Abstract
Microplastics have become a major environmental concern globally due to their potential impact on ecosystem function. They are known to be ubiquitously present, persistent and bio-accumulative, yet there is a lack of abundance quantifications in freshwater shorelines. Furthermore, there is little understanding of combined effects of seasonality and human population density on the extent of microplastic pollution. In this study, we assessed microplastics in sediment along a subtropical reservoir shoreline across three seasons and seven sites to understand microplastic pollution dynamics along a human population gradient. Multivariable analysis was used to assess relationships among substrate embeddedness, sediment organic matter, human population density and microplastic particle densities and characteristics. Microplastic densities were relatively high during the hot–dry season (mean range 120–6417 particles kg−1 dwt) whilst the hot–wet season had the lowest densities (mean range 5–94 particles kg−1 dwt). Microplastic abundances were positively correlated with population density, demonstrating the direct effects of human activity on microplastic contamination. These results highlight the need to further explore microplastic distribution patterns in freshwater ecosystems in the Southern Hemisphere. Furthermore, our findings suggest particular risk for fauna during low rainfall periods through microplastic concentration effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activity has resulted in pollution of aquatic ecosystems with synthetic polymers, i.e. plastics, which may be harmful to ecosystem function (Wagner and Lambert 2018). The light weight, high durability and low production costs of plastics make them ideal for different purposes, and has resulted in an increase in plastic use since the 1950s (GESAMP 2015). Microplastics (< 5 mm) that contaminate aquatic ecosystems are diverse in shape, size and origin (GESAMP 2015, 2019). Microplastics manufactured intentionally, either as resin pellets to produce larger items or indirectly in cosmetic products, such as facial scrubs and toothpastes, are called primary microplastics, whilst secondary microplastics disintegrate from larger plastic debris (Horton et al. 2017). Microbeads, fragments, foam and fibre are common categories used when identifying microplastics (Eriksen et al. 2013). Such characterisation can potentially be used to indicate microplastics origin, such as line/fibre which usually originates from fishing lines, clothing and/or other textiles (Wu et al. 2018). Microplastics can also be classified according to polymer types such as polyethylene (HD/LD–PE), polyethylene terephthalate (PET), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC) and polyamide fibres (nylon) (Andrady 2011; Zbyszewski et al. 2014).
The South African government categorised the plastic industry as an important sector for economic growth in enhancing export, innovation and recycling (DTI 2016). However, the country is eleventh globally as a main contributor of marine plastic debris, despite waste disposal protocols and legislation to promote recycling being in place (Verster et al. 2017). Excessive plastic production has placed a strain on aquatic ecosystems as unwanted microplastics enter aquatic environments through wastewater discharge, degradation of larger plastic items and user discards (Barnes et al. 2009). Thus, the widespread abundance of microplastics in the environment is directly due to human activities (Rillig 2012), yet there is currently a paucity of understanding into how microplastic pollution relates to human population densities. Furthermore, whilst much research has focused on investigating the source, fate, abundance and impact of microplastics in marine systems, only a few studies have been conducted within freshwater ecosystems (e.g. rivers, lakes, reservoirs) (Biginagwa et al. 2016; Horton et al. 2017; Nel et al. 2018; Hurley et al. 2018; Tibbetts et al. 2018). Accordingly, this emphasises the need for further studies in these ecosystems to close knowledge gaps.
Freshwater systems are an important conduit for microplastics between inland terrestrial inputs and marine environments (Mani et al. 2015). Microplastics enter water sources through various routes such as storm water (Silva-Cavalcanti et al. 2017; GESAMP 2019), wastewater discharge (Nel et al. 2018; GESAMP 2019) and littering (Dris et al. 2017; GESAMP 2019). Microbeads, for example, are typically buoyant in water bodies and can be desorbed upon entering gastrointestinal tracts, thereby affecting the pH and ion balance in organisms (Tanaka et al. 2013). Moreover, microplastic with sorbed co-contaminants such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and dichlorodiphenyltrichloroethane (DDT) can also be deposited in sediment surfaces. The aggregation of microplastic particles with organic matter in sediments can increase particle size and density, resulting in increased microplastic sedimentation rates (Long et al. 2015; Nel et al. 2019). Of greatest concern is the potentially wide range of effects microplastics pose to ecosystems and public health due to microplastic ingestion by aquatic organisms and trophic transference through entire food webs (Teuten et al. 2009; Browne et al. 2008; Farrell and Nelson 2013; Nel et al. 2018; Cuthbert et al. 2019).
