Abstract
For the purpose of enhancing the removal rate of nitrogen (N) and organic matters, intermittent aeration and carbon source were used in filled-and-drained vertical flow constructed wetlands (VFCWs). The results showed that the best removal of COD (74.16%), NH4+-N (93.56%), TN (86.88%), and NO3−-N (79.65%) was achieved in VFCW1 (aerated with carbon source system). Illumina MiSeq300 high-throughput sequencing showed that carbon source aerated system increases the diversity and richness of the microbial community. The copy numbers of nitrification functional genes (nxrA, amoA), denitrification functional genes (nirS, nirK, nosZ), and anammox functional gene (anammox 16S rRNA) displayed various changes when applied different aeration modes and additional carbon source to each system. An increase of the DO concentration and carbon source facilitated the absolute abundance of microbial nitrification and denitrification functional genes, respectively. All in all, these results demonstrate that carbon source combined with intermittent aeration is valid to improve the pollutant treatment performance in these systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High N and organic matters released into water bodies lead to deterioration of water quality via eutrophication (Fan et al. 2016). Compared with the traditional sewage treatment technology, the constructed wetlands (CWs) have the characteristics of stable effluent quality, low energy consumption, simple operation, and environmentally friendly performance (Hou et al. 2018). Thus, it is widely used throughout the world. Due to the high pollutant removal efficiency, good oxygen transfer performance for nitrification, and a small footprint, VFCWs are often applied to purify and treat various wastewaters (Pelissari et al. 2017). The CWs utilize a substrate–plant–microbial integration system under the combination of physical, chemical, and biological effects (Liu et al. 2018) to achieve efficient purification of sewage by precipitation and volatilization, microbial degradation, plant absorption, substrate adsorption, and filtration (Wang et al. 2016a, b; Jia et al. 2018). CWs perform beneficial treatment effect on organics and suspended solid (SS), but removal rate of N in wastewater shows enormous fluctuations (Wang et al. 2014). Consequently, enhancing N removal rate has being an issue in CW continuous wastewater treatment.
Microbial transformation (nitrification and denitrification) is one of the main N removal mechanisms in CWs (Mesquita et al. 2017). Nitrification is the process of oxidizing ammonia nitrogen (NH4+-N) to nitrate nitrogen (NO3−-N) by nitrifying bacteria under aerobic conditions (Zhou et al. 2017). Denitrification refers to the process in which nitrite nitrogen (NO2−-N) and nitrate nitrogen (NO3−-N) are converted into gaseous nitrogen (N2) by the transformation of denitrifying bacteria (Tan et al. 2017). The minimal utilization rate of dissolved oxygen (DO) is one of the main factors which lead to poor N removal in wetlands (Li et al. 2014). Adequate DO supply is the key to ensure the nitrification process by facilitating the multiplication of the nitrobacteria (Jia et al. 2018). The intermittent aeration operation mode of CWs can improve the DO level in the wetlands and create an alternate aerobic and anoxic environment to promote nitrification and denitrification, respectively, thereby achieving simultaneous efficient removal of NH4+-N and TN (Wu et al. 2015; Zhou et al. 2017). In addition, carbon source is one of the important inhibition factors which affect the denitrification in CWs during microbial heterotrophic metabolism (Saeed et al. 2018). When the COD/N ratio in wastewater is low, an exogenous carbon source is required to provide denitrifying electron donors (Zhou et al. 2017; Pan et al. 2017). Therefore, studying the combination of intermittent aeration and carbon source is essential for the enhancement of the N removal from wastewater in CWs.
The conversion, circulation, and removal of N and organic pollutants in CWs are closely linked to the microbial species, quantity, and spatial and temporal distribution in the systems (Zhu et al. 2014). Therefore, microorganism transformation is deemed to be the dominant route of N removal (Li et al. 2017). Moreover, the degradation of pollutants in water is mainly implemented by microorganisms, which act as the primary decomposer in the system (Faulwetter et al. 2009). It has a close relationship between the microbial biomass and purification functions in CWs. The increase of microorganism biomass leads to an improvement in the removal rate of pollutants (Hu et al. 2016). Previous study found that the amount of microorganisms in the sewage, especially the number of nitrifying and denitrifying bacteria, is positively correlated with the efficiency of N removal (Zhu et al. 2014). In addition, Wang et al. (2015) reported that the functional genes of nxrA (nitrite oxidoreductase), amoA (ammonia monooxygenase), anammox bacterial 16S rRNA (anammox bacteria), nirS (cd1-containing nitrite reductase), nirK (copper-containing nitrite reductase), and nosZ (nitrous oxide reductase) participated in microbial nitrification and denitrification processes. Up to now, there are few reports on the microbial responses during N removal through the operation of intermittent aeration combined with exogenous carbon addition in influent. Therefore, in order to reveal the mechanism and process of N removal from wetlands, it is highly necessary for us to determine the copy number of N transformation functional genes in the system by real-time quantitative PCR technology. Hence, a comprehensive understanding of the relationships between N transformation functional genes which indicate the varieties of microbial functions and N removal efficiency can help optimize the treatment performance of pollutants in VFCWs.
