Abstract
Fluoride (F) and cadmium (Cd) are two common water pollutants. There is low information about their co-exposure in low doses. So, in this study, we evaluated the combination effects of non-toxic doses of F and Cd and the possible mechanism of their combined interaction. Male rats were exposed to non-toxic doses of sodium fluoride (30 mg/l) and/or cadmium chloride (40 mg/l) in drinking water for 6 weeks. Then, liver tissues were separated and several factors including oxidative stress, mitochondrial toxicity, inflammation, apoptosis, and biochemical and histopathological changes were evaluated. Cd and F alone did not induce any significant changes in evaluated factors compared to control group, while significant elevation in liver enzymes as well as histopathological changes were observed in rats treated with F+Cd. Also, a remarkable increase in oxidative stress markers including reactive oxygen species, lipid peroxidation, and protein carbonyl and also decreasing glutathione and superoxide dismutase levels were detected following co-exposure to F and Cd. Furthermore, a combination of F and Cd resulted in mitochondrial dysfunction, swelling, as well as a reduction in mitochondrial membrane potential in isolated liver mitochondria. On the other hand, TNF-α, IL-1β, and NF-kB inflammatory genes were upregulated in the liver after combined exposure to F and Cd compared to individual treatments. Also, F+Cd treatment increased the Bax expression but decreased the expression of Bcl-2 significantly. These findings suggest that Cd and F can potentiate their individual toxic effects on the liver tissue through disruption of the cellular redox status, inflammation, and apoptosis pathway.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Humans are exposed to hundreds of chemicals in the environment including metals, pesticides, and polychlorinated biphenyls, which can affect their health (Whittaker et al. 2011). In the majority of toxicological studies, a single chemical has been used in highly predictable toxic dose. However, in the environment, people are encountered with simultaneous multiple exposure to low doses of these chemicals. So, it is rational to investigate the effects of chemical combinations rather than individuals (Yuan et al. 2014).
Cadmium (Cd) is a well-known environmental pollutant (Chen et al. 2018). It is not an essential element and does not play a physiological role in the human body, but it is widely found in soil, air, food, and water at various concentrations (Chen et al. 2016). Therefore, people are chronically exposed to Cd, raising concerns about its health effects. So, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) defined the provisional tolerable weekly intake (PTWI) for Cd as 400–500 μg/person/week (Satarug et al. 2000). Furthermore, the World Health Organization (WHO) estimated the safe level of Cd in drinking water as 1–3 μg/l (Fouad and Jresat 2015). Biological half-life of Cd is between 5 and 30 years, and also, it can be accumulated in multiple tissues. Therefore, chronic exposure to Cd even at biologically low levels is associated with irreversible damages to body organs, of which the liver, kidney, bone, and testes are the most sensitive (Khan et al. 2019). The International Agency for Research on Cancer (IARC) classified Cd into group 1 carcinogens (Andjelkovic et al. 2019).
Fluoride (F), as an inorganic anion, is the 13th most abundant element in the Earth’s crust. It is found in foods, water, coal, pesticides, bottled teas, and toothpastes. Water is the main source of human exposure to F (Mohammed et al. 2017; Sun et al. 2018). Also, in many countries, F has been added to the drinking water because of its role in the prevention of dental caries and maintenance of bone homeostasis (Alhusaini et al. 2018b). Therefore, many studies investigated the safe amount of F in drinking water. On this basis, the WHO has recommended the amount of 1.5 mg/l as a maximum acceptable limit of F level in drinking water (Tuyen et al. 2016). However, water with F concentration above the permissible limit of 1.5 mg/l has been recorded from several parts of the world such as China, India, Canada, Norway, Turkey, Mexico, and Brazil (Brindha and Elango 2011). In addition, based on research articles and some clinical overdose cases, the probable toxic dose (PTD) of F was defined as 5 mg/kg of body weight (Kanduti et al. 2016). Indeed, F is a cumulative toxicant and, in a long-time exposure, it can cause several damages to soft tissues such as the thyroid, liver, kidney, brain, and reproductive organ (Alhusaini et al. 2018b).
Co-exposure to F and Cd is non-avoidable due to their common presence in the environment, especially in drinking water. Based on experimental studies, both F and Cd show their tissue-toxic effects through induction of oxidative stress and inflammation. Oxidative stress has been defined as an imbalance between the production and re-neutralization of free radicals in the body (Shaki et al. 2019). Oxidative stress plays an essential role in the emergence of a number of chronic disorders such as diabetes and cancer by inducing inflammation (Acaroz et al. 2019). It was also reported that administration of Cd led to significant cardiotoxicity in rats through production of reactive oxygen species (ROS) and also by enhancing several inflammatory cytokine levels such as interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), and nuclear factor kappa B (NF-kΒ) (Zhang et al. 2018). Furthermore, in another study, a significant reduction in the endogenous antioxidants superoxide dismutase (SOD) and glutathione (GSH) and also an increase of inflammatory mediators were observed in the liver tissue of male rats in response to F treatment (Alhusaini et al. 2018a). ROS can also trigger NF-kΒ inflammatory pathway, which is involved in generation of several inflammatory mediators such as interleukin-6, nitric oxide (NO), and cyclooxygenase-2 (Hoesel and Schmid 2013).
