Abstract
Natural water sources are habitually marred by insidious anthropogenic practices and municipal wastewater discharges that contain either of xenobiotic pollutants and their sometimes more toxic degradation products, or both. Although wastewater is considered as both a resource and a problem, as explained in this review, it is however daunting that, while the global village is still struggling to decipher the mode of proper handling, subsequent discharge and regulation of already established aromatic contaminants in wastewater, there emanates some more aggressive, stealth and sinister groups of compounds. It is quite ironic that majority of these compounds are the ‘go through’ consumables in our present society and have been suspected to pose several health risks to the aquatic ecosystem, eliciting unfavourable clinical manifestations in aquatic animals and humans, which has heightened the uncertainties conferred on freshwater use and consumption of some aquatic foods. This review therefore serves to give a brief account on the metamorphosis of approach in detection of aromatic pollutants and ultimately their implications along the trophic chains in the community.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a strategic measure to reduce the pressure anthropogenic activities and undulating weather conditions bestowed on pristine water resources, worldwide, the increased use of other surrounding surface water and wastewater for recreation, irrigation and other suitable purposes has been strongly encouraged (WHO 2011). However, the overwhelming reports on the diverse classes of aromatic contaminants domiciled in wastewater, which have been consequently discharged into the natural water bodies in the past decade, have called for the proper handling, adequate use and disposal of wastewater. The constant exploitation of this group of pollutants in different communities has been evident in effluents from one or a conglomerate of the following: domestic, commercial establishments and health centres, industrial plants, stormwater and other urban run-off, agricultural practices, either dissolved or as suspended matter that forms wastewater (Corcoran et al. 2010).
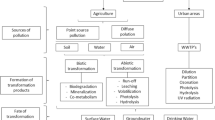
Indubitably, industrial outflows, seepages from agricultural practices and human domestic activities are the usual suspects of aromatic fouling of wastewater (Fig. 1), which could be reflected in municipal discharges, industrial and agricultural wastes, excretion of partially metabolized pharmaceuticals and accidental spills. Once released into the environment, these pollutants might be exposed to some physical and biochemical processes that are expected to enhance their elimination, but this is not always guaranteed. Subject to the phase in which they are in the environment (sediment, groundwater and surface water) or their receptacles respectively (wastewater treatment plants and drinking-water facilities), distinct spontaneous transformations could occur, sporadically producing degradation products that most times differ in their environmental impacts and ecotoxological profile.
Conversely, some transformation products might not only be more toxic than their corresponding parent compounds but also elicit greater environmental persistence. Furthermore, it is worth mentioning that many of these compounds, especially the halogenated aromatics, have been reported to be carcinogenic and teratogenic (Li and Mitch 2018) and are unpleasant to the aquatic environment and humans due to their fat solubility and bioaccumulation potential. In the light of the aforementioned, it is therefore necessitous to awaken our consciences with regard to the quandary we would be subjecting the coming generation to, from the environmental perspective.
Wastewater: a resource and a problem
According to a UNEP/UNHABITAT report, which was outlined in the document ‘Sick Water?’, Corcoran et al. (2010) defined wastewater as ‘a blend of any or all of: domestic effluents comprising of black- and greywater; outflows from commercial establishments and institutions (hospitals inclusive); industrial effluent, stormwater and other urban run-off; agricultural, horticultural and aquaculture effluent, either dissolved or as suspended matter’. Wastewater, from the effluent perspective is composed of about 99% water and 1% pendent, colloidal and dissolved solids (UN-WATER 2014). However, this composition is relative to the source and some other factors that are responsible for its deposition; for example, municipal wastewater has been reported to contain organic matter, nutrients, inorganic materials and solubilized minerals, malignant substances and disease-causing microorganism (Makarov and Ponomarev 2017).
Agricultural effluents are one of the most challenging sources of freshwater pollution. They could be generated in response to hydrological conditions at a given period; hence, they are regarded as non-point source of pollution because they can neither be controlled nor measured directly thereby posing regulation difficulties. The pollutants that constitute these effluents are chiefly sediment; nutrient, which are applied to farmland as fertilizer; animal manure and municipal wastewater; and chemical runoff from pesticides, herbicides and other agrochemicals such as residues of veterinary drugs. Furthermore, apart from its contribution to pollution, agriculture represents the sole leading consumer of freshwater resources, representing virtually 70% of global water withdrawals (Fig. 2). This statistic is really puzzling, especially when factors like population explosion, global warming and the inability to cultivate organic produce to feed a significant fraction of world population are considered.
Continental freshwater apportionments (source: FAO-AQUASTAT 2018)
In this accelerating industrial age, discharge of effluents from industries could either follow the diffuse mode, such as the one employed by the mining and agro-industries, or pipe-end discharges and, mostly, illegal tanker outflows. The diffuse mode is frequently, exceedingly polluting and intricate to sequester and treat; however, the end-of-pipe mode can be controlled, contained and treated, provided there is adequate legislature and law enforcement capacity to ensure compliance. Interestingly, some wastewaters are generated by small-scale industries that do not necessarily channel their waste to any foreseeable sewer but instead dump at the closest point of convenience. In consequence, it was declared at a World Water Development Report that only a small fraction of globally generated wastewater receives adequate treatment (UNESCO 2012) as only a few countries are able for effectively treat all wastewater generated (Table 1). This is consequent of the astronomical upsurge in urban populations and industrial activities, which is not concurrent with its wastewater regulation as a result of ageing, absent or unsophisticated sewage arrangement. Our thoughts have been further corroborated by Marzouk and Ali (2018), stating that treatment capacity typically depends on the income rank of the country, of which above 70% might be accomplished in economically viable countries compared with 8% in low-turnover countries (Table 1).
Owing to the controversies regarding wastewater treatment and handling, wastewater could be identified as both ‘a treasure resource’ and ‘a nuisance’. Wastewater could be considered as a treasure resource if the following avenues are sagaciously considered and utilized:
-
(i)
As a ceaseless source of water (specifically for agriculture and industry): here, FAO (2010) reports agriculture to be the principal user of reclaimed water in about 50 countries, with agricultural irrigation statistics falling between 20,000,000 and 45,000,000 ha worldwide (Sato et al. 2013), while environmental applications, recreation and urban and industrial reuse represent the remaining sectors that are addressed. However, occasionally, during scarcity of water, authorities might divert water that serves agricultural purposes to settlements because water serving urban and industrial purposes tend to supersede that used for most agricultural purposes in economic value; besides, human consumption is prioritized over other impending needs.
-
(ii)
As a source of nutrient and soil conditioner for agriculture: wastewater, being rich in nutrients, can reduce the intense application of chemical fertilizers. Phosphorus, an essential nutrient and constituent of fertilizers, derives its main source from phosphate rock, which is non-renewable, hence costly. Human feces in sludge form, however, which comprises about 0.5% phosphorus, serves two beneficial purposes such as improvement of phosphorus security and reduce pollution (Drangert et al. 2018, and references therein) and also function as soil conditioners/fertilizers often termed ‘biosolids’, which could be generated in diverse quantities from civic wastewater treatments plants (WWTPs) to individual settlements where environmental sanitation is observed. This could ultimately promote food security and also encourage the discontinuation of synthetic fertilizers.
-
(iii)
As a source of energy or heat: anaerobic digestion of the organic components of whole wastewater gives off a cocktail of methane and CO2, commonly used as biogas, an invaluable renewable energy fountain. This is common at some wastewater treatment setups in a specially designed compartment, the ‘digester’ or ‘stabilization ponds’. The prospects of ‘excreta sludge’ as a fuel was explored in advanced, as well as advancing countries; previously, Hafford et al. (2018) compared faecal sludge samples from Colorado (USA, North America) and Uganda (Africa). They observed that the energy content of urine-diverted dry faeces (20.0 MJ/kg) was comparable with biomass, which is approximately a fifth of the calorific values of diesel, natural gas. Interestingly, this value could be augmented, given that the dehydrated faecal sludge is co-combusted with some other potential energy-liberating biomass. This would serve as a cheaper source of energy to developing countries, which have overdependence on conventional sources of energy.
