Abstract
The present experimental work was conducted at different sites of district Bhakkar, a semiarid region of Pakistan, to assess whether the goats are suffering nickel deficiency or toxicity and what are the possible seasonal effects on the availability and translocation of nickel in food chain. A total of 27 forage and 320 goats according to four physiological stages [does (she goat), bucks (he goat), wether (castrated), juvenile (6 month)] were recruited for this study. To fulfill this objective, soil, forage, blood plasma, urine, and feces samples were collected in 4 seasons of the year at 2 sites and were analyzed by atomic absorption spectrophotometer for nickel concentration. Different indices BCF, EF, and PLI were also studied to check the metal transfer. The results showed that sites had significant (P < 0.05) effect on nickel concentration in soil, forage, and goats. On the other hand, season and site x season had nonsignificant (P > 0.05) effects on nickel level in soil and goats. The soil (0.68–0.71 mg kg−1), forage (3.41–3.70 mg/kg), and blood (0.21–0.28 mg/l) level was lower than the permissible limits, while feces (0.57–1.34 mg/kg) and urine (0.35–1.32 mg/l) had enough concentration of nickel. Sources showed significant (P < 0.05) effects on Ni level in all stages of goats. All stages of goats except Wether (castrated) showed low level of nickel in blood. Most fluctuations in nickel concentration were observed in (S1) summer (low) and spring (S4) (high) season as a whole, while overall site 2 had high level of nickel than site 1. Thus, nickel showed deficiency in soil, forage, as well as in all stages of goats except wether goats. Nickel containing mineral mixtures are essential for does (she goat), bucks (he goat), and juveniles (6 months old), so application of Ni containing fertilizers to the soil and forage of that region and supplementation of Ni mineral mixture for grazing ruminants should be done.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Minerals play a major role in animal metabolism and nutrition, but availability of these minerals from soil to forage and to animals varies greatly and depends on many factors. Health and production of ruminants are directly related to appropriate availability of mineral elements in the dietary food, but excessive amount of mineral does not sure the transfer of these elements to animals (Khan et al. 2010a). Grazing ruminants mostly fulfill their mineral requirements from the wild forages. Seasonal variation affects the maturity of forage and directly related to mineral formation in forage (Napolitano et al. 2011).
Trace mineral and also Ni have got much attention in recent years because of its major role in the proper metabolism of living system. Nickel is important because of its association with the activity of the enzyme urease. It also works as a cofactor for an enzyme functioning in nitrogen metabolism (Page and Feller 2015). Ni is essential for the activity of ruminal urease, alanine transaminase (ALT), and sorbite dehydrogenase (SDH) in the blood and liver. Enzymes (carbon monoxide dehydrogenase and methyl-coenzyme-M-reductase) required for ruminal fatty acid production are dependent on Ni for their optimum activity (National Research Council (NRC) 2001). Ni is absorbed by the plant roots in its cationic form and in the form of phytosiderophore complexes by roots of graminoids (Clemens 2010; Kramer 2010; Williams and Pittman 2010; White and Greenwood 2013).
Nickel plays a vital role for proper functioning of metabolic systems (Jinwal et al. 2009). Use of Ni containing chemical on large scale causes the toxicity of Ni in environment which may cause sever health problems for animals and also affect the absorbance of other minerals in plants like iron. High level of Ni in soil also causes to increase the Ni level in forage and animals feeding on this forage (Denkhaus and Salnikow 2002). Nickel is found in a very high concentration in contaminated soils. Its value ranges from 200 to 26,000 mg kg−1 as compared to the acceptable limit of 100–1000 mg kg−1 in natural unpolluted soils (Izosimova 2005).
Nickel has significant role in animals such as cows, sheep, and goats, and its deficiency also shows serious health effects in animals such as reduced hemoglobin, skin eruption, low number of offspring, decrease hematocrit values, anemia, and delayed gestation period (World Health Organization (WHO) 1991). Ni deficiency also slows down the activity of several enzymes (Fraga 2005). Physiological status of ruminants determines the uptake of mineral elements. Bucks grow rapidly than does, so bucks need more mineral than does and castrated goats (Anonymous 1980).
