecol Eng 25:183–190
Beaulieu JJ, Tank JL, Hamilton SK, Wollheim WM, Hall RO Jr, Mulholland PJ, Peterson BJ, Ashkenas LR, Cooper LW, Dahm CN, Dodds WK, Grimm NB, Johnson SL, McDowell WH, Poole GC, Valett HM, Arango CP, Bernot MJ, Burgin AJ, Crenshaw CL, Helton AM, Johnson LT, O'Brien JM, Potter JD, Sheibley RW, Sobota DJ, Thomas SM (2011) Nitrous oxide emission from denitrification in stream and river networks. PNAS 108:214–219
Bruesewitz DA, Hamilton DP, Schipper LA (2011) Denitrification potential inlake sediment increases across a gradient of catchment agriculture. Ecosystems 14:341–352
Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ 5(2):89–96
Camargo JA, Álvaro A (2006) Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environ Int 32(6):831–849
Choi JH, Kang H, Park SS (2009) Comparison of enzyme activities in vegetated and nonvegetated sediments. J Environ Eng 135(5):299–305
Compiling Team for Annals of Dongping County Shandong Province (2006) Annals of Dongping County (1986–2003). Zhonghua Book Company, Beijing. (in Chinese)
de Klein JJM, Overbeek CC, Juncher Jørgensen C, Veraart AJ (2017) Effect of temperature on oxygen profiles and denitrification rates in freshwater sediments. Wetlands 37:975–983
Deng HG, Zhang J, Chen SY, Yang L, Wang D, Yu S (2016) Metal release/accumulation during the decomposition of Potamogeton crispus in a shallow macrophytic lake. J Environ Sci 42(4):71–78
Deng HG, Zhang ZB, Liu T et al (2019) Short-term effects of exogenous nitrate on N2O and CH4 emission from greenbelt in urban riparian zone. J Liaocheng Univ (Nat Sci) 32(9):1–9 (in Chinese)
Eeuwijk FA, Kroonenberg PM (1998) Multiplicative models for interaction in three-way ANOVA, with applications to plant breeding. Biometrics 54(4):1315–1333
Eriksson PG, Weisner SE (1999) An experimental study on effects of submersed macrophytes on nitrification and denitrification in ammonium-rich aquatic systems. Am Soc Limnol Oceanogr 44(8):1993–1999
Fear JM, Thompson SP, Gallo TE (2005) Denitrification rates measured along a salinity gradient in the eutrophic Neuse River estuary, North Carolina, USA. Estuaries 28(4):608–619
Fernandes SO, Dutta P, Gonsalves MJ, Bonin PC, LokaBharathi PA (2016) Denitrification activity in mangrove sediments varies with associated vegetation. Ecol Eng 95:671–681
Fowler D, Coyle M, Skiba U, Sutton MA, Cape JN, Reis S, Sheppard LJ, Jenkins A, Grizzetti B, Galloway JN, Vitousek P, Leach A, Bouwman AF, Butterbach-Bahl K, Dentener F, Stevenson D, Amann M, Voss M (2013) The global nitrogen cycle in the twenty-first century. Philos Trans R Soc Lond 368(1621):20130164
Grasshof K, Ehrhard M, Kremling K (1983) Methods of seawater analysis, 2nd edn. Verlag Chemie, Weiheim
Guan BH, An SQ, Gu BH (2011) Assessment of ecosystem health during the past 40 years for Lake Taihu in the Yangtze River Delta, China. Limnology 12(1):47–53
He DJ, Xing YH, Jiang RX et al (2010) Distribution of nitrogen and phosphorus in water and eutrophication assessment of Dongping Lake. Environ Sci Technol 33(8):45–48, 61 (in Chinese)
Hupfer M, Dollan A (2003) Immobilisation of phosphorus by iron-coated roots of submerged macrophytes. Hydrobiologia 506-509(1):635–640
Laverman AM, Van Cappellen P, van Rotterdam-Los D et al (2006) Potential rates and pathways of microbial nitrate reduction in coastal sediments. FEMS Microbiol Ecol 58:179–192
Li C, Wang B, Ye C, Ba YX (2014) The release of nitrogen and phosphorus during the decomposition process of submerged macrophyte (Hydrilla verticillata Royle) with different biomass levels. Ecol Eng 70:268–274
Li B, Chen J, Zhen W et al (2018) Seasonal and spatial dynamics of denitrification rate and denitrifier community in constructed wetland treating polluted river water. Int Biodeterior Biodegradation 126:143–151
Lin L, Li YY, Li XY (2018) Acidogenic sludge fermentation to recover soluble organics as the carbon source for denitrification in wastewater treatment: comparison of sludge types. Front Environ Sci Eng 12(4):3. https://doi.org/10.1007/s11783-018-1043-x
Liu L, Wang DQ, Deng HG, Li Y, Chang S, Wu Z, Yu L, Hu Y, Yu Z, Chen Z (2014) The capability of estuarine sediments to remove nitrogen: implications for drinking water resource in Yangtze estuary. Environ Sci Pollut Res 21(18):10890–10899
