Abstract
The fast-growing discharge of effluents of engineered nanomaterials (ENM) and heavy metals in freshwater ecosystems raises concern in recent times. This study investigated the effects of the co-exposure between nanoparticles (TiO2 NPs) and lead (Pb) in a simplified freshwater food web model, including zooplankton (copepods sp.) and Clarias gariepinus on bioaccumulation and antioxidant activity. We carried out a chronic (28 days) semi-static bioassay by feeding individually fish with zooplankton exposed to TiO2 NPs (0.09 and 0.20 μM), Pb (0.01 and 0.04 μM), and their binary mixtures. The binary mixtures caused a significant (p < 0.05) decrease in malondialdehyde (1.64–2.01-fold), catalase (3.18–3.89-fold), glutathione reductase (1.37–1.46-fold), and glutathione peroxidase (1.19–1.89-fold) levels. Lead accumulated in the tissues had bioaccumulation factor between 0.40 and 1.42 in binary mixture. These results indicate that chronic exposure of TiO2 NPs could influence the BAF of Pb, neurotoxicity, changes of antioxidant enzymes, and retardation of food uptake. These findings raise concerns regarding the fate of higher trophic levels in polluted freshwater ecosystems with a binary mixture of engineer nanomaterials and heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The technological revolution has increased the number of manufactured products containing metal oxide engineered materials (ENM), leading to concerns regarding their ecological effects (Skjolding et al. 2016). Generally, these materials have a size in a nanoscale level between approximately 1 and 100 nm and have a much higher surface/volume ratio due to their small size (Oberdörster et al. 2005). Several industries use ENM for one application or the other, for instance, in the electronic and energy sector, ceramics, optics, packaging, paints, agriculture, textiles, and cosmetics (Hansen et al. 2008). The most commonly manufactured engineered nanomaterials is titanium dioxide nanoparticles (TiO2 NPS) (550–5500 metric tons) followed by SiO2, aluminum oxide (AlOx), zinc oxide (ZnO), carbon nanotubes (CNT), iron oxide (FeOx), cerium oxide (CeOx), and silver (Ag) (Skjolding et al. 2016). The yearly demand for TiO2 NPs is estimated at 2.5106 metric tons by 2025 in the USA (Robichaud et al. 2009). One will find TiO2 NPs manufactured and incorporated in various commercial products such as cosmetics and sunblock about 65%, inks, building materials, self-cleaning ceramics, paper making, paint, glass, and self-cleaning ceramics (Gazquez et al. 2014; Hansen et al. 2008; Davis 2009; Federici et al. 2007). With the increasing use of TiO2 NPs, it is likely to be released into aquatic environments through wastewater and effluents, thus causing pollution and a potential hazard to ecological safety. The level of toxicity of TiO2 NPs on aquatic organisms is a function of the specificity of the particle sizes, and it is likely to cause harmful effects such as oxidative stress (Qian et al. 2009; Miao et al. 2015; Kulacki and Cardinale 2012; Hall et al. 2009). Due to the large specific surface of TiO2 NPs, it is capable of interacting with heavy metal and ion adsorbed in aquatic ecosystems thereby forming NPs-complex that alter the toxicity and bioavailability of co-exposed metal in vivo in aquatic organisms (Wu et al. 2019; Vicaria et al. 2018; Wu et al. 2015). Therefore, the interaction between co-exposed NPs and heavy metal is of great concern for environmental risk assessment. Furthermore, understanding the potential impact of the interaction between TiO2 NPs and heavy metal on aquatic organisms is critical for the assessment and evaluation of their potential ecotoxicological effects.
