Abstract
Bio-waste materials from aquatic species are alternative sources of chitin and chitosan—high-value natural biodegradable and biocompatible polymers. More than 250,000 metric tons of shell, scale, and carapace waste are produced in the Philippines. An evaluation of the quality of raw chitin and chitosan yields from the bio-waste materials of Asian green mussel (Perna viridis), tropical oyster (Crassostrea iredalei), milkfish (Chanos chanos), tilapia (Oreochromis niloticus), and king mangrove crab (Scylla serrata) is needed for the sustainable sourcing. The mild extraction method done in this study showed significantly higher yields of chitin and chitosan for S. serrata and P. viridis (p = 0.001), with chemical structure confirmed through FTIR-ATR analysis. Elemental analysis showed pure extracts from S. serrata, P. viridis, and C. iredalei (N = 6.43–7.01%; DA = 98.7–104.1%). Extracts from the fish scales have high moisture content and glycoprotein contamination. Protein content, determined using UV-VIS spectrophotometry, was found to be significantly less in P. viridis and may be related to the fineness of particle size after grinding. It is recommended to improve the protocol to increase yield across all bio-waste materials, including additional tests to determine the quality of chitin and chitosan extracted, and to check water and oil holding capacities of the extracts to identify the best downstream applications of the varied chitin and chitosan qualities from each source.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Close to 8 million tonnes of shell and scale waste are produced per annum globally with 1.5 million tonnes produced in Southeast Asia (Fisheries FAO 2014). Mussel shell waste amounts to 65,628 to 93,541 t per year (Peña-Rodríguez et al. 2010) while oyster shell waste adds up to about 140,000 t (Kwon et al. 2004). In the Philippines, the growing production of the mangrove crab industry at 16,000 t (Quinitio et al. 2011), and the milkfish and tilapia industries at 300,000 tones (Guerrero and Guerrero 2004; Yap et al. 2007) have their consequent shell and scale waste. Unfortunately, improper bio-waste disposal poses significant environmental and health problems in the coastal regions (Mo et al. 2018). Alternative uses to these bio-waste materials contribute to increased sustainability of the aquaculture industries by transitioning to a circular economy, where waste is converted into a resource (Yu et al. 2015; Geissdoerfer et al. 2017).
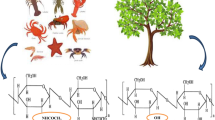
Chitin is an abundant biopolymer. It is the major component of invertebrate exoskeletons and fungi cell walls (Fadlaoui et al. 2019). Its structure is comprised of 2-acetamido-2-deoxy-β-d-glucose (NAG) monomers linked together by β(1➔4) linkages. It is highly valued because of its antimicrobial and bioadsorbent properties, making it useful in food production, as an additive in biomedical products, and in bioremediation (Hirano 1996; Shahidi et al. 1999; Srinivasa and Tharanathan 2007). Chitin, when deacetylated, leads to the formation of chitosan (Abdulkarim et al. 2013). Chitosan is a non-water-soluble, biodegradable compound that is made up of 2-amino-2-deoxy-(1➔β) residues (Rasti et al. 2017). It has anti-inflammatory properties, high mechanical and tensile strengths, and is known for its wide range of applications. It is used in the paper and textile industries, in the cosmetics industry, in nanotechnology, in tissue engineering, and in other biomedical applications (Dutta et al. 2004; Park and Kim 2010; Mladenova et al. 2011; Elieh-Ali-Komi and Hamblin 2016).
The shells of Asian green mussel (Perna viridis Linnaeus, 1758) and the tropical oyster (Crassostrea iredalei Faustino, 1932), the fish scales of milkfish (Chanos chanos Forsskål, 1775) and tilapia (Oreochromis niloticus Linnaeus, 1758), and the carapace of the mangrove crab (Scylla serrata Forsskål, 1775) are potential sources of chitin and chitosan (Abdulkarim et al. 2013; Kumari and Rath 2014; Solidum 2011).
The extraction of high-quality chitin and chitosan from a wide variety of bio-waste encourages alternative extraction sources for industrial and medical applications that can lessen waste production. A controlled comparative study on the chitin and chitosan yield and quality from multiple bio-waste materials allows for a clearer evaluation of the potential of each bio-waste type to serve as an industrial source. This increases the sustainability of industries dependent on chitin and chitosan by decreasing carbon footprint and providing improved means of waste management.