On the other hand, mitochondria are the major site for ROS production and the key intracellular target for Cd as well as F toxicity (Pan et al. 2018; Wei et al. 2018). Mitochondria are considered as the major site of oxidative phosphorylation and play an important role in mediating apoptosis. It is well documented that mitochondrial dysfunction due to excessive production of ROS can trigger the intrinsic apoptosis pathway (Dai et al. 2018).
The liver, due to its major role in detoxification of xenobiotics, is the most sensitive organ against chemical-induced toxicity. Simultaneous exposure to environmental chemicals can produce effects different from their individual exposure. Also, chemicals may synergistically increase the toxicity of each other even at low doses which are not considered to be toxic (Alese et al. 2018).
So, to the best of our knowledge, this study is the first attempt to evaluate the combination of toxic effects of Cd and F in a sub-chronic experimental model of hepatotoxicity using doses below the toxic levels. We also investigated the underlying cellular and molecular mechanisms based on oxidative stress, NF-kΒ, and mitochondrial apoptosis pathways.
Materials and methods
Animal treatment and study design
Male Wistar rats (200–250 g) were housed at a controlled room (on a 12-h light/dark cycle at a temperature of 22 °C ± 2 °C) with free access to food and water. All experimental procedures were conducted according to the ethical standards and protocols approved by the Committee of Animal Experimentation of Mazandaran University of Medical Sciences, Sari, Iran.
In the first step, for selecting non-toxic doses, animals were exposed to several doses of sodium fluoride (15 mg/l, 30 mg/l, 60 mg/l, and 120 mg/l) and cadmium chloride (20 mg/l, 40 mg/l, 60 mg/l, and 100 mg/l) in drinking water, and then several toxicity hallmarks in liver tissue including ROS generation, lipid peroxidation, protein carbonyl formation, GSH depletion, as well as mitochondrial function and serum ALT, AST, and ALP levels were investigated. These selected doses were below the common toxic doses, which were used in previous literatures. Finally, the highest doses which did not induce any significant toxic effects in the liver (30 mg/l for sodium fluoride and 40 mg/l for cadmium chloride) were considered as non-toxic doses and included in the study (data not shown).
Then, rats were divided into four groups of six rats for evaluation of combination toxicity and were treated as below for 6 weeks:
-
1.
Group 1, control (received only drinking water)
-
2.
Group 2, received Cd (as cadmium chloride 40 mg/l, dissolved in drinking water)
-
3.
Group 3, received F (as sodium fluoride 30 mg/l, dissolved in drinking water)
-
4.
Group 4, received cadmium chloride 40 mg/l + sodium fluoride 30 mg/l, dissolved in drinking water
At the end of 6 weeks, the animals were anesthetized and blood samples were immediately collected from their hearts. Then, blood serums were obtained by centrifugation (3500×g for 10 min). Liver tissues were separated, homogenized, and then centrifuged at 2000×g for 10 min. Supernatants were separated and centrifuged again at 10,000×g for 10 min. Then, supernatants were stored at − 80 °C until use for biochemical estimations. Liver tissues required for gene expression analysis were kept at − 80 °C in RNA-later solution. Liver tissues were fixed in 10% formalin solution for histopathological examination.
Assessment of liver oxidative stress
Primarily, protein concentrations were determined in samples through the Coomassie Brilliant Blue protein binding as explained by the Bradford method (Bradford 1976). Then, all samples were equalized in 1 mg protein/ml for evaluation of oxidative stress parameters. ROS formation was assessed using dichlorofluorescin diacetate (DCFH-DA) as an indicator (Vahidirad et al. 2018). Malondialdehyde (MDA) contents were estimated by reacting with thiobarbituric acid as a marker of lipid peroxidation (Zhang et al. 2008). Elman’s reagent–based assay was carried out to determine the GSH activity in the liver tissues (Ellman 1959). Protein oxidation was evaluated through measuring protein carbonyl production (μM) (Aebi 1984). SOD activity was estimated using Nasdox commercial kit (Navand Salamat, Iran).
Assessment of liver inflammation
Gene expression analysis of NF-kΒ, TNF-α, and IL-1β in the liver
Total RNA was separated from liver tissues using Hybrid-R kit (GeneAll, Seoul, South Korea). cDNA synthesis was performed by a commercial kit (Yekta Tajhiz Azama, Iran). Real-time PCR was done using a Corbett machine (Rotor-Gene 6000). The thermal profile for the PCR reaction was 95 °C for 15 min (denaturation), followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 15 s, followed by melting curve analysis. After PCR amplification, the ΔCt was determined. PCR primers for IL-1β, TNF-α, and NF-kΒ genes were prepared by Metabion International AG (Metabion GmbH, Germany) using the Primer-Blast program from the National Center for Biotechnology Information (NCBI). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was employed as an internal control and appeared to be unchanged by the different therapies (Almeer et al. 2018). The used primer pairs are listed in Table 1.