Liu W, Jiang X, Zhang Q et al (2018) Has submerged vegetation loss altered sediment denitrification, N2O production, and denitrifying microbial communities in subtropical lakes? Glob Biogeochem Cycles 32:1195–1207
Luo J, Tillman RW, Ball PR (2000) Nitrogen loss through denitrification in a soil under pasture in New Zealand. Soil Biol Biochem 32(4):497–509
Mi WJ, Zhu DW, Zhou YY, Zhou HD, Yang TW, Hamilton DP (2008) Influence of Potamogeton crispus growth on nutrients in the sediment and water of Lake Tangxunhu. Hydrobiologia 603(1):139–146
Monsen NE, Cloern JE, Lucas LV (2002) A comment on the use of Flushing time, residence time, and age as transport time scales. Limnol Oceanogr 47(5):1545–1553
Nelissen V, Saha BK, Ruysschaert G, Boeckx P (2014) Effect of different biochar and fertilizer types on N2O and NO emissions. Soil Biol Biochem 70:244–255
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Sparks D L, Page A L, Helmke P A, Loeppert R H, Soltanpour P N, Tabatabai M A, Sumner M E (ed) Methods of soil analysis. Part 3-chemical methods (Soil Science Society of America Book Series, No. 5). Madison, pp 961–1010
Nizzoli D, Welsh DT, Longhi D, Viaroli P (2014) Influence of Potamogeton pectinatus and microphytobenthos on benthic metabolism, nutrient fluxes and denitrification in a freshwater littoral sediment in an agricultural landscape: N assimilation versus N removal. Hydrobiologia 737:183–200
O’brien J, Hamilton SK, Podzikowski L et al (2012) The fate of assimilated nitrogen in streams: an in situ benthic chamber study. Freshw Biol 57(6):1113–1125
Palacin-Lizarbe C, Camarero L, Catalan J (2018) Denitrification temperature dependence in remote, cold, and N-poor lake sediments. Water Resour Res 54(2):1161–1173
Palacin-Lizarbe C, Camarero L, Hallin S, Jones CM, Catalan J (2020) Denitrification rates in lake sediments of mountains affected by high atmospheric nitrogen deposition. Sci Rep 10:3003
Rissanen A, Tiirola M, Ojala A (2011) Spatial and temporal variation in denitrification and in the denitrifier community in a boreal lake. Aquat Microb Ecol 64(1):27–40
Sand-Jensen K, Prahl C, Stokholm H (1982) Oxygen release from roots of submerged aquatic macrophytes. Oikos 38:349–354
Saunders DL, Kalff J (2001) Nitrogen retention in wetlands, lakes and rivers. Hydrobiologia 443(1–3):205–212
Scott JT, Mccarthy MJ, Gardner WS et al (2008) Denitrification, dissimilatory nitrate reduction to ammonium, and nitrogen fixation along a nitrate concentration gradient in a created freshwater wetland. Biogeochemistry 87(1):99–111
Seitzinger SP, Nielsen LP, Caffrey J, Christensen PB (1993) Denitrification measurements in aquatic sediments: a comparison of three methods. Biogeochemistry 23:147–167
SEPA (State Environmental Protection Administration) (2002) Water and wastewater monitoring and analysis method, 4th edn. China Science Press, Beijing (in Chinese)
Shi JH, Wang QD, Li XQ et al (2011) Aquatic ecosystem changes and eutrophication assessment in Dongping Lake. J Yangtze Univ (Natural Science Edition) 8(4):242–245 (in Chinese)
Shi JH, Li XQ, Dong GC et al (2015) Comprehensive evaluation of water environmental quality in Dongping Lake. J Dalian Ocean Univ 30(4):391–397 (in Chinese)
Shibata H, Branquinho C, Mcdowell WH et al (2015) Consequence of altered nitrogen cycles in the coupled human and ecological system under changing climate: the need for long-term and site-based research. Ambio 44(3):178–193
Singh RK, Agrawal M (2005) Atmospheric depositions around a heavily industrialized area in a seasonally dry tropical environment of India. Environ Pollut 138(1):142–152
Veraart AJ, de Bruijne WJJ, de Klein JJM, Peeters ETHM, Scheffer M (2011) Effects of aquatic vegetation type on denitrification. Biogeochemistry 104:267–274
Vila-Costa M, Pulido C, Chappuis E, Calviño A, Casamayor EO, Gacia E (2016) Macrophyte landscape modulates lake ecosystem-level nitrogen losses through tightly coupled plant-microbe interactions. Limnol Oceanogr 61:78–88
Wang Y, Ge FL, Liu XH et al (2006) Analysis of ionic composition characteristics of precipitation at Mount Taishan. China Environ Sci 26(4):422–426 (in Chinese)
Wang J, Sun XL, Cao SL et al (2014) Study on eco-environmental water demand of Dongping Lake. Yellow River 11:77–80 (in Chinese)
Wang DQ, Tan YJ, Yu ZJ et al (2015) Nitrous oxide production in river sediment of highly urbanized area and the effects of water quality. Wetlands, 2015 35(6):1213–1223