Lead (Pb) industries have existed for more than a hundred decades, despite the efforts by countries to regulate its use (Fitzsimmons 2017). In 2016, Pb production rose by 2.8% and 2.2% to 11.39 million metric tons in 2017 and it is expected to reach 11.6 million metric tons by 2020 (Fitzsimmons 2017). They used lead mainly for the production of paints, mines, gasoline, household paints, pigments, and pipes with significant health risks concentration on the biodiversity exposed to maximum residue limit (Soto-Jimenez et al. 2011). The large amount of lead in manufactured products can lead to their persistence in the environment as dust and can infiltrate several compartments where it becomes pervasive to human (Travis et al. 2007). However, lead does not break down and the result is that it becomes magnified in the environment. This manufactured product releases lead into the environment with harmful effects on both human and aquatic biodiversity (García-Lestón et al. 2010). As the use of manufactured Pb and TiO2 NPs increases, it seems inevitable that both compounds are being released into the environment and more especially in an aquatic environment where pollutants and contaminants from many sources are received (Leigh et al. 2012).
Recently, the impact of co-exposure between TiO2 NPs and Pb on zebrafish embryos revealed that the addition of Pb to TiO2 NPs further significantly increased metallothionein activity. However, neurodevelopment-related genes were downregulated. TiO2 NPs act as capable carriers of Pb and enhance its uptake, bioavailability, and toxicity (Wu et al. 2019; Wu et al. 2015). Moreover, the co-exposure of TiO2 NPs and Pb in fish Hoplias intermedius did not alter acetylcholinesterase activity but significantly increase metallothionein activity (Vicari et al., 2018).
With regard to previous studies on combined exposure between TiO2 NPs and Pb, it is worthy to note that studies on the potential effects of interaction between TiO2 NPs and Pb on trophic transfer/food chain are sparse. However, there exists an increasing concern due to the growing discharge of both compounds into the aquatic environment. Trophic transfer in this study is defined as the movement of pollutants up through the food web via the ingestion of prey organisms by predators (Soto-Jimenez et al. 2011). It remains unknown if the uptake of the prey (primary consumer/copepods) contaminated with a binary mixture between TiO2 NPs and Pb through dietary route by a secondary consumer (fish) enhanced the uptake and bioavailability of Pb and also enhanced antioxidant activities in fish. There exists little information on the bioaccumulation and trophic transfer through dietary uptake of contaminated freshwater organisms (Tangaa et al. 2016). The few available data on bioaccumulation and trophic transfer in the food chain mostly deal with individual heavy metals and nanoparticles, respectively (Soto-Jimenez et al. 2011; Skjolding et al. 2016; Carvalho 2014; Bour et al. 2015; Ates et al. 2014).
In order to evaluate and prevent environmental risks associated with the mixture of TiO2 and Pb on the aquatic food web, biological (oxidative stress) consequences need to be investigated since these compounds from run-off water become internalized in the cells of the highest trophic aquatic organism. Thus, this study aimed at examining the bioaccumulation of TiO2 NPs (Ti4+) and ionic lead (Pb2+) in C. gariepinus fry fed contaminated zooplankton (copepods sp.) with a binary mixture of TiO2 NPs and Pb and the selected antioxidant activities (SOD, MDA, CAT, AchE, GR, and GPx).
We selected the freshwater zooplankton (copepods sp.) and C. gariepinus lava because of their presence in freshwater bodies. The freshwater C. gariepinus is of commercial importance and also used as a model species for toxicological studies (Nguyen and Janssen 2002; Marimuthu et al. 2013; Mahmoud et al. 2012). Also, fish are useful sentinels for poor environmental conditions because of their perennial contact with the aquatic ecosystem. The health status of fish gives an insight on the lower trophic levels, and therefore, the use of fish has become common in addressing the issue of ecotoxicology (Matranga and Corsi 2012).
Materials and methods
Stock preparations of TiO2 NPs and Pb2+
We purchased titanium oxide nanoparticles (TiO2 NPs) anatase-rutile of purity (99.5%), particle size < 21 nm, CAS 13463-67-7 and N2O6Pb (CAS1009974-8) from Sigma-Aldrich (St. Louis, MO, USA). We also prepared the stock of TiO2 NPs (1 μg L−1) and N2O6Pb (1 μg L−1) in deionized water (pH = 7.5) while sonicating TiO2 NPs stock for 10 min at 25 °C, 40 40 Hz using the SB4200DTD Ultrasonic Cleaner model.
