Abstract
The critical issue generated by foaming in wastewater treatment plants (WWTPs) is a problem that is currently very common and shared, but which to date is treated mainly only at the management level. In this work, an experimental study with foam tests on real and synthetic waters was conducted using a laboratory scale plant and foaming power indices were calculated. To date, the estimation of foaming potential is mainly based on these indices which give information only on height/volume of foams but not on the type of foams, in terms of consistency and therefore stability. Tests showed that foaming power indices were highly variable with the same water: it was not possible to identify a single foaming potential value for each water. Two models were proposed to estimate the percentage increase in height of chemical foams produced following the introduction of air below the surface of a liquid. In terms of determination coefficient, the results obtained from the complex model were better: R2 was 0.82 for the simple linear model and 0.90 for the complex one. This approach has allowed to underline some critical aspects of foaming potential as it is determined today and the possible improvements applicable for a more objective evaluation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Studies on the formation of chemical and biological foaming in conventional activated sludge (CAS) plants are carried out towards the end of the 1960s (Jenkins et al. 2004; Fryer and Gray 2012; Capodici et al. 2014). Excessive foam formation in biological wastewater treatment plants (WWTPs) is a common and shared problem but it can lead to serious management difficulties and above all to the lowering of purification yields (Capodici et al. 2014). As already expressed for drinking water (Sorlini et al. 2015a, 2015b, 2019), the same approach should also be applied for WWTPs in order to optimize the currently existing processes. This would allow to cope with the increasingly presence of industrial contaminants in wastewater (WW) (Roy et al. 2018; Collivignarelli et al. 2019a) but also have a good quality sludge suitable for reuse in agriculture (Collivignarelli et al. 2015, 2019b; Ashekuzzaman et al. 2019). In order to optimize WWTP management, foam formation, quantification, and removal must be investigated. Foam is a set of stable bubbles, produced when air or other gases are introduced below the surface of the liquid which expands to enclose the gas with a liquid film called “lamellae” of the foam (Heard et al. 2008). As already widely discussed in the scientific literature (Davenport et al. 2000, 2008; De Los Reyes and Raskin 2002; Schilling and Zessner 2011; Capodici et al. 2014), foams can be chemical (white foams) or biological (brown foams). The formation of chemical foams is mainly due to the presence of synthetic surfactants and other surface-active compounds, i.e., synthetic detergents, fats, oils, greases but also biosurfactants (Blackall et al. 1991). For instance, in biological treatment, high concentration of methylene blue active substances produces foams and has a toxic effect on microorganisms, depolarizes the bacterial cell wall and reacts with the enzymes and other proteins essential to the proper functioning of bacterial cells (Aloui et al. 2009; Collivignarelli et al. 2019c).
Instead, the biological foams are generated following the growth of filamentous bacteria in the activated sludge and/or the presence of extracellular polymeric substances (EPS) (Nakajima and Mishima 2005; Di Bella et al. 2011; Petrovski et al. 2011; Di Bella and Torregrossa 2013; Capodici et al. 2014). Studies carried out in CAS processes have shown that the formation of biological foams is associated mainly with filamentous bacterial populations of Nocardioforms and Microthrix parvicella (Iwahori et al. 1997; Di Bella et al. 2011). Their proliferation is induced by WW properties, such as fats or oil content, and operational aspects such as high retention times of both sludge and sewage to be treated, low dissolved oxygen concentrations, high temperatures, low food/biomass ratio, high mixed-liquor suspended solid concentrations (Oerther et al. 2001; Jenkins et al. 2004; Xie et al. 2007; Di Bella et al. 2011). The EPSs are hydrophobic substances which have properties of surface-active agents, of both protein and polysaccharide nature, excreted by microorganisms, produced from cell lysis, and adsorbed organic matter from WW (Nakajima and Mishima 2005; Sheng et al. 2010; Capodici et al. 2014).