Wang J, Liu H, Lei Y et al (2017a) Nitrification and denitrification rates in a small tributary, Nanhe River, of Three Gorge Dam Reservoir during water collection and release events. Environ Sci 38(3):946–953
Wang AL, Liu J, Wang CY et al (2017b) Information extraction from Dongping Lake wetland with the density segmentation and object-oriented method. J Shandong Agric Univ (Natural Science Edition) 48(1):70–74 (in Chinese)
Wang XM, Hu MJ, Ren HC, Li J, Tong C, Musenze RS (2018) Seasonal variations of nitrous oxide fluxes and soil denitrification rates in subtropical freshwater and brackish tidal marshes of the Min River estuary. Sci Total Environ 616-617:1404–1413
Weiss RF, Price BA (1980) Nitrous oxide solubility in water and seawater. Mar Chem 8:347–359
Wu JZ, Chen NW, Hong HS, Lu T, Wang L, Chen Z (2013) Direct measurement of dissolved N2 and denitrification along a subtropical river-estuary gradient, China. Mar Pollut Bull 66(1–2):125–134
Wu JX, Fan R, Wang MC et al (2018) Denitrification ability of surficial sediments in Dianshan Lake and its environmental implications. Acta Sci Circumst 38(3):867–874 (in Chinese)
Wu S, Wu Z, Liang Z et al (2019) Denitrification and the controlling factors in Yunnan Plateau Lakes (China): exploring the role of enhanced internal nitrogen cycling by algal blooms. J Environ Sci 76(2):352–361
WWAP (2017) The United Nations World Water Development Report 2017. Wastewater: the untapped resource. Paris: UNESCO
Xing P, Kong FX, Chen KN et al (2008) Ammonia-oxidizing Bacteria community composition at the root zones of aquatic plants after ecological restoration. Environ Sci 29(8):2154–2159 (in Chinese)
Xu CL, Luo CL, Xue YJ et al (2016) Concentration and source assessment of dissolved organic and inorganic carbon and dissolved nitrogen in snow and rainfalls in Shandong Province. Acta Sci Circumst 36(2):658–666 (in Chinese)
Yang L, Chen S, Zhang J, Yu S, Deng H (2017) Environmental factors controlling the spatial distribution of subfossil Chironomidae in surface sediments of Lake Dongping, a warm temperate lake in North China. Environ Earth Sci 76(15):524
Yao L, Jiang XL, Chen CR, Liu G, Liu W (2016) Within-lake variability and environmental controls of sediment denitrification and associated N2O production in a shallow eutrophic lake. Ecol Eng 97:251–257
Yin GY, Hou LJ, Zong HB, Ding P, Liu M, Zhang S, Cheng X, Zhou J (2015) Denitrification and anaerobic ammonium oxidization across the sediment-water interface in the hypereutrophic ecosystem, Jinpu Bay, in the northeastern coast of China. Estuar Coasts 38(1):211–219
Yu Z, Li Y, Deng H et al (2012) Effect of Scirpus mariqueter on nitrous oxide emissions from a subtropical monsoon estuarine wetland. J Geophys Res 117:G02017
Zhang J, Deng HG, Chen SY et al (2011) Eco-environmental health risk assessment of Dongping Lake water-resources. J Saf Environ 11(6):111–115 (in Chinese)
Zhang F, Yi N, Di PP et al (2017) Nitrogen removal efficiency and control of bio-denitrification process of aquatic plants. J Ecol Rural Environ 33(2):174–180 (in Chinese)
Zhong J, Fan C, Liu G, Zhang L, Shang J, Gu X (2010) Seasonal variation of potential denitrification rates of surface sediment from Meiliang Bay, Taihu Lake, China. J Environ Sci 22(7):961–967
Zhong J, Yu J, Wang J, Liu D, Chen C, Fan C (2020) The co-regulation of nitrate and temperature on denitrification at the sediment-water interface in the algae-dominated ecosystem of Lake Taihu, China. J Soils Sediments 20:2277–2288. https://doi.org/10.1007/s11368-019-02558-2
Acknowledgments
This work was jointly supported by the National Natural Science Foundation of China (Grant No. 41977322, 41807430), the Natural Science Foundation of Shandong province (Grant no. ZR2014JL028), and Scientific Research Foundation of Liaocheng University (Grant No. 318011909).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 49 kb)
Rights and permissions
About this article
Cite this article
Deng, Hg., Zhang, J., Wu, Jj. et al. Biological denitrification in a macrophytic lake: implications for macrophytes-dominated lake management in the north of China. Environ Sci Pollut Res 27, 42460–42471 (2020). https://doi.org/10.1007/s11356-020-10230-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10230-3