This study addressed the effects of intermittent aeration combined with exogenous carbon source on N removal efficiency and microbial responses in VFCWs. The main purpose of this experiment were to: (1) explore the optimal removal rate of N and organic matters; (2) investigate the variety of microorganism community as influenced by operation conditions; and (3) quantify the affiliation between microbial responses and removal of corresponding pollutants.
Materials and methods
Description and operation of VFCWs
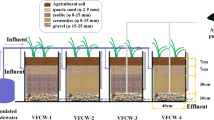
In this experiment, four filled-and-drained VFCWs of the same size were designed and placed outdoor in the University of Jinan, which were recorded as VFCW1 (aerated with carbon source addition), VFCW2 (aerated with non-carbon source addition), VFCW3 (non-aerated with carbon source addition), and VFCW4 (non-aerated with non-carbon source addition). All experimental systems were prepared by Plexiglass (50 cm in length, 40 cm in width, and 60 cm in height) with a working volume of 35 L. The diagrammatic sketch of the VFCW systems was displayed in Fig. 1. The systems were packed according to our previous study (Lai et al. 2020). The substrates were divided into four layers from top to bottom with 10-cm-thick soils, 10-cm-thick quartz sands (1–2 mm in diameter), 15-cm-thick zeolites (8–10 mm in diameter), and 15-cm-thick lava rock (8–10 mm in diameter). The arrangement of the substrates from small to large in size increases the utilization of aeration. Canna indica L. was planted in each system at a density of 12 plants. There are two pre-buried PVC pipes which were located in VFCW system center. One (10 cm in diameter) was applied for pH and DO value determination in situ, and the other (3 cm in diameter) was for artificial aeration, which was connected to an air pump and perforated on the pipe body at intervals of 10 cm.
This experiment began in June 2018 and lasted a total of 150 days. Before the artificial contaminated influent was imported, the systems have irrigated with secondary wastewater received from Everbright Water Co., Ltd. (Jinan, China) for one month for the proliferation of microorganisms. So, as to investigate the N removal efficiency of VFCWs, different aeration modes and carbon source addition were designed. As an external carbon source, glucose was added to the systems. Synthetic wastewater was made up of tap water (Jinan) and mainly contained the following components (per liter): 0.0049 g KNO3, 0.089 g NH4Cl, and 0.19 g glucose, leading to concentrations of 3.6 mg L−1 NO3−, 30.8 mg L−1 NH4+, and 196 mg L−1 COD, and the ratio of COD/N achieved in 5.87, which were applied to minimize inconstancy of the influent concentration. The detailed compositions in the synthetic wastewater have been demonstrated by Lai et al. (2020). The VFCW system which required aeration was intermittently aerated for 4 h at an air-flow rate of 1.0 ± 0.2 L min−1 every day. After every hydraulic retention time (HRT, 72 h) of the cycle, each VFCW was drained and immediately refilled with the wastewater, which formed a filled-and-drained batch mode. In the current operating mode, hydraulic loading rate (HLR) of the tested VFCW system during the whole study was 0.058 m3 (m−2 d−1).
Water sample collecting and testing
Influent and effluent samples from each VFCW were collected every 72 h, hereafter, transferred immediately to lab for analysis of COD, NH4+-N, TN, NO3−-N, and NO2−-N in the light of standard methods (APHA 2005). The DO and pH value were measured in situ with a digital DO meter (CyberScan DO110, USA) and pH glass meter (DENVER UB-7, USA), respectively.
Microbial communities and qPCR analysis
Sampling of microorganisms
The substrates samples were collected at the center and in four corners, positions of the VFCW systems, and homogenized to obtain a composite sample after 150 days of system operation. The sample was stored in a sterile plastic bag placed in an ice incubator and brought back to the laboratory for further processing. Then, the substrate samples were washed by vortexing. Lastly, it was centrifuged at 12,000g for 2 min to collect the sediment in sterile tubes (Du et al. 2017). Sediment of the substrate samples was stored at − 20 °C for further analysis and testing.
Illumina MiSeq high-throughput sequencing
E.Z.N.A. soil DNA kit (Omega, USA) was utilized to extract DNA from diverse microbial samples in accordance with the manufacturer’s instructions. The extracted DNA was detected by way of 2% agarose gel electrophoresis and preserved at − 20 °C until use. Microbial sample analysis by Illumina MiSeq high-throughput sequencing was provided by LC-Bio Technology Co., Ltd. (Hangzhou, China). Operational taxonomic units (OTUs) were clustered via Vsearch (version 2.3.4) at 97% sequences similarity. To understand microbial diversity and community richness, the indices of Simpson, Observed species, Shannon, and Chao 1 were calculated by Qiime (version 1.8.0). Taxonomic datum was allocated at the phylum level through ribosomal database project (RDP).