Studies focusing on microplastics occurrence and distribution in freshwater sediments are lacking within Southern Hemisphere tropical regions. Since microplastic pollution may have detrimental environmental effects, a comprehensive examination of the occurrence, characteristics and distribution of microplastics in a model subtropical reservoir was carried out. Such systems can serve as regional models for ecosystem types, potentially highlighting the sources, nature and extent of microplastic pollutants. Thus, the current study aimed to contribute to the limited body of knowledge on microplastics that currently exists for freshwater ecosystems in the Southern Hemisphere. More specifically, the study assessed the sediment microplastic pollution, in terms of composition, distribution and density, across three seasons. We hypothesised that sediment microplastic densities would show strong seasonal (hot–dry, hot–wet, cool–dry) and site (low and high population density) differences, with high microplastic loads in highly populated sites and during the hot–dry season, and with human population activities and substrate embeddedness strongly influencing the abundance of microplastics found along the reservoir shorelines.
Materials and methods
Study area
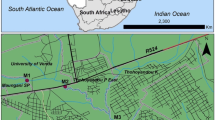
Nandoni reservoir (22°59′11″S, 30°36′16.19″E) is located between Mutoti and Budeli villages, within Thulamela municipality, approximately 10 km from the town of Thohoyandou, Limpopo province, South Africa, and is mainly used for domestic water supply and irrigation. The reservoir is 2215 m long, has a catchment area of 1380 km2 and a total capacity of 16.4 million m3. The region is generally characterised by warm, humid summers and cool–dry winters. The average temperatures in summer and winter are 23 °C and 17 °C, respectively. The average annual precipitation for the entire catchment varies between 610 and 800 mm, with a mean annual runoff of 519 million m3 (Heath and Classen 1990). The prevailing wind direction is from the east to southeast in both the summer and the winter months. The topography of the reservoir area falls under the Soutpansberg Group. It is comprised of low-lying, undulating terrain which is underlain by a gneiss sequence. The soil in most parts has been eroded due to continuous cultivation. Erosion in the reservoir basin occurs generally in areas of dissipative topography where erodible material is available.
The study was carried out over three seasons, i.e. hot–dry (September 2018), hot–wet (March 2019) and cool–dry (June 2019). Site selection was based on human population density along the reservoir shorelines, where seven sites were selected around the reservoir: four sites (site 1, Mulenzhe village (population density (PD) 2566, area 4.37 km2); site 4, Budeli village (PD 2362, area 3.56 km2); site 5, Muledane village (PD 1428, area 1.57 km2); site 6, Dididi village (PD 2312, area 2.66 km2)) were categorised as high PD sites and three sites (site 2, PD 6; site 3, PD 0; site 7, PD 4) were categorised as low PD sites (Fig. 1). Most of these sites were also areas of high deposition as highlighted by the substrate content, i.e. high amounts clay/silt to sandy soil being observed and no samples were collected during the hot–dry season for sites 5 to 7.
Sediment characteristics
Substrate embeddedness was determined according to Platts et al. (1983) through the assessment of the surface covered by fine sediment: 1 (> 75%), 2 (50–75%), 3 (25–50%), 4 (5–25%) and 5 (< 5%). The sediment organic matter (SOM) was determined using the Chan et al. (2001) modified Walkley–Black method.
Extraction and enumeration of sediment microplastics
Microplastics considered for this study were < 5 mm (GESAMP 2015, 2019), but > 63 μm (mesh size utilised). Two sediment samples (~ 1.5–2 kg) were collected per site on the littoral zone (i.e. one sample was made up of three sediment subsamples collected from three random spots, approximately 10 m apart) from the upper 5 cm sediment layer along the reservoir shoreline and stored in labelled clear-plastic ziplock bags. In the laboratory, the sediment samples were dried in an oven at 60 °C for 72 h until a constant weight was reached. After drying, each sediment sample was homogenised using a riffle splitter, and thereafter a sediment subsample of 0.5 kg was separated from the riffle splitter and sieved through a 500-μm mesh steel sieve to remove large organic matter particles and rocks. The sediment material retained on the sieve was analysed for large microplastics (500 μm–5 mm), for inclusion in the total microplastic count.
