Abstract
Acrylamide concentration in food products collected from the Tehran market was investigated by the aid of a dispersive liquid-liquid microextraction (DLLME) system coupled with gas chromatography-mass spectrometry (GC-MS). Also, the dietary exposure distribution and related potential risk for acrylamide ingestion were estimated by the Monte Carlo simulation (MCS). The highest and lowest mean concentration of acrylamide was detected in coffee and roasted nuts samples as 549 and 133 μg/kg, respectively. The mean acrylamide dietary exposure values for children (3–10 years), adolescents (11–17 years), adults (18–60 years), and seniors (61–96 years) were estimated to be 1.81, 1.02, 0.61, and 0.53 μg/kg body weight (BW)/day, respectively. In all age groups, except children, the estimated exposure in men and boys was higher than that in women and girls. Bread, despite containing low acrylamide content groups (157 μg/kg while compared with other, except roasted nuts), showed with the highest contribution rate in all age groups due to its high consumption rate. The estimated incremental lifetime cancer risk (ILCR) for all age groups was noted as greater than 10−4 indicating serious risk to the population. Moreover, the margin of exposure (MOE) values based on carcinogenicity showed health concern to all age groups (< 10,000). Regarding the non-carcinogenic risk, the target hazard quotient (THQ) was lower than 1, and MOE based on neurotoxicity was higher than 125 (safety thresholds), which represented negligible and ignorable risk in all age groups except in a small group of children and adolescents.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
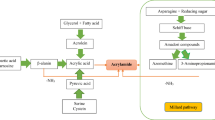
Acrylamide (2-propenamide), as a white crystal and odorless substance with excellent water solubility, is a vinylic compound with low molecular mass (71 Da) (Marconi et al. 2010; Altunay et al. 2016). It is a synthetic compound and, in the structure of polyacrylamide, has widespread applications in different industries such as drinking water purification, textile, cosmetics, paper processing, and mining (Taeymans et al. 2004; Elbashir et al. 2014) as well as synthesizing gel in electrophoresis (Oroian et al. 2015). Acrylamide is generated during heat treatment (like frying, roasting, and baking) of food products rich in proteins and carbohydrates at temperatures greater than 120 °C. It is considered as a Maillard reaction byproduct which is produced through the interaction of reducing sugars (especially glucose and fructose) and the amino acid asparagine (Shahrbabki et al. 2018). However, in the case of raw or boiled foods, no acrylamide has been detected (Amrein et al. 2005; Eerola et al. 2007; Zokaei et al., 2020). The major segments of acrylamide in human diet are potato- and cereal-based products as well as roasted coffee. According to a European Food Safety Authority (EFSA) statement, the acrylamide concentration was found about 23 to 7095 μg/kg in bread and coffee products, respectively (EFSA 2015). Furthermore, moderate (5–50 μg/kg) and higher (approximately 150–4000 μg/kg) amounts of acrylamide were detected in protein and carbohydrate heated foods (Khoshnam et al. 2010). Food and Agriculture Organization/World Health Organization (FAO/WHO) reported that dietary acrylamide exposure could vary between 0.3 and 0.8 μg/kg BW/day (WHO 2002a, b).
Long-term exposure to acrylamide through occupational and accidental pathways can cause some damages in the central and peripheral nervous systems of humans such as neural destructions (Marconi et al. 2010). Furthermore, sufficient evidence regarding neurotoxic, genotoxic, mutagenic, and carcinogenic effects is reported among experimental animals (Pacetti et al. 2015). After ingestion, acrylamide is rapidly absorbed from the gastrointestinal tract and translocated to all tissues and organs all over the body, and it can even enter into breast milk. Subsequently, in the liver, acrylamide oxidizes to an active epoxide named glycidamide (GA) via the CYP 450 2E1 enzymatic system. GA is considered as the main cancer-inducing metabolite of acrylamide (Alpözen and Üren 2013; Ayvaz and Rodriguez-Saona 2015; Kim et al. 2015). In this regard, the International Agency for Research on Cancer (IARC) classified acrylamide as a probable carcinogenic compound to humans (class 2A) (IARC 1994). Also, the WHO and the European Union (EU) established the upper residue limit of 0.5 and 0.1 μg/kg for acrylamide content in drinking water, respectively (Lim and Shin 2014). There is no permitted legal limit for acrylamide concentration in food products, although the EU set a benchmark level for some food products like potato chips and French fries which are 1000 and 600 μg/kg, respectively (Pacetti et al. 2015).
Several studies have been focused on investigating the formation, concentration, toxicology, and health dietary risk assessment of acrylamide in different food products (Dybing and Sanner 2003; Hogervorst et al. 2007; Hogervorst et al. 2008; Pelucchi et al. 2011; Olsen et al. 2012). Quantitative dietary acrylamide risk assessment is one of the most important methodologies for estimating exposure through diet and the probability of carcinogenic and non-carcinogenic effects of acrylamide in a defined population. The risk assessment findings may also provide the required information for risk managers to make effective decisions and some actions like establishing the mitigation strategies (Xu et al. 2014). Additionally, three different methods are mainly used to evaluate exposure to acrylamide which consist of the point estimate (deterministic) procedure, the semi-probabilistic method, and the probabilistic modeling named Monte Carlo simulation (MCS) (Skog and Alexander 2006). The latter one is the most promising model due to its capability in identifying uncertainties and variabilities in the long-term exposure estimations as well as in performing risk predictions that are used extensively in food science (Shahrbabki et al. 2018).
Therefore, the aim of this study was to investigate acrylamide concentration in the main heat-treated food products collected from Tehran’s market. Furthermore, for the first time, the acrylamide dietary exposure in Tehran, Iran, was estimated using the Monte Carlo simulation. The carcinogenic and non-carcinogenic risks also were assessed by the aid of the margin of exposure (MOE), incremental lifetime cancer risk (ILCR), and target hazard quotient (THQ) for different age groups through dietary exposure to acrylamide. Additionally, sensitivity analyses were performed to characterize the accuracy of important input variables on predicted risks.