Quantitative polymerase chain reaction
DNeasy PowerSoil Kit (MoBio, USA) was utilized to extract the microbial genomic DNA. Quantitative testing of related transformation functional genes (nxrA, amoA, nosZ, nirS, nirK, and anammox 16S rRNA) was conducted on a real-time quantitative PCR detection system (Bio-Rad, USA). Synthetic primers were presented in Table S1 and diluted according to the manufacturer’s (Nanjing Jinsirui Biotechnology Co., Ltd., Nanjing, China) instructions. The 20 μL reaction mixture had the following composition: 10 μL Maxima SYBR Green qPCR Master Mix (2×) (TaKaRa), 1 μL template DNA (sample DNA or plasmid DNA for standard curves), 0.4 μL in forward and reverse primers, and sterile water. Quantitative analysis was conducted using a procedure of three-step thermal cycle, followed by melting curve analysis. It is useful to note that all assays were performed in triplicate to reduce the variables.
Statistical analyses
Software SPSS 17.0 (SPSS Inc., Chicago, USA) was carried out for all statistical analyses performed by one-way analysis of variance (ANOVA). The statistically significant differences among treatment performances in three VFCW systems were considered at P < 0.05. Redundancy analysis (RDA) (Canoco 5.0) was used to illustrate the relationship between microorganisms and N removal.
Results and discussion
Overall removal performances of N and organic matters
N removal
During the experimental period, the average effluent concentration and removal efficiency of NH4+-N, NO3−-N, and TN were shown in Fig. 2 and Table 1, respectively. With varied aeration modes and exogenous carbon source, VFCW systems presented different removal efficiency in N removal. Overall, there are significant differences among the N removal rate in VFCWs. As shown in Fig. 2 and Table 1, NH4+-N removal efficiency could achieve above 84% under all treatments, and the average effluent NH4+-N concentration fall below 5 mg L−1, which was within the scope of Chinese wastewater discharge standard (GB18918-2002). The removal of NH4+-N mainly depends on microbial nitrification, which transforms NH4+-N to NO3−-N under sufficient oxygen conditions (Li et al. 2014). NH4+-N removal rate of the aerated VFCW1 (93.56%) and VFCW2 (95.38%) was higher than those of the non-aerated VFCW3 (84.45%) and VFCW4 (85.37%) under unanimous exogenous carbon source (P < 0.05). Research of Yang et al. (2017) found that NH4+-N removal rate was higher in aerated CWs, which was similar to our findings. In the present study, the removal of NH4+-N decreased with the addition of a carbon source under the identical aeration mode. Owing to the addition of the carbon source, increasing organic matters consume part of the oxygen and the insufficient supply of oxygen prevents the NH4+-N from being completely converted into NO3−-N (Zhou et al. 2019). Therefore, the reduction of NH4+-N removal may be due to hypoxia inhibition of nitrification (Zhu et al. 2014). The current study demonstrated that the combination of intermittent aeration and exogenous carbon source improved NH4+-N removal rate, which was more effective than the previous result (88%) achieved by Ding et al. (2014). Furthermore, the NH4+-N removal efficiency obtained by Zhao et al. (2016) was 84%, indicating the operation and setup in current VFCWs are conducive to the removal of NH4+-N.
As seen from Fig. 2b, a lower average effluent NO3−-N concentration was achieved in VFCW1 (0.75 mg L−1) and VFCW3 (0.63 mg L−1), suggesting the higher removal rate of NO3−-N is mainly attributed to good denitrification in VFCW system with carbon source addition. Previous studies have observed that insufficient carbon sources or excessive oxygen inhibited denitrification (Wu et al. 2016), which supports our results. NO3−-N removal mainly depends on microbial heterotrophic denitrification, which was conducted under anaerobic conditions (Pan et al. 2017). In this study, it revealed that the combined operation of intermittent aeration and carbon source addition provides oxygen condition and available carbon supply for aerobic nitrification and anaerobic denitrification of microorganisms, respectively. Thus, the highest NO3−-N removal rate was obtained in VFCW3 (82.91%) among all systems. In addition, the removal rate of NO3−-N under these running VFCWs were greater than those in non-aerated VFCWs packed with fly-ash brick (65%) (Liu et al. 2019) and the two-stage deep sequencing CW (NO3−-N concentration accumulation) (He et al. 2018). This finding manifested that the operating conditions of these systems facilitate the growth of heterotrophic denitrifying bacteria. The concentration of NO2−-N was all less than 0.60 mg L−1 during the whole operation period, indicating the nitrification and denitrification processes occurred thoroughly, although no statistically significant differences were discovered among four VFCW systems (P > 0.05).