Materials and methods
Sampling and sourcing of bio-waste
The P. viridis and C. iredalei shells, the S. serrata carapace, and the C. chanos and O. niloticus scales were obtained from different public markets and restaurants in Metro Manila, Philippines. The fish scales, mussel, and oyster shells were all acquired fresh and untreated. The collected mangrove crab carapaces were from steamed crabs that were heated at 90–120 °C at an average of 15–30 min. Five hundred grams (500 g) of each bio-waste material was collected and stored at 4 °C prior to processing.
Initial preparation of bio-waste materials prior to extraction and determination of moisture content
Collected materials were rinsed with a mild detergent to remove barnacles and the slimy layer from their surfaces. The detergent used was fully rinsed to remove impurities. The shells were blot-dried before their initial weight was determined.
The dry weights of the samples were recorded after oven-drying for 24 h at 60 °C. The moisture content of the shells was calculated using Formula 1. High moisture content in the bio-waste means there would be less potential chitin and chitosan that can be extracted per gram from the collected materials.
Isolation of chitin and chitosan from dried bio-waste
The dried bio-waste materials underwent three phases for the extraction of chitosan: demineralization, deproteinization, and deacetylation. The bio-waste materials were separated into three replicates of 50 g each. Particle sizes of the powderized bio-waste was determined through mechanical sieving that can differentiate particle sizes from 500 to 2000 um. The fish scales were separated into three replicates of 40 g each.
Demineralization was done using 1 M HCl solution at 75 °C for 2 h. The samples were filtered through a gravity filtration setup and were rinsed with distilled water. The demineralized samples were oven-dried at 60 °C for 24 h. Deproteinization was accomplished using a 1 M NaOH solution at 80 °C for 2 h. Deproteinized samples were filtered using a gravity filtration setup and rinsed with distilled water. The samples were again oven-dried at 60 °C for 24 h. The weight of the extracted chitin was recorded and half of each portion was set aside for analysis. Deacetylation of extracted chitin to acquire chitosan was done using 50% NaOH solution at 100 °C for 2 h. The samples were filtered through a gravity filtration setup and rinsed with distilled water. The samples were then oven-dried at 60 °C for 24 h. The weight of the extracted chitosan was recorded.
Evaluation of percentage chitin and chitosan yield
Formula 2 was used to calculate for the percentage chitin yield and Formula 3 for percentage chitosan yield.
A one-way ANOVA at a 95% confidence level was used to determine if the difference of the means of the raw chitin and chitosan yields among the bio-waste materials was significant. Tukey post hoc tests were used whenever significant differences in ANOVA were encountered.
Characterization of isolated chitin and chitosan
Fourier transform infrared spectroscopy-attenuated total reflection (FTIR-ATR) was performed through GASMET FTIR spectrophotometry, using commercial chitin and chitosan from Merck as control. Measurement of transmittance was done at a function of the wavenumber from 4000 to 600 cm−1 with an 8-cm−1 resolution (Abdel-Rahman et al. 2015). The number of scans was set to 32.
Elemental analysis of chitin extracts to check for %C, N, and H was performed using the Fisons - EA-1108 CHNS-O Element Analyzer. Formula 4 was used to compute for the degree of deacetylation (Sajomsang and Gonil 2010):
Protein content was determined using UV-VIS spectroscopy using a Shimadzu UV160A UV-VIS spectrophotometer at a wavelength of 564 nm. Solutions were set at a concentration of 0.5 mg/mL. Protein content (%P) was computed using Formula 5 (Abdel-Rahman et al. 2015).