The phenomenon of biological and chemical foam formation in WWTPs is a problem that negatively affects the management of the processes and the quality of the treated effluent (Capodici et al. 2014; Collivignarelli et al. 2020). This operative problem occurs simultaneously in both aerobic and CAS processes (aeration basins and clarifier) (Davenport et al. 2008; Di Bella et al. 2011; Guo et al. 2015), in thermophilic aerobic digesters (Collivignarelli et al. 2017) and in the anaerobic processes of biogas production (Jiang et al. 2018), such as mesophilic anaerobic sludge digesters (Subramanian and Pagilla 2015). In the CAS treatment plants, the foams appear on the surface of the aeration basins in the form of stable air bubbles enclosed in a liquid film (Capodici et al. 2014). On the surface of the aeration basin, at the mixed liquor-air interface where the foam is concentrated, there is a high concentration of suspended solids inside the foams. The biomass is retained by the foam bubbles and this causes a decrease in the concentration of biomass inside the mixed liquor. Therefore, the biological reactor decreases its ability of remove pollutants (Pujol et al. 1991; Fryer and Gray 2012). The primary consequence is a worsening of the quality of the effluent and a formation of bad smells due to the excessive formation of foams (Di Bella et al. 2011). The discomfort generated by foaming in WWTPs is a problem that is currently very common and shared, but which to date is treated mainly only at a management level and still too little studied through a shared scientific method. The determination of volume/height of foams is not enough to assess the problematic organically and systematically. It is also necessary to dwell on the type of foams that has formed since foams of different consistency and stability cause different problems. Foam stability, defined by Blackall et al. (1991), as the foam collapse time after the stopping of aeration. A more labile foam (which is destroyed faster) is objectively less alarming from a management point of view.
The purpose of this study is to examine and investigate the problem of chemical foams through the methodological approach of research. Starting from the calculation of foaming potential, real and synthetic waters were compared and subsequently, an applicative model for the quantification of foams was proposed and validated. Through the model, it was possible to confirm the different weights of the variables present in the foaming potential formula. This finding is very significant considering that in literature, the information on this aspect is almost absent. This approach has allowed to underline (i) some critical aspects of foaming potential, as it is determined today, (ii) the possible improvements applicable for a more objective evaluation and (iii) the need to provide an evaluation method that can be used as a management tool for WWTPs.
Materials and methods
Description of the pilot plant
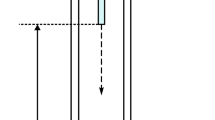
Usually, to evaluate the formation of foams simulating the conditions of aeration in CAS treatment plants, a series of foamability tests are carried out (Di Bella and Torregrossa 2013). The experimentation was performed with the plant represented in Fig. 1, applying the aeration method already used in literature by Patist et al. (1998); Fryer et al. (2011); Jiang et al. (2018). The sample was inserted into a graduated plexiglass cylinder (35 mm of internal diameter and 1000 mm height) positioned vertically and fitted with a porous bottom stone. To generate foams, a predetermined air flow rate as introduced from the bottom of the cylinder for a specific interval of aeration time using an air pump (25 W) coupled with a flowmeter.
Characteristics of waters and operative conditions
The behavior of two real waters (RWs) and three synthetic waters (SWs) with different concentrations of sodium dodecyl sulfate (SDS) (80 mg L−1, 120 mg L−1, and 160 mg L−1) were tested. It is an anionic surfactant and is a known foaming agent (Folmer and Kronberg 2000). In the following experiments, it was used as a “reference white” due to its marked foaming effect and because it consists of known molecular composition. The operative parameters of the experimental tests and the characteristics of tested waters, including the chemical oxygen demand (COD), are reported in Table 1.
Experimental tests and data processing methods
The initial height reached by the liquid in the cylinder (H0) was measured and the air flow has been kept constant for the chosen aeration time. After turning off the air diffuser, the level of the foam-liquid interface (H1) and the maximum level reached by the foams (H2) were measured. The last measure of H1 corresponded to H0. The different heights are represented in Fig. 1.
Foam power
The foaming power test proposed by Nakajima and Mishima (2005) allowed to establish the foaming power index (FP1) as the consumed sample volume (mL) from foaming by 1 L of aeration through the Eq. (1):
where:
- H0:
-
the level of the interface between air and water before aeration (cm)
- H1:
-
the level of the interface between water and foams after an aeration interval ΔT (cm)
- S:
-
the area of the cylinder (cm2)
- Q:
-
the aeration flow rate (Lair min−1)
- ΔT:
-
the aeration period (min)
This approach has also been used by Capodici et al. (2014), Di Bella et al. (2011), and Di Bella and Torregrossa (2013) to assess the foaming potential of membrane bioreactor activated sludge.