Data about nickel toxicity and deficiency related to soil, forage, and ruminant with respect to physiological status is not enough. Considering the importance of Ni for soil, forage, and ruminant, current study was designed to assess possible seasonal and spatial effects on the Ni level in blood plasma, urine, and feces of different physiological stages of goats and in soil and forage on which goats fulfill their nutritional requirements. This study will provide a baseline data for formulation of Ni containing mineral mixture for ruminants. Current work will also be helpful for maintaining appropriate level of Ni in soil-plant-animal continuum by considering the current results.
Materials and methods
Design of experiment
RCBD (Randomized Complete Block Design) was followed. The present investigation was conducted at two sites of district Bhakkar, Pakistan (Tehsil Bhakkar and Tehsil Darya Khan), having average annual temperature 24.6 °C and average rainfall 213 mm (Fig. 1). Each site was divided into 10 plots, and goats were divided into four groups or stages [does (female), bucks (male), wether (castrated), juvenile (6 month)] 10 in each group, and sampling of blood from these different goat stages were collected. All goats were 1 year old except juvenile which were 6 months old. All sampling was done in four different seasons, viz., (S1) summer, (S2) winter, (S3) autumn, and (S4) spring. Forages were also listed on which ruminants fulfill their feeding requirements (Table 1).
Soil
Soil samples (10 g each) were collected from every 10 plot of site where forage/fodders were collected and made into one composite sample. The selected places were dug up to 12- to 15-cm deep partially containing all the layers by stainless steel auger. Five composite samples were prepared and dried in air and put in forced air oven for 48 h at a temperature of 72 °C. These collected samples were air dried, stored in labeled sealed paper bags, and placed in incubator for 5 days at a temperature of 70 °C (Rhue and Kidder 1983).
Forages and fodders
The available forage and fodder samples (on which the goats graze) were collected from the same site from where soil samples were taken by using sterilized apparatus. Then collected samples were air dried, stored in labeled sealed paper bags, and placed in incubator for 5 days at a temperature of 70 °C (Sanchez 1976).
Blood serum samples collection
Blood samples were collected from four categories of the goats [does, bucks, wether, and juvenile (10 in each group)]. A total of 20 ml blood was taken from the jugular vein of each goat in heparin vile sterile plastic test tubes which was placed in slanting position for an hour to let the serum ooze out. After blood collection, the serum was separated from plasma by centrifugation. Then the serum was aspirated carefully with a pipette, put in small labeled voiles, and placed in the freezer at − 20 °C till analysis (Kamada et al. 2000).
Urine
After cleaning and washing the external genitalia of the animals with toilet soap and lukewarm water, catheters were fixed in urethra of goats. Urine was collected into clean glass beakers or bags. These samples were kept at − 20 °C for further investigation (Kamada et al. 2000).
Feces
Fecal sample of each animal was collected manually from the rectum of the animals and put in small plastic bags, oven-dried at 55 °C for 96 h, and stored in polyethylene bags for further analysis (Kamada et al. 2000).
Sample size calculation for animals
The sample size for the ruminants used in this study was calculated by using the equation for a study comparing two means (Eng 2003) as shown: N = 4σ2 (Zcrit + Zpwr)/D2.
Therefore, the total number of samples for the four seasons was:
Sample preparation and analytical procedure
Two ml of liquid samples (blood serum and urine) and 2 g of solid samples (soil, forages, fodder, and feces) were immersed in 20 ml of acid solution composed of H2SO4, HClO4, and HNO3 in a ratio of 1:2:5. The mixture was subjected to intense heating under fume hood with temperature gradually rising from 100 to 250 °C until the volume reduced to about 2–3 ml. Sample was cooled to room temperature, filtered, and diluted with distilled water to bring the volume to 60 ml. This diluted sample was used for analysis of minerals (Kamada et al. 2000).
