Abstract
In leather industries, raw hides/skins are always preserved before being processed into leather. Salting method of preservation is the general and age old popular practice of preservation used in these industries. The main drawbacks of this method are the generation of huge amounts of pollution load, in terms of total dissolved solids (TDS), total suspended salts (TSS), and chlorides; and ecological damage which occurs as a result of these waste effluents being discharged into the ground. Therefore, finding cheaper and eco-friendly methods of preservation has become a major necessity for these industries. In this manuscript, we have used ethanolic extract of Aegle marmelos for preservation which totally eliminates salt. The efficacy of this method was assessed by evaluating parameters such as microbial count, nitrogen content, and collagen content of the skin samples, and biological oxygen demand (BOD), chemical oxygen demand (COD), TDS, and TSS of the waste effluents collected during processing of leather. It was found that this method showed a remarkable reduction in pollution loads like BOD (46%), COD (3-fold), TDS (many folds), and increased values of collagen content. Thus, we could conclude that preservation using A. marmelos was found to be more effective and eco-friendly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Leather can be defined as durable and flexible materials created via the tanning of putrefying animal raw hides and skins. It is usually used in the manufacture of jacket, coats, parts, shoes, backpart, briefcases, bags, purses, etc. The goat skins of North Bihar and Bengal are known to possess fine grains and hence are considered one of the finest raw materials for the production of high-quality leather (SINET).
India is one of the leading countries in leather export business with a largest livestock population (Rob 2020) and is having at least 2702 authorized slaughterhouses. Around 2200 tanneries present across the country process the hides and skins obtained as a byproduct from the meat industry into leather after appropriate curing or preservation (Shilpa 2014). Though leather industry is one of the major revenue earning areas in India, it is equally a polluting one. It is very difficult and hard to treat the solid and liquid wastes obtained from leather industries. Tannery effluent exhibits very high values of biological oxygen demand (BOD), chemical oxygen demand (COD), suspended solids (SS), and total dissolved solids (TDS) (Davis and Scroggie 1973).
Common effluent treatment plants (CETPs) that have been established to treat the effluent discharged by tanneries, are efficiently eliminating SS and also bringing down the COD and BOD within the permissible limits (Mariappan 1997; Vinod et al. 2003). Yet, it is very difficult to reduce the TDS values; it is always high even in treated effluents. The major portion of TDS is contributed by two steps involved in leather making, the curing, i.e., the preservation of skins and hides, and the pickling. Both these steps require common salt in large quantities. Common salt or sodium chloride is the major culprit for the increased TDS in the effluents discharged from leather industries, which in turn affects the fertility of the soil and quality of the ground water (Kavitha and Ganapathy 2015). Hence, leather industries are forced to look for alternative methods for the different steps involved in leather making.
Preservation is the first step in leather making (Bailey 1997). As soon as the skins and hides are flayed, they have to be preserved before they are transported to the tanneries. The generally used preservative is common salt (Bienkiewickz 1983).
Several methods of preservation of skins and hides are currently available. To mention a few: preserving the raw skins and hides using chemicals like boric acid (Hughes 1974; Kanagaraj et al. 2005a), sodium metabisulfite, silica gel, and potassium chloride; antibiotics (Berwick et al. 1990; Vankar and Dwivedi 2009), gamma radiation, and green chemistry–based preservation using plant extracts; preservation of skins by refrigeration (Wu et al. 2017). A combination of sodium chloride, potassium chloride, and oxalic acid has been used for preservation of skins/hides and patented (Martin 2014). Short-term preservation of hides using vacuum under controlled temperature has also been reported in recent times (Gudro et al. 2014). Kanagaraj et al. (2014) have studied the bacteriocin extracted from Lactobacillus plantarum as a potent agent for short-term preservation of skins/hides.
Ten percent common salt along with different concentrations of de-oiled neem cake has been used to preserve raw skins (Vedaraman et al. 2016). Etissa and Anja (2018) have demonstrated the constraints in the preservation and transportation of hides and skins in Ethiopia.
Many plant-based formulations have been reported for preserving skins and hides (Sivabalan and Jayanthi 2009; Vijayalakshmi et al. 2009). We have also reported the efficacy of the nut extract of Semecarpus anacardium for the short-term preservation of skins/hides (Iyappan et al. 2013). Tamil Selvi et al. (2015) have used the leaf extract of Tamarindus indica for preservation of skins and hides. Leaf paste of Azadirachta indica along with different salts like NaCl, KCl, and ZnCl2 has been used for short-term preservation of skins (Sobur et al. 2015). Abul et al. (2018) have used the leaf paste of Moringa oleifera along with low concentration of common salt for preservation of goat skins. Thus, green chemistry–based preservation methods are getting attention due to their effectiveness in reducing pollution load. In this background, we have exploited ethanolic extract of Aegle marmelos for potential preservation of skins for the first time.