To prevent contamination, prior to all analyses, the entire laboratory was cleaned with all surfaces and equipment rinsed with milliQ distilled water. No air-conditioners or fans were utilised in the lab during the study to minimise the risk of potential air-borne microplastic particle transport. Each sieved 0.5 kg subsample was placed into a clean 5-L beaker and a 63-μm mesh filtered hypersaturated saline solution (100 g coarse salt L−1) was added. The mixture was stirred vigorously to allow the release and suspension of trapped plastic particles, before allowing the denser sediment to settle out for 3–24 h, depending on the soil type. After this time, the supernatant was filtered through a 63-μm mesh and the entire process was repeated five times so that all microplastics could be quantified (Nel et al. 2018). To further reduce potential air-borne microplastic particle contamination, all samples were covered with a small tray. The microplastics on the 63-μm mesh sieve were carefully rinsed with distilled water into 50-mL polystyrene jars, before the samples were visually sorted under an Olympus dissecting microscope at ×50 magnification, whereby all possible microplastic particles were enumerated according to colour (i.e. colours: red/pink, white, black/blue, yellow/orange). Particles were deemed to be microplastics if they possessed unnatural colouration (e.g. bright colouration, multi-coloured) and/or unnatural shape (e.g. sharp edges, perfectly spherical; Hidalgo-Ruz et al. 2012). As visual inspection alone was not adequate to characterise and exhaustively quantify microplastics, further physical analysis was utilised (Mintenig et al. 2017; GESAMP 2019). Therefore, a vibrational Platinum-ATR Fourier-transform infrared spectroscopy (FT-IR) (Bruker Alpha model, Germany) technique was employed on selected microplastic particles for confirmation. This technique offers available libraries for microplastic polymer identification and is more efficient for dense samples, as in the present study (Picó and Barceló 2019). The number of microplastic particles was estimated as number of microplastic particles kg−1 of dry weight (dwt).
To test microplastic recovery rates, soil samples (n = 3, 0.5 kg) were collected from non-impacted terrestrial sites about 30–50 cm underground. The samples were each spiked with 0.1 g (~ 309 particles or 77 particles L−1) ultra-high molecular weight, surface-modified multi-coloured polyethylene powder, 125-μm particle size (Sigma-Aldrich, UK) and homogenised (well-mixed) before being separated similar to field samples using a hypersaturated saline solution. The recovery rates ranged between 88 and 95% (mean 92%) of the microplastic particles.
Data analysis
All microplastic particle data were log (x + 1) transformed to homogenise variances. Correlation analysis was used to assess for relationships of the environmental variables (substrate embeddedness, SOM), population densities and microplastic abundances using SPSS v16.0 (SPSS Inc. 2007).
We tested whether abundances of combined microplastic particle types (i.e. colours: red/pink, white, black/blue, yellow/orange) differed among seasons and sites. Distance-based permutational analysis of variance (PERMANOVA; Anderson 2001; McArdle and Anderson 2001) based on Euclidean distance dissimilarities was carried in PRIMER v6 add-on package PERMANOVA+ (Anderson et al. 2008) to determine the differences in microplastic particle types and abundances among study sites and/or seasons. Each term in the analysis was tested using 9999 permutations of the correct relevant permutable units (Anderson and Braak 2003), with significant terms investigated using posteriori pairwise comparisons with the PERMANOVA t statistic (Anderson et al. 2008). Spatiotemporal variation in microplastic particles was analysed using non-metric multi-dimensional scaling (n-MDS) based on Bray-Curtis similarity measures.
Results
Sediment characteristics
Shoreline substrate embeddedness, determined according to Platts et al. (1983), was rated 1 (> 75% fine sediment) for sites 1, 3 and 6, with sites 2 and 5 exhibiting a rating of 2 (50–75% fine sediment), site 4 a rating of 3 (25–50% fine sediment) and site 7 a rating of 5 (< 5% fine sediment). Sediment organic matter was generally high in sites with high fine sediment content (range 10.6–26.7%) compared with low fine sediment sites (1.5–7.6%). A significant interaction between sites and seasons on SOM was detected (F = 4.488, p = 0.004). No significant relationships were observed for microplastic loads and substrate embeddedness (r = − 0.23, p = 0.174) and population density (r = 0.10, p = 0.098) but microplastic load was significantly positively correlated (r = 0.63, p = 0.021) with SOM, suggesting that sites with high SOM (e.g. sites 1, 3, 6) had greater microplastic loads.