Standard solutions are required for drawing standard curve during mineral analysis. For the preparation of standard, stock solutions of 1000 ppm were made by dissolving calculated amount of nickel bromide (Ni) in distilled water. Ten ml of this solution was diluted up to exactly 100 ml to prepare a solution of 100 ppm. This solution was used for preparing standard solution of required concentration.
Digested samples were used for nickel detection. Ni detection in all samples was done by using Atomic Absorption Spectrophotometer Perkin-Elmer AAS-5000 (Perkin-Elmer Corp., 1980) after wet digestion.
Bioconcentration factor
Bioconcentration is find out by using the formula devised by Cui et al. (2004):
Pollution Load Index (PLI)
Pollution Load Index is determined by the following formula (Liu et al. 2005):
Enrichment Factor (EF)
Enrichment Factor is calculated by formula of Buat-Menard and Chesselet (1979).
Statistical analysis
Ggplot2 package in software R was used for the formation of combined box plot. Statistix 8.1 was used for performing analysis of variance (ANOVA) along with least significant (0.05) difference (LSD) test (Steel et al. 2006).
Results and discussion
Soil
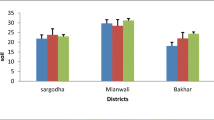
Analysis of variance of Ni values in soil samples showed significant (P < 0.05) effects in site but nonsignificant effects in season and site x season (Table 2). Ni values in the soil samples were ranged from 0.68 to 0.71 mg/kg at both sites in all seasons. Lowest Ni concentration was observed in spring (S1) season, at site 1, and highest concentration was observed in (S2) summer season, at site 2 (Fig. 2). Ni values in soil was below the permissible limit (75 mg/kg) given by European Union (2002). Present investigation showed that Ni concentration in soil was lower than the values reported by Abida et al. (2009). The present Ni values in the soil samples were similar to the values reported by Yahaya et al. (2009) but higher than the values described by Orisakwe et al. (2017).
Ni concentration depends on the parent material in soil and weathering processes, and the primary source of Ni in soil is igneous rock. Use of some phosphate fertilizers is also considered as a source which releases Ni as a pollutant in the environment. Deficiency of Ni in soil occurs mostly by leaching and runoff, and deficiency can be diminished by the addition of agricultural chemicals or the introduction of a high amount of Ni soil in deficit nickel soil (Chauhan et al. 2008). Low nickel level was present in soil which contains sedimentary rocks, sand stone, and limestone, while high amount was present in igneous rocks. The mobility of Ni in soil totally depends on soil composition, texture, and minerology (Kabata-Pendias 2001). Association of nickel with other element also exists very strongly. High level of cobalt and iron in soil means that nickel level is also higher in soil (Kabata-Pendias and Mukherjee 2007). Low level of Ni in the present investigation might be due to basic structure of sandy soil in the study area.
Forages
Analysis of variance showed that site, season, and site x season had significant (P < 0.05) effects on Ni concentration in forages (Table 2). Ni values in forages were observed between 3.41 and 3.70 mg/kg at all sites in all seasons. Summer (S1) season of site 1 was responsible for minimum Ni concentration, and spring (S4) season of site 2 was responsible for maximum Ni concentration in forages (Fig. 2). Ni level in forage samples in the present study was lower than the critical level (5 mg/kg) recommended by National Research Council (NRC) (1980) but was higher than the values reported by Sadeghi et al. (2014). It can be said that the present Ni values in forages meet the minimum Ni requirement for livestock established by Anke et al. (1983). Root is the main source of Ni uptake in plants, but in higher plants, Ni is always available to plant through aerial parts even in toxic and deficit Ni conditions (Wood 2010). Naturally Ni is added to environment from many sources. These sources include volcanic emission, forest fire, dust, coal combustion, sewage sludge, waste material, and fuel oil. These all sources, soil mineralogy, and plant species determine the availability of Ni to plant (Iyaka 2011). Soil pH and organic matter show basic role in the availability of Ni to plants. Nickel availability in some grasses is inversely proportional to soil pH, so it means that high pH of soil decreases the nickel availability to plants (Harasim and Filipek 2015). High organic matter also reduces the nickel uptake in plant from soil because nickel particles bind tightly with organic matter. High soil pH and organic matter in soil cause nickel deficiency in plant (Weng et al. 2004). Ni level in present study was in permissible limits because there was no source of Ni toxicity around the study area and nickel in soil was also in limits.