Aegle marmelos (Family: Rutaceae) is a small- or medium-sized deciduous tree with sharp axillary thorns. The plant is commonly known as bael, golden apple, stone apple, wood apple, etc. In Tamil, it is called as Vilvum. The antimicrobial and biological activities of this plant are well recorded (Sudha and Radhika 2007). All parts of the plants are used for healing of several diseases by menfolk. Different parts of the plant are known to possess several biological activities (Kausik et al. 2019). The plant has been reported to possess many terpenoids, alkaloids, coumarins, tannins, flavonoids, vitamins, etc. (Kausik et al. 2019).
In this study, for the first time, we have reported the role of ethanolic extract of A. marmelos on preservation of skins used to make leathers.
Materials and methods
Skins
Freshly flayed goat skins of average weight 1 kg and average area of 5 sq. ft. that were collected from a local slaughter house located at Perambur, Chennai, India, were used in the study.
Chemicals
Commercial-grade sodium chloride was purchased from Sdfine-Chem Ltd., Mumbai, India. l-Hydroxyproline and chloramine-T were obtained from Sigma Chemical Co. (St. Louis, USA). p-Dimethyl aminobenzaldehyde was procured from Loba Chemie, Mumbai, India. Methyl cellosolve was obtained from Merck, Darmstadt, Germany. All other chemicals and solvents used in the present study were procured from standard agencies and were of analytical grade.
Preparation of the plant extract
A total of 100 g of the shade dried leaves of A. marmelos was mixed with 500 ml of 50% ethanol and allowed to extract in a rotary shaker at 200 rpm for 24 h. The extract was then filtered through muslin cloth and centrifuged at 3000 rpm for 10 min. The solvent in the obtained supernatant was evaporated to get a viscous paste and stored at 4 °C in air tight bottles until further use. The yield of the extract was 11.8%.
Antimicrobial activity of A. marmelos extract
Antimicrobial activity of A. marmelos was assessed by agar well diffusion method. About 20 ml of sterilized Muller Hinton Agar was autoclaved and poured into a sterile petri plate, and after solidification, 100 μl of fresh culture of microbes was swabbed on the respective plates. A well was created to which 100 μl (1 mg/ml concentration) of the plant extract was added. The plates were incubated for 24 h at 37 °C. After incubation, the diameter of inhibitory zones formed around each wells was measured in millimeters and recorded.
Preservation of skins using A. marmelos extract
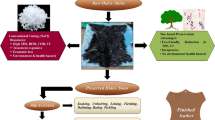
Two raw goat skins were taken. One was salted with 40% common salt (salting method of preservation) and the other one was applied with different concentrations of (1–10%) A. marmelos extract to study the extent of preservation instigated by both the commercial salt and the plant extract. The salted skin was used as control for carrying out a comparative analysis of both the skins. Both the skins were preserved for different days. Skins were shuffled every day and assessed periodically for physical changes like odor, hair slip, and growth of worms which are signs of decay (Sivaparvathi and Nandy 1974). Apart from this, the parameters analyzed were as follows: moisture content, microbial count, total nitrogen, total collagen, BOD, COD, TDS, and TSS. All experiments were repeated for at least three times to check the reproducibility. After 30 days, the preserved skins were taken for soaking and processed into the final leather product to assess their physical properties. From the results, the concentration of A. marmelos extract for optimum preservation was found to be 5%.
Viable count of microorganisms in salted and saltless preserved skins
Skin samples (5 g) were cut at different periods of preservation and soaked separately by shaking the bottles in an orbital shaker at 200 rpm for 30 min. The soak liquor was diluted in the ratio of 1:10 with sterile water and kept for shaking to get a uniform suspension of bacteria. Molten nutrient agar was poured in sterile petri plates to which 0.1 ml of the diluted solution was added and shaken gently to get uniform distribution of the bacteria. Petri plates were incubated at 37 °C for 48 h (Cruickshank 1965). The bacterial population was determined and expressed as CFU/per g of skin.