Fryer and Gray (2012), and later Jiang et al. (2018), proposed an alternative approach to the quantification of foaming potential. Instead of evaluating the lowering of the foaming liquid interface, the height reached by foams in the column following aeration (H2) was measured in (cm). In this case, the foaming power index, called FP2, can be quantified using Eq. (2):
Applicative model
The study has been developed based on a two-level factorial experiment to evaluate the influence of some operative parameters on foam formation, especially those that appear in the equations of foaming potential. In order to quantify the foams with a value of management interest, the percentage difference between the level of the interface reached by foams at the end of the aeration (H2) and the initial level of the interface between air and water (H0) has been calculated as follows (Eq. 3):
In order to bypass the interferences caused by different molecules, the model was developed referring to a SW with SDS. The foamability of a sample (as the volume of foams generated during the test) depends on the composition of the liquid to be tested, on temperature, and foam generation method (Fryer et al. 2011). Therefore, for the development of the factorial, the experimentation focused on four operative parameters: the interface level between the air and the water before aeration (H0), the aeration time (t), the aeration flow normalized to the base surface of the cylinder (Q/A), and the SDS concentration of the solution (SDS). The correlation between the increasing of the real percentage increase of the height of foams (∆HR) and the estimated values (∆HE) has been investigated by two linear models.
The simple linear model was evaluated with no constant: the hypothesis was ∆HE = ∆HR for ∆HR = 0. The following linear model was applied (Eq. 4):
where Q/A is the aeration flow divided by the cylinder base surface [NL min−1 cm−2], t is the aeration time [min], H0 is the level of the interface between air and water before the aeration [cm], SDS is the concentration of SDS surfactant in the solution [mg L−1], and a [min cm2 NL−1], b [min−1], c [cm−1], and d [L mg−1] are the weights of the above operative parameters. Furthermore, a more complex linear model was proposed (Eq. 5). In this case, the correlation between the two- and three-level operative parameters is taken into consideration.
where a [min cm2 NL−1], b [min−1], c [cm−1], d [L mg−1], e [cm2 NL−1], f [min cm NL−1], g [min cm2 mg−1], h [min−1 cm−1], i [L mg−1 min−1], l [L mg−1 cm−1], m [cm NL−1], n [cm2 mg−1], o [L min−1 cm−1 mg−1], and p [cm min mg−1] represent the weights of the operative parameters.
The parameters of both linear and non-linear models were optimized minimizing the squared error sum (∆HE − ∆HR)2. Each variable of the model was also normalized to assume a value between 0 and 1. The normalization was made by dividing each variable by the greater value assumed by the variable itself. In this way, at the end of the model development, the normalized parameters were obtained.
In order to (i) evaluate the variability of the FP indices around the mean value obtained from the tests and (ii) make a comparison in absolute terms between the parameters of the simple linear model, a continuous probability distribution has been calculated. The normal distribution is characterized by the probability density function reported by Eq. (6). The probabilities of a normal distribution depend only on the values assumed by the two parameters μ (average value of the sample of data) and σ (standard deviation of the sample of data). The distribution is symmetric with respect to μ and is asymptotic to the axis on both sides. The average value is the center of the distribution and characterizes the position of the curve with respect to the ordinate axis. As the average changes, the curve translates along the abscissa axis, but its shape remains unchanged. The σ parameter characterizes the shape of the curve as it represents the dispersion of the values around the maximum of the curve, as σ changes, the curve changes its shape by flattening or rising.
Results and discussion
Foam potential
Real waters
The two RWs have completely different chemical characteristics and consequently, they produce qualitatively different foams:
-
RW1: considerable formation of very stable foams that can easily reach the maximum height of the pilot plant and which slowly collapse.
-
RW2: formation of less thick and much fewer stable foams. The suspension of ventilation leads to a rapid collapse of foams.
The results of the foaming power tests, which were conducted on both RWs samples, are shown in Fig. 2. The foaming potential should be an intrinsic quantity to the water; therefore, for each tested water, a single value associated with it should be found. Considering the same water, it was possible to observe a significant variability of values of the calculated foaming power indices, both for FP1 (Fig. 2a) and FP2 (Fig. 2b). For both RWs tested, in order to evaluate the dispersion of data samples, the normal probability distribution is shown in Fig. 2, also reporting the number of tests carried out for each type of water. For RW1, the confidence interval was 133 ± 39 mL Lair−1 and 883 ± 199 mL Lair−1, respectively for FP1 and FP2. For RW2, the results for FP1 and FP2 were the following: 71 ± 25 mL Lair−1 and 759 ± 94 mL Lair−1. In both cases, FP2 showed a more marked variability than FP1. The RW1 had a mean value of FP1 about twice that of RW2. For FP2, the average values of the two RWs were comparable. Based on these results, it is not immediate and intuitive to define and distinguish the real behaviors of RW1 and RW2. Changing the operative conditions (such as the aeration time or the airflow), with the same water, identifying a single foaming potential value for each water was not possible. This aspect was related to the weight of the parameters of the equations used to calculate the indices and it is better explained below with reference to SWs.