Goats
Ni values in goats showed the significant (P < 0.05) variations in sites but nonsignificant (P > 0.05) variations in season and site x season (Table 2). Level of Ni in goats varied from 0.38 to 0.98 mg/kg in all seasons of both sites. Lowest level of Ni in goats was observed in summer (S1) season at site 1, and highest level was also observed in summer (S1) season but at site 2 (Fig. 2). Analysis of variance for Ni also showed significant (P < 0.05) variation in source but nonsignificant (P > 0.05) variations in stage and source x stage. All sources and stages showed the range of Ni in goats from 0.24 to 0.99 mg/kg (Table 3). Minimum Ni value was observed in blood of wether, and maximum Ni value was observed in feces of bucks.
Box plot data of Ni values for blood plasma showed that the range of Ni from 0.21 to 0.28 mg/L. minimum level of Ni was revealed in summer (S1) season of site 1, and maximum was also revealed in summer (S1) season but at site 2. Ni values in feces varied from 0.57 to 1.34 mg/kg at both sites throughout the season. Summer (S1) season of both sites showed the lowest and highest level of Ni in feces, respectively. Ni values in urine in goats were observed between 0.35 and 1.32 mg/L at both sites of all season. Maximum and minimum level of Ni in urine was observed at site 2 and site 1, respectively (Fig. 3).
Ni plays essential role in protein synthesis and numerous metabolic processes of organisms, so Ni imbalance leads toward many serious problems in animals (Sidhu et al. 2005). Ni level in blood plasma samples of the present study was higher than the level of blood Ni given by Milam et al. (2017) but lower than the standard limits (0.4 mg/L) described by National Research Council (NRC) (1980). Ni concentration was also higher than the study of Orisakwe et al. (2017). In the present study, Ni values in feces were higher than the values of Ni described by Adesoye et al. (2014).
Nickel with association of other nutrient also plays considerable role for proper biological functioning of numerous metabolic processes (Jinwal et al. 2009). Accretion of Ni in various tissues of animal body was also reported because availability of Ni salts in diet of animals causes its accretion in the tissues of animal body. Absorption of Ni in gastrointestinal tract was also reported, but its amount depends on feeding diet of animal (Youde 2002). In animals, Ni can replace or interact with 13 essential elements like cobalt, copper, iron, potassium, sodium, etc. So long exposure of nickel is associated with nasal, lung tumor, mild nausea, and chest pain (Cempel and Nikel 2006; Iyaka 2011). Nickel value in the present study was reported high, and it might be due to toxicity of Ni through particles of polluted air, e.g., nickel containing dust and smoke.
Bioconcentration factor
BCF for Ni in forages in all seasons at both sites was present in between 4.65 and 5.03. Minimum BCF of Ni in forages was observed in season 1 at site 2, and maximum BCF of Ni was observed in season 2 at site 1. Ni BCF of goats varied from 0.111 to 0.271 at all sites in all seasons. Minimum BCF of Ni in goats was observed in summer (S1) season at site 1, and maximum BCF of Ni in goats was observed in winter (S2) season at site 2 (Table 4).