Results and discussion
As an archipelago, the Philippines has bountiful aquatic resources and has the highest seafood consumption in Southeast Asia (Sioen et al. 2009). The market demands for fresh and preserved seafood are consistently increasing at a rate of 3.53% per annum (Fabinyi 2016; Crona et al. 2016). This economic boon provides for potential bio-waste sources of chitin and chitosan. The infrastructure and policy for proper solid waste management of seafood waste should comply with Republic Act 9003 or the Ecological Solid Waste Management Act of 2000, but implementation is varied across locations and managing units with local government units (LGU) still unable to prevent illegal dumping of bio-waste in landfills, citing limitations in budget for lapses (Sapuay 2005). This presents the need for alternative means to handle bio-waste and for new income streams in communities that deal with high amounts of bio-waste materials. Potential sources of targeted bio-waste in large quantities are wet markets, supermarkets, and canning facilities in seafood hotspots, including the provinces of Cavite, Bataan, Cagayan, Roxas, and Lanao del Norte (Van Duijn et al. 2012; Gongona 2018).
Bio-waste sources and pre-processing assessment
The collection of all types of bio-waste materials used in this study was simple and direct, with the target weight per type of material gathered within 5 working days. The shells of Perna viridis and Crassostrea iredalei and the scales of Chanos chanos and Oreochromis niloticus were acquired raw in sufficient amounts. The fish scales proved to be the easiest to acquire, with the target amount collected within 1 day (Fig. 1). There were no sources for raw Scylla serrata carapaces found. Instead, they were collected from seafood restaurants and catering companies where the amount collected was limited by the number of orders they received per day.
Despite the ease of collecting fish scales, they were shown to have the highest moisture content (Fig. 2). This poses issues in cost recovery of collection and transportation because only 37.4% of C. chanos and 34.4% of O. niloticus were available after dehydration. Alternative drying processes may result to higher yield, such as 72 h of sun drying or 24-h exposure to lower temperatures ranging from 45 to 56 °C (Zaku et al. 2011). The lowest moisture content was from C. iredalei, and the loose chalkiness of the initial ground materials from their shells supports this. The low moisture content implies higher potential amounts of chitin and chitosan, although it is expected that the bulk of the dry weight from this bio-waste is calcium carbonate (Dumapig and Hapinat 2016).
Grinding the dried bio-waste materials resulted to a variety of particle sizes. The dried scales from C. chanos and O. niloticus had the highest particle sizes at 1200–1500 um, P. viridis at 900–1000 um, and S. serrata at 800 um. Crassostrea iredalei particles were the finest at < 500 um. The difference in the particle sizes after grinding may have been caused by glycoprotein residues that prevent the particulates from separating (Chateigner et al. 2000). The thickness of the shells may have also affected how the samples were pulverized as they were manually ground down. Scylla serrata carapace and P. viridis shells are thinner, causing them to crack and provide less contact. The thicker calcium carbonate–rich shells of C. iredalei, on the other hand, have a chalky texture that resulted to finer particles.
Quantity and quality of extracted chitin
The higher yields of chitin from P. viridis and S. serrata (p = 0.001) bio-waste were observed, with little variation across replicates (coefficient of variance = 2.3–3.7) (Fig. 3). Crassostrea iredalei also has a significantly higher yield compared to the fish scales, although significantly less than P. viridis and S. serrata (p = 0.006; p = 0.011).
It is notable that Crassostrea spp. is a known source of chitinase, an enzyme that degrades chitin (Badariotti et al. 2007; Okada et al. 2013). Chitinase is produced in the mantle of most chitin-bearing organisms and is a protective mechanism against chitin-coated pathogens. Ideally, traces of chitinase were removed upon cleaning of the shells. Traces of remaining chitinase should have denatured during the drying process and after deproteinization. The loss in chitin could have happened if the enzyme was activated during the collection of bio-waste materials and transport to the laboratory.
Greater yield from the fish scales could also be achieved through an alternative extraction procedure that extends demineralization and deproteinization to 36 h, including a KMnO4 and oxalic acid treatment (Kumari and Rath 2014).
The lesser yield in the fish scales may be caused by the high amounts glycoprotein that were retained on the surface of the fish scales, which was observed as a shiny glaze on the surface of the scales after drying. These substances prevented the successful separation of chitin from the other scale components leaving them in the discarded materials of the extraction procedure.
The high yields from P. viridis shells and S. serrata carapace indicates that the extraction technique suits the maximization of the chitin yield from the materials. It implies that standardized procedures may be applied to both sources, reducing the need to separate the two bio-waste materials upon collection.