Determination of moisture content
Samples of cured skins were taken and the hair was removed and weighed and the moisture content was determined by drying in a drying chamber at 50–60 °C for 5–6 h (Bureau of Indian Standards 1971) and the reduction in weight was calculated.
Determination of nitrogen content
The cured samples of known weight (5 g) were kept in 50 ml of distilled water and shaken well in a bottle at 30 rpm for 3 h to extract the nitrogenous matter soluble in water. The extract was then filtered through a filter paper and digested, and nitrogen content was analyzed by the Kjeldahl method (Bureau of Indian Standards 1971).
Measurement of pollution load generated in leather processing
The skin samples, after different time points of preservation, were subjected to soaking. The soaking liquor was collected and analyzed for BOD, COD, TDS, SS, and chlorides using standard analytical procedures (Eaton et al. 1995).
Estimation of total collagen in the control and experimental skins
The total collagen content of salted and saltless preserved skins was estimated by measuring the hydroxyproline content by the method of Woessner (1979), after removing the fat in the skin samples by immersing them in a mixture of chloroform:methanol (2:1) two to three times.
Results and discussion
Preservation is the very first step in the processing of leather production. Being a biological source with full of nutrients and moisture, skins are good media for microorganisms to grow. The growth of these organisms, if not prevented, will start degrade the skins and hides which in turn affect the quality of the leather, which the tanners will come to know only after passing through several steps of leather making. This would lead a great loss to the tanners.
Extensive research is being carried out to replace the chemicals used for preservation to avoid pollution load (Kanth et al. 2009; Rai et al. 2009; Tamil Selvi et al. 2015; Sivakumar et al. 2010; Murugan et al. 2013; Sivakumar et al. 2016; Wu et al. 2017; Md. Minhaz et al. 2019). In this present study, we have explored the possibility of using a leaf extract of A. marmelos, as an alternative to salt for the curing process.
Table 1 shows the standardization of optimum concentration of A. marmelos extract for ambient preservation of skins. Five percent of the extract was found to be effective in preserving the skins for 1 month whereas 4% concentration preserved the skins for 20 days. There was no hair slip or odor observed for 5% and 10% concentration even after 30 days. Thus, 5% of the extract was used to continue with further experiments.
Moisture content is one of the important factors that could be used to assess the ability of curing agents for preservation. Conventionally, 40–50% of common salt is spread on the flesh side of the skins and hides. The salt facilitates dehydration of skin/hide to bring down the water content from 60 to 25% to curb the bacterial growth by osmotic process, as their functions would be prevented in lower moisture content (Sivakumar et al. 2019).
The moisture content of the preserved skins at different time point intervals is shown in Table 2. The moisture content of the salt-treated skins was drastically reduced from 74 to 65% on day 1 itself (14% reduction), whereas there was not much change in the moisture content of the experimental skins on day 1 (5.7%). Even on 7th day, the moisture content of the experimental skins was maintained at 45% whereas it was only 40% in salt-preserved skins. The greater reduction in moisture content in salt-treated skins might be due to the hypertonic nature of salt (Lakshmi and Mitra 2006). High concentration of salt extracts water from the cells and thus the moisture reduces. The driving force for the diffusion of water from the skin into the solution is provided by the higher osmotic pressure of the hypertonic solution, i.e., salt. It has been reported that the moisture content could reduce to 34% after 2 days (Kanagaraj et al. 2005b). Bacteria can propagate only in the presence of critical moisture in the skins and hides. Preservation by common salt helps to dehydrate the water below the minimum requirement for the bacteria to grow (Kanagaraj and Chandrababu 2002).
Bacterial count in the preserved skins is shown in Table 3. The main purpose of performing bacterial count was to determine the number of bacteria present in the new method of preservation without salt. The bacterial count was 9 × 1010 in the case of salt curing method on day 1 whereas it was 6 × 1010 in A. marmelos–treated skins. At the end of 7 days, the bacterial count has been reduced to 6 × 1010 in salt-treated skins whereas it was 6 × 108 in the extract-treated preservation. The difference in the reduction in the microbial content in the salt-preserved skins might be due to the reduction in moisture content which is known to be directly proportional to the bacterial count (Kanagaraj et al. 2001). The decreased microbial count in the experimental group might be due to the antimicrobial property of the plant extract (Kausik et al. 2019).
Antimicrobial activity of the plant extract against S. aureus, B. subtilis, K. pneumoniae, and E. coli is illustrated in Table 4. From the table, it could be observed that the extract is effective at a concentration of 100 μg and more effective against B. subtilis and S. aureus.