Materials and methods
Sample collection
Different food samples, in total, 195 samples of food products including 25 samples of traditional and industrial bread, 42 samples of other bakery products (cakes and biscuits), 26 samples of confectionery products (pastries and chocolates), 50 samples of snacks (potato-based and corn-based), 16 samples of coffee powder, 24 samples of roasted nuts (almonds, pistachios, hazelnuts, peanuts, and edible seeds), and 12 samples of fast foods (pizza, hamburger, and sausage), were collected in 2017–2018 from randomly selected local supermarkets, stores, and restaurants all over Tehran. The samples were transferred to the laboratory and preserved at a refrigerator until the experiments. All experiments were done in triplicate.
Acrylamide measurement
Pretreatment section
Regarding differences in sample types, the samples of bread, bakery, and confectionery products were dried in room temperature and then milled using a grinder to get a homogenous refined powder. For samples rich in fat (like snacks, roasted nuts, some fast foods, and bakery products), after grinding, 3 mL of n-hexane (in 2 stages) was added for removing fat and was shook thoroughly and finally, the upper phase was discarded.
Sample preparation
This step was accomplished based on a former work (Nematollahi et al. 2019). Briefly, 1 g of each food sample was weighed and 200 μL of acetamide (internal standard) was added. In the next step, 10 mL of potassium hydroxide (1 M):ethanol (80:20) was added. After shaking and centrifuging at 2683g for 5 min, the upper clear aqueous phase was separated, and then its pH value was decreased to 3 with HCl. Then, 1 mL of potassium hexaferrocyanide (Carrez solution I) and 1 mL of zinc acetate (Carrez solution II) were added to precipitate proteins and carbohydrates. After shaking and centrifuging another time, the supernatant was separated. Then, 2 mL of HCl (1 M) and 60 μL of xanthydrol were added for derivatization of acrylamide. This solution was shaken completely for 5 min and was stored in darkness at an ambient temperature for 30 min to complete the derivatization procedure. After that, 2 mL of K2HPO4 (2 M) and 2 mL of KOH (2 M) were added, and the pH value of the samples was changed to approximately 7. Finally, the solution was centrifuged at 2683.2g for 5 min, and the upper cloudless aqueous part was separated. In dispersive liquid-liquid microextraction (DLLME) stage, 80 μL of extracting solvent (tetrachloroethylene) and 300 μL of dispersing solvent (ethanol) were used. After shaking and centrifuging at 2683.2g for 5 min, the extracting solvent containing acrylamide is gathered at the bottom of the volumetric flask. Finally, 1 μL of this part is introduced to the GC-MS.
A 7890A GC system from Agilent Technologies (Palo Alto, CA, USA) with a triple-axis detector equipped with a split/splitless injector is used for GC-MS analyses. This system was coupled with a 5975C inert MSD network mass selective detector. Separation of chemical substances was carried out by an HP-5 MS capillary column with 5% phenyl siloxane/95% methyl polyorganosiloxane, with an interior diameter and film thickness of 30 m × 0.25 mm and 0.25 μm, respectively. The oven temperature program was isothermal at 100 °C for 1 min, risen to 300 °C at 20 °C min−1, and again isothermal for 10 min. Helium (He) gas was applied as a carrier gas at a stable flow rate of 0.8 mL min−1. The injector and auxiliary temperature were adjusted at 290 °C and 280 °C, respectively. One microliter of the bottom phase of the solution was introduced in a splitless manner with a split proportion of 1:50. The selected ion monitoring (SIM) achievement form was utilized for the measurement of acrylamide and acetamide (internal standard). The quantification procedure was performed by supervising peaks at m/z 207 and 234 for acrylamide and 180 and 239 for acetamide. The retention times for acrylamide and acetamide were 10.27 and 9.95 min, respectively, and the ratio of acrylamide/acetamide peak area was used in the calibration curve to calculate acrylamide concentration in the samples. Good separation and clean chromatogram are presented in Fig. 1 in the absence of food matrix interference.
Method validation
The present method (DLLME-GC-MS) was validated by considering linearity, recovery, repeatability (RSD%), enrichment factor (EF), limit of detection (LOD), and limit of quantitation (LOQ) under experimental conditions. The calibration curve showed the linearity at the range of 5–500 ng mL−1. The correlation coefficient (R2) calculated approximately 0.998. The LOD and LOQ were defined as a chromatographic signal at the lowest concentration of the analyte that can be shown 3 and 10 times higher than the background noise, respectively. LOD and LOQ were observed as 0.6 ng mL−1 and 2 ng mL−1, respectively. The enrichment factor is defined as the ratio between the concentration of the compound in the organic phase and the initial concentration in the aqueous solution. The EF of the recommended procedure was 116. The repeatability (RSDs), calculated based on comparative peak areas of six replicate extraction methods from snack samples and the associated RSD, was calculated to be 4.5–12% while the reproducibility obtained was lower than 8%. The recovery percentage of acrylamide was determined to be 95% by measuring up the level of acrylamide standard added to the samples (50 ng g−1) with the level after the microextraction process. Also, a standard addition method has been used to approve the efficiency of the proposed method.
Food intake data
To assess the Tehran population acrylamide dietary exposure, food intake data was taken from “The Tehran Lipid and Glucose Study (TLGS).” This program is a society-based forthcoming (cohort) study, accomplished on a sample of populations in 13 regions of Tehran, Iran (Mirmiran et al. 2011). It firstly planned to preclude non-communicable diseases (NCDs) using a plan to support beneficial lifestyles and decrease the risk aspects of noted diseases, started in 1999, and standard follow-up was carried out every 3 years. Of 7291 individuals (3354 men and 3937 women) aged 3–96 years, the participants of the Sixth TLGS Program Survey were randomly used for dietary assessment. These individuals consisted of 228 children (3–10 years), 412 adolescents (11–17 years), 5439 adults (18–60 years), and 1212 seniors (61–96 years). The research method used was the Food Frequency Questionnaire (FFQ) containing approximately 150 food items in the period from 2017 April to April 2018. The food consumption rate was extracted in grams/person/day.