FTIR-ATR of the chitin isolates show similar peaks for commercial chitosan and extracts from S. serrata, indicating minimal to no significant difference (Fig. 4). Extracts from P. viridis and C. iredalei had lower peaks at 3510–3410 cm−1 which may be caused by the hydroxyl and secondary amine groups undergoing stretching vibrations, which is typical for chitin. For P. viridis and C. iredalei, the peaks were observed at 1650 cm−1 and 1550 cm−1 indicating a ß-chitin conformation as expected from molluscs (Rasti et al. 2017). For C. chanos, O. niloticus, and S. serrata, the peaks were observed at 1650 cm−1, 1620 cm−1, and 1554 cm−1, which indicates that the samples are in the α-chitin conformation (Kaya et al. 2015; Kumari et al. 2015). Other notable structural features of chitin were observed in all samples (Table 1).
Lower %N values of the extracts from the C. chanos and O. niloticus scales and S. serrata carapace (Table 2) imply the presence of residual water in the extracts (Kaya et al. 2016). All samples except for P. viridis had %DA values higher than 100% which indicates the incomplete removal of inorganic material during demineralization (Sajomsang and Gonil 2010). The P. viridis samples also had the lowest protein contamination. Efficiency of demineralization and deproteinization is affected by particle size of the powderized substance, with ideal particle size from 800 to 1000 um (Abdel-Rahman et al. 2015). This parameter was only seen in the P. viridis and the S. serrata powderized bio-waste.
Quantity and quality of extracted chitosan
Scylla serrata and P. viridis had the highest yield of chitosan (p = 0.002) with minimal variation (coefficient of variance = 3.2–4.8) (Fig. 5). The deacetylation process for these bio-waste materials made use of the same concentration of NaOH, but adjustments may be done to maximize the removal of acetyl groups and minimize depolymerization (Hossain and Iqbal 2014). The reduced yield may have been caused by excessive washing after demineralization since chitosan is partially soluble to weak acids (Zhang et al. 2003).
The FTIR-ATR results indicated high deacetylation of the samples due to the low adsorption band at 1594 cm−1 (Fig. 6). Distinct peaks were found at 1658 cm−1, 1590 cm−1, and 1378 cm−1 for the amide groups of α chitosan for C. chanos, O. niloticus, and S. serrata, while P. viridis and C. iredalei only had peaks at 1650 cm−1 and 1380 cm−1. These features were consistent in indicating that the chitosan extracts were in ß-conformation (Kaya et al. 2015; Kumari et al. 2015). Differences in conformation often affect clinical applications, including antimicrobial activity (Jung and Zhao 2013). Notable features found in chitosan were found in all samples (Table 3).
Elemental analysis of extracted chitosan confirmed high deacetylation (Table 4), with ideal values close to 75% (Kumari et al. 2015). The high deacetylation of the samples may be due to the concentration of NaOH and the nature of the chitosan source. A lower concentration of NaOH ranging from 40 to 60% generated ideal results in other experiments (Abdel-Rahman et al. 2015; Kumari et al. 2017). Crustacean species have been known to produce highly deacetylated chitosan with standard extraction procedures (No and Hur 1998), but no similar trend has been found for fish scales and mollusc shells. The higher deacetylation leaves behind more amino groups allowing it to be more chemically reactive (Hussain et al. 2013). Protein content remains high for the fish scales and low for P. viridis. Protein contamination is still attributed to inefficient deproteinization that also affected chitin quality.
Conclusion
This study compared the yields of chitin and chitosan across five common types of bio-waste in the Philippines. The highest quality and yield for both chitin and chitosan came from the P. viridis shells and S. serrata carapace due to these bio-waste materials being better suited to the drying techniques used, the achievement of ideal particle size during grinding, and a compatibility with the reagent concentrations for demineralization and deacetylation. The yields from fresh and preserved C. iredalei samples may be compared to check chitinase activity. Grinding must also be modified to result to bigger particle sizes. Due to their abundance, fish scales from C. chanos and O. niloticus, still hold great promise but also require the most optimization to maximize yield. Improvements in the drying process to minimize water contamination and early removal of glycoprotein, which prevents proper grinding, are needed. Ideal reagent concentrations for demineralization and deacetylation are is also needed to remove protein contamination. Further structural and quality analysis of the extracted chitin and chitosan using X-ray diffraction analysis, electron microscopy, and thermogravimetric analysis are recommended. The water and oil holding capacities of the extracts may be checked to determine the best downstream applications, from food supplements to water treatment.