Preparation and characterization of TiO2 NPs
Before the experiment, we carried out X-ray diffraction analysis with X-ray diffractometer (Xpertpro, Panalytical, Phillips) equipped with filtered Cu Kα radiation operated at 40 Kv and 40 mA. We recorded the XRD patterns from 10 to 80° 2θ with a scanning speed of 0.526° per minute while also ascertaining the chemical and crystal structure or the compound. We used Zeta plus (Brookhaven 22001) to detect the nanoparticle’s zeta potential after the dispersion of nanoparticles in deionized water. The initial hydrodynamic size of TiO2 NPs was analyzed in deionized water by dynamic light scattering (DLS) incorporated in Malvern zetasizer Nano ZS90 Analyzer (Malvern Instruments Ltd., Spectris company) (Matouke and Mustapha 2018). We performed electron microscopic analysis (Xpert Pro) to display the aggregation pattern further.
Experimental setup
Chronic toxicity test
We performed acute toxicity of individual compounds TiO2 NPs and N2O6Pb according to an OECD standard protocol (OECD 211, 1984). The sublethal concentrations derived were TiO2 NPs (0.09 and 0.20 μM) and Pb2+ (0.01 and 0.04 μM) and were used for further chronic test. Before the experiment, averagely thirty-five (35) copepods/mL were isolated using a mesh size of 50 μm and transferred in aerated containers (500 mL glass beakers containing 250 mL test solution containing C. ellipsoids as food). We maintained the culture at constant temperature (25 ± 2 °C) with a natural photoperiod (12 h light:12 h dark cycle). Test containers were monitored every 24 h for microalga cell counting under a hemocytometer.
Cyclopoid copepod exposure
We used the copepods donated by the Limnology laboratory of the National Institute of Freshwater and Fisheries (NIFFR), New Bussa, Nigeria. The cyclopoid copepod sp. obtained was continuously cultured in aerated semi-static borehole water without pollutants for 48 h. The copepod sp. was after that exposed for 48 h to TiO2 NPs and Pb2+ alone and in combination. We maintained a constant temperature (25 ± 2 °C) in the laboratory with a natural light-dark cycle, as described by Matouke and Mustapha (2018).
The copepods of approximately 300 μm long were isolated and transferred in glass aquaria containing exactly 10 L of borehole freshwater. Copepods were then exposed for 2 days to the sublethal concentrations dissolved individually or in combination in the medium: TiO2 NPs (0.09 and 0.20 μM), Pb (0.01 and 0.04 μM), and binary mixtures (0.01, 0.09 μM); (0.01, 0.20 μM); (0.04, 0.09 μM); (0.04, 0.20 μM). After 48 h of exposure to contaminated water, we observed no mortality; copepods, thirty-five (35) individuals/mL were collected, rinsed with fresh water, and transferred to tanks containing C. gariepinus fry (4 days old) as food.
Acclimatization and training of C. gariepinus
We hatched Clarias gariepinus (4 days old; 0.33 ± 0.09 g) in the Hatchery laboratory of NIFFR. We kept the fry in three (3) replicates in glass aquaria of 24 × 12 × 12 cm (25 fries per tank) filled with 10 L of freshwater and connected to an aerator (Uniclife 4 watt 4-LPM2) throughout the experiment. Before the experiment, fish were kept in their experimental conditions (25 ± 2 °C; 12:12 h light:dark) with two-third daily water renewal. We conducted all animal protocols in this study under the supervision and approval of the ethical board committee (UERC/LSC/029) of the University of Ilorin, Ilorin, Nigeria.
Trophic transfer of TiO2 NPs and Pb from copepods sp. to C. gariepinus
We conducted trophic transfer experiments consisting of 28-day uptake to determine if a food chain transfer of Ti4+ and Pb2+ from contaminated copepods to C. gariepinus can occur.