Acrylamide dietary exposure estimation
Dietary exposure to acrylamide was estimated for different age groups in both genders (children, adolescents, adults, and seniors) in the Tehran population. Acrylamide dietary exposure (DE) expressed as microgram/kilogram BW/day for each age group was calculated as follows:
where Ci is the concentration of acrylamide in food i (μg/kg), IRi is the intake rate of food i (g/day) for each age group, and BW is the body weight (kg) of each person according to the age groups. Ci, IRi, and BW, which all followed log-normal distribution, were used for acrylamide dietary exposure calculation by a probabilistic approach (Monte Carlo simulation program) (Eq. 1) (Abtahi et al. 2018; Nabizadeh et al. 2018). Based on food consumption and acrylamide concentration for each food group (i), dietary exposure of food group (i) was calculated, then summed and divided by BW × 1000 (correction factor) to achieve a reliable distribution for total food groups (ED). For each age category, one Monte Carlo simulation with 10,000 repetitions was done.
Carcinogenic risk evaluation
To evaluate carcinogenic risk correlated to acrylamide dietary exposure, the margin of exposure (MOE) approach based on experimental animal data has been used, although it is a common approach in risk characterization for substances that are both carcinogenic and genotoxic. For the carcinogenic effect of acrylamide, the Joint FAO/WHO Expert Committee on Food Additive (JECFA) suggested two distinct values of benchmark dose lower limit for a 10% increase in tumor initiation (BMDL10) as 310 μg/kg BW/day for initiation of mammary tumors in female rats and 180 μg/kg BW/day for Harderian gland tumors in mice (FAO/WHO 2011), whereas the EFSA used the values of BMDL10 170 μg/kg BW/day for neoplastic outcomes in mice (EFSA 2015). Thus, for assessing a risk description of carcinogenic effects by acrylamide exposure, the MOE (dimensionless), based on Eq. 2, was calculated for all age groups:
where MOE (dimensionless) is the margin of exposure, DE is the acrylamide dietary exposure (μg/kg BW/day), and BMDL10 is the benchmark dose lower limit 10% (μg/kg BW/day) (Fakhri et al. 2018; Atamaleki et al. 2019; Fakhri et al. 2019a, b; Gholami et al. 2019).
Furthermore, the incremental lifetime cancer risk (ILCR) proposed by the Environmental Protection Agency (EPA) related to acrylamide dietary exposure was calculated based on Eq. 3:
where ILCR (dimensionless) is the additional possibility of cancer over human lifetime, Ci is the concentration of acrylamide in food i (μg/kg), IRi is the intake rate of food i (g/day) for each age group, BW is the body weight (kg) of each person according to age groups, ED is the exposure duration (year) (for children, adolescents, adults, and seniors; ED = 8, 7, 43, and 36, respectively), EF is the exposure frequency (365 days/year), SF is the slope factor of acrylamide (0.5 per [(mg/kg)/day], CF is the correction factor (10−6 mg/μg), ADAF is the age-dependent adjustment factor (dimensionless) (ADAF for 3–15 years is 3 and for ages greater than 16 is 1 (EPA 2010)), and AT is the average time for carcinogens (25,550 days). We considered Ci, IRi, and BW as log-normal distributions in Eq. 3 probabilistically.
Non-carcinogenic risk evaluation
MOE, also, for non-carcinogenic effects is used. A value of BMDL10 of 430 μg/kg BW/day was proposed by EFSA for neurotoxicity (peripheral neuropathy in rats) (EFSA 2015). MOE for neurotoxicity effect is calculated by Eq. 2.
The target hazard quotient (THQ) is also used for non-carcinogenic risk of dietary acrylamide exposure by Eq. 4 (Ali et al. 2019; Fakhri et al. 2019a, b):
where THQ (dimensionless) is the target hazard quotient, DE is the acrylamide dietary exposure (μg/kg/day), and RFD is the oral reference dose for acrylamide (2 μg/kg/day) (EPA 2010).
Uncertainty analysis
The Monte Carlo simulation (MCS) method with 10,000 iterations using the Crystal Ball (version 7.2., Oracle) was performed to calculate the uncertainties in the risk characterization step. The estimated results of acrylamide dietary exposure and the related risk assessment showed the effects of input parameters on the risk estimation (Shahsavani et al. 2017; Fathabad et al. 2018; Keramati et al. 2018; Rahmani et al. 2018). So, a sensitivity evaluation was carried out to find the most effective input factors on the estimated results (exposure and risk) (Dadar et al. 2017). Rank correspondence between every input parameter and the estimated risk (output) was assessed, and afterward by squaring the risk variance and standardizing it to 100%, the effect of each input parameter on the output variance was calculated.
Results and discussion
Concentrations of acrylamide in foods
The concentrations of acrylamide in 195 food samples purchased from the Tehran market are illustrated in Fig. 2. Based on the food groups, the highest concentration of acrylamide was found in coffee samples (549 μg/kg), followed by potato- and corn-based snacks (465 μg/kg), confectionery products (367 μg/kg), bakery products (199 μg/kg), fast foods (192 μg/kg), and bread (157 μg/kg), whereas the lowest acrylamide level was observed in roasted nuts (133 μg/kg).
The mean content of acrylamide ranged widely from 33 to 2029 μg/kg product. Figure 2 shows considerable variations even between different food types and brands of the same group. These observed differences were similarly reported by many studies in Poland, the Netherlands, and Sweden (Konings et al. 2003; Svensson et al. 2003; Mojska et al. 2010), whereas in these studies, the highest level of acrylamide was found in potato chips. The calculated acrylamide concentration in this study was similar to that of the EFSA report while the highest concentration of acrylamide was discovered in coffee substitute (1499 μg/kg) and coffee powder (522 μg/kg). High acrylamide amount, also, was detected in snacks (389 μg/kg) and other fried potato products (308 μg/kg) (EFSA 2015).