Bioconcentration factor for Ni in present findings was greater than 1 indicating greater transfer of this metal from soil to plant. The values of BCF in current work were higher than the values of previous studies (Jan et al. 2010; Khan et al. 2010b; Mahmood and Malik 2014). Iqbal et al. (2016) reported lower values of BCF (0.34–0.93) as compared to present findings.
Pollution Load Index
Results depicted that PLI for Ni was present between 0.079 and 0.086 in all sampling seasons at site 1 and site 2. Minimum value of PLI was observed in summer (S1) and spring (S3) season at site 1, and maximum value of PLI was also observed in summer (S1) season at site 2 (Table 4). The values of PLI for Ni in current work were lower than the reference values of Ni (9.06 mg/kg) given by Singh et al. (2010). Rabee et al. (2011) recorded high values of PLI for Ni (0.16–0.38) as compared to present findings. The values of PLI for Ni are less than 1, which shows that study soil is unpolluted.
Enrichment Factor
Data of EF for Ni varied from 0.62 to 0.68 at all sites in all seasons. Summer (S1) season showed the lowest level of EF for Ni at site 2, and winter (S2) season of site 1 showed highest level of EF for Ni (Table 4). Ni E.F analyzed in present study was lower from the E.F reported by Ezemokwe et al. (2017) and Rizo et al. (2011). Nickel E.F was assigned in deficient E.F level as described by Barbieri (2016).
Conclusion
Results showed that soil and forage had deficit of nickel. Summer season possessed the highest level of Ni in goats, and all stages of goats showed low level of nickel in blood, so Ni containing mineral mixtures is essential for does (she goat), bucks (he goat), wether (castrated), and juveniles (6 months old). Indices values were also lower from the pollution level and showed deficiency of element. However, application of Ni containing fertilizers to the soil and forage of that region and supplementation of Ni mineral mixture for grazing ruminants should be done.
References
Abida B, Ramaiah M, Irfanulla K, Veena K (2009) Analysis of heavy metals concentration in soil and litchens from various localities of Hosur Road, Bangalore, India. J Chem 6(1):13–22
Adesoye AM, Adekola FA, Olukomaiya KO, Olukomaiya OO, Iwuchukwu PO (2014) Evaluation of physical properties and heavy metal composition of manure of some domestic animals. Int J Inno Sci Res 9(2):293–296
Anke M, Groppel G, Nordmann S, Kronemann H (1983) In: Anke M, Baumann W, Braunlich H, Bruckner C (eds) 4th Spurenelement symposium Lithium. Abteilung Wissenschaftliche Publikationen, Friedrich Schiller Universtate, Jena, p 1
Anonymous (1980) The nutrient requirements of ruminant livestock. Commonwealth Agricultural Bureaux, Farnham Royal, Slough, Academic Press, London, UK
Barbieri M (2016) The Importance of enrichment Factor (EF) and Geoaccumulation Index (Igeo) to evaluate the soil contamination. J Geol Geophys 5(1):237–242
Buat-Menard P, Chesselet R (1979) Variable influence of atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth Planet Sci Lett 42:399–411
Cempel M, Nikel G (2006) Nickel: a review of its sources and environmental toxicology. Pol J Environ Stud 15(3):375–382
Chauhan SS, Thakur R, Sharma GD (2008) Nickel: its availability and reactions in soil. J Indus Poll Contl 24(1):57–62
Clemens S (2010) Zn – a versatile player in plant cell biology. In: Hell R, Mendel R-R (eds) plant cell monographs 17, cell bio. Met nut spring, Berlin, pp 281–298
Cui YJ, Zhu YG, Zhai RH, Chen DY, Huang YZ, Qui Y, Liang JZ (2004) Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China. Environ Int 30:785–791