Assessment of nitric oxide level in the liver
Nitric oxide was evaluated using the Zistfanteb kit (Cib Biotech, Iran) based on the Griess reaction (Habibi et al. 2018).
Assessment of mitochondrial toxicity in the liver
Mitochondrial preparation
Liver mitochondria were prepared using differential centrifugation (Shaki et al. 2012). Mitochondria were extracted just after rats were anesthetized, and fresh isolated mitochondria were used. Indeed, liver tissues were homogenized and the nuclei and broken cell debris were sedimented through centrifuging at 2000×g for 10 min at 4 °C. Then, the supernatant was subjected to a further centrifugation at 10,000×g for 10 min. The mitochondrial sediment was suspended in Tris HCl buffer (0.05 M Tris HCl, 0.25 M sucrose, 20 mM KCl, 2 mM MgCl2, and 1 mM Na2HPO4, pH of 7.4) (Shaki et al. 2012).
Evaluation of mitochondrial ROS generation
Mitochondrial ROS level was measured using DCFH-DA as an indicator. DCFH-DA is hydrolyzed by cellular esterases to dichlorofluorescin (DCFH), which can be oxidized by ROS to dichlorofluorescein (DCF), which is a fluorescent product (Kalyanaraman et al. 2012). Briefly, DCFH-DA was added to fresh isolated mitochondrial samples and, after the 10-min incubation, the fluorescence intensity of DCF was determined using a fluorescence spectrophotometer (Shimadzu RF5000U) at 485 nm excitation and 520 nm emission wavelengths (Habibi et al. 2018).
Evaluation of mitochondrial function
Mitochondrial function was evaluated by measuring the reduction of tetrazolium salt (MTT). Briefly, MTT (0.4%) was added to 100 μl of fresh isolated mitochondrial suspensions (1 mg protein/ml) and then incubated at 37 °C for 30 min. The product of formazan crystals was dissolved in 150 μl dimethyl sulfoxide, and the absorbance was measured with an ELISA reader (ELX800; BioTek, USA) at 570 nm wavelength (Shokrzadeh et al. 2015).
Estimation of the mitochondrial membrane potential
Mitochondrial uptake of the cationic fluorescent dye, rhodamine 123, has been used to estimate mitochondrial membrane potential (MMP). Ten micromolars of rhodamine 123 was added to the fresh isolated mitochondrial fraction (1 mg protein/ml) solution in MMP assay buffer (220 mM sucrose, 68 mM d-mannitol, 10 mM KCl, 5 mM KH2PO4, 2 mM MgCl2, 50 μM EGTA, 5 mM sodium succinate, 10 mM HEPES, and 2 μM rotenone). The fluorescence intensity of released rhodamine in the outer space of mitochondria was monitored using a fluorescence spectrophotometer (Shimadzu RF5000U) at the excitation and emission wavelengths of 490 nm and 535 nm, respectively (Arab-nozari et al. 2019). Increasing the fluorescence intensity shows reduction in MMP.
Determination of the mitochondrial swelling
Analysis of mitochondrial swelling in the isolated liver mitochondria was estimated through changes in light scattering as monitored spectrophotometrically at 540 nm. Briefly, fresh isolated mitochondria were suspended in swelling buffer (70 mM sucrose, 230 mM mannitol, 3 mM HEPES, 2 mM Tris-phosphate, 5 mM succinate, and 1 μM rotenone). The absorbance was read using an ELISA reader (Tecan; Rainbow Thermo, Austria). Decreasing absorbance indicates an increase in mitochondrial swelling (Hosseini et al. 2013).
Evaluation of apoptosis in liver tissue
Expression levels of Bax and Bcl-2 genes were evaluated through the real-time PCR method as described in the section “Gene expression analysis of NF-kΒ, TNF-α, and IL-1β in the liver.” The primer pairs are listed in Table 1.
Serum biochemical evaluation
Serum levels of ALT, AST, and ALP were determined as one of the important indicators of liver tissue damage using commercial kits (Pars Azmun, Iran).
Liver histopathological examination
Liver tissue samples were fixed in 10% formalin, dehydrated and embedded in paraffin, and then sectioned. The deparaffinized sections were consistently stained with hematoxylin and eosin for light microscopy (Abarikwu et al. 2017).
Statistical analysis
Results are presented as mean ± SD. Data from various evaluations were investigated by one-way analysis of variance followed by Tukey’s post hoc test using a statistical program (SPSS, version 17.0). In this study, p values < 0.05 were considered statistically significant.