We harvested copepods sp. exposed to TiO2 NPs (0.09 and 0.20 μM), Pb (0.01 and 0.04 μM), and binary mixtures (0.01, 0.09 μM); (0.01, 0.20 μM); (0.04, 0.09 μM); (0.04, 0.20 μM) for 48 h in each glass aquarium with a plastic net of mesh size of 50 μm and washed three times with 500 mL of freshwater, and transferred into various C. gariepinus aquaria as fish food. We fed C. gariepinus with the contaminated copepods sp. ad libitum for 28 days. Water was aerated with an aerator daily and then siphoned every 24 h. During the experiment, we used three replicates of nine (9) treatments containing twenty-five (25) fry each while taking fish samples on day 0 as a control (aquarium without chemical) and after 28 days (exposure period).
We anesthetized fry with 80 mg L−1 of benzocaine (Morato-Fernandes et al. 2013); they were measured and weighed before further fish tissue (muscles) analysis. Fry tissue samples were collected and examined for Ti4++ and Pb2+. To analyze the food chain transfer of TiO2 NPs from copepods sp. to C. gariepinus, bioaccumulation factor (BAF) of Ti4+ and Pb2+ was computed as the ratio concentrations between fry and copepods (Arnot and Gobas 2006).
Chemical analysis
To determine the quantity of TiO2 NPs and Pb2+ in copepods and fish samples, we used the modified method by Zhang et al. (2007). The sampled fish were thoroughly washed with distilled water and then dried to constant weight at 70 °C in an oven overnight. These dried samples were digested using pure 0.5% (v/v) HNO3 (Sigma-Aldrich, Inc., St. Louis, MO, USA) in a glass beaker and then evaporated to dryness. TiO2 NPs released by digestion were decomposed into titanium (IV) ion by heating with 5 ml of the sulfuric acid-ammonium sulfate solution (3 M). The Ti4+ and Pb2+ concentrations in digested samples were then determined by atomic absorption spectrophotometer (AAS) with wavelength 283.3 nm for Pb and air acetylene oxidizing flame. However, Ti4+ used argon atmospheric purge gas with a wavelength of 365.4 nm. We evaporated the water samples to dryness and then analyzed for Ti4+ and Pb2+ following Zhang et al. (2007).
Antioxidant responses of fish (muscles)
Superoxide dismutase
We determined superoxide dismutase (SOD, EC. 1.15.1.1) activity by tetrazolium reduction method (Beauchamp and Fridovish 1971). We defined one SOD unit as the amount of enzyme required to inhibit 50% of the nitroblue tetrazolium photo-reduction rate. Each sample was divided into three fractions in tubes—the measuring tube, the light control tube, and the dark control tube, respectively. A mixture of 550 mmol L−1 potassium phosphate buffer (pH 7.8), 130 mmol L−1 methionine solution, 750 μmol L−1 NBT solution, 20 μmol L−1 riboflavin solution, 100 μmol L−1 EDTA-Na2, distilled water were added to the enzyme solution in the measuring tube. We added the same volume of distilled water to other tubes. We placed the tubes under 1000 Luminous Fluorescent color reaction for 15 min, covered with a black material to terminate the reactions. We used the dark control tube as a blank control and measured the absorbance at 560 nm.
Lipid peroxidation (MDA)
A 0.2-mL supernatant was added to 2 mL of (1:1:1 ratio) TCA-TBA-HCl reagent (thiobarbituric acid 0.37%, 0.24 N HCl, and 15% tricarboxylic acid) and boiled at 100 °C for 15 min and allowed to cool. Flocculant materials were removed by centrifuging at 4000 rpm for 10 min (Hodges et al. 1999). We removed the supernatant and measure the absorbance read at 532 nm.