Denkhaus E, Salnikow K (2002) Nickel essentiality, toxicity and carcinogenicity. Crit Rev Oncol Hematol 42:35–56
Eng JMD (2003) Sample size estimation: how many individuals should be studied? Radiol 227:309–313
European Union (2002) Heavy Metals in Wastes, European Commission on Environment. http://ec.europa.eu/environment/waste/studies/pdf/heavy_metalsreport.pdf
Ezemokwe DE, Ichu CB, Okoro JN, Opara AI (2017) Evaluation of heavy metal contamination of soils alongside Awka-Enugu road, southeastern Nigeria. Asian J Environ Eco 4(1):1–11
Food and Agricultural Organization (FAO/WHO) (2001). Codex Alimentarius Commission. Food additive and contaminants. Joint FAO/ WHO food standards program, ALINORM 01/ 12A, pp. 1–289
Fraga CG (2005) Relevance, essentiality and toxicity of trace elements in human health. Mol Asp Med 26:235–244
Harasim P, Filipek T (2015) Nickel in the environment. J Elem 20(2):525–534
Iqbal HH, Taseer R, Anwar S, Mumtaz M, Qadir A, Shahid N (2016) Human health risk assessment: heavy metal contamination of vegetables in Bahawalpur, Pakistan. Bull Environ Stud 1(1):10–17
Iyaka YA (2011) Nickel in soils: a review of its distribution and impacts. Sci Res Essays 6(33):6774–6777
Izosimova A (2005) Modeling the interaction between calcium and nickel in the soil-plant system. Landbauforschung Volkenrode FAL Agricul Resear 288(2):100
Jan FA, Ishaq M, Ihsanullah I, Asim S (2010) Multivariate statistical analysis of heavy metals pollution in industrial area and its comparison with relatively less polluted area: a case study from the city of Peshawar and district Dir Lower. J Haz Mat 176(1):609–616
Jinwal A, Dixit S, Malik S (2009) Some trace elements investigation in ground water of Bhopal and Sehore district in Madhya Pradesh: India. J Appl Sci Environ Manag 13:47–50
Kabata-Pendias A (2001) Trace elements in soils and plants, 3rd edn. CRC, Boca Raton, pp 1–448
Kabata-Pendias A, Mukherjee AB (2007) Trace elements from soil to human, vol 1. Springer- Verlag, Berlin
Kamada H, Nishimura HK, Krongyuti P, Sukkasame P, Phoengpong N, Intramanee N (2000) Selenium status of soil, herbage and beef cattle in Southern Thailand. Asian Aus J Anim Sci 13:757–760
Khan ZI, Ashraf M, Ahmad K, Valeem EE (2010a) Periodic evaluation of potassium transfer from soil and forage to small ruminants on an experimental station in southern Punjab, Pakistan. Pak J Bot 42:1353–1360
Khan S, Rehman S, Khan AZ, Khan MA, Shah MT (2010b) Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, Northern Pakistan. Ecotox Environ Saft 73(7):1820–1827
Kramer U (2010) Metal hyper accumulation in plants. Annu Rev Plant Biol 61:517–534
Liu WH, Zhao JZ, Ouyang ZY, Soderlund L, Liu GH (2005) Impacts of sewage irrigation on heavy metals distribution and contamination in Beijing, China. Environ Int 31:805–812
Mahmood A, Malik RN (2014) Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arab J Chem 7(1):91–99
Milam C, One MB, Dogara RK, Yila EY (2017) Assessment of heavy metals (As, Cd, Cr, Cu, Ni, Pb and Zn) in blood samples of sheep and rabbits from Jimeta-Yola, Adamawa State, Nigeria. Int J Adv Pharma Bio Chem 6(3):160–166
Napolitano F, Girolami A, Pacelli C, Braghieri A (2011) Activity budgets and forage selection of Podolian cattle, a semiwild bovine breed. Int Schol Res Network. https://doi.org/10.5402/2011/972804
National Research Council (NRC) (1980) Mineral tolerance of domestic animals. National Research Council/National Academy of Science. Washington D.C, USA: pp. 345–363
National Research Council (NRC) (2001) Nutrient requirements of domestic animals. National Academy of Science Press, Washington, DC, p 1