Foaming power of RWs: FP1 (a) and FP2 (b) indices. Boxplots represent the distance between the first and third quartiles, while whiskers are set as the most extreme (lower and upper) data point not exceeding 1.5 times the quartile range from the median. Black curves represent the normal distributions of the data. (n number of tests)
Synthetic waters
In Fig. 3, foaming power trends (FP1 and FP2 indices) of solutions with different SDS concentrations are reported. Changing the concentration of surfactant during the experimental tests, SW produced foams of different consistency and stability. As in the case of tests carried out on RWs, the experimental results showed how the foaming potential calculated with the indices FP1 (Fig. 3a) and FP2 (Fig. 3b) was very variable keeping constant the concentration of surfactant. For each SDS solution tested, the normal probability distribution is shown in Fig. 3 with the aim of assessing the dispersion of the data sample. The number of tests carried out is also indicated. For each SW, confidence intervals were determined. The results by increasing SDS concentration were the following: 30 ± 4 mL Lair−1, 27 ± 4 mL Lair−1, 37 ± 4 mL Lair−1 and 406 ± 22 mL Lair−1, 417 ± 37 mL Lair−1, 502 ± 30 mL Lair−1, for FP1 and FP2 respectively. Comparing the solutions with different concentrations of SDS, there was no significant variation in the mean value, for both the indices used. As with real waters RWs described above, it was not possible to diversify the different solutions of surfactant (80 mg L−1, 120 mg L−1 e 160 mg L−1) based on the results obtained on the FP indices. This result was related with the aspect that the foaming potential could depend differently on the air flow rate, and the aeration time was not considered. Instead, in the proposed equations of FP1 and FP2, all variables had the same weight equal to 1. Therefore, an applicative model has been proposed (i) to define the real weight of these variables and (ii) to provide a tool that can be used as a management instrument for WWTPs.
Foaming power of SWs: FP1 (a) and FP2 (b) indices. Boxplots represent the distance between the first and third quartiles, while whiskers are set as the most extreme (lower and upper) data point not exceeding 1.5 times the quartile range from the median. Black curves represent the normal distributions of the data. (n number of tests
Applicative model
In this section, the results of a two-level factorial experiment are shown to study the influence of the operative parameters considered (shown in Table 1) on the height reached by foams following aeration. For the determination of a reliable model, a large number of data were used, corresponding to as many laboratory tests (n = 180). These data (∆HR) referred to different operative conditions of sample volume (level of interface between air and water before aeration), air flow rate, aeration time, and SDS concentration of the solution, with the variation of a single variable in turn. The purpose of this model was to assess the effects of the tested operative parameters (model variables) on ΔH. It was important to analyze the weight of each variable in order to understand which operative parameters most influenced the phenomenon studied. The water used for the development of the model was a solution of surfactant SDS, with different SDS concentration, as shown in Table 1.
The results of the simple linear model (Eq. 4) and of the complex linear model (Eq. 5) are reported in Table 2.
In order to define the real weight of the variables on ΔH after aeration, all data used for the models have been normalized (Table 2).
In Fig. 4, the correlation between ∆HR and ∆HE is shown for both models. For the linear model described by Eq. (4), the slope of the interpolation line was about 0.97 (Fig. 4a). This result suggested that the operative parameters taken into consideration took an important role in the foaming phenomenon. The linear determination coefficient (R2) to evaluate the goodness of adaptation of the linear model to the observed data has been defined. For construction it varied from zero to one, expressing a good degree of linear adaptation if its value was close to one. R2 was 0.82 for the simple model (Fig. 4a).
Regarding the more complex model (Eq. 5), the slope of the interpolation line was near 0.98 and R2 was equal to 0.90 (Fig. 4b). In terms of R2, the results obtained from the complex model were better than the first model. Considering the interpretation problems related to the second model and the too high number of variables with respect to the experimental data available, it is preferable to use the simple linear model, which can already provide a reliable estimate of ∆H under defined operating conditions.