Contrariwise, from the agricultural perspective, wastewater might speedily be accepted as a resort owing to its influence as a nutrient-rich domain on crops, neglecting challenges the employment of reclaimed water for irrigation poses to the community. This observation has been corroborated by Qadir et al. (2010), who pointed out that many farmers are ignorant of conceivable negative impacts consequent of wastewater; worse still, many consumers of agricultural produce and related products in Africa may not be concerned about the water sources used in their production. For example, a study conducted in Ghana showed that health risk awareness on the use of wastewater for edible crop irrigation and processing was high amongst sales persons and consumers but low amongst farmers (Antwi-Agyei et al. 2016). Therefore, it is necessary that public awareness programmes on health implications and alleviation precepts of wastewater pollutants could be of public health significance. Some of the potential problems associated with improper handling of wastewater are outlined as follows:
-
(i)
Eutrophication: this is resultant of seepage of excess dissolved nutrients, especially nitrates and phosphates of the wastewater into freshwater, consequently encouraging the growth and decomposition of oxygen-depleting plant life such as algal blooms, which may release toxins into water, thereby increasing the biological oxygen demand (BOD) and resulting in the harm of aerobic organisms in the aquatic ecosystem, decreased biodiversity, changes in speciation and preponderance and a drastic reduction in water geniality. The deterioration of water quality as a result of eutrophication is reported to instigate reduced biodiversity in aquatic bodies by virtually a third globally, with Far East Asia, South Asia, Europe and Southern Africa enlisted endangered zones (OECD 2012);
-
(ii)
Contamination of groundwater with heavy metals: during the course of irrigation, nutrients, heavy metals and salts could be leached into the natural and relatively untapped reservoir of groundwater, which in many countries serves as the last resort of potable water. However, it should be noted that leaching of pollutants into groundwater is dependent on the soil profile and hydrological characteristics of the particular environment;
-
(iii)
The creation of habitat for disease vectors: wastewater, expressly that of domestic origin, could contain high concentrations of pathogens laden excreta, chiefly in countries that have a history of the prevalence of intestinal parasites and diarrhoeal diseases. Infections could manifest sequel to direct contact with untreated wastewater, even as from wastewater-tainted potable water, food (fruits as well as vegetables, whose edible vegetative parts are sprinkled with untreated wastewater during irrigation) and recreational water (WHO 2006);
-
(iv)
Build-up of toxic organic pollutants: this might be due to constant irrigation with untreated or moderately treated wastewater or the incessant haphazard discharge of untreated effluents from tankers into the natural water bodies. There has been a lot of work done over the past decade on the classification, incidence, deposition patterns, environmental impacts and legislation concerning the handling and disposal of many organic pollutants (National Toxicology Program 2014).
Organic pollutants: a nemesis of our industrial advancement
The drinking water industry, in the past decade, has become perplexed about the occurrence of organic contaminants in supposedly potable water caches. In earlier times, preference was given to pesticides but was subsequently diverted toward other organic micropollutants whose concentrations were observed to be on the increase in water reservoirs. The procedural complexities encountered in the discharge of these micropollutants into the municipal aquatic milieu result from the partial elimination in wastewater treatment plants and the subsurface seepages of sewers (Schirmer and Schirmer 2008). Interestingly, some physiology–modulatory compounds viz. endocrine disruptors and pharmaceuticals and personal care products (PPCPs), which symbolize a conglomerate of biologically active agents, are being used in copious amounts in human and veterinary medicine, universally. This has called for unending debate premised on the ostensive risk accompanying the presence of the minutest concentration of these organic pollutants within aquatic milieu, which might be biomagnified, sequel to absorption and bioaccumulation in terrestrial and aquatic ecosystems. A typical example of its bioaccumulation is further illustrated (Fig. 3), which studies the bioaccumulation of polychlorinated biphenyl (PCB) in the marine food chain. To this end, the United Nations Environment Programme regarded them as ‘persistent organic pollutants’ (POP) at the Stockholm Convention (UNEP, 2013).
In a given aquatic matrix, organic pollutants may exist in a variety of phases: as loosely dissolved, as slurry or associated with sedimentary material. It is worth mentioning that the sorption coefficient of an organic pollutant onto a particular sediment might be hinged on both the sediment’s properties and the named aromatic compound. In general, commonly reported organic pollutants or aromatic contaminants discharged into wastewater comprise the following:
-
(i)
Persistent compounds resultant of fractional combustion of fossil fuel which enter the municipal wastewater collection system through agglomeration onto paved surfaces through run-off (PAHs and polychlorinated dibenzodioxins and furans: PCDD/Fs).
-
(ii)
Run-off of impurities in wood preservatives namely creosote (PAHs) and pentachlorophenol (PCP) (e.g. polychlorinated dibenzodioxins and furans: PCDD/Fs) into urban wastewater.
-
(iii)
The mobilization of persistent compounds through volatilization from soil, subsequent accumulation and transfer to urban wastewater in run-off (e.g. PAHs, PCBs and PCDD/Fs).
-
(iv)
Aromatic materials that are prohibited from use/manufacture but are transferred by run-off to urban wastewater through domestic sources that are not well monitored (e.g. chlorinated pesticides).
-
(v)
Sewer discharges of compounds that are applied directly in industrial processes or domestically, which leach from plastics and surfaces during routines and are conveyed with run-off (e.g. di(2-ethylhexyl)phthalate DEHP, PBDEs).
-
(vi)
Detergent lees (e.g. LASs, NPEs) and textile dyes.
-
(vii)
Endogenous hormones, artificial steroids and PPCPs.
-
(viii)
Aromatic compounds with endocrine-disrupting potential.
Polynuclear aromatic hydrocarbons and halogenated aromatics
Customarily, this class of pollutants is deposed in the environment by biogenic, petrogenic and pyrogenic sources (Stout et al. 2015), where they adhere to surfaces, most especially sediments. Once they are adsorbed to an environmental matrix, they may be degraded or transformed, which determines their distribution and concentration, respectively. However, a report reveals that high molecular weight PAHs are liable to be more stable, less water soluble but highly lipophilic, which increases their environmental persistence and toxicity (Rengarajan et al. 2015).
Although the production of most PAHs is not commercially driven, some are manufactured in pure forms and exist as colorless, white or pale yellow solids (Rengarajan et al. 2015). Initially, appropriate detection of these pollutants in environmental matrices was cumbersome and imprecise; however, following the enthusiasm about the invention of sophisticated separation techniques and analytical methods with higher resolving power, gainful insights have been given on the origin of even infinitesimal concentrations (parts-per-trillion or parts-per-quadrillion) of PAHs in the environment, most especially the priority pollutants, based on their varying degrees of alkylation. This development has, in the long run, assisted the environmental organizations and other regulatory agencies, worldwide, through the exemplary creation of the list of 16 PAHs (Fig. 4) by the Environmental Protection Agency (USEPA 2011), which has since served as a microcosm of a broader array of this group of pollutants in the environment.
Halogenated aromatics, together with some PAHs, form majority of persistent organic pollutants (POPs) that have been perpetually enlisted by the Environmental Protection Agency (EPA). Although 12 organic compounds were initially confirmed as the ‘murky twelve’ at the 2001 ‘Stockholm Convention on Persistent Organic Pollutants’ (Stockholm Convention on Persistent Organic Pollutants 2001), a more recent meeting in 2009 (Stockhlom Convention 2009) further increased the list of delimited aromatic pollutants. They generally derive applications that span production of organopesticides for agricultural and horticulture practices, as flame retardants additives in appliance, textiles and plastics (thermoplastics), dielectric and covalent fluids in electrical equipments, and pharmaceutically, as ingredients of formulations for treatment of lice and scabies. Although majority of the halogenated aromatics were produced with deliberate exactitude to address several industrial and commercial cravings, some have been formed naturally from forest fires and volcanic eruptions (Srogi 2008), some have been found as contaminants of desired aromatic products, some as by-products of chemical reactions, low temperature thermal reactions, as effluents of pulp bleaching and also as final effluents of wastewater treatment plants sequel to the chlorination of secondary effluents, which contain traces of aromatic contaminants.