Sediment microplastics
Control samples contained no microplastics. As such, microplastics encountered in samples were considered from the collection site and not an aspect of laboratory contamination. Overall, up to four different microplastic colour types (i.e. red/pink, white, black/blue and yellow/orange) were observed, with white microplastics being the dominant colour at all sites (Fig. S1). Generally, high microplastic numbers were observed during the hot–dry season (mean range 120–6417 particles kg−1 dwt) compared with low numbers observed during the hot–wet season (mean range 5–94 particles kg−1 dwt) (Fig. 2). Overall, the high population density sites had high microplastic densities (mean 833.9 ± 634.9 particles kg−1 dwt) compared with the low population density sites (mean 77.5 ± 27.4 particles kg−1 dwt). High population density sites 1 (mean 6417 ± 4407 particles kg−1 dwt) and 4 (1414 particles kg−1 dwt) during the hot–dry season recorded highest microplastic abundances (Fig. 2).
Using PERMANOVA, significant differences in microplastic abundances were observed among sites (Pseudo F = 2.469, p(MC) = 0.006), seasons (Pseudo F = 7.453, p(MC) < 0.001) and site × season (Pseudo F = 2.564, p(MC) = 0.001). The significant ‘site × season’ interaction indicated greater microplastic density differences among sites as seasons changed, with higher microplastic abundance being observed during the hot–dry season (Fig. 2). Pairwise comparisons highlighted significant differences in microplastic colour abundances for sites 1 vs 4 (t = 2.477, p = 0.025), 2 vs 4 (t = 3.208, p = 0.006) and 3 vs 4 (t = 3.040, p = 0.007). Furthermore, pairwise significant seasonal differences in microplastic colour abundances were also observed for the hot–dry vs hot–wet (t = 3.255, p = 0.002), hot–dry vs cool–dry (t = 2.710, p = 0.009) and hot–wet vs cool–dry (t = 1.981, p = 0.024), with high microplastic densities being observed for the hot–dry season.
Low human population density category sites had generally reduced microplastic numbers (mean range 17–193 particles kg−1 dwt) compared with sites categorised as highly populated density sites (mean range 40–3915 particles kg−1 dwt) (Fig. 2). No significant differences were observed in microplastic densities within the two population group (i.e. low, high) areas (F = 1.330, p = 0.365) and seasons (F = 1.140, p = 0.467). However, a significant interaction effect (F = 4.156, p = 0.026) was observed between population groups and season indicating that changes in population group activities across seasons resulted in a change in microplastic abundances.
The n-MDS ordination based on microplastic numbers for all sites discriminated slightly among seasons (stress values of 0.07 indicated a useful two-dimensional representation of the groups; Fig. 3). The overlap observed among seasons, especially during hot–wet and cool–dry seasons, could be attributed to reduced activity (i.e. reduced laundry washing) along the reservoir shoreline (Fig. 3). Selected polymers of all microplastic types were identified using vibrational FT-IR technique, resulting in eight polymer types: 20.2% polypropylene, 22.7% polyethylene, 30.9% polystyrene, 9.7% polyvinyl chloride, 5.8% polyester, 4.5% high-density polyethylene, 3.9% polydimethylsiloxane and 2.3% poly(lauryl acrylate).
Discussion
Whilst microplastics continue to accrue across all habitat types globally, there is currently little information on microplastic pollution in freshwaters, and particularly in subtropical regions. In many African remote areas, rural populations collect water from freshwater resources. It therefore becomes a problem when water sources for domestic and agricultural use are polluted with contaminants such as microplastics, owing to negative effects on water quality, food security and population well-being (Verster et al. 2017). We anticipated microplastics to be present at our study sites owing to their widespread distribution within freshwater and marine environments (Nel et al. 2018; Ngupula et al. 2014; Silva-Cavalcanti et al. 2017).