In the current study, the average effluent concentration of TN was 7.08 mg L−1 and removal efficiency ranged from 72.9 to 86.9% (Fig. 2c and Table 1). Aeration modes significantly varied the TN removal efficiency (P < 0.05). In VFCW3 and VFCW4, low oxygen supply inhibited microbial nitrification in the system and led to insufficient NO3−-N for denitrification, resulting in a low TN removal rate (10–11% lower than that with intermittent aeration). Yang et al. (2017) reviewed the previous results and indicated that the average removal rate of TN in the systems of aeration was greater than the non-aeration systems (97.03 to 90.41%). Similar research results also appeared in the study by Zhou et al. (2017), which concluded that hypoxia inhibited the removal of TN. Compared with VFCW2, removal rate of TN in VFCW1 significantly increased due to the addition of a carbon source (P < 0.05). A significantly higher TN removal rate was also found in VFCW3 than VFCW4 (P < 0.05) (Table 1). The addition of a carbon source promoted the heterotrophic denitrification process, thereby increasing the removal efficiency of TN (Zhi and Ji 2014). Recent findings from Wu et al. (2018) achieved analogous conclusions, suggesting the removal efficiency of TN reached a higher value when the cattail litter was used as an exogenous carbon source. In addition, the removal efficiency of TN under these running VFCWs was greater than those in biochar-intermittent aerated VFCW (53%) (Zhou et al. 2017), the bioaugmentation VFCW (74%) (Zhao et al. 2016), and polyvinyl alcohol immobilized nitrifier CW (43%) (Wang et al. 2016a, b). The highest TN removal efficiency (86.88%) was obtained in VFCW1 (intermittently aerated with carbon source) (Table 1), which verified that carbon source supplement in combination with artificial intermittent aeration enhanced the TN removal efficiency. In the existing studies, there is always a huge difference in the highest TN removal efficiency, which may be caused by various operating conditions, for instance the types of the CWs, climate environment, cultivated plants, and substrate filling materials (Gargallo et al. 2017). Therefore, studies on a wider range of designed continuous water are needed to assess the impact of operational conditions, especially on the effects of aeration combined with carbon source addition on microbial functions, which are associated with the N removal.
COD removal
As showed in Table 1, the DO concentration in VFCW1 (5.75 mg L−1) and VFCW2 (5.58 mg L−1) increased significantly due to the intermittent aeration. In contrast, low DO was detected in VFCW3 (3.54 mg L−1) and VFCW4 (3.20 mg L−1), which resulted in an anaerobic environment with a lack of oxygen supply. Zhou et al. (2017) concluded that DO is a crucial factor in the degradation of organic matters. Figure 3 showed the average COD removal rate and concentration in effluent in four experimental VFCWs. Among the four VFCWs, the COD removal efficiency in intermittent aeration systems (VFCW1 and VFCW2) was much higher than non-intermittent aeration systems (VFCW3 and VFCW4). An adequate supply of oxygen provided by the intermittent aeration led to higher COD removal efficiency. The results resemble with the study conducted by Yang et al. (2017), who investigated that the average concentration of effluent COD was higher in CWs with non-aeration (72.76 mg L−1). In this study, COD removal efficiency was 74.16% in VFCW1, which was greater than that in VFCW2 (62.51%). Similar trends were also observed in VFCW3 (57.82%) and VFCW4 (53.58%). Previous studies have investigated that the removal rate of COD increased when the amount of exogenous carbon source increased in the influent in the VFCW systems (Jia et al. 2018). Therefore, we have confirmed that the addition of a carbon source enhances the removal of COD in the wastewater. Furthermore, we also found that the removal rate of COD in bioaugmentation VFCW was 65% (Zhao et al. 2016), which was lower than that of intermittently aerated external carbon addition VFCW (74%) in this study, indicating the operation conditions in this system are conducive to the removal of organic matters.
Nitrogen concentrations of influent and effluent ((a) NH4+-N, (b) NO3−-N, and (c) TN) in four VFCWs throughout the whole experimental period. VFCW1, aerated with carbon source CW; VFCW2, aerated with non-carbon source CW; VFCW3, non-aerated with carbon source CW; VFCW4, non-aerated with non-carbon source CW
Microorganism communities
Microbial community diversity and abundance
Illumina Miseq high-throughput sequencing was conducted to analyze the impact of aeration modes and carbon source addition on microbial community structure on substrates of biofilm in CWs. The microbial richness (Chao1 index) and diversity (Shannon index and Simpson index) in different systems were shown in Table S2 (Supplementary Information). The observed species represent the number of OTUs in the samples. The observed species and Chao1 index were greater in aeration system (VFCW1 and VFCW2) than corresponding values of non-aeration systems (VFCW3 and VFCW4), which indicated that aeration increased the microbial richness. Moreover, higher Shannon and Simpson indices were also obtained in aeration system, indicating the microbial diversity was facilitated by aeration. Among the two aerated systems, the values of observed species (3569.33), Chao1 index (5435.32), Shannon index (9.62), and Simpson index (0.99) were higher in VFCW1 than the corresponding values of VFCW2 (3438, 5250.37, 9.45, and 0.99), respectively. Similar trends were shown in VFCW3 and VFCW4, which pointed out that carbon sources increase the richness and diversity of microorganisms. In summary, the indices of Shannon, Chao1, and Simpson in these four VFCW systems were significantly higher than those of ofloxacin CWs (Tong et al. 2019) and free water surface CWs (Zheng et al. 2018). It proved that a combination of intermittent aeration and exogenous carbon source was beneficial to microbial growth and the increase of bacterial diversity in the VFCW systems.