Of all the halogenated aromatic implicated as persistent organic pollutants, the chlorinated aromatics are of utmost concern due to the stability of the carbon-chlorine bond toward hydrolysis. This feature contributes greatly to their bioaccumulation potential; however, the chlorine attached to an aromatic ring is less readily hydrolysed as compared with aliphatic fused one. Even so, bromophenyls with at least five substitutions could bioaccumulate rather insignificantly than chlorobiphenyls, whereas extremely chlorinated biphenyl compounds have a tendency to accumulate more in the environment than the scantily chlorinated biphenyls. However, metabolism and subsequent excretion rates are accelerated for less chlorinated aromatics than their copiously chlorinated confrère.
Consequently, chlorinated pesticides having molecular mass above 236 g mol−1 have been famous for absorbency in biotic tissues as well as bioconcentration in upper trophic level organisms of the aquatic ecosystem; this has been attributed to their stability to photolytic and biological degradation. A typical example is permethrin, a pyrethroid, which is traditionally adopted as an insecticide and pesticide. Studies have shown that it has a high lipophilicity and is also stable to photolytic and biological degradation. In a recent study, excretions of metabolites from permethrin (0.8–462.5 μg/L) were observed after cutaneous contacts with permethrin-impregnated wares (Maule et al. 2019). Apart from the disturbing reports about the dangers it poses to aquatic and other domestic animals, a critical evaluation of the side effects it could confer on humans makes it a salient risk if its excessive use is not discontinued. While the commercial industries and government agencies have generally portrayed pyrethroid reagents as innocuous, they have been associated with male reproduction defects (Jurewicz et al. 2015), respiratory issues (Ye et al. 2016) and cognitive dysfunction (Gunier et al. 2017). Interestingly, this is not the earliest occurrence of haloaromatic contamination of our environment. Sequel to the ban placed on DDT [1,1,1-trichloro-2,2-di(4-chlorophenyl) ethane] by most developed countries in the early 1970s, associated halogenated aromatics were still being discovered in disturbing concentrations in the environment. Although this had ultimately led to the creation of a chart of persistent organic pollutants at the 2001 Stockholm convention, the annexation of newly found pollutants in the convention held in 2009, and also motivated the voluntary phase-out appeal by the US EPA in 2011, these mitigation efforts have not been conveyed to the developing and third world countries, who might not be able to be at pace with the developed countries toward achieving a greener environment, economically; therefore, it anticipated that the use of halogenated aromatics for disease prevention might not be restricted, globally.
Pollutants of emerging environmental concern
Although much attention has been conferred on majority of the persistent organic pollutants in wastewater, natural surface waters and water caches with respect to their prospect, detection and regulation to safeguard water quality, swelling concern is being allotted to a group of mostly polar compounds, which are not only capable of effortless solution and transport through the water cycle (Tran et al. 2018) but are also seemingly surreptitiously evade most conventional treatment and some advanced treatment technologies. They are branded ‘emerging organic pollutants’ because, at the time, they had not been regulated due to the lack of or scanty information regarding their distribution patterns and toxicity, inappropriate detection and analysis techniques, or both. However, in contemporary times, due to the sophistication of analytical approaches, more than 700 emerging pollutants have been characterized from the European aquatic environment (www.norman-network.net), and this statistic is expected to expand due to novel processes, as regards chemical synthesis and transformation products from degradation of already existing pollutants, that are anticipated in the not too distant future. Basically, emerging contaminants include a wide assortment of newly synthesized substances as well as the environmentally tacit ones whose significance is now being highlighted; specifically PPCPs, agricultural and veterinary merchandise, food additives and preservatives, adjuncts of industrial produce and fabricated nanomaterials (Lapworth et al. 2012). Moreover, a series of heuristic studies have revealed their presence in different water matrices, using state-of-the-art analytical equipment (Table 2). Notwithstanding, as our knowledge of these emerging aromatic pollutants increase, so also does the list expand continually. This purports the ever cumbersome tasks that faces engineers in the design of suitable methodology for their near eradication.
Pharmaceuticals and personal care products
Customarily, profuse debate and debacle has been accorded the conventional ‘priority pollutants’ or persistent organic pollutants, which are only a small fraction of the total spectrum of possible pollutants, and their environmental reverberations, much to the negligence of the pharmaceutical and personal care products (PPCPs) whose presence in surface water and wastewater effluents were detected at about the same decade. However, a pioneering revelation presented by establishment of the nexus between a synthetic contraceptive, ethinyl estradiol, and male fish feminization (Jokbling et al. 1998) has compelled the adequate attention of researchers and environmental agencies alike. Subsequently, studies on PPCP compounds and their metabolites have revealed a great deal about their ubiquity in environmental matrices (Wilkinson et al. 2018).
Thus far, PPCP residues isolated from wastewater include therapeutic lozenges, lotions and suspensions for physical discomforts, depression, lipid regulation and colds; birth control tablets; hair foods; cleaning supplies and pesticides. In a collective sense, they simply comprise a large, heterogeneous group of organic compounds such as prescription drugs and antibiotics, non-prescribed or ‘over the counter’ drugs and active constituents of daily personal care products like preservatives and toiletries, which are in comprehensive demand, worldwide. The PPCPs have been regularly discovered within swift flowing and still water, groundwater (Sorensen et al. 2015), innate potable water founts and treated supplies (Tröger et al. 2018). Kolpin et al. (2002), at the apogee of the twentieth century (1999–2000), investigated the existence of pharmaceuticals and associated wastewater-related pollutants in predisposed streams in the USA, and thereafter observed that they were associated with streams that serve as sinks for agricultural, domestic and industrial wastewater. On the other hand, important PPCP effluxes might be sourced from pharmaceutical and chemical manufacturing plants, health care centres, long-term care conveniences, veterinary operations, landfill leachate, septage tank haulers and runoff from animal slaughtering and feeding, and operations where large quantities of antibiotics and other drugs are used. Interestingly, one of the largest sources of PPCPs is the typical household in a society where flushing of unused medications and chemicals down the drain and the use of more pungent deodorants, disinfectants and contraceptives—that will augment the amount of PPCPs in the environment—are still in practice.
Although majority of pharmaceuticals, unlike the PAHs and halogenated aromatics, are highly hydrophilic rather than lipophilic, fragrances and musks, due to their lipophilic nature, tend to be adsorbed on skin surfaces for long periods and are washed off with body oils and dirt during showers. Western Europe has, over recent years, served as the hub of wastewater pollution by PPCPs, as the average of over 300 mg active ingredients, most of which are dominated by 60 named compounds, are consumed habitually per inhabitant (Ortiz de García et al., 2013). Up until now, more than 200 different pharmaceuticals, alone, have been implicated in river waters globally (Hughes et al. 2013). From a ratiocinative purview, it is worthy of note that the concentrations of these pollutants in wastewater is a function of the epidemiology, pharmacogenomics, pharmacological and socio-economic practices of the populace. Interestingly, these pharmaceuticals, once ingested, could be partially metabolized and excreted as fractions of the non-metabolized original molecule and conjugated forms of the parent compounds as metabolites, either in feces or urine. Although methyl triclosan, the dissociated form of the disinfectant, triclosan, was revealed as being photodegradable, Lindstrom and his colleagues in 2002 identified both triclosan and methyl triclosan, its primary degradant, in the surface waters of Switzerland thereby classifying the latter as equally persistent (Lindstrom et al. 2002).