These are the major organisms present in the skin which secrete proteolytic and collagenolytic enzymes which affect the quality of the skins and in turn the final product, i.e., the leather. It has been reported that the microorganisms predominantly present in the hides and skins were Staphylococcus spp., Micrococcus spp., Bacillus spp., and Corynebacterium spp. along with Staphylococcus albus, Streptococcus pyogenes, Pseudomonas aeruginosa, Bacillus subtilis, and Corynebacterium pyogenes (Mohamed et al. 2016; Richard et al. 2019).
The raw hides and skins contain protein and water which are essential for the growth of microorganisms. The microorganisms use the protein and water present in the skins and hides as medium to grow by decomposing the protein. This is termed as putrefaction. These microorganisms (particularly bacteria) degrade collagen. Among different types of bacteria like aerobic, facultative, and anaerobic, anaerobic bacteria are very hazardous as they are involved in the degradation of collagen at specific sites and convert it into amino acids. This affects the quantity of collagen present in the skins and hides and thus the quality of leather ultimately.
Figure 1 illustrates the collagen content of the skins cured by both salt- and extract-treated methods. Collagen is the major protein present in the skins of any animal. Being a structural protein with three polypeptides, collagen plays a significant role in the stability and elasticity of the skins. Any changes occur in the collagen content during the process of leather making would definitely affect the quality of the leather.
Even though there were no significant changes observed during the initial stages of preservation, a 32% reduction in the collagen content in salt-preserved skins was observed in day 15 samples when compared with plant extract–preserved skins. This biochemical parameter strongly substantiates that preservation by plant extract is a better method than salt preservation. Kannan et al. (2010) have reported that the difference in the collagen content of the skins might be due to the difference in the moisture content. They have observed that the hydroxyproline content of skin samples preserved by sodium chloride was lower when compared with drying method.
Table 5 shows the TKN values of preserved skins. TKN is the sum of all organic nitrogen and ammonia present in the sample. Animal skins are usually made up proteins which are present mainly in the form of collagen, keratin, elastins, globulins, etc. The amount of nitrogen present in the skin sample gives an approximate indication of the quality of the leather. The more the amount of nitrogen present, the better the quality of the leather. The total extractable nitrogen gradually rose in salt-preserved skins with increased duration of preservation, whereas in the skins preserved by the method using A. marmelos extract, it gradually decreased. The saltless preservation showed a value of 2.28 g/kg when compared with the corresponding salt-cured skin (5.52 g/kg) after 30 days.
Measuring hydrothermal stability becomes mandatory to substantiate the quality of preservation. Hydrothermal stability can be measured as shrinkage temperature, which is an index of any structural changes in the collagen. When the shrinkage temperature is higher, the stability of the collagen is more and vice versa.
The changes in the shrinkage temperature of the preserved skins after different days are shown in Fig. 2. It could be noted that there is no significant changes in the shrinkage temperature of both the salt-preserved and the plant extract–preserved skins substantiating that the preservation quality of the leaf extract is on par with the conventional method of preservation and there is no harmful effect established on the skin matrix by the new method of preservation.
The reduction in pollution load assessed by measuring BOD, COD, TDS, and TSS of soak liquors of control and extract-treated skins is shown in Table 6. There is more than 45% reduction in the BOD in A. marmelos–treated skins when compared with controls. Also, a threefold decrease could be observed in COD levels. TDS, the main culprit which spoils the ground water, is markedly reduced in the experimental group (from 270 to 3 g) when compared with control. TSS is also very much reduced in the experimental group.
This green chemistry–based method of preservation has a major advantage. As this method totally eliminates salt, the water used for soaking process is completely saved. Instead of three washes normally used to remove the salt completely, only one wash is enough to remove the remnants of the plant extract as we removed the plant extract manually before soaking. The extract could then be used as manure for plants.
The water, becoming precious nowadays, is very much conserved in this method of preservation. Valeika (2016) have shown that treatment of a mixture of water, sodium hydroxide, hydrogen peroxide, and acetic anhydride preserved hides for 20 days at 22 ± 1 °C. They have mentioned that leather produced from this oxidative method of preservation had shown slightly lower tensile and grain strength when compared with the leather produced by conventional preservation. But in our case, A. marmelos extract preserves skins for more than 30 days without allowing the skins for putrefaction. The antimicrobial activity of the plant extract exhibited by the plant due to the presence of many phytochemicals might be the main reason for potential preservation of skins in the absence of salt.