The calculated values of the weights (a, b, c, and d) in the simple linear model showed the positive effects on the value of ∆H of the aeration flow rate and of the aeration time. As the concentration of SDS surfactant in the solution increased, there was a small increase in ∆H because the corresponding coefficient “d” assumed a value much lower than 1. Therefore, the greater dependence of ∆H was associated with the aeration flow rate. It was also observed that as the level of the interface between air and water before aeration increased, there was a decrease in ∆H.
The normal or Gaussian distribution was used to analytically define how the values assumed by the variables of the simple linear model were distributed. The symbol f(X) was used to denote the mathematical expression of the probability density function and X was the value assumed by the parameters a, b, c, and d of the simple linear model (Fig. 5). Each normal distribution was obtained by calculating the parameters based on random groups of data. Each point of the curves corresponds to a causal set of tests. From each of these groups of experimental tests, the values of the parameters and the normal distributions were calculated.
The skewness and kurtosis of the distributions are shown in Table 3. The skewness was negative for all distributions. The distribution of the parameter “c” had a skewness close to zero; therefore, there was a marked symmetry (a perfectly symmetrical data set has a skewness of 0 as the normal distribution). The dataset relative to the last parameter was lower symmetrical, having a skewness approximately equal to − 2. For the distributions “a” and “b,” the kurtosis was close to zero because they had all the data in each tail as they did in the peak. A kurtosis was less than zero for the “c” distribution and assumed a high positive value for the distribution of the parameter “d”.
Conclusions
In this work, an experimental study with quantitative tests on chemical foams produced by RWs and SWs was conducted using a pilot plant on the laboratory scale. The indices of foaming power suggested by literature present several critical aspects.
As demonstrated in this paper, FPs give only information on how many foams are produced in terms of height or volume but not on consistency and stability of foam. Therefore, FPs should not be used individually to define the foaming potential of water. Moreover, considering that FPs of the same water were very variable changing operative conditions, identify a single foaming potential value for each water was not possible. This problem was related with the weight of the parameters of the equations used to calculate the indices.
The proposed alternative linear model showed that keeping constant the concentration of surfactant in the tested solution, the quantity of foams produced (and the FP) was not a constant value that describes the solution but a function mainly related to the aeration flow rate, the aeration time, and the initial height before aeration (a = 0.748 min cm2 NL−1, b = 0.458 min−1 and c = − 0.646 cm−1). In terms of determination coefficient, the results obtained from the complex model were better (0.90) compare with the linear ones (0.82): the use of the simple linear model, which can already provide a reliable estimate of the level reached by foams, is suggested. Starting from the total absence of a model, the simple linear one proposed in this work, represents the first step for the future development of models that take into consideration several variables, such as the viscosity of the fluid, the density of the fluid, and the concentration of others surfactants.
The future goal could be to identify a method for assessing the foaming potential that: (i) best represents the intrinsic nature of the parameter and (ii) it is simple to calculate and quick to apply, especially in view of easy applicability from a management and operative point of view for WWTPs.