Microplastics
Plastics were once considered as biochemically inert materials with innocuous effect on the physiology of living organisms due to their high molecular weight, which prevents their penetration through the cell membrane (Teuten et al. 2009). In the 1940s, Yarsley and Couzens heralded the beginning of the ‘plastic age’ with much optimism as it was being introduced as a substitute for metals, which were prone to rust. This long existing premonition has allowed its use as a sole or auxiliary component in many short lifetime products and today’s upgrade-and-dispose predisposed electronic goods; moreover, the invaluable properties of plastic viz. malleability, durability, light weight and downmarket protracts its application in the manufacture of toys, automobile parts, kitchen utensils and even laboratory materials. In a 2009 review on plastics recycling, Hopewell and his colleagues submitted that about 50% of plastic derives single-use disposable functions viz. packaging as disposable consumer items, a figure motivated by its improved physical and chemical qualities. Conversely, these aforementioned qualities make its disposal exigent as they possess the ability to persist in the environment (Science for Environment Policy 2011); these challenges are bound to linger, due to the popularity of plastic products in emerging economies coupled with the simplicity of their waste management infrastructure.
Measurement of the state of plastic waste has been so far very cumbersome; albeit EU-27, Norway and Switzerland generated an estimated 24.9 megatonnes of plastic waste (Mudgal et al. 2011), also its dispersal is hard to resolve due to the emanation of a new type of plastic, ‘microplastic’. Though, the derivation of the term was hinted by Ryan and Moloney (1990) in their submission of the investigation outcomes of South African beaches, it was made formal by Thompson and coinvestigators (Thompson et al. 2004) where they reported the spatial distribution and build-up of about 50 μm plastic bits and fibres on shorelines and in the water column. Appropriate definitions for microplastics have since then varied amongst authors who have either set upper limits at 5 mm (Arthur et al. 2009) or 1 mm (Costa et al. 2010); where the definition of the former stands since it was ratified at the National Oceanic and Atmospheric Administration (NOAA) Technical Memorandum in the USA. These particles have been generated as a result of prolonged physical, chemical and biological disintegration of larger entities such that smaller particles are successively formed (Law and Thompson, 2014). An example is the representation infra, which displays microplastics gathered from sediment samples around the sewer of a named laboratory and some environs where our investigation was carried out, which had been left unattended for a considerable period of time (Fig. 5). This discovery corroborates the aforementioned reflections of Law and Thompson (2014) that plastics may generally be degraded into bits after consistent stress from the exogenous environmental factors.
However, some have been deliberately designed as microbeads or -pellets apropos of use as abrasives and exfoliants in domestic cleaning and beauty care products (Yurtsever and Yurtsever, 2019), which are meant to be rinsed down the drain regardless of the biodegradability of the resulting material. A previous study conducted by Rochman et al. (2015), advocating the ban on microbeads—which are plastic—reveals that an estimated 8 trillion microbeads per day are discharged into aquatic habitats in the USA, which are enough to overlay well over 300 tennis courts, with about 800 trillion supposedly being trapped in the sludge of WWTPs used as biosolids and landfills and are ultimately washed back to waterbodies through runoff. This daunting discovery is further escalated by the conception that these particles could serve as sponges, sorbing persistent bioaccumulative and toxic chemicals (Fig. 6) coupled with the plausible release of monomers, oligomers and potentially toxic additives (Teuten et al. 2009; Law and Thompson 2014). The consequence of this subject matter has since motivated grants from research agencies as well as researchers, non-profit groups and other institutions globally, in order to identify other aftereffects.
The degeneration of plastics in to microplastics and the direct transfer of microplastics in beauty care products into the aquatic environment, their distribution in the various compartments and their potentials to act as sponges on which micropollutants and microorganisms could be adsorbed (adapted from Leslie 2014 with slight modification)
Plastic leachates: bisphenol A and phthalate
Although deemed seemingly inert, biochemically, plastics whose formation undergoes incomplete polymerization may allow additives, which serve to improve properties or enhance durability to migrate from the matrix during aberrant environmental conditions. These additives could be, of utmost certainty, environmentally harrowing since they both affect degradation times of plastics as well as convey potentially hazardous substances to biota. Bisphenol A and phthalates, which were at their inception not solely intended for the manufacture of plastics, are established synthetic chemicals, which are seemingly dissimilar in application, except plastic manufacture (Koch and Calafat 2009). Bisphenol A is well represented amongst the highest volumes of chemicals produced worldwide; about 3,800,000 tons were formed in 2006 (WHO/FAO 2010). It is almost exclusively employed for fabrication of the polycarbonate plastic, which is used as food storage, beverage cans and various plastic consumer merchandise such as toys, plumbings, coverings for electronics and as precursor in the manufacture of monomers of epoxy resin linings of metal cans of processed fruits and vegetables, optical lenses, dental sealants and composites used for fillings, medical equipments and tubing, even as a constituent of printer inks and receipt paper coating termed ‘carbonless paper’. From the aforementioned processes bisphenol A partakes, it is apparent that a large percentage are inevitably human-participatory, which further connotes the high susceptibility of humans to their aftereffects.
Phthalates, whose application is a function of the length of their ester chains (dialkyl or alkylaryl), are additives mostly applied as plasticisers, which enhances the flexibility and resilience of PVCs, making up 40% (Koch and Calafat 2009); low-molecular weight phthalates have been employed as solvents for personal care products, lacquers, varnishes and coatings, which in some cases serve to aid timed dispensations in certain pharmaceuticals. Their ubiquity therefore accounts for the global production of at least 8.0 million tons of phthalate esters annually (Wang et al. 2015); moreover, their particularly high production in the Far East Asian Countries (China and Japan) confirms their status as a flagship in emerging technologies over the last decade. Moreover, due to predilection for their products and derivatives amongst arguably most developed and developing countries in households and industry, bisphenol A and phthalates are conceivably resident in raw sewage. For example, Wilk et al. (2019) observed a high concentration of phthalates and bisphenol A (ca. 738 μg L−1 and 957 μg L−1) in raw wastewater collected from passenger ships. Also, apart from spontaneous disintegration of polycarbonate plastics, other routes which bisphenol A pass into the surroundings are through landfill leachates, wastewater, which is depicted as its major point source, and treated sewage. They are channelled into wastewater treatment facilities either as direct discharge from sewers and stormwater runoff, or both; hence, their status as part of major secondary sources of bisphenol A pollution. About 2202 μg L−1 concentration of bisphenol A was collected from a municipal solid waste plant (Wilk et al. 2019).
The paper industry is a major supporter of bisphenol A in wastewater; this occurs either during paper making or recycling because bisphenol A provides a reactive agent during temperature sensitive paper moulding. Studies conducted in more than 120 wastewater treatment plants worldwide inferred that considerable quantities of bisphenol A were present in influent and effluent, biosolids inclusive. Also an average range of ca. 30–40 μg/g dw, nine different phthalate esters was observed in sludges from 46 wastewater treatment plants (Zhu et al. 2019). The recurrent detection of bisphenol A in surface water could be chiefly because of its incessant discharge into surroundings, amongst claims of its environmental persistence. Phthalates likewise have been ascertained in landfill leachates, surface water, groundwater, wastewater and, somewhat unsurprisingly, drinking water (Liu et al. 2013). In a 2009 study conducted in Spain, wastewater cocktails contained phthalate ester concentrations ranging between 39 and 531 μg L−1; however, the major highlight was its presence in domestic wastewaters as the largest constituent (67.3 to 69.1 μg L−1) with about 153 μg L−1 influent matter (Sánchez-Avila et al. 2009). This derisive discovery therefore not only confirms the role played by the seemingly benign domestic routines but also serves as a herald of future pollution despite the embargo placed on most of these substances and their derivatives.
Bioplastics: a reliable long-term option for sustainability?