The present study highlights that human population density with regard to activities interacted with seasonal variation to influence the abundance and distribution of microplastics in reservoir sediments. In particular, the study highlights that microplastic abundances along the focal shoreline were significantly higher in areas with high human population density under certain seasons. The study further attributes the possible microplastic sources might be from laundry washing (Fig. 4), cosmetic and cleaning products, as well as discarded litter which breaks down into microplastics, since no significant differences were observed for population density alone and microplastic loads. Thus, this lack of significant relationship highlights that the pollution likely mixes within the lake and results in more homogenous distribution of microplastics.
Sediment microplastic densities showed a seasonal difference along the shoreline, with high microplastic abundances being observed during the hot–dry season and this was most likely due to reduced river water flow as a result of low precipitation and increased pressure to do laundry by the lakeside. Whereas during the hot–wet season, people normally capture rainwater and wash at their houses away from the lake shore. The high abundance of microplastics during the hot–dry season suggests that microplastics were temporarily stored in sediments before being redistributed in other seasons. Indeed, Nel et al. (2018) suggested that freshwater sediments are temporary sinks for microplastics. In contrast to the current study (mean 616 particles kg−1 dwt), Fischer et al. (2016) recorded low average sediment microplastic values of 112 and 234 particles kg−1 dwt in two Italian lakes: Bolsena and Chiusi, respectively. Thus, one can assume that Nandoni reservoir is heavily polluted during the hot–dry season, with the hot–wet and cool–dry seasons showing low to moderate microplastic contamination. Nandoni reservoir can be considered a microplastic exporter as it acts as a sink during the hot–dry season and when flows increase during the hot–wet season, it may become a microplastic source to other areas, thereby reducing microplastics in the reservoir.
We also suspect little input from catchment wastewater discharges as the area is mostly rural-based and uses pit latrines; hence, most microplastics do not reach aquatic ecosystems or water treatment works, as indicated by low microplastic densities at the reservoir mouth sites i.e. sites 5 and 6. However, some may reach the reservoir from the breakdown of macroplastics (Dalu et al. 2019). Whilst hydrodynamics are known to significantly influence the deposition and distribution of plastics on marine shorelines (GESAMP 2019), the influence of these factors is likely to be less pronounced in smaller freshwater environments which are subject to reduced hydrodynamics from, for example, wave action. Results showed that highly populated areas had greater plastic numbers, reaching 6417 particles kg−1 dwt, whilst sites with low population densities had low plastic numbers ranging between 9 and 265 particles kg−1 dwt. Therefore, it is likely that microplastic contamination levels in the absence of proper waste management infrastructures can have non-localised effects in such impounded freshwater ecosystems (Lambert et al. 2017; Verster et al. 2017; Tibbets et al. 2018). Studies by Andrady (2011) have highlighted that densely populated areas are considered a major land-based source of microplastic pollution through the breaking down of directly or indirectly discarded plastic debris and poorly regulated discharge of domestic effluent. Thus, it is assumed that more plastics will enter from densely human populated areas, which suggests that human density is a strong determinant of the amount of microplastic input, whilst residence time is the determining factor of microplastic distribution (Mahoney 2017).
Microplastic colour helped to indicate their potential origin in the present study. Recovered microplastics were found in a variety of colours, with white being the dominant colour. In some studies (e.g. Su et al. 2016), microplastic colours such as blue, white and black were identified, with white also being the dominant colour. Microplastic colours indicate the parent plastic product, and some colours change to white due to degradation process, making white the dominant colour; in turn, these particles may be ingested by aquatic biota (Lambert et al. 2017). Polystyrene foam microplastics were also identified which could have originated from disposable food containers and cups. Zhang et al. (2019) and Nel et al. (2018, 2019) implied that the higher organic matter in the sediment soil samples strongly influenced the microplastic recovery. Our results, however, showed that sites with high SOM generally had higher microplastics as compared with sites with low SOM content.
Whilst the present study demonstrates high prevalence of microplastics in a subtropical reservoir, further in-depth studies in Austral freshwaters are required to understand the presence of microplastics, and other key drivers of differences. In particular, meteorological and hydrodynamic effects on pollutant concentrations and distributions require further examination, owing to the effects on other shoreline systems (GESAMP 2019). Nonetheless, our findings suggest key spatiotemporal context-dependencies are important drivers of differences in microplastic abundances, with differences emergent across seasons according to human population densities close to reservoir shorelines. In turn, our empirical results serve to inform lab-based exposure studies, as these often use unrealistic microplastic concentrations to quantify ecological impacts.