Results
Effects of treatments on liver oxidative stress markers
As shown in Table 2, exposure to the combination of F and Cd demonstrated a significant (p < 0.05) increase in ROS, MDA, and protein carbonyl levels, while SOD and GSH levels were reduced compared to control and individual groups. In contrast, animals, which were exposed to F or Cd alone, showed no differences in above parameters compared to their control animals.
Effects of treatments on liver inflammation
Effects of treatments on liver TNF-α, IL-1β, and NF-kB gene expression levels
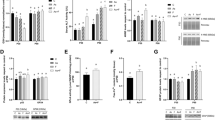
Figure 1 shows the effects of F or/and Cd on the gene expression of liver pro-inflammatory cytokines (TNF-α, IL-1β) and also NF-kB. Expression of TNF-α, IL-1β, and NF-kB was markedly upregulated (p < 0.05) in the liver of the rats treated with a combination of F and Cd compared to the animals exposed to F alone or Cd alone, while exposure to F and Cd alone did not significantly affect the expression of these markers.
Effect of treatments on IL-1β, TNF-α, and NF-kΒ gene expression in rat liver tissue. Groups: C control, F fluoride, Cd cadmium, F+Cd fluoride + cadmium. Values are expressed as mean ± SD. ***p < 0.001, significantly different compared to the C group. #p < 0.05, ##p < 0.01, and ###p < 0.001, significantly different compared to the F group. $p < 0.05 and $$$p < 0.001, significantly different compared to the Cd group
Effects of treatments on liver nitric oxide levels
NO level was significantly increased in the liver of rats treated with F+Cd in comparison with individual and control groups (p < 0.05), while F or Cd treatment alone did not show a significant change in the NO levels in comparison with the control group (Fig. 2).
Effect of treatments on nitric oxide levels in rat liver tissue. Groups: C control, F fluoride, Cd cadmium, F+Cd fluoride + cadmium. Values are expressed as mean ± SD. ***p < 0.001, significantly different compared to the C group. #p < 0.05, significantly different compared to the F group. $p < 0.05, significantly different compared to the Cd group
Effects of treatments on isolated liver mitochondria
Effects of treatments on liver mitochondrial ROS formation
As shown in Fig. 3a, ROS formation was increased in rats that received a combination of F and Cd compared to control and individual groups in isolated liver mitochondria, while F or Cd alone could not induce significant ROS generation compared to control rats.
Effect of treatments on mitochondrial function (panel a) and ROS production (panel b) in isolated liver mitochondria. Groups: C control, F fluoride, Cd cadmium, F+Cd fluoride + cadmium. Values are expressed as mean ± SD. ***p < 0.001, significantly different compared to the C group. ##p < 0.01 and ###p < 0.001, significantly different compared to the F group. $p < 0.05 and $$$p < 0.001, significantly different compared to the Cd group
Effects of treatments on liver mitochondrial function
The mitochondrial function was evaluated by the MTT test. The yellow tetrazolium salt (MTT) was metabolized by a mitochondrial succinate dehydrogenase enzyme to yield a purple formazan reaction product, which is an indicator of mitochondrial function. As shown in Fig. 3b, the combination of F and Cd significantly decreased mitochondrial function in comparison with control and F or Cd alone groups (p < 0.05). However, individual exposure to F or Cd did not specify marked alteration in mitochondrial function.
Effects of treatments on liver mitochondrial membrane potential
MMP is a sensitive indicator of the mitochondrial membrane condition. MMP collapse results in an increase in rhodamine release from the mitochondria to the outer space. Therefore, an increase in florescence intensity of rhodamine can be considered as an indicator of MMP loss and mitochondrial damage. As shown in Fig. 4a, F+Cd treatment significantly increased rhodamine fluorescence intensity (decreased the MMP) (p < 0.05). Additionally, there was no significant difference between F and Cd alone in comparison with the control group.
Effect of treatments on rhodamine fluorescence intensity (panel a) and swelling (panel b). Rhodamine fluorescence intensity was determined as an indicator of mitochondrial membrane potential condition. Mitochondrial swelling was assessed by reading the absorbance at 540 nm wavelength as described in the Materials and methods. **p < 0.01, significantly different compared to the C group. #p < 0.05, significantly different compared to the F group. $p < 0.05 and $$p < 0.01, significantly different compared to the Cd group
Effects of treatments on liver mitochondrial swelling
We assayed mitochondrial swelling, as an indicator of mitochondrial membrane permeability. Mitochondrial swelling results in more light scattering and ultimately reduced the detected absorbance. As shown in Fig. 4b, co-exposure to F and Cd resulted in significant (p < 0.05) mitochondrial swelling in comparison with control and individual F or Cd groups. In contrast, treatment of F or Cd alone had no significant effect on mitochondrial swelling.
Effects of treatments on liver apoptosis
As shown in Fig. 5, the expression levels of anti-apoptotic Bcl-2 were significantly downregulated, while those of apoptotic Bax were significantly upregulated in the liver tissue of the rats treated with F plus Cd (p < 0.05). However, no significant alteration occurred in the expression levels of Bcl-2 and Bax following treatment with F or Cd alone in comparison with the control group.