Owing to the public concern about the environmental consequences of plastic consumption, aggressive research has been devoted toward biobased products, which is causing environomic revolutions amongst some developed countries, worldwide. The introduction of bioplastic was thought to avert the challenges of non-biodegradability of conventional synthetic plastics, which are made from offshoots of fossil fuel processing and vitally contribute to global warming and build-up of toxic compounds, when thermally destroyed/degraded (Emadian et al. 2017). Furthermore, its low turnover of CO2 emission, synthesis from natural sources such as plants and microorganisms (bacteria, algae and fungi), makes it a sensible alternative and hence bears eloquence to increasing acceptability. However, with respect to biodegradability, not all bioplastics might be as readily degradable as anticipated; this is because their degradation depends on abiotic and biotic factors of their immediate environment and also the chemical constituents of the named biomaterial (Thakur et al. 2018 and references therein). In fact, their eventual degradation in a landfill, however slow, would require adherence to stringent environmental conditions and would contribute to the generation of greenhouse gases (GHGs), where they are deprived of oxygen. This implies ‘just because it’s biodegradable does not mean it’s good. If it goes to landfill it breaks down to methane. Only a percentage is captured; in theory, bioplastics are good, but in practice there a lot of barriers’, as echoed by Peter Skelton of Wrap, the UK Government-funded Waste and Resources Action Programme. Correspondingly, Michael Warhurt, a resources campaigner at Friends of the Earth recounted ‘It is just not possible to capture all the methane from landfill sites; a significant percentage leaks to the atmosphere’. Altogether, if the bioplastics are from plant-based chemicals, it could spell a greater pollution challenge on the environment than what the already existing conventional synthetic plastic presents. This is because their production life cycle assessment entails process and chemicals that could ultimately do more harm than good to the environment. For example, outcomes from a 2010 study on life cycle assessment on bioplastics suggested that their production resulted in greater amounts of pollution, due to the chemicals employed in growth of feedstocks and their chemical processing. Furthermore, the hybrid plastic produced, B-PET, was found to have the highest potential for toxic effects on the ecosystem (Tabone et al. 2010). Interestingly, annual global production of bioplastics has reflected the use of non-biodegradable (83.6%) biobased starter chemicals over their biodegradable (16.4%) confrère (Fig. 7); this implies the struggle to rid our environment of plastic wastes still lingers. Moreover, our aquatic ecosystems might not be free of plastic pollution anytime soon as there are little or no preceding information regarding the biodegradation of bioplastics in aquatic milieu, to my knowledge; potential alternatives and solutions would be addressed in a forthcoming review.
Effects of aromatic pollutants on the community: the environmental health skirmish
In spite of the efforts made by technological, legislative and regulatory arms in the domain of waste management, the global community still struggle with the palliation of majority of established and emerging organic pollutants. Consequential of the discharge, transboundary transport and subsequent accumulation of the different classes of pollutants mentioned earlier in this review, the environment remains very much despoiled, owing to the imbalance created by the perceptible extinction in the amount of lotic and lenthic natural denizens, and their mutagenic and degenerative effects. Environmental contaminants may affect many different organisms and biological processes at different levels of organization. Generally speaking, the effect of organic pollutants on the community can be observed from four perspectives; the decomposers (bacteria and fungi), the primary producers (algae and planktons), the consumer animals (invertebrates and vertebrates) and the human perspective. The cascade of events that occurs is usually dependent on the fauna stratification and constituents of the cocktail contaminants as they co-exist in water bodies in form of synergism, addition or antagonism.
The decomposers
Although the biodegradative effects of decomposers have been succinctly reviewed, recently (Wick and Chatzinotas 2019), a process that is dependent on the biodiversity and abiotic factors of the immediate community, the toxicity of organic pollutants on these pollutants, is worth mentioning since they serve as vital members of the freshwater trophic cycle and hence indirectly dictate the biodiversity and population dynamics of the aquatic ecosystem. This is partly because (1) they are the critical to the decomposition of leaf litter in riparian vegetation, which serves as food for detritivores in freshwater food webs. Furthermore, an expressive inter-dependence between bacterial production and phytoplankton primary production could be conceived, (2) their status as the quickest of the natural populations of the trophic cascade to respond to environmental changes conferred by toxicants, an attribute which makes them suitable tools of environmental pollution biomonitoring (a typical example is Vibrio fischeri, which is used to monitor environmental pollution based on bioluminescence). For example, in a recent study, the toxicity of 36 single-benzene ring compounds toward Vibrio fischeri was established, where toxicities, EC50 (mg L−1) such as 2,4-dichlorophenol 4.56 mg L−1 and 7.45 mg L−1 were observed for p-chlorophenol and benzene sulfonic acid, respectively (Cvetnic et al. 2019). In another study, exposure of sea bacterium Rheinheimera sp. BAL341 to a cocktail of PAH, alkanes and organophosphate esters resulted in a drastic and pronounced decrease in species abundance and proliferation, which also provoked shifts in its gene expression profile (Karlsson et al. 2019).
Primary producers
Primary producers are solely dependent on photosynthesis for the conversion of inorganic nutrients into new organic forms and could be affected by the competition of photosystem II (PSII) inhibitors (Organic pollutants) with photoreceptors, hence inhibiting energy transfer: This could drastically affect their biomass proliferation and species diversity. This is corroborated by the investigation of Beaulieu et al. (2020), where the inimical effects of environmentally relevant concentrations of some herbicides on phytoplankton cultures, especially their PSII organization, was elucidated after 12 to 24 h treatments. Furthermore, owing to the relationship between the primary producers and other invertebrates and macrophytes, the latter group is adversely affected.
Consumer animals
The freshwater invertebrates, due to their status as primary consumers of phytoplankton, represent a cardinal link in trophic systems, especially in the monitoring of safety level of polluted freshwater. Ecotoxicology studies have revealed that some aquatic invertebrates manifest sensitivities to toxicity through reduced ingestion activity, longevity, survival and fecundity, immobility, phototaxis, genotoxicity and inhibition of enzyme production (Booth et al. 2019). On the other hand, vertebrates, notably fishes, which are the most diverse and abundant of the vertebrate group, are most vulnerable to aromatic toxicity at their juvenile or developmental stages. The exposure of fishes to organic pollutants may cause alterations in reproduction, growth and development. Even low doses (0.5–10 μg L−1) have been reported to elicit toxic, mutagenic and carcinogenic effects, respiratory difficulties, impairment of hormonal balance and nutrient metabolism (Muthulakshmi et al. 2018) with morphological pathologies and behavioural changes (Bautista et al. 2019). Furthermore, several anomalies in morpho-functional features of fish digestive tract were observed on exposure to organic xenobiotics (Adamovsky et al. 2018), which may change the activities of the enzymes responsible for the digestion of essential components of their food.
Humans
Ultimately, the effect of organic xenobiotic pollutants cannot be overemphasized in humans as there are diverse articles of good repute regarding the physiological and biochemical phenomena encountered, sequel to their integration into the human using animal model studies (Lee et al. 2014). Hypothetically, effects on humans may include skin irritations, juvenile behavioural problems, leukaemia, neurotoxicity, immunotoxicity, reproductive diseases, hormonal dysfunction, slow convalescence rates in bacterial infections due to acquired antibiotics resistance and worse, still, death.
The climax of the effect of environmental organic pollutants on humans in this present age is portrayed in metabolic anomalies such as Type 2 diabetes and obesity. At least US$ 548 billion was expended in 2013 courtesy of global health concerns, and this spending status is only bound to increase as the years wear on (International Diabetes Federation 2014). Although putative factors like obesity, family history of diabetes, lack of physical activity and genetic predisposition are the usual suspects, they cannot satisfactorily explain its explosion worldwide. Accumulated evidences have surmised the link between organic pollutants and diabetes, high blood glucose levels, insulin resistance and obesity; a 19-year prospective assessment showed that incidence of type 2 diabetes increased in congruence with hexachlorobenzene levels in the plasma (Lee et al. 2014). One of several mechanisms that trigger diabetogenesis is the disruption of glucose homeostasis by reduced absorption of glucose by the adipose tissue, liver and pancreas, accompanied with decreases in insulin generation and emission by beta cells (Magliano et al. 2014). The development of obesity, which is sometimes inferred as a determinant factor in the susceptibility to diabetes, has long been attributed to the traditional assertion ‘calorie stuffing with reduced exercise and energy expenditure’ (Sayer and Hill 2019); other hypotheses such as inadequate sleep, gut bacterial overgrowth, genetic predisposition and viral theories have similarly been expounded.