Acrylamide levels in snack groups ranged from 116 to 1183 μg/kg while the corresponding values found in potato chips and French fries were 510.63 μg/kg and 496.16 μg/kg, respectively. However, based on previous investigations, among corn-based snack samples, the concentration of acrylamide in potato-based food products such as French fries and potato chips was the highest (Konings et al. 2003; Mojska et al. 2010; Sirot et al. 2012) which can be correlated with the presence of high levels of acrylamide reactants (reducing sugars and asparagine) in potato and the use of high temperatures for processing such as frying. The variations in the concentrations of acrylamide among most studies can be associated with the type and variety of potato, as well as processing time and temperature. It is reported that acrylamide content in European countries has decreased in potato chips as a popular snack from 2002 to 2011 that declined from 763 to 358 μg/kg (EFSA 2015).
Moreover, the acrylamide content in bread presented a wide range from 77 to 415 μg/kg (Fig. 2), an indication that the amount of acrylamide in traditional flatbreads (169 μg/kg) was higher than that in industrial bread (97 μg/kg) due to using flour with a high extraction rate and using higher temperatures in the processing of the traditional bread (Nematollahi et al. 2019). In Iran, traditional bread types such as Sangak and Barbari are more popular than leavened industrial types. In many studies, the acrylamide level in different types of bread reported was lower than that in the current investigation mainly due to differences in bread as well as the methods used for baking (Mojska et al. 2010; EFSA 2015). Similarly, acrylamide concentrations in bread from Syria were measured to be 119 to 263 μg/kg (Alyousef et al. 2016), while the average acrylamide levels in flatbread investigated in Italy and Turkey were higher than those of the current study (230 and 225 μg/kg, respectively) (Cengiz and Gündüz 2013). However, the acrylamide concentration of bread samples in Poland was lower than that of our study (3.6–163 μg/kg) (Roszko et al. 2020).
In the case of other bakery products, the acrylamide content was reported in a range of 89 to 584 μg/kg, while in the case of wafers, biscuits, crackers, cakes, and cookies, the acrylamide content levels were determined to be 234, 211, 191, 177, and 156 μg/kg, respectively (Fig. 2). These diversities in acrylamide content levels could be attributed to differences in ingredients and processing procedures, especially baking time and temperatures (Nematollahi et al. 2019). Some similar findings were reported by EFSA except in the case of wafers and biscuits (201 μg/kg), crackers (231 μg/kg), and cakes (66 μg/kg) (EFSA 2015).
A range from 117 to 2029 μg/kg for acrylamide concentration was associated with confectionery products, including different kinds of pastries and chocolate (Fig. 2), which can be correlated with the variations in their formulation (using ingredients rich in acrylamide precursors such as embryo of wheat grain), high temperature employed in their production, and processing methods. A similar result regarding the acrylamide content in pastry products collected from a Poland market was demonstrated previously (198 μg/kg) (Mojska et al. 2010).
A range of 33 to 251 μg/kg nominated to acrylamide concentrations in roasted nuts was demonstrated (Fig. 2), while the concentrations of acrylamide in roasted almonds, pistachios, peanuts, edible seeds, and hazelnuts were determined to be 177, 145, 131, 108, and 91 μg/kg, respectively. A few number of studies considering the acrylamide concentration in roasted nuts were published. Roasting as a heating process results in the improvement of flavor, color, and texture of these kinds of products. The highest amount of acrylamide in roasted almonds could be attributed to the presence of higher amounts of asparagine in comparison to other nuts (Nematollahi et al. 2020). Our results were similar to some of the previous investigations such as those of Chen et al. (2008).
Regarding coffee, a range of 106 to 1710 μg/kg was reported based on different brands of coffee samples (Fig. 2), while the acrylamide level in coffee samples of Syria (114–164 μg/kg) was lower (Alyousef et al. 2016), whereas in Romany, a higher level from 48 to 6968 μg/kg in an average of 1115 μg/kg was reported (Oroian et al. 2015). According to an EFSA report, an average acrylamide content of 522 μg/kg was demonstrated (EFSA 2015). These differences could be related to types of coffee and differences in roasting time and temperatures.
The acrylamide concentrations in fast foods including hamburgers, sausages, and pizzas were detected to be 287, 165, and 123 μg/kg, respectively, with a range of 50–386 μg/kg (Fig. 2). In Italy, the measured acrylamide concentration in hamburgers and pizzas was similar to the present study (90–250 μg/kg and 127 μg/kg, respectively) (Altissimi et al. 2017), while a notable difference regarding the corresponding values in the Netherlands was noted (less than 15 μg/kg) (Konings et al. 2003). Similar to other food products, these differences are derived from divergences in ingredients used in formulation and processing operations.
Acrylamide dietary exposure
The mean intake rate of 7 food groups containing acrylamide in different age groups of the Tehran population is presented in Table 1. The highest and lowest intake rates (g/day) in all age groups were observed for bread and coffee, respectively. The highest amounts of bread, fast foods, snacks, and roasted nuts consumption (g/day) were demonstrated in adolescents with an average of 182.23, 27.33, 25.23, and 6.50 g/day, respectively. However, children 3–10 years had the highest consumption rate in the case of bakery (32.32 g/day) and confectionery (8.12 g/day) products. The highest coffee consumption rate (1.79 g/day) was reported for adults.