Microbial community composition
The identified top 10 bacteria phyla with relative abundance over 1% were illustrated in Fig. 4 to evaluate the impacts of aeration modes and carbon source addition on bacterial community composition. There were three major phyla with different relative abundance that appeared in all systems, in which Proteobacteria was the dominant phylum. The Proteobacteria in each system accounted for 36.98, 46.03, 38.17, and 35.00%, respectively. It has been reported that Proteobacteria played an important role in the transformation and cycling of carbon, N, and sulfur (Ansola et al. 2014; Wang et al. 2016a, b). Actinobacteria accounted for 20.58% in VFCW1, 12.04% in VFCW2, 16.71% in VFCW3, and 15.93% in VFCW4, followed by Acidobacteria which accounted for 10.90, 10.47, 11.10, and 11.01% of the above-mentioned four systems, respectively. Previous investigation indicated that Bacteroidetes and Actinobacteria could produce antibiotics to suppress plant pathogens and promote denitrification (Zhang et al. 2017). Figure 4 showed that the relative abundance of Actinobacteria in carbon source addition systems (VFCW1 and VFCW3) was significantly greater than systems (VFCW2 and VFCW4) with non-carbon source, which indicated that carbon source addition facilitated the growth of heterotrophic denitrifying bacteria. Previous study reported that Acidobacteria had the ability to degrade phosphorus and organics (Wang et al. 2016a, b). Furthermore, it should be noted that in the four systems, Firmicutes dominated higher abundance of 5.68, 3.05, 6.14, and 2.11%, respectively. Chen et al. (2017) disclosed that Firmicutes was a strictly heterotrophic denitrifying bacterium, which led to better NO3−-N removal efficiency in carbon source addition systems (VFCW1 and VFCW3).
N transformation functional genes
The number of functional genes provides information on the number of related microorganisms (Zhang et al. 2018). Throughout the current experiment, the absolute abundance of anammox bacterial (anammox bacterial 16S rRNA), nitrifying bacteria (amoA, nxrA), and denitrifying bacteria (nosZ, nirK, and nirS) were quantified to analyze microorganisms on the surface of the substrates in each VFCW.
Two genes of nxrA and amoA were used to evaluate the microbial abundance which participate in the nitrification process (Dionisi et al. 2002; Hu et al. 2016). AmoA gene codes ammonia monooxygenase, representing the transformation procedure from NH4+-N to NO2−-N (Johnston et al. 2019). Nitrite oxidase (nxrA) gene indicates the process of nitrite oxidation (NO2−-N converted to NO3−-N) (Attard et al. 2010). The absolute abundance of amoA gene was higher in aerated CW systems of VFCW1 and VFCW2 (2.64 × 105 and 3.98 × 105 copies g−1), than non-aerated CW systems of VFCW3 and VFCW4 (1.20 × 105 and 1.63 × 105 copies g−1) (P < 0.05) (Fig. 5a and Table S3). In the study of Yang et al. (2017), the copy number of amoA gene in the limited aeration CW (7.53 × 104 copies g−1) was greater than that of the non-aerated CW (1.25 × 104 copies g−1), indicating that the aeration enhanced microbial activity related to nitrification. The absolute abundance of nxrA displayed similar tendencies to those described by amoA. In aerated systems, the copy numbers of nxrA were 5.78 × 104 and 3.00 × 105 copies g−1, which were greater than the values in non-aerated VFCW3 (2.05 × 104 copies g−1) and VFCW4 (3.27 × 104 copies g−1). Aeration promoted the activity of aerobic microorganisms and increased the abundance of aerobic-nitrifying bacteria, which is consistent with the increase of amoA and nxrA gene in the aeration system. The result was congruent with the previous research, which found that the copy number of amoA and nxrA increased with oxygen supply (Pan et al. 2017). In this study, it was found that the addition of a carbon source (VFCW1 and VFCW3) reduced the absolute abundance of functional genes amoA and nxrA (Table S3). The possible reason was that microbial nitrification was inhibited due to excess oxygen consumption (Chen et al. 2019).
The absolute abundance of related functional genes in each VFCW system: (a) nitrification bacteria (amoA and nxrA); (b) anammox 16S rRNA; (c) denitrification bacteria (nirS, nirK, and nosZ). VFCW1, aerated with carbon source CW; VFCW2, aerated with non-carbon source CW; VFCW3, non-aerated with carbon source CW; VFCW4, non-aerated with non-carbon source CW
Anammox 16S rRNA marks the anammox process which transforms NH4+-N and NO2−-N to N2 by anammox bacteria under anaerobic conditions (Tsushima et al. 2007). Figure 5b showed the highest copy number (2.00 × 107 copies g−1) obtained in VFCW4 (non-aerated with non-carbon source). The absolute abundance of anammox 16S rRNA in non-aerated systems (VFCW3 and VFCW4) was nearly 1 time greater than those in aerated systems (VFCW1 and VFCW3), suggesting anaerobic conditions are more conducive to the anammox process. Li et al. (2019) found that the contribution of anaerobic anammox 16S rRNA with the removal of NH4+-N increased due to the low concentration of DO in the systems. The copy number of anammox 16S rRNA declined as the addition of a carbon source, but the results did not show significant differences between treatments (P > 0.05). Therefore, we concluded that anaerobic anammox bacteria was barely affected by the carbon source addition under aerated or non-aerated operational conditions in this study. Our findings are consistent with the investigation conducted by Hu et al. (2016), who reported that anammox generated mainly in anaerobic area of the VFCWs.