In addition to the aforementioned pathways to obesogenesis, there is emerging evidence that environmental contaminants are likely thresholds for obesity as they are capable of altering the balance between energy intake and utilization. Consumption and bioaccumulation of a number of environmental pollutants such as phthalates, bisphenol A and POPs (Reaves et al. 2015) have been implicated, so far, as vehicles for obesogenesis. Out of several mechanisms by which these pollutants initiate the chronic metabolic condition, obesity, the following are remarkable: the downregulation of lipid homeostasis regulators (Kamura et al. 2018); P450 enzymes stimulation, which subjects the liver to synthesize copious amounts of cholesterol and triglycerides than necessary; alteration of lipid build-up and adipogenesis; oxidative stress induction in endothelial cell through increased inflammatory processes; disruption of serum lipid homeostasis (Suarez-Lopez et al. 2019); enhancement of adipocyte hyperplasia; disruption of energy balance controlling systems; and interaction with the nervous system (Heindel and Blumberg 2019), some of which may contribute to the progression of other diseases (Ljunggren et al. 2014; Magliano et al. 2014), and ultimately, mortality. Bearing in mind the biochemical and physiological dysfunctions outlined as syndromes of cytological xenotoxicity, it is palpable that a major factor influencing bioaccumulation and/or the pathology of majority of environmental contaminants is their lipophilicity. Therefore, it would be sagacious to discontinue the use of packaging materials that contain traces of these pollutants in the food industry, especially in the packaging of fatty foods and oily foods. Therefore, the use of sustainable and environment-friendly packaging materials should be deliberated.
Conclusion and personal reflections
Despite the claims for better and pristine natural water sources, it is however overwhelming that diverse classes of aromatic pollutants are obliviously released into the environment. In summary, reports of wastewater still containing certain levels of pollutants surmise that they may yet serve as thresholds for insuperable existence of cocktails of these pollutants in our natural water bodies as presumed by a plethora of investigations. This however poses a puzzling stance at potable water miners, wastewater professionals and community as a whole. Major highlights in the review revealed the following: the sources of the pollutants, which are uncannily heterogeneous; the somewhat stealth and sinister nature of these contaminants once haphazardly released into the environment; and the constant transformation of parent pollutants in the different environmental matrix, as dictated by certain environmental conditions. These may therefore make fingerprinting cumbersome, though assessed with modestly sophisticated analytical equipment of our age. Moreover, there are many emerging compounds that have not been fittingly monitored up to the regulation level, hence the existing conflicts in policies around the globe. It is fair to say that the manufacturing industries at large are the malefactors; however, more scrutiny should be directed toward the general public (consumers) as they indirectly influence the performance of the industry. A typical example in the pressure saddled on the pharmaceutical and personal care products where the aggressive quest to quick solutions to present human needs have driven the industries to manufacture goods whose constituents may not be convenient for the receiving ecosystem, microplastics to be specific. To that effect, some countries have moved to ban the use of these products and also instruct that eco-friendly and environmentally benign, natural supplements be employed. The production and use of bioplastics has been encouraged worldwide, in search of greener alternatives to the traditional plastics; even so, its aftereffects, in the long run, could be worse than what our ecosystem is currently experiencing from traditional plastics wastes. Also, the incidence of presumed “biodegradable bioplastics” might become an excuse for the further littering of our environments.
Although the issue of regulatory inefficiencies and socio-economic factors contribute to the occurrence of aromatic pollutants in the ecosystem, prized attention should be devoted to studying the nature of the pollutants as many are somewhat surreptitious in conventional water treatment facilities. Seeing that some treatment methods so far applied have not only failed to successfully eliminate the pollutants but have also generated more toxic by-products and advanced methods proposed so far are cost intensive and require the expertise of a trained professional for equipment maintenance and near reproducibility in treatment outcomes, we can only sensitize and encourage ourselves on the debarment of these malevolent products while the search for greener ones continue, constantly monitor the adherence to tenets as regards wastewater management in every sphere of the community and lastly improve or upgrade the wastewater treatment facilities.
References
Adamovsky O, Buerger AN, Wormington AM, Ector N, Griffitt RJ, Bisesi JH Jr, Martyniuk CJ (2018) The gut microbiome and aquatic toxicology: an emerging concept for environmental health. Environ Toxicol Chem 37(11):2758–2775
Agunbiade FO, Moodley B (2016) Occurrence and distribution pattern of acidic pharmaceuticals in surface water, wastewater, and sediment of the Msunduzi River, Kwazulu-Natal, South Africa. Environ Toxicol Chem 35:36–46
Arthur C, Baker J, Bamford H (2009). In: Proceedings of the International Research Workshop on the Occurence, Effects and Fate of Microplastic Marine Debris. NOAA Technical, Memorandum NOS-OR&R-30
Bautista NM, Pothini T, Meng K, Burggren WW (2019) Behavioural consequences of dietary exposure to crude oil extracts in the Siamese fighting fish (Betta splendens). Aquat Toxicol 207:34–42
Beaulieu M, Cabana H, Huot Y (2020) Adverse effects of atrazine, DCMU and metolachlor on phytoplankton cultures and communities at environmentally relevant concentrations using Fast Repetition Rate Fluorescence. Sci Total Environ 712:136239
Booth SR, Patten K, New L (2019) Response of estuarine benthic invertebrates to field applications of insecticide. Estuar Coast Shelf Sci 218:86–94
Chokwe TB, Okonkwo JO, Sibali LL, Ncube EJ (2012) Optimization and simultaneous determination of alkylphenol ethoxylates and brominated flame retardants in water after SPE and heptafluorobutyric anhydride derivatization followed by GC/MS. Chromatogr 75:1165–1176
Clara M, Windhofer G, Weilgony P, Gans O, Denner M, Chovanec A, Zessner M (2012) Identification of relevant micropollutants in Austrian municipal wastewater and their behaviour during wastewater treatment. Chemosphere 87:1265–1272