These results strongly substantiate that this method of preservation is very eco-friendly and could be effectively used as an alternative for salt curing.
Conclusion
The efficacy of A. marmelos for better preservation of skins was evaluated by carrying out bacterial count, pollution load, total extractable nitrogen, shrinkage temperature, etc. This preservation method using A. marmelos presents an environmental support to overcome the problems produced by using salt. The advantage of this method is the total elimination of salt though there is no compromise in the quality of the leather when compared with leather made from salt-preserved skins.
References
Abul M, Abdul MM, Mehedi H (2018) Leaf paste aided goat skin preservation: significant chloride reduction in tannery. J Environ Chem Eng 6:4423–4428
Bailey DG (1997) Evergreen hide market ready. Leather Manuf 115:22–26
Berwick PG, Gerbi SA, Russel AE (1990) Antibiotics to control green hide biodeterioration for short term preservation. J Soc Leather Technol Chem 74:142–150
Bienkiewickz K (1983) Physical chemistry of leather manufacture. Kriege Publishing Company, Florida
Bureau of Indian Standards (1971) Chemical testing of leather. Bureau of Indian Standards, New Delhi, pp 2–80
Cruickshank R (1965) Determination of bacterial count method. In: Medical microbiology, 11th edn. Livingstone, Edinburgh, pp 768–769
Davis MH, Scroggie JG (1973) Investigation of commercial chrome tanning systems. J Soc Leath Tech Ch 57:81–83
Eaton AD, Clesceri LS, Greenberg AE (1995) Standard methods of the examination of water and wastewater, 19th edn. APHA, Washington, DC
Etissa G, Anja A (2018) Preservation, transportation and constraints of hide and skin in Woliso woreda, South West Shoa Zone, Oromia region Ethiopia. MOJ Ecol Environ Sci 3:162–165
Gudro I, Valeika V, Sirvaitytė J (2014) Short term preservation of hide using vacuum: influence on properties of hide and of processed leather. PLoS One 9(11):1–9 e112783
Hughes IR (1974) Temporary preservation of hides using boric acid. J Soc Leath Tech Ch 58:100–103
Iyappan K, Ponrasu T, Sangeethapriya V, Gayathri VS, Suguna L (2013) An eco-friendly method for short term preservation of skins/hides using Semecarpus anacardium nut extract. Environ Sci Pollut Res Int 20:6324–6330
Kanagaraj J, Chandrababu NK (2002) Alternatives to salt curing techniques – a review. J Sci Ind Res 61:339–348
Kanagaraj J, Chandra Babu NK, Sadulla S, Suseela Rajkumar G, Visalakshi V, Chandra Kumar N (2001) Cleaner techniques for the preservation of raw goat skins. J Clean Prod 9:261–268
Kanagaraj J, John Sundar V, Muralidharan C, Sadulla S (2005a) Alternatives to sodium chloride in prevention of skin protein degradation-a case study. J Clean Prod 13:825–831
Kanagaraj J, Sastry TP, Rose C (2005b) Effective preservation of raw goat skins for the reduction of total dissolved solids. J Clean Prod 13:959–964
Kanagaraj J, Tamil Selvi A, Senthilvelan T, Chandra Babu NK, Chandrasekaran B (2014) Evaluation of new bacteriocin as a potential short-term preservative for goat skin. Am J Microbiol Res 2:86–93
Kannan K, Kumar M, Rao J, Nair B (2010) A novel approach towards preservation of skins. J Am Leather Chem Assoc 105:360–368
Kanth S, Keerthi S, Selvi A, Saravanan P, Rao J, Nair B (2009) Studies on the use of Sesuvium portulacastrum. J Am Leather Chem Assoc 10:25–32
Kausik B, Sumanta M, Padilam S (2019) An eye-catching review of Aegle marmelos L. (golden apple). Pharm J 11:207–224
Kavitha PR, Ganapathy GP (2015) Tannery process and its environmental impacts a case study: Vellore District, Tamil Nadu, India. J Chem Pharm Sci 8:759–764
Lakshmi M, Mitra RB (2006) A cleaner production method for the synthesis of bronopol: a bactericide that is useful in leather making. J Clean Prod 14:536–538
Mariappan M (1997) Environmental protection initiatives in Indian tanneries – a perspective view. Proceedings of the 31st Leather Research Industry Get – Together, Central Leather Research Institute, Adyar, India, 150p
Martin CP (2014) Reduced salt preservation process for skins and hides. WO2014191862A1