Catalase
We determined catalase (CAT) activity based on ultraviolet spectrophotometry according to the method of Xu et al. (1997). We added 10 μL of the sample to 3.0 mL of H2O2 phosphate buffer, pH 7.0 (0.16 mL of 30% H2O2 to 100 mL of 0.067 mol phosphate buffer), and we measured the change in H2O2 absorbance within the 60s at 250 nm with a UV spectrophotometer. We defined one unit of enzyme activity as the amount of the enzyme that decreased 1 μmol H2O2 per min.
We analyzed each of the samples in triplicates using the conventional methods mentioned above.
Acetylcholinesterase
We estimated acetylcholinesterase (AChE) activity in tissues (1:10) homogenate in (0.1 M) phosphate buffer, PH. In this study, the homogenate was centrifuged 10.0000g for 20 min at 4 °C. The supernatant obtained was separated and used to test AChE activity. Normalization of AChE to protein was carried out and expressed as nmol min−1 protein. However, protein concentration was determined using the Bradford method (Bradford 1976) with bovine serum albumin as the standard (Kim and Kang 2016).
Glutathione reductase
In this study, glutathione reductase (GR) activity estimation followed the method described by Smith et al. (1988). GR activity was measured as a result of the change of absorbance at 412 nm due to the reduction of 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) by GSH in 2-nito-5 thiobenzoic acid.
Glutathione peroxidase
Glutathione peroxidase (GPx) activity followed the method of Hafeman and Lang (1997). We measured the rate of reaction by the decrease in GSH, which was determined by measuring the reaction product of DTNB and GSH (absorbance measured at 423 nm).
Statistical analysis
We repeated all experiments three times independently, and data were recorded as the mean with standard error (SE) to reflect the sampling fluctuation of the tool used. The normality and homogeneity of data were validated by Levene’s test and the Kolmogoroy-Smirnov test. A one-way analysis of variance with Tukey’s multiple comparisons ad hoc test was applied to evaluate the differences between the control and the treatment groups, respectively. Two-way analysis of variance was also used to measure the significant interaction between both compounds and antioxidant by using Origin 8.1 Pro Lab software for data analysis. In all data analyses, p value less than 0.05 was considered statistically significant.
Results
Characterization of TiO2 NPs
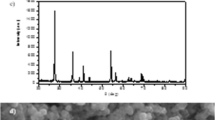
X-ray diffractograms of TiO2 NPs had shown the presence of eleven (11) most distinct diffracted peaks (62.72, 62.07, 55.11, 53.92, 48.07, 38.50, 37.78, 36.88, and 25.33) which could account for their tetragonal structure (supplementary 1). The texture of the nanomaterial also revealed predominant anatase (82%) and rutile (31%) phase. Scanning electron micrographs (SEM) showed individual spherical particles. The average particle size calculated from the full width at half maximum (FWHM) of the distinct peaks (62.73°) using the Scherrer formula was determined to be 22.66 ± 0.18 nm. The result is slightly larger than 21 nm reported by the manufacturer (Matouke et al. 2018). We revealed the zeta potential (mV) of TiO2 NPs and TiO2 NPs + Pb to be − 8.372 ± 1.78, and − 14.27 ± 9.50, respectively, making them all unstable. Matouke and Mustapha (2018) described the hydrodynamic size distribution of TiO2 NPs as in supplementary 1.
Bioaccumulation of lead (Pb2+) and titanium dioxide (TiO2 NPs) in fish
Bioaccumulation factors (BAF) of lead (Pb) in C. gariepinus fed with contaminated copepods (Table 1) showed that binary mixture Pb (0.04) + TiO2 NPs (0.09) μM had the highest BAF of Pb2+ in fish. The individual treatment TiO2 NPs (0.09) and TiO2 NPs (0.20) μM had the highest BAF 3.75 and 2.63. However, we observed in the binary mixture a decrease of BAF compare with individual TiO2 NPs. In addition, the highest BAF (2.21) in binary mixture was also observed in Pb (0.04) + TiO2 NPs (0.09) μM (Table 1). This result generally revealed that TiO2 NPs highly bioaccumulated in C. gariepinus exposed to TiO2 NPs alone compared with Pb (TiO2 NPs and Pb separately). However, we added Pb to TiO2 NPs; the BAF of TiO2 NPs decreased while Pb bioavailability (uptake) increased in the mixture (Pb (0.04) + TiO2 NPs (0.09) μM, the BAF was not concentration-dependent in fish food (Table 1).