Figure 5c showed the functional genes involved in microbial denitrification (nosZ, nirK, and nirS). NirK and nirS genes implement the nitrite reduction, which convert NO2−-N to N2O and NO (Throbäck et al. 2004; Kandeler et al. 2006). Nitrous oxide reductase (nosZ) gene, an indicator of complete heterotrophic denitrification, transforms N2O and NO to N2 (Henry et al. 2006). This present research displayed that the copy numbers of nosZ, nirK, and nirS were 6.10 × 105, 7.74 × 105, and 2.17 × 106 copies g−1 in VFCW1, 5.50 × 105, 5.55 × 105, and 1.60 × 106 copies g−1 in VFCW2, 7.26 × 105, 7.93 × 105, and 3.07 × 106 copies g−1 in VFCW3, and 6.09 × 105, 5.96 × 105, and 2.53 × 106 copies g−1 in VFCW4, respectively. The results demonstrated that the absolute abundance of nosZ, nirK, and nirS genes in aeration systems was lower than that of the non-aeration systems. Aeration inhibits microbial heterotrophic denitrification due to the changes of anaerobic conditions (Yang et al. 2017). However, due to the addition of carbon sources, the copy number of nosZ, nirK, and nirS genes showed opposite results to the effects of aeration (P < 0.05). The probable reason was that supplementing the exogenous carbon source facilitated the growth of denitrifying bacteria, while the carbon limitation attenuated the denitrifying activity (Hou et al. 2018). Zhi and Ji (2014) recorded analogous conclusions in preceding studies. Furthermore, contrary to the research by Hu et al. (2016), a significantly higher absolute abundance of nosZ gene was obtained in this experiment, which means that complete denitrification may occur.
Relationship between N transformation and microorganisms
To further investigate the relationship between N transformation and microorganisms, an overview of the values of microbial compositions and functional genes was performed by RDA analysis. As Fig. 6 illustrated, the removal efficiency of COD, TN, NH4+-N, and NO3−-N appeared unique distribution between microbial characteristics. The angles between N and functional genes suggested that nosZ, nirK, and nirS were mainly positively associated with NO3−-N and COD removal, but they were negatively correlated with removals of NH4+-N. Moreover, amoA and nxrA were positively correlated with the removal efficiency of NH4+-N and were mainly located in the aeration system VFCW2 (region represented by circles), indicating intermittent aeration promoted nitrification. Arrow position indicate that carbon source system VFCW3 (region represented by diamonds) was mainly associated with Firmicutes and Actinobacteria, which played a crucial role in heterotrophic denitrification process (Chen et al. 2017).
The relationship between N removal and microorganisms revealed by redundancy analysis (down triangles represent VFCW1 with aerated and carbon source; circles represent VFCW2 with aerated and non-carbon source; diamonds represent VFCW3 with carbon source and non-aerated; stars represent VFCW4 with non-carbon source and non-aerated)
Conclusion
The combined impacts of intermittent aeration and exogenous carbon source addition in VFCWs significantly enhanced N and organic matter removal in the current study. Optimal average NH4+-N, TN, NO3–-N, and COD removal efficiency (93.56, 86.88, 79.65, and 74.16%) were obtained in an aerated system accompanied by carbon source addition (VFCW1), which contained the highest richness and diversity of microbial community. The increase in the DO concentration and carbon source elevated the copy number of functional genes in microbial nitrification (nxrA and amoA) and heterotrophic denitrification (nosZ, nirK, and nirS), respectively. Overall, the appropriate combination of artificial intermittent aeration and exogenous carbon source addition is an effective operation to remove N and organics from the VFCW systems.
References
Ansola G, Arroyo P, Sáenz de Miera LE (2014) Characterisation of the soil bacterial community structure and composition of natural and constructed wetlands. Sci Total Environ 473–474:63–71
APHA (2005) Standard methods for the examination of water and wastewater, twenty-firsted. American Public Health Association, Washington, D.C.