References
Abdel-Rahman RM, Hrdina R, Abdel-Mohsen AM, Fouda MM, Soliman AY, Mohamed FK, Mohsin K, Pinto TD (2015) Chitin and chitosan from Brazilian Atlantic Coast: isolation, characterization and antibacterial activity. Int J Biol Macromol 80:107–120
Abdulkarim A, Isa MT, Abdulsalam S, Muhammad AJ, Ameh AO (2013) Extraction and characterisation of chitin and chitosan from mussel shell. Civ Env R 3(2):108–109
Badariotti F, Thuau R, Lelong C, Dubos MP, Favrel P (2007) Characterization of an atypical family 18 chitinase from the oyster Crassostrea gigas: evidence for a role in early development and immunity. Dev Comp Immunol 31(6):559–570
Chateigner D, Hedegaard C, Wenk HR (2000) Mollusc shell microstructures and crystallographic textures. J Struct Geol 22(11–12):1723–1735
Crona BI, Basurto X, Squires D, Gelcich S, Daw TM, Khan A, Havice E, Chomo V, Troell M, Buchary EA, Allison EH (2016) Towards a typology of interactions between small-scale fisheries and global seafood trade. Mar Policy 65:1–10
Dumapig MD, Hapinat HL (2016) Lime from Selected Shellfishes: Treatment for Acidic Soil:26(1)
Dutta PK, Dutta J, Tripathi VS (2004) Chitin and chitosan: chemistry, properties and applications. JSIR 63(1):20–31
Elieh-Ali-Komi D, Hamblin MR (2016) Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int J Adv R 4(3):411
Fabinyi M (2016) Producing for Chinese luxury seafood value chains: different outcomes for producers in the Philippines and North America. Mar Policy 63:184–190
Fadlaoui S, El Asri O, Mohammed L, Sihame A, Omari A, Melhaoui M (2019) Isolation and characterization of chitin from shells of the freshwater crab Potamon algeriense. Progress on Chemistry and Application of Chitin and its Derivatives 24:23–35
Fisheries FAO (2014) The state of world fisheries and aquaculture. Food and Agriculture Organization of the United Nations. http://www.fao.org/3/a-i3720e.pdf. Accessed 28 December 2019
Geissdoerfer M, Savaget P, Bocken NM, Hultink EJ (2017) The circular economy–a new sustainability paradigm? J Cleaner production 143:757–768
Gongona E (2018) Philippine fisheries profile 2018(preliminary copy). Bureau of Fisheries and Aquatic Resources On-Line Information System. https://www.bfar.da.gov.ph/publication. Accessed 28 December 2019
Guerrero III RD, Guerrero LA (2004) Brackishwater culture of tilapias in the Philippines: an assessment. In 6th ISTA (6):14
Hirano S (1996) Chitin biotechnology applications. In Biotechnology annual review Vol. 2. Elsevier, pp. 237-258
Hossain MS, Iqbal A (2014) Production and characterization of chitosan from shrimp waste. J Bangl Agric Univ 12(1):153–160
Hussain MR, Iman M, Maji TK (2013) Determination of degree of deacetylation of chitosan and their effect on the release behavior of essential oil from chitosan and chitosan-gelatin complex microcapsules. Int J Adv Eng Appl 6(4):4–12
Jung J, Zhao Y (2013) Impact of the structural differences between α-and β-chitosan on their depolymerizing reaction and antibacterial activity. J Agr Food Chem 61(37):8783–8789
Kaya M, Lelešius E, Nagrockaitė R, Sargin I, Arslan G, Mol A, Baran T, Can E, Bitim B (2015) Differentiations of chitin content and surface morphologies of chitins extracted from male and female grasshopper species. PLoS One 10(1):e0115531
Kumari S, Rath PK (2014) Extraction and characterization of chitin and chitosan from (Labeo rohit) fish scales. Proc Mat Sci 6:482–489
Kumari S, Annamareddy SHK, Abanti S, Rath PK (2017) Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int J Biol Macromol 104:1697–1705