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46
Anderson M, Braak CT (2003) Permutation tests for multi-factorial analysis of variance. J Stat Comput Simul 73:85–113
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62:1596–1603
Barnes DKA, Gilgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc B 364:1985–1998
Biginagwa FJ, Mayoma BS, Shashoua Y, Syberg K, Khan FR (2016) First evidence of microplastics in the African Great Lakes: recovery from Lake Victoria Nile perch and Nile tilapia. J Great Lakes Res 42(1):146–149
Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC (2008) Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ Sci Technol 42(13):5026–5031
Chan KY, Bowman A, Oates A (2001) Oxidizible organic carbon fractions and soil quality changes in an Oxic Paleustalf under different pasture leys. Soil Sci Soc Am J 66:61–67
Cuthbert RN, Al-Jaibachi R, Dalu T, Dick JT, Callaghan A (2019) The influence of microplastics on trophic interaction strengths and oviposition preferences of dipterans. Sci Total Environ 651:2420–2423
Dalu T, Malesa B, Cuthbert RN (2019) Assessing factors driving the distribution and characteristics of shoreline macroplastics in a subtropical reservoir. Sci Total Environ 696: 133992
Dekiff JH, Remy D, Klasmeier J, Fries E (2014) Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ Pollut 186:248–256
Department of Trade and Industry (DTI) (2016) Industrial Policy Action Plan: IPAP 2016/17 2018/19. Government of South Africa, Pretoria
Dris R, Gasperi J, Mirande C, Mandin C, Guerrouache M, Langlois V, Tassin B (2017) A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ Pollut 221:453–458
Eriksen M, Mason S, Wilson S, Box C, Zellers A, Edwards W, Farley H, Amato S (2013) Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar Pollut Bull 77:177–182
Farrell, P. and Nelson, K., 2013. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environmental pollution, 177, pp.1-3.
Fischer EK, Paglialonga L, Czech E, Tamminga M (2016) Microplastic pollution in lakes and lake shoreline sediments - a case study on Lake Bolsena and Lake Chiusi (central Italy). Environ Pollut 213:648–657
GESAMP (2015) Sources, fate and effects of microplastics in the marine environment: a global assessment. In: Kershaw PJ (ed) (IMO/FAO/UNESCO-IOC/UNIDO/WMO/ IAEA/UN/UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection). Rep. Stud. GESAMP No. 90. 96
GESAMP (2019) Guidelines for the monitoring and assessment of plastic litter in the ocean. (Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection). Rep. Stud. GESAMP no. 99. 123
Heath RGM, Classen M (1990) An overview of the pesticide and metal levels present in populations of the larger indigenous fish species of selected South African rivers. WRC Rep No. 428, Water Research Commission, Pretoria
Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M (2012) Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol 46:3060–3075
Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C (2017) Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ 586:127–141
Hurley R, Woodward J, Rothwell JJ (2018) Microplastic contamination of river beds significantly reduced by catchment–wide flooding. Nature Geosci 11:251–257
Lambert S, Scherer C, Wagner M (2017) Ecotoxicity testing of microplastics: Considering the heterogeneity of physicochemical properties. Integr Environ Assess Manag 13(3):470–475. https://doi.org/10.1007/s11356-020-08640-4
Long M, Moriceau B, Gallinari M, Lambert C, Huvet A, Raffray J, Soudant P (2015) Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Mar Chem 175:39–46
Mahoney T (2017) The concentration of microplastics compared to relative population proximity and basin residence times in Hood Canal and Whidbey Basin in Puget Sound, WA. PhD Thesis. University of Washingtown, Seattle WA
Mani T, Hauk A, Walter U, Burkhardt-Holm P (2015) Microplastics profile along the Rhine River. Scientific Reports 5(1):1–7
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance based redundancy analysis. Ecology 82:290–297
Mintenig SM, Int–Veen I, Löder MGJ, Primpke S, Gerdts G (2017) Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res 108:365–372