Effect of treatments on Bax and Bcl-2 gene expression in rat liver tissue. Groups: C control, F fluoride, Cd cadmium, F+Cd fluoride + cadmium. Values are expressed as mean ± SD. **p < 0.01 and ***p < 0.001, significantly different compared to the C group. #p < 0.05 and ##p < 0.01, significantly different compared to the F group. $p < 0.05 and $$$p < 0.001, significantly different compared to the Cd group
Effects of treatments on serum biochemical indicators of hepatotoxicity
Exposure to F or Cd demonstrated no significant alterations in the ALT, AST, and ALP levels in comparison with the control group. On the other hand, the levels of these enzymes were significantly (p < 0.05) increased following exposure to F+Cd in comparison with the F or Cd individual exposure (Table 3).
Effects of treatments on liver histopathological features
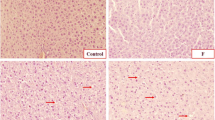
As shown in Fig. 6, the hepatic tissue of the control group showed a normal liver architecture with normal lobular pattern. The examined liver tissue of the F+Cd-treated rats showed sinusoidal dilation, inflammatory cell infiltration, and proliferation of Kupffer cells. On the other hand, we were not able to observe any significant changes within the liver anatomical structure of rats treated with these two chemicals, individually.
Representative photomicrographs of liver sections from rats treated with control (C), fluoride (F), cadmium (Cd), and fluoride + cadmium (F+Cd). (Liver sections were fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin-eosin). Light microscopic examination (× 40 objective lens) of tissues. a Tissue sections from control rat. b F+Cd-administered rat tissue sections. c Cd-treated rat tissue sections. d Tissue sections from F-treated rat
Discussion
Recently, there are increasing toxicological research studies focusing on the interaction between chemicals in the environment (Philippot et al. 2019). In this study, non-toxic doses of two water pollutants (Cd and F) were employed alone and in combination in order to investigate whether the toxic effects of these chemicals were enhanced by their combination in comparison with their alone effects.
Experimental studies declared that both Cd and F could induce oxidative stress in the liver tissues. For example, Saleh (2018) reported that exposure to Cd for 4 weeks led to elevation of lipid peroxidation and reduction in endogenous antioxidants (GSH and SOD) in the liver of male rats (Saleh 2018). In another study, it was shown that F administration increased ROS levels, which resulted in oxidation of membrane lipids and GSH as well as reduction of SOD and catalase levels in the liver of mice (Lu et al. 2017). Our results showed that the combination of non-toxic doses of F and Cd resulted in significant generation of ROS and also oxidative damage to cellular macromolecules such as lipids and proteins. Free radicals can promote breakdown of membranes and oxidation of membrane phospholipids to form MDA as a hallmark of tissue oxidative damage (Abu-El-Zahab et al. 2019).
In addition, ROS is capable of targeting cellular proteins. During this event, ROS react with amino acid chains of the protein molecule, producing a detectable marker of protein oxidative damage, called protein carbonyl (Baskaran et al. 2018).
On the other hand, in our study, F+Cd treatment resulted in a remarkable decline in the activity of two pivotal endogenous antioxidants (GSH and SOD), which have clear protective effects against oxidative stress. GSH is considered as a primary indicator of oxidative damage to organs. It is a reservoir of cysteine and participates in immediate cell response to xenobiotics (Bharti et al. 2014). It has been found that both F and Cd can react with sulfhydryl group in the GSH structure, destroying its normal function (Almeer et al. 2018; Chouhan et al. 2013). On the other hand, SOD is the first line of cell defense against deleterious effects of ROS by converting superoxide anion radicals into hydrogen peroxide and molecular oxygen (Faraji et al. 2019). F acts as a competitive inhibitor of SOD and alters its function by binding with CU++ at the binding site of this enzyme (Jana et al. 2018). Furthermore, Cd can affect the stability of SOD, due to its high affinity to thiol groups in SOD enzyme (Elkhadragy et al. 2018).
We also found that combined exposure to non-toxic levels of F and Cd showed an increase in the hepatic NF-kΒ, IL-1β, and TNF-α expression as well as NO level. These were consistent with the results of previous studies, which reported the ability of F and Cd to induce inflammatory mediators in different tissues (Alhusaini et al. 2018a; Almeer et al. 2018). Based on explanations of mechanistic studies, oxidative stress is involved in triggering several inflammatory processes in cells, in which the NF-kΒ signaling pathway is the most notable (Hoesel and Schmid 2013). The NF-kΒ is a cytosolic protein complex, which is responsible for controlling the expression of many inflammatory mediators. IL-1β and TNF-α are the most important stimulating signaling molecules in the induction of canonical pathway of NF-kB. After activation, NF-kB p65 sub-unit is released from cytosolic inhibitors and translocates to the nucleolus and subsequently upregulates the expression of a large number of inflammatory target genes such as interleukin-6, nitric oxide synthase, and cyclooxygenase (Hoesel and Schmid 2013). Accumulated evidences have suggested that oxygen-derived free radicals can promote NF-kB upregulation through overproduction of IL-1β and TNF-α cytokines (Wang et al. 2019). So, it can be concluded that the elevation of these inflammatory markers in our study may be due to the occurrence of oxidative stress in liver tissue.