To estimate acrylamide dietary exposure, two types of data were employed: concentration data (Fig. 2) and consumption data (Table 1). The estimated dietary exposure to acrylamide for each age group of the Tehran population is presented in Table 2, while a significant difference (P < 0.05) was observed among the 4 studied age groups. As it is clear, the highest and lowest acrylamide exposure was estimated for adolescents (55.06 μg/day) and seniors (37.11 μg/day), respectively. As shown in Table 1, adolescents consume the highest amount of food containing acrylamide while compared with other groups. After calculating the acrylamide dietary exposure (μg/kg BW/day), the highest and lowest estimated acrylamide dietary exposure was attributed to children (1.81 μg/kg BW/day) and seniors (0.53 μg/kg BW/day), respectively. The average acrylamide dietary exposure values for adolescents and adults were estimated to be 1.02 and 0.61 μg/kg BW/day, respectively, which can be associated with lower body weight of children and adolescents besides the higher consumption rate of food containing acrylamide. Our results, also, showed that in all age groups except children, acrylamide dietary exposure was significantly (P < 0.05) higher in boys and the male population compared with girls and the female population, in good agreement with EFSA reports, which reported that acrylamide dietary exposure levels (P95) for adults and children were reported to be 0.2–6.2 μg/kg BW/day and 1.4–5.2 μg/kg BW/day, respectively (EFSA 2015), while the monitoring studies carried out by the European Commission from 2007 showed that acrylamide dietary intake for mean and high consumers (P90–P99) estimated to be 0.3–2 and 0.6–5.1 μg/kg BW/day, respectively (EFSA 2012). Similarly, Mills et al. (2008) reported that acrylamide dietary exposure values for English and Irish adults were 0.61 and 0.59 μg/kg BW/day, respectively (Mills et al. 2008), although Zajac et al. (2013) estimated the mean and high acrylamide dietary exposure to be 0.85 and 1.70 μg/kg BW/day, respectively (Zajac et al. 2013). Higher amounts were estimated for Turkish and Lebanese (Ölmez et al. 2008; Hariri et al. 2015). In contrast, in several investigations, the estimated acrylamide dietary exposure was lower compared with the current investigation (Svensson et al. 2003; Chen et al. 2008; Mojska et al. 2010; Altissimi et al. 2017).
These differences in reported acrylamide dietary exposure among different countries could be correlated with the divergences in the concentration of acrylamide in various foodstuffs. Another important reason is differences in diet habits and traditional food processing methods among different cultures. Figure 3 exhibits the contribution percentage of each food group in the dietary exposure to acrylamide in 4 studied age groups of the Tehran population. In all age groups, bread contributed with the highest share proportion owing to the highest consumption rate. Share percentages of bread in children, adolescents, adults, and seniors were calculated as 49%, 53%, 63%, and 75%, respectively. Coffee, despite showing the highest amount of acrylamide, due to the lowest consumption rate per day, in all age groups, supplied only 1–2% share proportion. After bread, snacks (potato- and corn-based) occupied the second rank with given share percentage in the range of 6% for seniors to 21% for children. Confectionery and bakery products showed a similar contribution proportion in all age groups except for children (5–9% for each product). The contribution rate of bakery products in this group was approximately twofold (15%) compared with confectionery products. In contrast, in Norway, coffee supplied the highest contribution percentage, followed by potato products, completely vice versa with this study (Dybing and Sanner 2003). In the USA, most acrylamide intake resulted from potato chips, French fries, and bread (EFSA 2012). In Canada, also, 50% of the contribution rate were associated with French fries (Normandin et al. 2013). In China, surprisingly, vegetable products with 54% share percentage were the most important acrylamide source in food in 2014, while, similar to the present study, the share percentage of flour products in acrylamide exposure was higher than that of other products (53%) followed by potato products (20%) (Chen et al. 2008). In Turkey, similar to this study, bread supplied approximately 50% of the share percentage in children (Cengiz and Gündüz 2013). Furthermore, the acrylamide dietary exposure in the Polish population through bread was 31% in the group of children, and 49% in the adults followed by crisps and French fries supplied 14% exposure to acrylamide around adults (> 19 years) and in both children and adolescents (7–18 years) reported 42%. Furthermore, unlike this study, in the Polish population, coffee was an important source of acrylamide dietary exposure (27% in adults) except for children (1–6 years) (Mojska et al. 2010).
Risk assessment
The incremental lifetime cancer risk (ILCR) was estimated to evaluate the extra possibility of human cancer during life (70 years for carcinogenicity). The cumulative probability and frequency distributions of ILCR for all age categories in Tehran are presented in Fig. 4. The mean values of ILCR in children, adolescents, adults, and seniors were predicted to be 3.1 × 10−4, 1.3 × 10−4, 1.89 × 10−4, and 1.37 × 10−4, respectively. For high consumers (P95), the estimated ILCR values were 7.07 × 10−4, 2.79 × 10−4, 4.17 × 10−4, and 3.17 × 10−4 for noted age groups, respectively.
An ILCR of 10−6 or fewer indicates the level of risk is acceptable and insignificant, an ILCR more than 10−4 implies serious health risk, and an ILCR between 10−6 and 10−4 shows potential and considerable risk (Xia et al. 2010). Therefore, according to estimated ILCR, a serious risk in all age groups particularly in children was noted.
Results of Dybing and Sanner (2003) compelled with those of our study (6 × 10−4). Chen et al. (2012) reported the ILCR of approximately 10−5–10−6 for acrylamide intake through fried potato products in adolescents 13–18 years (Chen et al. 2012). Eslami et al. (2018) demonstrated a serious risk for children and adults due to potato Tah-dig consumption (ILCR more than 10−3 and 10−4, respectively) (Shahrbabki et al. 2018).
MOE using BMDL10 reported by EFSA and JECFA was based on tumor induction in mice and rats, while in the current study, it is calculated to evaluate the carcinogenic risk of acrylamide intake (FAO/WHO 2011; EFSA 2015). Figure 5 displays the estimated MOE based on (a) BMDL10 = 170 μg/kg BW/day, (b) BMDL10 = 180 μg/kg BW/day, and (c) BMDL10 = 310 μg/kg BW/day based on neoplastic effects in mice, Harderian gland tumors in male mice, and mammary tumors in female rats, respectively. As shown in Fig. 5, MOE values related to carcinogenicity effects in experimental animals within all of the age groups are far lower than the safety limit of 10,000. The reported results of several published studies were similar to the values of the present study (Sirot et al. 2012; Normandin et al. 2013; Wyka et al. 2015; Esposito et al. 2017).
The non-carcinogenic risk of dietary acrylamide exposure was calculated by the MOE values based on BMDL10 = 430 μg/kg BW/day recommended by EFSA. Furthermore, THQ based on a reference dose of 2 μg/kg BW/day for acrylamide was calculated to assess the non-carcinogenic risk. The estimated MOE and THQ for the Tehran population are presented in Fig. 6. If the values of MOE for neurotoxicity are lower than 125 (safety threshold) and THQ values are higher than 1, a potential concern and considerable risk of non-carcinogenicity can be expected (EFSA 2015; Shahrbabki et al. 2018).