Corcoran E, Nellemann C, Baker E, Bos R, Osborn D, Savelli H, (eds) (2010) Sick water? The central role of wastewater management in sustainable development. A Rapid Response Assessment. UNEP/UNHABITAT
Costa MF, Ivar do Sul JA, Silva-Cavalcanti JS, MCB A, Spengler A, Tourinho PS (2010) On the importance of size of plastic fragments and pellets on the strandline: a snapshot of a Brazilian beach. Environ Monit Assess 168:299–304
Cunha SC, Pena A, Fernandes JO (2015) Dispersive liquid–liquid microextraction followed by microwave-assisted silylation and gas chromatography-mass spectrometry analysis for simultaneous trace quantification of bisphenol A and 13 ultraviolet filters in wastewaters. J Chromatogr A 1414:10–21
Cvetnic M, Persic DJ, Kovacic M, Ukic S, Bolanca T, Rasulev B, Kusic H, Bozic AL (2019) Toxicity of aromatic pollutants and photooxidative intermediates in water: a QSAR study. Ecotoxicol Environ Saf 169:918–927
Drangert J-O, Tonderski K, McConville J (2018) Extending the European Union waste hierarchy to guide nutrient-effective urban sanitation toward global food security – opportunities for phosphorus recovery. Front Sustain Food Syst 2:3
Emadian SM, Onay TT, Demirel B (2017) Biodegradation of bioplastics in natural environments. Waste Manag 59:526–536
FAO (2010) The wealth of waste. The economics of wastewater use in agriculture. FAO Water Reports 35. Food and Agriculture Organization, Rome
FAO (2018) AQUASTAT website. Food and Agriculture Organization of the United Nations (FAO). Website accessed on [2018/12/14]
Gunier RB, Bradman A, Harley KG, Kogut K, Eskenazi B (2017) Prenatal residential proximity to agricultural pesticide use and IQ in 7-year-old children. Environ Health Perspect 125:057002. https://doi.org/10.1289/EHP504
Hafford LM, Ward BJ, Welmer AW, Linden K (2018) Faecal sludge as a fuel: characterization, cofire limits, and evaluation of quality improvement measures. Water Sci Technol 98(12):2437–2448
Heindel JJ, Blumberg B (2019) Environmental obesogens: mechanisms and controversies. Ann Rev Pharmacol Toxicol 59(1):89–106
Hughes SR, Kay P, Brown LE (2013) Global synthesis and critical evaluation of pharmaceutical data sets collected from river systems. Environ Sci Technol 47:661–677. https://doi.org/10.1021/es3030148
International Diabetes Federation (2014) Diabetes Atlas. 6th ed. Available from: http://www.idf.org [accessed 28 October 2015]
Jokbling S, Nolan M, Tyler CR, Brighty G, Sumpter JP (1998) Widespread sexual disruptions in wild fish. Environ Sci Technol 32(17):2498–2506
Jurewicz J, Radwan M, Weilgomas B, Sobala W, Piskunowicz M, Radwan P, Bochenek M, Hanke W (2015) The effect of environmental exposure to pyrethroids and DNA damae in human sperm. Syst Biol Reprod Med 61:37–43
K’oreje KO, Demeestere K, De Wispelaere P, Vergeynst L, Dewulf J, Van Langenhove H (2012) From multiresidue screening to target analysis of pharmaceuticals in water: development of a new approach based on magnetic sector mass spectrometry and application in the Nairobi River basin, Kenya. Sci. Total Environ 437:153–164
K’oreje KO, Vergeynst L, Ombaka D, De Wispelaere P, Okoth M, Van Langenhove H, Demeestere K (2016) Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu City, Kenya. Chemosphere 149:238–244
Kamura K, Shin J, Kiyonari H, Abe T, Shioi G, Fukuhara A, Sasaki H (2018) Obesity in yap transgenic mice is associated with TAZ downregulation. Biochem Biophysic Res Commun 505(3):951–957
Karlsson CM, Cerro-Gálvez E, Lundin D, Karlsson C, Vila-Costa M, Pinhassi J (2019) Direct effects of organic pollutants on the growth and gene expression of the Baltic Sea model bacterium Rheinheimera sp. BAL 341. Microb Biotechnol 12(5):892–906
Koch HM, Calafat AM (2009) Human body burdens of chemicals used in plastic manufacture. Philos Trans Royal Soc B 364:2063–2078
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36(6):1202–1211
Langford KH, Reid MJ, Fjeld E, Øxnevad S, Thomas KV (2015) Environmental occurrence and risk of organic UV filters and stabilizers in multiple matrices in Norway. Environ Int 80:1–7
Langford KH, Thomas KV (2008) Inputs of chemicals from recreational activities into the Norwegian coastal zone. J Environ Monit 10:894–898
Lapworth D, Baran N, Stuart M, Ward R (2012) Emerging organic contaminants in groundwater: a review of sources, fate and occurrence. Environ Pollut 163:287–303
Law KL, Thompson RC (2014) Microplastics in the seas. Science 345:144–145
Lee DH, Porta M, Jacobs DR Jr, Vandenberg LN (2014) Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev 35:557–601
Leslie HA (2014) Review of microplastics in cosmetics. Institute for Environmental Studies [IVM]
Li X-F, Mitch WA (2018) Drinking water disinfection byproducts (DBPs) and human health effects: multidisciplinary challenges and opportunities. Environ Sci Technol 52:1681–1689
Lindstrom A, Buerge IJ, Poiger T, Bergqvist PA, Muller MD, Buser HR (2002) Occurrence and environmental behaviour of the bacteriacide triclosan and its methyl derivative in surface waters and in wastewater. Environ Sci Technol 36:2322–2329
Liu Y, Chen Z, Shen J (2013) Occurrence and removal characteristics of phthalate esters from typical water sources in Northeast China. J Anal Methods Chem:1–8
Ljunggren SA, Helmfrid I, Salihovic S, van Bavel B, Wingren G, Lindahl M, Karlsson H (2014) Persistent organic pollutants distribution in lipoprotein fractions in relation to cardiovascular disease and cancer. Environ Int 65:93–99. https://doi.org/10.1016/j.envint.2013.12.017
Magliano DJ, Loh VH, Harding JL, Botton J, Shaw JE (2014) Persistent organic pollutants and diabetes: a review of the epidemiological evidence. Diabetes Metab 40:1–14. https://doi.org/10.1016/j.diabet.2013.09.006
Maruya KA, Dodder NG, Sengupta A, Smith DJ, Lyons JM, Heil AT, Drewes JE (2016) Multimedia screening of contaminants of emerging concern (CECS) in coastal urban watersheds in Southern California (USA). Environ Toxicol Chem 35:1986–1199
Marzouk M, Ali M (2018) Mitigating risks in wastewater treatment plants PPPs using minimum revenue guarantee and real options. Util Policy 53:121–133
Maule AL, Scarpaci MM, Proctor SP (2019) Uninary concentrations of permethrin metabolires in US Army personnel in comparison with the US adult population, occupationally exposed cohorts, and other general populations. Int J Hyg Environ Health 222:355–363
Mudgal S, Lyons L, Bain J, et al. (2011) Plastic waste in the environment – Revised Final Report for European Commission DG Environment. Bio Intelligence Service. http://www.ec.europa.eu/environment/waste/studies/pdf/plastics.pdf
Muthulakshmi S, Hamideh PF, Habibi HR, Maharajan K, Kadirvelu K, Mudili V (2018) Mycotoxin zearalenone induced gonadal impairment and altered gene expression in the hypothalamic-pituitary-gonadal axis of adult female zebrafish (Danio rerio). J Appl Toxicol 38:1388–1397
NTP (National Toxicology Program) (2014) Report on carcinogens, Thirteenth Edition. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service. http://ntp.niehs.nih.gov.