Kwon HB, Lee CW, Jun BS, Weon SY, Koopman B (2004) Recycling waste oyster shells for eutrophication control. Resour Conserv Recy 41(1):75–82
Mladenova EK, Dakova IG, Karadjova IB (2011) Chitosan membranes as sorbents for trace elements determination in surface waters. Env Sci Poll Res 18(9):1633
Mo KH, Alengaram UJ, Jumaat MZ, Lee SC, Goh WI, Yuen CW (2018) Recycling of seashell waste in concrete: a review. Constr Build Mater 162:751–764
No HK, Hur EY (1998) Control of foam formation by antifoam during demineralization of crustacean shell in preparation of chitin. J Agric Food Chem 46(9):3844–3846
Okada Y, Yamaura K, Suzuki T, Itoh N, Osada M, Takahashi KG (2013) Molecular characterization and expression analysis of chitinase from the Pacific oyster Crassostrea gigas. Comp Biochem Phys B 165(2):83–89
Park BK, Kim MM (2010) Applications of chitin and its derivatives in biological medicine. Int J Mol Sci 11(12):5152–5164
Peña-Rodríguez S, Fernández-Calviño D, Nóvoa-Muñoz JC, Arias-Estévez M, Núñez-Delgado A, Fernández-Sanjurjo MJ, Álvarez-Rodríguez E (2010) Kinetics of Hg(II) adsorption and desorption of calcined mussel shells. J Hazard Mater 180:622–627
Quinitio ET, de la Cruz JJ, Eguia MRR, Parado-Estepa FD, Pates G, Lavilla-Pitogo CR (2011) Domestication of the mud crab Scylla serrata. Aquacult Int 19(2):237–250
Rasti H, Parivar K, Baharara J, Iranshahi M, Namvar F (2017) Chitin from the mollusc Chiton: extraction, characterization and chitosan preparation. Iran J Pharm Res 16(1):366–379
Sajomsang W, Gonil P (2010) Preparation and characterization of α-chitin from cicada sloughs. Mater Sci Eng C 30(3):357–363
Sapuay GP (2005) Ecological solid waste management act of 2000 (RA 9003): a major step to better solid waste management in the Philippines. In International Conference on Integrated Solid Waste Management in Southeast Asian Cities, Siem Reap, pp 51–59
Shahidi F, Arachchi JKV, Jeon YJ (1999) Food applications of chitin and chitosans. Trends Food Sci Technol 10(2):37–51
Sioen I, De Henauw S, Van Camp J, Volatier JL, Leblanc JC (2009) Comparison of the nutritional–toxicological conflict related to seafood consumption in different regions worldwide. Regul Toxicol Pharmacol 55(2):219–228
Solidum JN (2011) Shell wastes for aerial lead monitoring. Int Journal 2(6):17–24
Srinivasa PC, Tharanathan RN (2007) Chitin/chitosan—safe, ecofriendly packaging materials with multiple potential uses. Food reviews international 23(1):53–72
Van Duijn AP, Beukers R, van der Pijl W (2012) The Philippine seafood sector: a value chain analysis. Wageningen University, Netherlands, CBI/LEI
Yap WG, Villaluz AC, Soriano MGG, Santos MN (2007) Milkfish production and processing technologies in the Philippines. Milkfish Project Publication Series 2(96):26–31
Yu F, Han F, Cui Z (2015) Assessment of life cycle environmental benefits of an industrial symbiosis cluster in China. Env Sci Poll Res 22(7):5511–5518
Zaku SG, Aguzue SEO, Thomas SA (2011) Extraction and characterization of chitin; a functional biopolymer obtained from scales of common carp fish (Cyprinus carpio L.): a lesser known source. Afr J Food Sci 5(8):478–483
Zhang C, Ping Q, Zhang H, Shen J (2003) Synthesis and characterization of water-soluble O-succinyl-chitosan. European Polymer J 39(8):1629–1634
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cadano, J.R., Jose, M., Lubi, A.G. et al. A comparative study on the raw chitin and chitosan yields of common bio-waste from Philippine seafood. Environ Sci Pollut Res 28, 11954–11961 (2021). https://doi.org/10.1007/s11356-020-08380-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08380-5