Md. Minhaz U, Nizam U, Thamina A, Yead M, Sobur A, Md. Jawad H, Sumaiya M, Md. Mokarom H, Nasima AM, Md. Latiful B, Ahmad IM, Sayed MS (2019) Eco-friendly goat skin preservation: an approach for TDS, salinity reduction in tannery waste water. J Ecol Resour 3:1–8
Mohamed HAA, Van Klink EGM, El Hassan SM (2016) Damage caused by spoilage bacteria to the structure of cattle hides and sheep skins. Int J Anim Health Livest Prod Res 2:39–56
Murugan D, Rai CL, Sivarajan M (2013) Moisture desorption characteristics of raw sheep and goat skins: a tool for eco-friendly method of preservation. J Am Leather Chem Assoc 108:32–40
Rai CL, Surianarayanan MA, Sivasamy A, Chandrasekaran F (2009) Pollution prevention by salt free preservation of raw goatskins through stepwise drying. Int J Environ Eng 1:295–305
Richard OO, John OO, Joshua NE (2019) The role of leather microbes in human health. In: Chauhan NS (ed) Role of microbes in human health and diseases. Intech Open, London, pp 1–18
Rob C (2020) World Cattle Inventory: ranking of countries https://beef2live.com/story-world-cattle-inventory-ranking-countries-0-106905
Shilpa G (2014) An in-depth study of India’s leather industry with special reference to export prospects of leather products. Int J Adv Res Manag Soc Sci 3:56–67
Sivabalan V, Jayanthi A (2009) A study to reduce salt usage in preservation of skins and hides with alternate use of plant extract. J Agric Biol Sci 4:43–46
Sivakumar V, Balakrishnan PA, Muralidharan C, Swaminathan G (2010) Use of ozone as a disinfectant for raw animal skins—application as short-term preservation in leather making. Ozone Sci Eng 32:449–455
Sivakumar V, Mohan R, Rangasamy T, Muralidharan C (2016) Antimicrobial activity of Myrobalan (Terminalia chebula Retz.) nuts: application in raw skin preservation for leather making. Ind J Nat Prod Resour 7:65–68
Sivakumar V, Resmi M, Muralidharan C (2019) Alternative methods for salt free / less salt short term preservation of hides and skins in leather making for sustainable development – a review. Text Leather Rev 2:46–52
Sivaparvathi M, Nandy SC (1974) Evaluation of preservatives of skin preservation. J Am Leather Chem Assoc 69:349–362
Sobur A, Fatema TZ, Arup S, Md. Abul H (2015) Short term preservation of goat skin with indigenous to reduce pollution load in leather processing Int. J. Curr Ther Res 7:21158–21161
Sudha R, Radhika J (2007) Antibacterial screening of Aegle marmelos, Lawsonia inermis and Albizzia libbeck. Afr J Tradit Complement Altern Med 4:199–204
Tamil Selvi A, Kanagaraj J, Saravanan P (2015) Preservation of goatskin using Tamarindus indica leaf extract - green process approach. J Soc Leather Technol Chem 99:107–114
Valeika V (2016) Short-term preservation of hides and skins using peracetic acid. J Am Leat Chem Assoc 111:1–9
Vankar PS, Dwivedi AK (2009) Sulphates for skin preservation - a novel approach to reduce tannery effluent salinity hazards. J Hazard Mater 163:207–212
Vedaraman N, Sandhya KV, Brindha V, Tamil Selvi A, Velappan A, John Sundar V, Kanagaraj J, Muralidharan C (2016) De-oiled neem cake as potential bio-additive for low-salt raw skin preservation: a process for salinity reduction in tanneries. Int J Environ Sci Technol 13(6):1563–1572
Vijayalakshmi K, Judith R, Rajkumar S (2009) A novel plant based formulations for short term preservation of animal skins. J Sci Ind Res 68:699–707
Vinod T, Sandeep G, Purnendu B (2003) Case studies on biological treatment of tannery effluents in India. J Air Waste Manage Assoc 53:976p
Woessner JR Jr (1979) The determination of hydroxy proline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93:440–447
Wu J, Zhao L, Liu X, Chen W, Haibin G (2017) Recent progress in cleaner preservation of hides and skins. J Clean Prod 148:158–173
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuttalam, I., Nagarajan, V. & Lonchin, S. An eco-friendly saltless method of preservation of skins using A. marmelos extract. Environ Sci Pollut Res 27, 23707–23713 (2020). https://doi.org/10.1007/s11356-020-08633-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08633-3