OECD (2012) Water quality and agriculture – meeting the policy challenge. OECD Studies on Water. OECD Publishing
Oliveira TS, Murphy M, Mendola N, Wong V, Carlson D, Waring L (2015) Characterization of pharmaceuticals and personal care products in hospital effluent and waste water influent/effluent by direct-injection LC-MS-MS. Sci Total Environ 518–519:459–478
Ort C, Lawrence MG, Reungoat J, Eaglesham G, Carter S, Keller J (2010) Determining the fraction of pharmaceutical residues in wastewater originating from a hospital. Water Res 44:605–615
Ortiz de García S, Pinto Pinto G, García Encina P, Irusta Mata R (2013) Consumption and occurrence of pharmaceutical and personal care products in the aquatic environment in Spain. Sci Total Environ 444:451–465
Prebihalo S, Brockman A, Cochran J, Dorman FL (2015) Determination of emerging contaminants in wastewater utilizing comprehensive two-dimensional gas-chromatography coupled with time-of-flight mass spectrometry. J Chromatogr A 1419:109–115
Qadir M, Wichelns D, Raschid-Sally L, McCornick PG, Drechsel P, Bahri A, Minhas PS (2010) The challenges of waste- water irrigation in developing countries. Agric Water Manag 97:561–568
Reaves DK, Ginsburg E, Bang JJ, Fleming JM (2015) Persistent organic pollutants and obesity: are they potential mechanisms for breast cancer promotion? Endocr Relat Cancer 22:R69–R86
Rengarajan T, Rajendran P, Nandakumar N, Lokeshkumar B, Rajendran P, Nishigaki I (2015) Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac J Trop Biomed 5(3):182–189
Ribeiro AR, Maia A, Santos M, Tiritan ME, Rosa Ribeiro CM (2016) Occurrence of natural contaminants of emerging concern in the Douro River Estuary, Portugal. Arch Environ Contam Toxicol 70:361–371
Rochman CM, Kross SM, Armstrong JB, Bogan MT, Darling ES, Green SJ, Smyth AR, Veríssimo D (2015) Scientific evidence supports a ban on microbeads. Environ Sci Technol 49:10759–10761
Ryan PG, Moloney CL (1990) Plastic and other artifacts on South Aafrican beaches – temporal trends in abundance and composition. S Afr J Sci 86(7–10):450–452
Sánchez-Avila J, Bonet J, Velasco G, Lacorte S (2009) Determination and occurrence of phthalates, alkylphenols, bisphenol A, PBDEs, PCBs and PAHs in an industrial sewage grid discharging to a municipal wastewater treatment plant. Sci Total Environ 407:4157–4167
Sato T, Qadir M, Yamamoto S, Endo T, Zahoor A (2013) Global, regional, a country level need for data on wastewater generation, treatment, and use. Agric Water Manag 130:1–13
Sayer RD, Hill JO (2019) Exercise in the treatment of obesity. In: Sbraccia P, Finer N (eds) Obesity. Endocrinology. Springer, Cham
Schirmer K, Schirmer M (2008) Who is chasing whom? A call for a more integrated approach to reduce the load of micro-polltants in the environment. Water Sci Technol 57(1):145–150
Science for Environment Policy (2011) Plastic waste: ecological and human health impacts. European Commision Directorate-General Environment. http://ec.europa.eu/environment/integration/research/newsalert/index_en.htm
Sorensen JPR, Lapworth DJ, Nkhuwa DCW, Stuart DC, Bell RA, Chirwa M, Kabika J, Liemisa M, Chibesa M, Pedley S (2015) Emerging contaminants in urban groundwater sources in Africa. Water Res 72:51–63
Srogi K (2008) Levels and congener distributions of PCDDs. PCDFs and dioxin-like PCBs in environmental and human samples: a review. Environ Chem Letters 6:1–28
Stockholm Convention (2009) The new POPs under the Stockholm Convention. http://chm.pops.int/Implementation/NewPOPs/TheNewPOPSs/tabid/672/Default.aspx
Stockholm Convention on Persistent Organic Pollutants (2001) United Nations Environment Programme. http://www.pops.int/
Stout SA, Emsbo-Mattingly SD, Douglas GS, Uhler AD, McCarthy KJ (2015) Beyond 16 priority pollutant PAHs: a review of PACs used in environmental forensic chemistry. Polycycl Aromat Compd 35(2–4):285–315
Suarez-Lopez JR, Clemesha CG, Porta M, Gross MD, Lee D-H (2019) Organochlorine pesticides and polychlorinated biphenyls (PCBs) in early adulthood and blood lipids over 23-year follow-up. Environ Toxicol Pharmacol 66:24–35
Tabone MD, Cregg JM, Beckman EJ, Landis AE (2010) Sustainability metrics: life cycle assessment and green design in polymers. Environ Sci Technol 44:8264–8269
Teuten EL, Saquing JM, Knappe DRU, Barlaz MA, Jonsson S, Björn A, Rowland SJ, Thompson RC, Galloway TS, Yamashita R, Ochi D, Watanuki Y, Moore C, Viet PH, Tana TS, Prudente M, Boonyatumanond R, Zakaria MP, Akkhavong K, Ogata Y, Hirai H, Iwasa S, Mizukawa K, Hagino Y, Imamura A, Saha M, Takada H (2009) Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc B 364:2027–2045
Thakur S, Chaudhary J, Sharma B, Verma A, Tamulevicius S, Thakur VK (2018) Sustainability bioplastics: opportunites and challenges. Curr Opin Green Sustain Chem 13:68–75
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AWG, McGonigle D, Russell AE (2004) Lost at sea: where is all the plastic? Science 304:838–838
Tran NH, Reinhard M, Gin KY-H (2018) Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res 133:182–207
Tröger R, Klöckner P, Ahrens L, Wiberg K (2018) Micropollutants in drinking water from source to tap – method development and application of a multiresidue screening method. Sci Total Environ 627:1404–1432
UNEP (2013) Stockholm convention [Online]. Available: http://chm.pops.int/TheConvention/ThePOPs/tabid/673/Default.aspx
UNESCO (2012) Managing water under uncertainty and risk. The United Nations World Water Development Report 4. United Nations Educational, Scientific and Cultural Organization, Paris
UN-Water (2014) A post-2015 global goal for water: synthesis of key findings and recommendations from UN-Water. January 2014. http://www.un.org/waterforlifedecade/pdf/27_01_2014_un-water_paper_on_a_post2015_global_goal_ for_water.pdf
USEPA (2011) U.S. Environmental Protection Agency. An exposure assessment of Polybrominated Diphenyl ethers. http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=210404
Valcárcel Y, Alonso SG, Rodríguez-Gil JL, Maroto RR, Gil A, Catalá M (2011) Analysis of the presence of cardiovascular and analgesic/anti-inflammatory/antipyretic pharmaceuticals in river-and drinking-water of the Madrid Region in Spain. Chemosphere. 82(7):1062–1071
Wang M, Yang XD, Bi WT (2015) Application of magnetic graphitic carbon nitride nanocomposites for the solid-phase extraction of phthalate esters in water samples. J Sep Sci 38:445–452
WHO (2006) WHO guidelines for the safe use of wastewater, excreta and greywater. World Health Organization, Geneva
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva ISBN:978-92-4-154815-1
WHO/FAO (2010) Joint FAO/WHO expert meeting to review toxicological and health aspects of bisphenol A. Summary Report in cluding Report of Stakeholder Meeting on Bisphenol A
Wick LY, Chatzinotas A (2019) Capacity of ecosystems to degrade anthropogenic chemicals. In: Schröter M, Bonn A, Klotz S, Seppelt R, Baessler C (eds) Altas of Ecosystem Services. Springer, Cham
Wilk BK, Fudala-Ksiazek S, Szopińska M, Luczkiewicz A (2019) Landfill leachates and wastewater of maritime origin as possible sources of endocrine disruptors in municipal wastewater. Environ Sci Pollut Res 26(25):25690–25701
Wilkinson JL, Hooda PS, Swinden J, Barker J (2018) Spatial (bio)accumulation of pharmaceuticals, illicit drugs, plasticers, perfluorinated compounds and metabolites in river sediment, aquatic plants and benthic organisms. Environ Pollut 234:864–875
Ye M, Beach J, Martin JW, Senthilselvan A (2016) Urinary concentrations of pyrethroid metabolites and its association with lung function in a Canadian general population. Occup Environ Med 73(2):119–126
Yu B, Zhang R, Liu P, Zhang Y, Zhang Y, Bai Y (2015) Determination of nine hydroxylated polybrominated diphenyl ethers in water by precolumn derivatization-gas chromatography mass spectrometry. J Chromatogr A 1419:19–25
Yurtsever M, Yurtsever U (2019) Use of a convolutional neural network for the classification of microbeads in urban wastewater. Chemosphere 216:271–290
Zhu Q, Jia J, Zhang K, Zhang H, Liao C (2019) Spatial distribution and mass loading of phthalate esters in wastewater treatment plants in China: an assessment of human exposure. Sci Total Environ 656:862–869
Acknowledgements
The South Africa Medical Research Council (SAMRC) and the Govan Mbeki Research and Development Centre (GMRDC), University of Fort Hare, South Africa, are highly appreciated for their support. Prof. A. I. Okoh and Prof U. U. Nwodo are duly acknowledged for their advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Responsible Editor: Hongwen Sun
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Unuofin, J.O. Garbage in garbage out: the contribution of our industrial advancement to wastewater degeneration. Environ Sci Pollut Res 27, 22319–22335 (2020). https://doi.org/10.1007/s11356-020-08944-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08944-5