Attard E, Poly F, Commeaux C, Laurent F, Terada A, Smets BF, Recous S, Le Roux X (2010) Shifts between Nitrospira- and Nitrobacter-like nitrite oxidizers underlie the response of soil potential nitrite oxidation to changes in tillage practices. Environ Microbiol 12(2):315–326
Chen C, Xu XJ, Xie P, Yuan Y, Zhou X, Wang AJ, Lee DJ, Ren NQ (2017) Pyrosequencing reveals microbial community dynamics in integrated simultaneous desulfurization and denitrification process at different influent nitrate concentrations. Chemosphere 171:294–301
Chen D, Gu X, Zhu W, He S, Huang J, Zhou W (2019) Electrons transfer determined greenhouse gas emissions in enhanced nitrogen-removal constructed wetlands with different carbon sources and carbon-to-nitrogen ratios. Bioresour Technol 285:121313. https://doi.org/10.1016/j.biortech.2019.121313
Ding Y, Wang W, Song X, Wang G, Wang Y (2014) Effect of spray aeration on organics and nitrogen removal in vertical subsurface flow constructed wetland. Chemosphere 117:502–505
Dionisi HM, Layton AC, Harms G, Gregory IR, Robinson KG, Sayler GS (2002) Quantification of Nitrosomonas oligotropha-like ammonia-oxidizing bacteria and Nitrospira spp. from full-scale wastewater treatment plants by competitive PCR. Appl Environ Microbiol 68(1):245–253
Du L, Chen Q, Liu P, Zhang X, Wang H, Zhou Q, Xu D, Wu Z (2017) Phosphorus removal performance and biological dephosphorization process in treating reclaimed water by integrated vertical-flow constructed wetlands (IVCWs). Bioresour Technol 243:204–211
Fan J, Zhang J, Guo W, Liang S, Wu H (2016) Enhanced long-term organics and nitrogen removal and associated microbial community in intermittently aerated subsurface flow constructed wetlands. Bioresour Technol 214:871–875
Faulwetter JL, Gagnon V, Sundberg C, Chazarenc F, Burr MD, Brisson J, Camper AK, Stein OR (2009) Microbial processes influencing performance of treatment wetlands: a review. Ecol Eng 35:987–1004
Gargallo S, Martín M, Oliver N, Hernández-Crespo C (2017) Biokinetic model for nitrogen removal in free water surface constructed wetlands. Sci Total Environ 587–588:145–156
He S, Wang Y, Li C, Li Y, Zhou J (2018) The nitrogen removal performance and microbial communities in a two-stage deep sequencing constructed wetland for advanced treatment of secondary effluent. Bioresour Technol 248:82–88
Henry S, Bru D, Stres B, Hallet S, Philippot L (2006) Quantitative detection of the nosZ gene encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl Environ Microbiol 72(8):5181–5189
Hou J, Wang X, Wang J, Xia L, Zhang Y, Li D, Ma X (2018) Pathway governing nitrogen removal in artificially aerated constructed wetlands—impact of aeration mode and influent chemical oxygen demand to nitrogen ratios. Bioresour Technol 257:137–146
Hu Y, He F, Ma L, Zhang Y, Wu Z (2016) Microbial nitrogen removal pathways in integrated vertical-flow constructed wetland systems. Bioresour Technol 207:339–345
Jia L, Wang R, Feng L, Zhou X, Lv J, Wu H (2018) Intensified nitrogen removal in intermittently-aerated vertical flow constructed wetlands with agricultural biomass: effect of influent C/N ratios. Chem Eng J 345:20–30
Johnston J, Lapara T, Behrens S (2019) Composition and dynamics of the activated sludge microbiome during seasonal nitrification failure. Sci Rep 9:4565. https://doi.org/10.1038/srep4565
Kandeler E, Deiglmayr K, Tscherko D, Bru D, Philippot L (2006) Abundance of narG, nirS, nirK, and nosZ genes of denitrifying bacteria during primary successions of a glacier foreland. Appl Environ Microbiol 72(9):5957–5962
Lai X, Zhao Y, Pan F, Yang B, Wang H, Wang S, He F (2020) Enhanced optimal removal of nitrogen and organics from intermittently aerated vertical flow constructed wetlands: relative COD/N ratios and microbial responses. Chemosphere 244:125556. https://doi.org/10.1016/j.chemosphere.2019.125556
Li F, Lu L, Zheng X, Ngo HH, Liang S, Guo W, Zhang X (2014) Enhanced nitrogen removal in constructed wetlands: effects of dissolved oxygen and stepfeeding. Bioresour Technol 169:395–402
Li B, Chen H, Li N, Wu Z, Wen Z, Xie S, Liu Y (2017) Spatio-temporal shifts in the archaeal community of a constructed wetland treating river water. Sci Total Environ 605–606:269–275
Li J, Hu Z, Li F, Fan J, Zhang J, Li F, Hu H (2019) Effect of oxygen supply strategy on nitrogen removal of biochar-based vertical subsurface flow constructed wetland: intermittent aeration and tidal flow. Chemosphere 223:366–374
Liu X, Zhang Y, Li X, Fu C, Shi T, Yan P (2018) Effects of influent nitrogen loads on nitrogen and COD removal in horizontal subsurface flow constructed wetlands during different growth periods of Phragmites australis. Sci Total Environ 635:1360–1366
Liu F, Fan J, Du J, Shi X, Zhang J, Shen Y (2019) Intensified nitrogen transformation in intermittently aerated constructed wetlands: removal pathways and microbial response mechanism. Sci Total Environ 650:2880–2887
Mesquita MC, Albuquerque A, Amaral R, Nogueira R (2017) Seasonal variation of nutrient removal in a full-scale horizontal constructed wetland. Energy Procedia 136:225–232
Pan J, Qi S, Sun Y, Jiang Y, Zhao N, Huang L, Sun Y (2017) Nitrogen removal and nitrogen functional gene abundances in three subsurface wastewater infiltration systems under different modes of aeration and influent C/N ratios. Bioresour Technol 241:1162–1167