Abbreviations
- CAS:
-
Conventional activated sludge
- COD:
-
Chemical oxygen demand
- EPS:
-
Extracellular polymeric substances
- FP1:
-
Foaming power index calculated as consumed sample per liter of supplied air
- FP2:
-
Foaming power index calculated as foam volume produced per liter of supplied air
- R 2 :
-
Determination coefficient
- RW:
-
Real water
- SDS:
-
Sodium dodecyl sulfate
- SW:
-
Synthetic water
- WW:
-
Wastewater
- WWTP:
-
Wastewater treatment plant
- ∆HR :
-
Real percentage increase of the height of foams
- ∆HE :
-
Estimated percentage increase of the height of foams
References
Aloui F, Kchaou S, Sayadi S (2009) Physicochemical treatments of anionic surfactants wastewater: effect on aerobic biodegradability. J Hazard Mater 164:353–359. https://doi.org/10.1016/j.jhazmat.2008.08.009
Ashekuzzaman SM, Forrestal P, Richards K, Fenton O (2019) Dairy industry derived wastewater treatment sludge: generation, type and characterization of nutrients and metals for agricultural reuse. J Clean Prod 230:1266–1275. https://doi.org/10.1016/j.jclepro.2019.05.025
Blackall LL, Harbers AE, Hayward AC, Greenfield PF (1991) Activated sludge foams: effects of environmental variables on organism growth and foam formation. Environ Technol (United Kingdom) 12:241–248. https://doi.org/10.1080/09593339109385001
Capodici M, Di Bella G, Nicosia S, Torregrossa M (2014) Effect of chemical and biological surfactants on activated sludge of MBR system: microscopic analysis and foam test. Bioresour Technol 177:80–86. https://doi.org/10.1016/j.biortech.2014.11.064
Collivignarelli MC, Abbà A, Padovani S et al (2015) Recovery of sewage sludge on agricultural land in lombardy: current issues and regulatory scenarios. Environ Eng Manag J 14:1477–1486. https://doi.org/10.30638/eemj.2015.159
Collivignarelli MC, Castagnola F, Sordi M, Bertanza G (2017) Sewage sludge treatment in a thermophilic membrane reactor (TMR): factors affecting foam formation. Environ Sci Pollut Res 24:2316–2325. https://doi.org/10.1007/s11356-016-7983-4
Collivignarelli MC, Abbà A, Carnevale Miino M, Arab H, Bestetti M, Franz S (2019a) Decolorization and biodegradability of a real pharmaceutical wastewater treated by H2O2-assisted photoelectrocatalysis on TiO2 meshes. J Hazard Mater 387:121668. https://doi.org/10.1016/j.jhazmat.2019.121668
Collivignarelli MC, Canato M, Abbà A, Carnevale Miino M (2019b) Biosolids: what are the different types of reuse? J Clean Prod 238:117844. https://doi.org/10.1016/j.jclepro.2019.117844
Collivignarelli MC, Carnevale Miino M, Baldi M, Manzi S, Abbà A, Bertanza G (2019c) Removal of non-ionic and anionic surfactants from real laundry wastewater by means of a full-scale treatment system. Process Saf Environ Prot 132:105–115. https://doi.org/10.1016/j.psep.2019.10.022
Collivignarelli MC, Baldi M, Abbà A, Caccamo FM, Carnevale Miino M, Rada EC, Torretta V (2020) Foams in wastewater treatment plants: from causes to control methods. Appl Sci 10:2716. https://doi.org/10.3390/app10082716
Davenport RJ, Curtis TP, Goodfellow M, Stainsby FM, Bingley M (2000) Quantitative use of fluorescent in situ hybridization to examine relationships between mycolic acid-containing actinomycetes and foaming in activated sludge plants. Appl Environ Microbiol 66:1158–1166. https://doi.org/10.1128/AEM.66.3.1158-1166.2000
Davenport RJ, Pickering RL, Goodhead AK, Curtis TP (2008) A universal threshold concept for hydrophobic mycolata in activated sludge foaming. Water Res 42:3446–3454. https://doi.org/10.1016/j.watres.2008.02.033
De Los Reyes FL, Raskin L (2002) Role of filamentous microorganisms in activated sludge foaming: relationship of mycolata levels to foaming initiation and stability. Water Res 36:445–459. https://doi.org/10.1016/S0043-1354(01)00227-5
Di Bella G, Torregrossa M (2013) Foaming in membrane bioreactors: identification of the causes. J Environ Manag 128:453–461. https://doi.org/10.1016/j.jenvman.2013.05.036
Di Bella G, Torregrossa M, Viviani G (2011) The role of EPS concentration in MBR foaming: analysis of a submerged pilot plant. Bioresour Technol 102:1628–1635. https://doi.org/10.1016/j.biortech.2010.09.028
Folmer BM, Kronberg B (2000) Effect of surfactant-polymer association on the stabilities of foams and thin films: sodium dodecyl sulfate and poly(vinyl pyrrolidone). Langmuir 16:5987–5992. https://doi.org/10.1021/la991655k