As illustrated in Fig. 6 for all age groups, the estimated MOE and THQ were higher and lower than safety concerns (125 and 1, respectively) except for P95 children and adolescents. Therefore, the non-carcinogenic risk (neurotoxicity) of acrylamide dietary exposure in the Tehran population is negligible. Our results are in good agreement with previous studies (Sirot et al. 2012; Esposito et al. 2017).
Sensitivity analysis
Figure 7a–d shows the sensitivity analysis results concerning the impressions of food intake, acrylamide concentration, and people’s body weight on estimated ILCR for children (3–10 years), adolescents (11–17 years), adults (18–60 years), and seniors (61–96 years), respectively. The most effective variable that contributed to the estimated risk is the intake rate in all age groups. The effect of intake rate in adults and seniors (61–65.1%) is more than that in children and adolescents (48.1–48.3%). In children and adolescents, BW has the most influence after intake rate (27.6–36%), whereas in adults and seniors, acrylamide concentration is in the second place concerning effect (24.9–26%). Therefore, based on the sensitivity analysis, the accuracy and precision of the health risk assessment could be expanded by developing the accuracy of intake rate, concentration, and body weight, particularly the accuracy of intake rate.
Conclusion
Acrylamide concentration was determined in 7 important groups of heated foods collected from Tehran’s market, while the mean contents of acrylamide ranged from 33 to 2029 μg/kg. Acrylamide dietary exposure distributions are estimated by the Monte Carlo simulation for all age groups of the Tehran population. The highest and lowest acrylamide exposure is calculated for children and seniors to be 1.81 and 0.53 μg/kg/day, respectively. Obviously, the estimated dietary exposure to acrylamide is approximately threefold in children compared with adults, due to their lower weight. Bread, despite having low amounts of acrylamide, is the most important contributor in all age groups (more than 50%). Our results showed that all age groups are in serious carcinogenic risk while the risk of non-carcinogenicity is negligible except in a small group of children and adolescents (P95). Concerning health due to acrylamide dietary exposure, especially in children, there is a need for further control and modify diet habits and food processing to lower acrylamide exposure mainly through Iranian traditional bread.
References
Abtahi M, Fakhri Y, Oliveri Conti G, Ferrante M, Taghavi M, Tavakoli J, Heshmati A, Keramati H, Moradi B, Amanidaz N (2018) The concentration of BTEX in the air of Tehran: a systematic review-meta analysis and risk assessment. Int J Environ Res Public Health 15(9):1837
Ali S, Fakhri Y, Golbini M, Thakur SK, Alinejad A, Parseh I, Shekhar S, Bhattacharya P (2019) Concentration of fluoride in groundwater of India: a systematic review, meta-analysis and risk assessment. Groundw Sustain Dev 100224
Alpözen E, Üren A (2013) Determination of acrylamide levels of “Izmir gevregi” and effects of cooking parameters on acrylamide formation. J Agric Food Chem 61(30):7212–7218
Altissimi MS, Roila R, Branciari R, Miraglia D, Ranucci D, Framboas M, Haouet N (2017) Contribution of street food on dietary acrylamide exposure by youth aged nineteen to thirty in Perugia, Italy. Ital J Food Saf 6(3)
Altunay N, Gürkan R, Orhan U (2016) A preconcentration method for indirect determination of acrylamide from chips, crackers and cereal-based baby foods using flame atomic absorption spectrometry. Talanta 161:143–150
Alyousef HA, Wang H, Al-Hajj NQM, Koko MY (2016) Determination of acrylamide levels in selected commercial and traditional foods in Syria. Trop J Pharm Res 15(6):1275–1281
Amrein TM, Lukac H, Andres L, Perren R, Escher F, Amadò R (2005) Acrylamide in roasted almonds and hazelnuts. J Agric Food Chem 53(20):7819–7825
Atamaleki A, Yazdanbakhsh A, Fakhri Y, Mahdipour F, Khodakarim S, Khaneghah AM (2019) The concentration of potentially toxic elements (PTEs) in the onion and tomato irrigated by wastewater: a systematic review; meta-analysis and health risk assessment. Food Res Int 125:108518
Ayvaz H, Rodriguez-Saona LE (2015) Application of handheld and portable spectrometers for screening acrylamide content in commercial potato chips. Food Chem 174:154–162
Cengiz MF, Gündüz CPB (2013) Acrylamide exposure among Turkish toddlers from selected cereal-based baby food samples. Food Chem Toxicol 60:514–519
Chen F, Yuan Y, Liu J, Zhao G, Hu X (2008) Survey of acrylamide levels in Chinese foods. Food Addit Contam 1(2):85–92
Chen M-J, Hsu H-T, Lin C-L, Ju W-Y (2012) A statistical regression model for the estimation of acrylamide concentrations in French fries for excess lifetime cancer risk assessment. Food Chem Toxicol 50(10):3867–3876
Dadar M, Adel M, Nasrollahzadeh Saravi H, Fakhri Y (2017) Trace element concentration and its risk assessment in common kilka (Clupeonella cultriventris caspia Bordin, 1904) from southern basin of Caspian Sea. Toxin Rev 36(3):222–227
Dybing E, Sanner T (2003) Risk assessment of acrylamide in foods. Toxicol Sci 75(1):7–15
Eerola S, Hollebekkers K, Hallikainen A, Peltonen K (2007) Acrylamide levels in Finnish foodstuffs analysed with liquid chromatography tandem mass spectrometry. Mol Nutr Food Res 51(2):239–247