Pelissari C, Guivernau M, Viñas M, De Souza SS, García J, Sezerino PH, Ávila C (2017) Unraveling the active microbial populations involved in nitrogen utilization in a vertical subsurface flow constructed wetland treating urban wastewater. Sci Total Environ 584–585:642–650
Saeed T, Muntaha S, Rashid M, Sun G, Hasnat A (2018) Industrial wastewater treatment in constructed wetlands packed with construction materials and agricultural by-products. J Clean Prod 189:442–453
Tan E, Hsu TC, Huang X, Lin HJ, Kao SJ (2017) Nitrogen transformations and removal efficiency enhancement of a constructed wetland in subtropical Taiwan. Sci Total Environ 601–602:1378–1388
Throbäck IN, Enwall K, Jarvis Å, Hallin S (2004) Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Ecol 49(3):401–417
Tong X, Wang X, He X, Xu K, Mao F (2019) Effects of ofloxacin on nitrogen removal and microbial community structure in constructed wetland. Sci Total Environ 656:503–511
Tsushima I, Kindaichi T, Okabe S (2007) Quantification of anaerobic ammonium-oxidizing bacteria in enrichment cultures by real-time PCR. Water Res 41(4):785–794
Wang Z, Liu C, Liao J, Liu L, Liu Y, Huang X (2014) Nitrogen removal and N2O emission in subsurface vertical flow constructed wetland treating swine wastewater: effect of shunt ratio. Ecol Eng 73:446–453
Wang H, Ji G, Bai X (2015) Enhanced long-term ammonium removal and its ranked contribution of microbial genes associated with nitrogen cycling in a lab-scale multimedia biofilter. Bioresour Technol 196:57–64
Wang W, Ding Y, Wang Y, Song X, Ambrose RF, Ullman JL, Gong J (2016a) Treatment of rich ammonia nitrogen wastewater with polyvinyl alcohol immobilized nitrifier biofortified constructed wetlands. Ecol Eng 94:7–11
Wang P, Zhang H, Zuo J, Zhao D, Zou X, Zhu Z, Jeelani N, Leng X, An S (2016b) A hardy plant facilitates nitrogen removal via microbial communities in subsurface flow constructed wetlands in winter. Sci Rep 6:33600. https://doi.org/10.1038/srep33600
Wu H, Fan J, Zhang J, Ngo HH, Guo W, Hu Z, Liang S (2015) Decentralized domestic wastewater treatment using intermittently aerated vertical flow constructed wetlands: impact of influent strengths. Bioresour Technol 176:163–168
Wu H, Fan J, Zhang J, Ngo HH, Guo W, Hu Z, Liang S (2016) Intensified organics and nitrogen removal in the intermittent-aerated constructed wetland using a novel sludge-ceramsite as substrate. Bioresour Technol 210:101–107
Wu S, Gao L, Gu J, Zhou W, Fan C, He S, Huang J, Zhang X, Cheng Y, Wu Z, Wang Z (2018) Enhancement of nitrogen removal via addition of cattail litter in surface flow constructed wetland. J Clean Prod 204:205–211
Yang Z, Yang L, Wei C, Wu W, Zhao X, Lu T (2017) Enhanced nitrogen removal using solid carbon source in constructed wetland with limited aeration. Bioresour Technol 248:98–103
Zhang H, Wang R, Chen S, Qi G, He Z, Zhao X (2017) Microbial taxa and functional genes shift in degraded soil with bacterial wilt. Sci Rep 7:39911. https://doi.org/10.1038/srep39911
Zhang X, Hu Z, Ngo HH, Zhang J, Guo W, Liang S, Xie H (2018) Simultaneous improvement of waste gas purification and nitrogen removal using a novel aerated vertical flow constructed wetland. Water Res 130:79–87
Zhao X, Yang J, Bai S, Ma F, Wang L (2016) Microbial population dynamics in response to bioaugmentation in a constructed wetland system under 10 °C. Bioresour Technol 205:166–173
Zheng Y, Dzakpasu M, Wang X, Zhang L, Ngo HH, Guo W, Zhao Y (2018) Molecular characterization of long-term impacts of macrophytes harvest management in constructed wetlands. Bioresour Technol 268:514–522
Zhi W, Ji G (2014) Quantitative response relationships between nitrogen transformation rates and nitrogen functional genes in a tidal flow constructed wetland under C/N ratio constraints. Water Res 64:32–41
Zhou X, Wang X, Zhang H, Wu H (2017) Enhanced nitrogen removal of low C/N domestic wastewater using a biochar-amended aerated vertical flow constructed wetland. Bioresour Technol 241:269–275
Zhou X, Wang R, Liu H, Wu S, Wu H (2019) Nitrogen removal responses to biochar addition in intermittent-aerated subsurface flow constructed wetland microcosms: enhancing role and mechanism. Ecol Eng 128:57–65
Zhu H, Yan B, Xu Y, Guan J, Liu S (2014) Removal of nitrogen and COD in horizontal subsurface flow constructed wetlands under different influent C/N ratios. Ecol Eng 63:58–63
Acknowledgments
The study received the support of the National Natural Science Foundation of China (31870606, 41877424, 41801089) and the Natural Science Foundation of Shandong Province, China (ZR2017MD022, ZR2018MD002). It was also supported by the founding project of Jinan Environmental Research Academy (2017214).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alexandros Stefanakis
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Lai, X., Zhao, Y., Pan, F. et al. Enhanced nitrogen removal in filled-and-drained vertical flow constructed wetlands: microbial responses to aeration mode and carbon source. Environ Sci Pollut Res 27, 37650–37659 (2020). https://doi.org/10.1007/s11356-020-09915-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09915-6