Nel HA, Dalu T, Wasserman RJ (2018) Sinks and sources: assessing microplastic abundance in river sediment and deposit feeders in an Austral temperate urban river system. Sci Total Environ 612:950–956
Nel HA, Dalu T, Wasserman RJ, Hean JW (2019) Colour and size influences plastic microbead underestimation, regardless of sediment grain size. Sci Total Environ 655:567–570
Ngupula GW, Kayanda RJ, Mashafi CA (2014) Abundance, composition and distribution of solid wastes in the Tanzanian waters of Lake Victoria. Afr J Aquat Sci 39:229–232
Picó Y, Barceló D (2019) Analysis and prevention of microplastics pollution in water: current perspectives and future directions. ACS Omega 4:6709–6719
Platts WS, Megahan WF, Minshall WG (1983) Methods for evaluating stream, riparian, and biotic conditions. General Technical Report INT-138, USDA Forest Service. Ogden, UT, Rocky Mountain Research Station
Rillig MC (2012) Microplastic in terrestrial tcosystems and the soil. Environ Sci Technol 46:6453–6454. https://doi.org/10.1007/s11356-020-08640-4
Silva-Cavalcanti JS, Silva JDB, de França EJ, de Araújo MCB, Gusmao F (2017) Microplastics ingestion by a common tropical freshwater fishing resource. Environ Pollut 221:218–226
SPSS Inc. (2007) SPSS Release 16.0.0 for Windows. Polar engineering and consulting. SPSS Inc., Chicago (IL)
Su L, Xue Y, Li L, Yang D, Kolandhasamy P, Li D, Shi H (2016) Microplastics in Taihu Lake, China. Environ Pollut 216:711–719
Tanaka K, Takada H, Yamashita R, Mizukawa K, Fukuwaka MA, Watanuki Y (2013) Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar Pollut Bull 69:219–222
Teuten EL, Saquing JM, Knappe DRU, Barlaz MA, Jonsson S, Bjorn A, Rowland SJ, Thompson RC, Galloway TS, Yamashita R (2009) Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc B 364:2027–2045
Tibbetts J, Krause S, Lynch I, Sambrook Smith GH (2018) Abundance, distribution, and drivers of microplastic contamination in urban river environments. Water 10:1597
Verster C, Minnaar K, Bouwman H (2017) Marine and freshwater microplastic research in South Africa. Integr Environ Assess Manag 13(3):533–535
Wagner M, Scherer C, Alvarez–Muñoz D, Brennholt N, Bourrain X, Buchinger S, Fries E, Grosbois C, Klasmeier J, Marti T, Rodriguez–Mozaz S, Urbatzka R, Vethaak AD, Winther–Nielsen M, Reifferscheid G (2014) Microplastics in freshwater ecosystems: what we know and what we need to know. Environ Sci Eur 26:12
Wagner M, Lambert S (2018) Freshwater microplastics. Springer International Publishing, Cham, Switzerland
Wu C, Zhang K, Xiong X (2018) Microplastic pollution in inland waters focusing on Asia. In: Freshwater microplastics. Springer, Cham, pp 85-99
Zhang SS, Ding JN, Razanajatovo RM, Jiang H, Zou H, Zou WB (2019) Interactive effects of polystyrene microplastics and roxithromycin on bioaccumulation and biochemical status in the freshwater fish red tilapia (Oreochromis niloticus). Sci Total Environ 646:1431–1439
Zbyszewski M, Corcoran PL (2011) Distribution and degradation of fresh water plastic particles along the beaches of Lake Huron, Canada. Water Air Soil Pollut 220:365–372
Zbyszewski M, Corcoran PL, Hockin A (2014) Comparison of the distribution and degradation of plastic debris along shorelines of the Great Lakes, North America. J Great Lakes Res 40:288–299
Funding
Financial support for this study was granted by the National Research Foundation of South Africa Thuthuka (NRF, UID: 117700) and the University of Venda Niche (SES/18/ERM/10) grants to TD. RNC received funding from the Department for the Economy, Northern Ireland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
Any opinions, findings, conclusions or recommendations expressed in this material are those of the authors, and the NRF does not accept any liability in this regard.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 201 kb)
Rights and permissions
About this article
Cite this article
Mbedzi, R., Cuthbert, R.N., Wasserman, R.J. et al. Spatiotemporal variation in microplastic contamination along a subtropical reservoir shoreline. Environ Sci Pollut Res 27, 23880–23887 (2020). https://doi.org/10.1007/s11356-020-08640-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08640-4