Previous studies reported mitochondrial toxicity and also apoptosis induced by F as well as Cd (Pan et al. 2018; Wei et al. 2018; Zhang et al. 2018). In the present study, we observed significant mitochondrial swelling and loss of MMP in F+Cd-treated rats compared to control and individual groups, which can reflect mitochondrial dysfunction. Exposure to oxidant chemicals can induce oxidation of –SH groups in the mitochondrial outer membrane, leading to formation of disulfide cross-link between thiol groups and opening of mitochondrial membrane transition pore. This event is associated with the release of cytochrome c to the cytosol and activation of caspase-9 and caspase-3, which finally results in cell death (He and Lemasters 2002). Furthermore, we found that intoxication with a combination of F and Cd upregulated Bax (pro-apoptotic) and downregulated Bcl-2 (anti-apoptotic) proteins. Indeed, mitochondria are the primary site of ROS generation in organs. It is well documented that excessive ROS can promote apoptosis through mitochondria-dependent pathway. So, inhibition of Bcl-2 and also induction of Bax result in opening of the mitochondrial permeability transition pore and induction of apoptosis as mentioned above (Wang et al. 2019).
Furthermore, ROS-induced lipid peroxidation is also accompanied with the rupture and loss of integrity of hepatocyte membrane, which appears with releasing ALT, AST, and ALP enzymes to the bloodstream (Chen et al. 2011). As shown in the study of Alhusaini et al. (2018a) and Elbaghdady et al. (2018), marked elevation in serum ALT, AST, and ALP was observed in F- or Cd-treated animals, which was in accordance with our findings in the combined F+Cd group.
As evident from our histopathological findings, co-exposure to F+Cd resulted in sinusoid dilatation and proliferation of Kupffer cells. Activation of these cells induces release of many inflammatory mediators such as NO, IL-1β, and TNF-α in hepatocytes. These pathological changes were parallel with increasing oxidative stress and inflammation in the liver of rats treated with F+Cd.
Conclusions
For the first time, the present study revealed that the combination of non-toxic doses of F and Cd has the ability to induce toxicity in the liver tissue of rats. We speculated that F and Cd can potentiate their individual toxicity through different mechanistic pathways including mitochondrial damage, oxidative stress, apoptosis, and inflammation. Therefore, the combination of F and Cd in the environment can be considered as a potential health risk to humans and animals.
References
Abarikwu SO, Njoku R-C, Lawrence CJ, Charles IA, Ikewuchi JC (2017) Rutin ameliorates oxidative stress and preserves hepatic and renal functions following exposure to cadmium and ethanol. Pharm Biol 55:2161–2169
Abu-El-Zahab HS, Hamza RZ, Montaser MM, El-Mahdi MM, Al-Harthi WA (2019) Antioxidant, antiapoptotic, antigenotoxic, and hepatic ameliorative effects of L-carnitine and selenium on cadmium-induced hepatotoxicity and alterations in liver cell structure in male mice. Ecotoxicol Environ Saf 173:419–428
Acaroz U, Ince S, Arslan-Acaroz D, Gurler Z, Demirel HH, Kucukkurt I, Eryavuz A, Kara R, Varol N, Zhu K (2019) Bisphenol-A induced oxidative stress, inflammatory gene expression, and metabolic and histopathological changes in male Wistar albino rats: protective role of boron. Toxicol Res-UK 8:262–269
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126
Alese M, Agbaje M, Alese O (2018) Cadmium induced damage in Wistar rats, ameliorative potentials of progesterone. J Trace Elem Med Biol 50:276–282
Alhusaini A, Faddaa L, Ali HM, Hassan I, El Orabi NF, Bassiouni Y (2018a) Amelioration of the protein expression of Cox2, NFκB, and STAT-3 by some antioxidants in the liver of sodium fluoride–intoxicated rats. Dose-Response 16:1–8
Alhusaini AM, Faddah LM, El Orabi NF, Hasan IH (2018b) Role of some natural antioxidants in the modulation of some proteins expressions against sodium fluoride-induced renal injury. Biomed Res Int 2018:1–9
Almeer RS, Alarifi S, Alkahtani S, Ibrahim SR, Ali D, Moneim A (2018) The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of Nrf2 expression. Biomed Pharmacother 106:1490–1498
Andjelkovic M, Buha Djordjevic A, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Jovanovic M, Boricic N, Wallace D (2019) Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int J Environ Res Public Health 16:274