Fryer M, Gray NF (2012) Foaming Scum Index (FSI) - A new tool for the assessment and characterisation of biological mediated activated sludge foams. J Environ Manag 110:8–19. https://doi.org/10.1016/j.jenvman.2012.05.009
Fryer M, Eoghan OF, Gray NF (2011) Evaluating the measurement of activated sludge foam potential. 424–444. https://doi.org/10.3390/w3010424
Guo F, Wang ZP, Yu K, Zhang T (2015) Detailed investigation of the microbial community in foaming activated sludge reveals novel foam formers. Sci Rep 5:1–9. https://doi.org/10.1038/srep07637
Heard J, Harvey E, Johnson BB, Wells JD, Angove MJ (2008) The effect of filamentous bacteria on foam production and stability. Colloids Surf B: Biointerfaces 63:21–26. https://doi.org/10.1016/j.colsurfb.2007.10.01
Iwahori K, Taki H, Miyata N, Fujita M (1997) Analysis of Nocardia amarae profiles in actual foaming activated sludge plant with viable cell count measurement. J Ferment Bioeng 84:98–102. https://doi.org/10.1016/S0922-338X(97)82795-X
Jenkins D, Richard MG, Daigger GT (2004) Manual on the causes and control of activated sludge bulking, foaming, and other solids separation problems
Jiang C, Qi R, Hao L, McIlroy SJ, Nielsen PH (2018) Monitoring foaming potential in anaerobic digesters. Waste Manag 75:280–288. https://doi.org/10.1016/j.wasman.2018.02.021
Nakajima J, Mishima I (2005) Measurement of foam quality of activated sludge in MBR process. Acta Hydrochim Hydrobiol 33:232–239. https://doi.org/10.1002/aheh.200400575
Oerther DB, De Los Reyes FL, De Los Reyes MF, Raskin L (2001) Quantifying filamentous microorganisms in activated sludge before, during, and after an incident of foaming by oligonucleotide probe hybridizations and antibody staining. Water Res 35:3325–3336. https://doi.org/10.1016/S0043-1354(01)00057-4
Patist A, Huibers PDT, Deneka B, Shah DO (1998) Effect of tetraalkylammonium chlorides on foaming properties of sodium dodecyl sulfate solutions. Langmuir 14:4471–4474. https://doi.org/10.1021/la980169e
Petrovski S, Dyson ZA, Quill ES, McIlroy SJ, Tillett D, Seviour RJ (2011) An examination of the mechanisms for stable foam formation in activated sludge systems. Water Res 45:2146–2154. https://doi.org/10.1016/j.watres.2010.12.026
Pujol R, Duchene P, Schetrite S, Canler JP (1991) Biological foams in activated sludge plants: characterization and situation. Water Res 25:1399–1404. https://doi.org/10.1016/0043-1354(91)90118-A
Roy U, Manna S, Sengupta S, et al (2018) Dye removal using microbial biosorbents. 253–280. https://doi.org/10.1007/978-3-319-92162-4_8
Schilling K, Zessner M (2011) Foam in the aquatic environment. Water Res 45:4355–4366. https://doi.org/10.1016/j.watres.2011.06.004
Sheng GP, Yu HQ, Li XY (2010) Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review. Biotechnol Adv 28:882–894. https://doi.org/10.1016/j.biotechadv.2010.08.001
Sorlini S, Biasibetti M, Collivignarelli MC, Crotti BM (2015a) Reducing the chlorine dioxide demand in final disinfection of drinking water treatment plants using activated carbon. Environ Technol (United Kingdom) 36:1499–1509. https://doi.org/10.1080/09593330.2014.994043
Sorlini S, Collivignarelli MC, Castagnola F, Crotti BM, Raboni M (2015b) Methodological approach for the optimization of drinking water treatment plants’ operation: a case study. Water Sci Technol 71:597–604. https://doi.org/10.2166/wst.2014.503
Sorlini S, Collivignarelli MC, Carnevale Miino M (2019) Technologies for the control of emerging contaminants in drinking water treatment plants. Environ Eng Manag J 18:2203–2216
Subramanian B, Pagilla KR (2015) Mechanisms of foam formation in anaerobic digesters. Colloids Surf B: Biointerfaces 126:621–630. https://doi.org/10.1016/j.colsurfb.2014.11.032
Xie B, Dai XC, Xu YT (2007) Cause and pre-alarm control of bulking and foaming by Microthrix parvicella-a case study in triple oxidation ditch at a wastewater treatment plant. J Hazard Mater 143:184–191. https://doi.org/10.1016/j.jhazmat.2006.09.006
Acknowledgments
The authors wish to thank Mariano Chiesa for his support to the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Responsible Editor: Marcus Schulz
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Collivignarelli, M.C., Carnevale Miino, M., Caccamo, F.M. et al. Evaluation of foaming potential for water treatment: limits and developments. Environ Sci Pollut Res 27, 27952–27960 (2020). https://doi.org/10.1007/s11356-020-09143-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09143-y