EFSA (2012) Update on acrylamide levels in food from monitoring years 2007 to 2010. EFSA J 10(10):2938
EFSA (2015) Scientific opinion on acrylamide in food. EFSA J 13(6):4104
Elbashir AA, Omar MMA, Ibrahim WAW, Schmitz OJ, Aboul-Enein HY (2014) Acrylamide analysis in food by liquid chromatographic and gas chromatographic methods. Crit Rev Anal Chem 44(2):107–141
EPA, U. (2010). Toxicological review of acrylamide (CAS no. 79-06-1) in support of summary Information on the Integrated Risk Information System (IRIS). EPA/635/R-07/009F, US EPA Washington, DC
Esposito F, Nardone A, Fasano E, Triassi M, Cirillo T (2017) Determination of acrylamide levels in potato crisps and other snacks and exposure risk assessment through a margin of exposure approach. Food Chem Toxicol 108:249–256
Fakhri Y, Mohseni-Bandpei A, Conti GO, Ferrante M, Cristaldi A, Jeihooni AK, Dehkordi MK, Alinejad A, Rasoulzadeh H, Mohseni SM (2018) Systematic review and health risk assessment of arsenic and lead in the fished shrimps from the Persian gulf. Food Chem Toxicol 113:278–286
Fakhri Y, Ghorbani R, Taghavi M, Keramati H, Amanidaz N, Moradi B, Nazari SH, Shariatifar N, Khaneghah AM (2019a) Concentration and prevalence of aflatoxin M1 in human breast milk in Iran: systematic review, meta-analysis, and carcinogenic risk assessment: a review. J Food Prot 82(5):785–795
Fakhri Y, Rahmani J, Oliveira CAF, Franco LT, Corassin CH, Saba S, Rafique J, Khaneghah AM (2019b) Aflatoxin M1 in human breast milk: a global systematic review, meta-analysis, and risk assessment study (Monte Carlo simulation). Trends Food Sci Technol 88:333
FAO/WHO (2011). "Evaluation of certain contaminants in food: seventy-second [72nd] report of the Joint FAO/WHO Expert Committee on Food Additives."
Fathabad AE, Shariatifar N, Moazzen M, Nazmara S, Fakhri Y, Alimohammadi M, Azari A, Khaneghah AM (2018) Determination of heavy metal content of processed fruit products from Tehran’s market using ICP-OES: a risk assessment study. Food Chem Toxicol 115:436–446
Gholami Z, Abtahi M, Golbini M, Parseh I, Alinejad A, Avazpour M, Moradi S, Fakhri Y, Mousavi Khaneghah A (2019) The concentration and probabilistic health risk assessment of nitrate in Iranian drinking water: a case study of Ilam city. Toxin Rev:1–10
Hariri E, Abboud MI, Demirdjian S, Korfali S, Mroueh M, Taleb RI (2015) Carcinogenic and neurotoxic risks of acrylamide and heavy metals from potato and corn chips consumed by the Lebanese population. J Food Compos Anal 42:91–97
Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA (2007) A prospective study of dietary acrylamide intake and the risk of endometrial, ovarian, and breast cancer. Cancer Epidemiol Biomarkers Prev 16(11):2304–2313
Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA (2008) Dietary acrylamide intake and the risk of renal cell, bladder, and prostate cancer. Am J Clin Nutr 87(5):1428–1438
IARC (1994). "IARC monographs on the evaluation of carcinogenic risks to humans." Lyon, France 60: 389–433
Keramati H, Ghorbani R, Fakhri Y, Khaneghah AM, Conti GO, Ferrante M, Ghaderpoori M, Taghavi M, Baninameh Z, Bay A (2018) Radon 222 in drinking water resources of Iran: a systematic review, meta-analysis and probabilistic risk assessment (Monte Carlo simulation). Food Chem Toxicol 115:460–469
Khoshnam F, Zargar B, Pourreza N, Parham H (2010) Acetone extraction and HPLC determination of acrylamide in potato chips. J Iran Chem Soc 7(4):853–858
Kim TH, Shin S, Kim KB, Seo WS, Shin JC, Choi JH, Weon K-Y, Joo SH, Jeong SW, Shin BS (2015) Determination of acrylamide and glycidamide in various biological matrices by liquid chromatography–tandem mass spectrometry and its application to a pharmacokinetic study. Talanta 131:46–54
Konings E, Baars A, Van Klaveren J, Spanjer M, Rensen P, Hiemstra M, Van Kooij J, Peters P (2003) Acrylamide exposure from foods of the Dutch population and an assessment of the consequent risks. Food Chem Toxicol 41(11):1569–1579
Lim H-H, Shin H-S (2014) A new derivatization approach with d-cysteine for the sensitive and simple analysis of acrylamide in foods by liquid chromatography–tandem mass spectrometry. J Chromatogr A 1361:117–124
Marconi O, Bravi E, Perretti G, Martini R, Montanari L, Fantozzi P (2010) Acrylamide risk in food products: the shortbread case study. Anal Methods 2(11):1686–1691
Mills C, Tlustos C, Evans R, Matthews W (2008) Dietary acrylamide exposure estimates for the United Kingdom and Ireland: comparison between semiprobabilistic and probabilistic exposure models. J Agric Food Chem 56(15):6039–6045
Mirmiran P, Hosseini-Esfahani F, Jessri M, Mahan LK, Shiva N, Azizi F (2011) Does dietary intake by Tehranian adults align with the 2005 dietary guidelines for Americans? Observations from the Tehran Lipid and Glucose Study. J Health Popul Nutr 29(1):39
Mojska H, Gielecińska I, Szponar L, Ołtarzewski M (2010) Estimation of the dietary acrylamide exposure of the Polish population. Food Chem Toxicol 48(8):2090–2096
Nabizadeh S, Shariatifar N, Shokoohi E, Shoeibi S, Gavahian M, Fakhri Y, Azari A, Khaneghah AM (2018) Prevalence and probabilistic health risk assessment of aflatoxins B 1, B 2, G 1, and G 2 in Iranian edible oils. Environ Sci Pollut Res 25(35):35562–35570