Arab-nozari M, Zamani E, Latifi A, Shaki F (2019) Mitochondrial toxicity of aluminium nanoparticles in comparison to its ionic form on isolated rat brain mitochondria. Bratisl Lek Listy 120:516–522
Baskaran R, Priya LB, Kumar VS, Padma VV (2018) Tinospora cordifolia extract prevents cadmium-induced oxidative stress and hepatotoxicity in experimental rats. J Ayurveda Integr Med 9:252–257
Bharti VK, Srivastava R, Kumar H, Bag S, Majumdar A, Singh G, Pandi-Perumal S, Brown GM (2014) Effects of melatonin and epiphyseal proteins on fluoride-induced adverse changes in antioxidant status of heart, liver, and kidney of rats. Adv Pharmacol Sci 2014:1–6
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brindha K, Elango L (2011) Fluoride in groundwater: causes, implications and mitigation measures. In: Fluoride properties, applications and environmental management, vol 1. Nova, pp 111–136
Chen AC, Arany PR, Huang Y-Y, Tomkinson EM, Sharma SK, Kharkwal GB, Saleem T, Mooney D, Yull FE, Blackwell TS (2011) Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One 6:e22453
Chen J, Du L, Li J, Song H (2016) Epigallocatechin-3-gallate attenuates cadmium-induced chronic renal injury and fibrosis. Food Chem Toxicol 96:70–78
Chen N, Su P, Wang M, Li Y-M (2018) Ascorbic acid inhibits cadmium-induced disruption of the blood-testis barrier by regulating oxidative stress-mediated p38 MAPK pathways. Environ Sci Pollut Res 25:21713–21720
Chouhan S, Kushwaha P, Kaul R, Flora SJ (2013) Co-administration of selenium but not iron prevents fluoride toxicity in rats. Biomed Prev Nutr 3:113–120
Dai X, Xing C, Cao H, Luo J, Wang T, Liu P, Guo X, Hu G, Zhang C (2018) Alterations of mitochondrial antioxidant indexes and apoptosis in duck livers caused by molybdenum or/and cadmium. Chemosphere 193:574–580
Elbaghdady HAM, Alwaili MA, El-Demerdash RS (2018) Regenerative potential of bone marrow mesenchymal stem cells on cadmium chloride-induced hepato-renal injury and testicular dysfunction in Sprague Dawley rats. Ecotoxicol Environ Saf 164:41–49
Elkhadragy MF, Al-Olayan EM, Al-Amiery AA, Moneim AEA (2018) Protective effects of Fragaria ananassa extract against cadmium chloride-induced acute renal toxicity in rats. Biol Trace Elem Res 181:378–387
Ellman M (1959) A spectrophotometric method for determination of reduced glutathione in tissues. Anal Biochem 74:214–226
Faraji T, Momeni HR, Malmir M (2019) Protective effects of silymarin on testis histopathology, oxidative stress indicators, antioxidant defence enzymes and serum testosterone in cadmium-treated mice. Andrologia 51:e13242
Fouad A, Jresat I (2015) Thymoquinone therapy abrogates toxic effect of cadmium on rat testes. Andrologia 47:417–426
Habibi E, Arab-Nozari M, Elahi P, Ghasemi M, Shaki F (2018) Modulatory effects of Viola odorata flower and leaf extracts upon oxidative stress-related damage in an experimental model of ethanol-induced hepatotoxicity. Appl Physiol Nutr Metab 44:521–527
He L, Lemasters JJ (2002) Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett 512:1–7
Hoesel B, Schmid JA (2013) The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 12:86
Hosseini M-J, Shaki F, Ghazi-Khansari M, Pourahmad J (2013) Toxicity of arsenic (III) on isolated liver mitochondria: a new mechanistic approach. Iran J Pharm Res 12:121–138
Jana L, Maity PP, Perveen H, Dash M, Jana S, Dey A, De SK, Chattopadhyay S (2018) Attenuation of utero-toxicity, metabolic dysfunction and inflammation by soy protein concentrate in rats exposed to fluoridated water: consequence of hyperlipidemia in parallel with hypohomocysteinemia. Environ Sci Pollut Res 25:36462–36473
Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ II, Ischiropoulos H (2012) Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52:1–6
Kanduti D, Sterbenk P, Artnik B (2016) Fluoride: a review of use and effects on health. Mater Sociomed 28:133–137
Khan R, Ali S, Mumtaz S, Andleeb S, Ulhaq M, Tahir HM, Khan MKA, Khan MA, Shakir HA (2019) Toxicological effects of toxic metals (cadmium and mercury) on blood and the thyroid gland and pharmacological intervention by vitamin C in rabbits. Environ Sci Pollut Res 26:16727–16741
Lu Y, Luo Q, Cui H, Deng H, Kuang P, Liu H, Fang J, Zuo Z, Deng J, Li Y (2017) Sodium fluoride causes oxidative stress and apoptosis in the mouse liver. Aging (Albany NY) 9:1623–1639