Orisakwe OE, Oladipo OO, Ajaezi GC, Udowelle NA (2017) Horizontal and vertical distribution of heavy metals in farm produce and livestock around lead-contaminated goldmine in Dareta and Abare, Zamfara state, Northern Nigeria. J Environ Public Health 2017:1–12
Page V, Feller U (2015) Heavy metals in crop plants: transport and redistribution processes on the whole plant level. Agronom 5(3):447–463
Rabee AM, Al-Fatlawy YF, Nameer M (2011) Using Pollution Load Index (PLI) and Geoaccumulation Index (Igeo) for the assessment of heavy metals pollution in Tigris river sediment in Baghdad region. Al-Nahrain J Sci 14(4):108–114
Rhue RD, Kidder G (1983) Analytical procedures used by the IFAS extension soil testing laboratory and the interpretation of results. Soil Science Department, University of Florida, Gainesville
Rizo OD, Hernandez IC, Lopez JA, Arado OD, Pino NL (2011) Chromium, cobalt and nickel contents in urban soils of Moa, northeastern Cuba. Bull Environ Contam Toxicol 86(2):189–193
Sadeghi SAT, Sadeghi MH, Dashtizadeh M (2014) Determination of heavy metals such as lead, nickel and cadmium in coastal range forage of Tangistan, Bushehr Province, Iran Province, Iran. Bull Environ Pharm Life Sci 3(3):266–270
Sanchez PA (1976) Properties and management of soils in the tropics. Wiley, New York, p 1
Sidhu P, Garg ML, Morgenstern P, Vogt J, Butz T, Dhawan DK (2005) Ineffectiveness of nickel in augmenting the hepatotoxicity in protein deficient rats. Nutr Hosp 20:378–385
Singh A, Sharma RK, Agrawal M, Marshall FM (2010) Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Fd Chem Toxicol 48:611–619
Steel RGD, Torrie JH, Dickey DA (2006) Principles and procedures of statistics. A biometrical approach. 3rd of McGraw Hill company, New York
Weng LP, Wolthoorn A, Lexmond TM, Temminghoff EJ, Van RWH (2004) Understanding the effects of soil characteristics on phytotoxicity and bioavailability of nickel using speciation models. Environ Sci Technol 38(1):156–162
White PJ, Greenwood DJ (2013) Properties and management of cationic elements for crop growth. In: Gregory PJ, Nortcliffe S (eds) Soil conditions and plant growth, 12th edn. Blackwell Publishing, Oxford, pp 160–194
Williams LE, Pittman JK (2010) Dissecting pathways involved in manganese homeostasis and stress in higher plants. In: Hell R, Mendel R-R (eds) Plant Cell Monographs 17, Cell Biol. Met. Nutr. Springer, Berlin, pp 95–117
Wood BW (2010) Nickel deficiency symptoms are influenced by foliar Zn:Ni and Cu:Ni concentration ratio. Acta Hortic 868:163–169
World Health Organization (WHO) (1991) Nickel environmental health criteria 108, World Health Organization, Geneva, Switzerland
Yahaya MI, Mohammad S, Abdullahi BK (2009) Seasonal variations of heavy metals concentration in abattoir dumping site soil in Nigeria. J Appl Sci Environ Manag 13(4):9–13
Youde H (2002) An experimental study on the treatment and prevention of shimao zheng (fleece eating) in sheep and goats in the Haizi area of Akesai County in China. Vet Res Commun 26:39–48
Acknowledgments
Authors are grateful to the Higher Education Commission, Pakistan, for providing funding through research project no. 20-3546/NRPU/R&D/HEC/14/536. We are also thankful to local farmers of area for providing help in sampling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bashir, H., Ahmad, K. & Khan, Z.I. Level and speciation of nickel in some forages in relation to spatial and temporal fluctuations. Environ Sci Pollut Res 27, 23793–23800 (2020). https://doi.org/10.1007/s11356-020-08321-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08321-2