Nematollahi A, Kamankesh M, Hosseini H, Ghasemi J, Hosseini-Esfahani F, Mohammadi A (2019) Investigation and determination of acrylamide in the main group of cereal products using advanced microextraction method coupled with gas chromatography-mass spectrometry. J Cereal Sci 87:157
Nematollahi A, Kamankesh M, Hosseini H, Hadian Z, Ghasemi J, Mohammadi A (2020) Investigation and determination of acrylamide in 24 types of roasted nuts and seeds using microextraction method coupled with gas chromatography–mass spectrometry: central composite design. J Food Measur Charact 14:1–12
Normandin L, Bouchard M, Ayotte P, Blanchet C, Becalski A, Bonvalot Y, Phaneuf D, Lapointe C, Gagné M, Courteau M (2013) Dietary exposure to acrylamide in adolescents from a Canadian urban center. Food Chem Toxicol 57:75–83
Ölmez H, Tuncay F, Özcan N, Demirel S (2008) A survey of acrylamide levels in foods from the Turkish market. J Food Compos Anal 21(7):564–568
Olsen A, Christensen J, Outzen M, Olesen PT, Frandsen H, Overvad K, Halkjær J (2012) Pre-diagnostic acrylamide exposure and survival after breast cancer among postmenopausal Danish women. Toxicology 296(1–3):67–72
Oroian M, Amariei S, Gutt G (2015) Acrylamide in Romanian food using HPLC-UV and a health risk assessment. Food Additives & Contaminants: Part B 8(2):136–141
Pacetti D, Gil E, Frega NG, Álvarez L, Dueñas P, Garzón A, Lucci P (2015) Acrylamide levels in selected Colombian foods. Food Additives & Contaminants: Part B 8(2):99–105
Pelucchi C, Galeone C, Talamini R, Negri E, Polesel J, Serraino D, La Vecchia C (2011) Dietary acrylamide and pancreatic cancer risk in an Italian case–control study. Ann Oncol 22(8):1910–1915
Rahmani J, Fakhri Y, Shahsavani A, Bahmani Z, Urbina MA, Chirumbolo S, Keramati H, Moradi B, Bay A, Bjørklund G (2018) A systematic review and meta-analysis of metal concentrations in canned tuna fish in Iran and human health risk assessment. Food Chem Toxicol 118:753–765
Roszko MŁ, Szczepańska M, Szymczyk K, Rzepkowska M (2020) Dietary risk evaluation of acrylamide intake with bread in Poland, determined by two comparable cleanup procedures. Food Addit Contam Part B Surveill 13(1):1–9
Shahrbabki PE, Hajimohammadi B, Shoeibi S, Elmi M, Yousefzadeh A, Conti GO, Ferrante M, Amirahmadi M, Fakhri Y, Khaneghah AM (2018) Probabilistic non-carcinogenic and carcinogenic risk assessments (Monte Carlo simulation method) of the measured acrylamide content in Tah-dig using QuEChERS extraction and UHPLC-MS/MS. Food Chem Toxicol 118:361–370
Shahsavani A, Fakhri Y, Ferrante M, Keramati H, Zandsalimi Y, Bay A, Hosseini Pouya SR, Moradi B, Bahmani Z, Mousavi Khaneghah A (2017) Risk assessment of heavy metals bioaccumulation: fished shrimps from the Persian Gulf. Toxin Rev 36(4):322–330
Sirot V, Hommet F, Tard A, Leblanc J-C (2012) Dietary acrylamide exposure of the French population: results of the second French Total Diet Study. Food Chem Toxicol 50(3–4):889–894
Skog K, Alexander J (2006) Acrylamide and other hazardous compounds in heat-treated foods. Publishing, Woodhead
Svensson K, Abramsson L, Becker W, Glynn A, Hellenäs K-E, Lind Y, Rosen J (2003) Dietary intake of acrylamide in Sweden. Food Chem Toxicol 41(11):1581–1586
Taeymans D, Wood J, Ashby P, Blank I, Studer A, Stadler RH, Gonde P, Eijck P, Lalljie S, Lingnert H (2004) A review of acrylamide: an industry perspective on research, analysis, formation, and control. Crit Rev Food Sci Nutr 44(5):323–347
WHO (2002a) FAO/WHO consultation on the health implications of acrylamide in food. Summary report of a meeting held in Geneva, 25–27 June 2002. World Health Organization, Geneva
WHO (2002b) Health implications of acrylamide in food: report of a Joint FAO/WHO Consultation, WHO Headquarters, Geneva, Switzerland, 25-27 June 2002. World Health Organization, Geneva
Wyka J, Tajner-Czopek A, Broniecka A, Piotrowska E, Bronkowska M, Biernat J (2015) Estimation of dietary exposure to acrylamide of Polish teenagers from an urban environment. Food Chem Toxicol 75:151–155
Xia Z, Duan X, Qiu W, Liu D, Wang B, Tao S, Jiang Q, Lu B, Song Y, Hu X (2010) Health risk assessment on dietary exposure to polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Sci Total Environ 408(22):5331–5337
Xu Y, Cui B, Ran R, Liu Y, Chen H, Kai G, Shi J (2014) Risk assessment, formation, and mitigation of dietary acrylamide: current status and future prospects. Food Chem Toxicol 69:1–12
Zajac J, Bojar I, Helbin J, Kolarzyk E, Potocki A, Strzemecka J, Owoc A (2013) Dietary acrylamide exposure in chosen population of South Poland. Ann Agric Environ Med 20(2):351
Zokaei M, Kamankesh M, Abedi AS, Moosavi MH, Mohammadi A, Rezvani M et al (2020) Reduction in acrylamide formation in potato crisps: application of extract and hydrocolloid-based coatings. J Food Prot 83(5):754–761
Acknowledgments
This research has been assisted by the Department of Food Science and Technology, National Nutrition and Food Technology Research Institute, Faculty of Nutrition Sciences, Food Science and Technology, and Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. We thankfully acknowledge their support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nematollahi, A., Kamankesh, M., Hosseini, H. et al. Acrylamide content of collected food products from Tehran’s market: a risk assessment study. Environ Sci Pollut Res 27, 30558–30570 (2020). https://doi.org/10.1007/s11356-020-09323-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09323-w