Mohammed AT, Mohamed AA-R, Ali H (2017) Pulmonary apoptotic and oxidative damaging effects of triclosan alone or in combination with fluoride in Sprague Dawley rats. Acta Histochem 119:357–363
Pan Y-X, Luo Z, Zhuo M-Q, Wei C-C, Chen G-H, Song Y-F (2018) Oxidative stress and mitochondrial dysfunction mediated Cd-induced hepatic lipid accumulation in zebrafish Danio rerio. Aquat Toxicol 199:12–20
Philippot G, Stenerlöw B, Fredriksson A, Sundell-Bergman S, Eriksson P, Buratovic S (2019) Developmental effects of neonatal fractionated co-exposure to low-dose gamma radiation and paraquat on behaviour in adult mice. J Appl Toxicol 39:582–589
Saleh AS (2018) Evaluation of hepatorenal protective activity of Moringa oleifera on histological and biochemical parameters in cadmium intoxicated rats. Toxin Rev 38:338–345
Satarug S, Haswell-Elkins MR, Moore MR (2000) Safe levels of cadmium intake to prevent renal toxicity in human subjects. Br J Nutr 84:791–802
Shaki F, Hosseini M-J, Ghazi-Khansari M, Pourahmad J (2012) Toxicity of depleted uranium on isolated rat kidney mitochondria. BBA Gen Subjects 1820:1940–1950
Shaki F, Arab-Nozari M, Elahi P, Ghasemi M, Habibi E (2019) Ameliorative effect of Viola odorata. L. ethyl acetate extract against nephrotoxicity induced by chronic ethanol exposure in rats. JMUMS 29:1–13
Shokrzadeh M, Zamani E, Mehrzad M, Norian Y, Shaki F (2015) Protective effects of propofol against methamphetamine-induced neurotoxicity. Toxicol Int 22:92–99
Sun Z, Li S, Yu Y, Chen H, Ommati MM, Manthari RK, Niu R, Wang J (2018) Alterations in epididymal proteomics and antioxidant activity of mice exposed to fluoride. Arch Toxicol 92:169–180
Tuyen TN, Quyen NDV, Ngoc TM, Hai HVM, Quang TN, Sy HT, Dai Lam T (2016) Treatment of fluoride in well-water in Khanhhoa, Vietnam by aluminum hydroxide coated rice husk ash. Green Process Synth 5:479–489
Vahidirad M, Arab-Nozari M, Mohammadi H, Shaki F (2018) Protective effect of edaravone against nephrotoxicity and neurotoxicity of acute exposure to diazinon. JMUMS 28:175–182
Wang H, Zhang R, Song Y, Li T, Ge M (2019) Protective effect of ganoderma triterpenoids on cadmium-induced testicular toxicity in chickens. Biol Trace Elem Res 187:281–290
Wei Q, Luo Q, Liu H, Chen L, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X (2018) The mitochondrial pathway is involved in sodium fluoride (NaF)-induced renal apoptosis in mice. Toxicol Res 7:792–808
Whittaker MH, Wang G, Chen X-Q, Lipsky M, Smith D, Gwiazda R, Fowler BA (2011) Exposure to Pb, Cd, and As mixtures potentiates the production of oxidative stress precursors: 30-day, 90-day, and 180-day drinking water studies in rats. Toxicol Appl Pharmacol 254:154–166
Yuan G, Dai S, Yin Z, Lu H, Jia R, Xu J, Song X, Li L, Shu Y, Zhao X (2014) Toxicological assessment of combined lead and cadmium: acute and sub-chronic toxicity study in rats. Food Chem Toxicol 65:260–268
Zhang D, Hou L, Peng W (2018) Tangeritin attenuates oxidative stress, apoptosis and inflammation in cadmium-induced cardiotoxicity in rats by activating Nrf2 signaling pathway. Trop J Pharm Res 17:2421–2426
Zhang F, Xu Z, Gao J, Xu B, Deng Y (2008) In vitro effect of manganese chloride exposure on energy metabolism and oxidative damage of mitochondria isolated from rat brain. Environ Toxicol Pharmacol 26:232–236
Acknowledgments
This study was extracted from the PhD thesis of Milad Arab-Nozari.
Funding
This study was supported by a grant from the Research Council of Mazandaran University of Medical Sciences, Sari, Iran (Grant Number: 1484).
Author information
Authors and Affiliations
Contributions
Fatemeh Shaki designed the research. Milad Arab-Nozari, Ebrahim Mohammadi, Mohammad Shokrzadeh, and Fereshteh Talebpour Amiri collected the data. Fatemeh Shaki and Nematollah Ahangar analyzed and interpreted the data. Milad Arab-Nozari and Fatemeh Shaki prepared the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arab-Nozari, M., Mohammadi, E., Shokrzadeh, M. et al. Co-exposure to non-toxic levels of cadmium and fluoride induces hepatotoxicity in rats via triggering mitochondrial oxidative damage, apoptosis, and NF-kB pathways. Environ Sci Pollut Res 27, 24048–24058 (2020). https://doi.org/10.1007/s11356-020-08791-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08791-4