Antioxidant activities in C. gariepinus
Superoxide dismutase in C. gariepinus
Decreased (superoxide dismutase) SOD levels in treated groups compared with the control were 1.93-, 1.60-, 2.10-, 2.28-, 2.02-, 2.09-, 1.72-, and 1.79-fold for Pb (0.01), Pb (0.04), TiO2 NPs (0.09), TiO2 (0.20), Pb (0.01) + TiO2 NPs (0.09), Pb (0.01) + TiO2 NPs (0.20), Pb (0.04) + TiO2 NPs (0.09), and Pb (0.04) + TiO2 NPs (0.20) μM treatment groups, respectively. Furthermore, there was significant decrease p < 0.05 of SOD levels. However, no significant p > 0.05 effect on the interaction of binary mixture in fish fed contaminated zooplankton was observed (Table 2).
Malondialdehyde in C. gariepinus
Figure 1b showed that malondialdehyde (MDA) increased significantly compared with the control with 1.41-, 1.72-, 1.43-, 1.78-, 1.64-, 1.84-, 1.97-, and 2.01-fold in fish fed Pb (0.01), Pb (0.04), TiO2 NPs (0.09), TiO2 NPs (0.20), Pb (0.01) + TiO2 NPs (0.09), Pb (0.01) + TiO2 NPs (0.20), Pb (0.04) + TiO2 NPs (0.09), and Pb (0.04) + TiO2 NPs (0.20) μM, respectively. Furthermore, there was a significant interaction p < 0.05 leading to the increase of MDA in fish fed both compounds (Table 2).
a SOD response of C. gariepinus after 28 days. F = 3669, p<0.0001. b MDA response of C. gariepinus after 28 days. F = 1175, p < 0.0001. c Catalase response of C. gariepinus after 28 days. F = 125,013, p < 0.0001. d Acetylcholinesterase response of C. gariepinus after 28 days. F = 6181, p < 0.0001. e GRx response of C. gariepinus after 28 days. F = 149, p < 0.0001. f GPx response of C. gariepinus after 28 days. F = 152.8, p < 0.0001. *Statistical significant difference from control at p < 0.05; #Statistical significant difference from Pb and TiO2 NPs co-exposure at p < 0.05. Threshold replicate were included (n = 3). Error bars indicate standard error (SE)
Catalase C. gariepinus
The inhibitory effects of Pb2+ and TiO2 NPs singly and in combination on CAT level in fish after dietary exposure are as shown in Fig. 1c. Catalase 5.5 mmol g−1 was the highest in the control of C. gariepinus compared with all other treated groups. Catalase responses 3.94 and 3.96 mmol g−1 increased with the increasing concentration of Pb (0.01) and Pb (0.04) μM respectively. Catalase activity 4.06 and 4.13 mmol g−1 increased with the increasing concentration of TiO2 NPs (0.09) and TiO2 NPs (0.20) μM, respectively. The significant decrease of CAT in contaminated fish compared with the control was observed to be 1.63-, 1.63-, 1.59-, 1.56-, 1.66-, 1.66-, 1.77-, and 2.02-fold lower for Pb (0.01), Pb (0.04), TiO2 NPs (0.09), TiO2 NPs (0.20), Pb (0.01) + TiO2 NPs (0.09), Pb (0.01) + TiO2 NPs (0.20), Pb (0.04) + TiO2 NPs (0.09), and Pb (0.04) + TiO2 NPs (0.20) μM, respectively. Individual compound and their interaction were found to significantly p < 0.05 inhibit CAT (Table 2).
Acetylcholinesterase in C. gariepinus
Figure 1d showed that AChE (11.96 mmol g−1) was the highest in the control of C. gariepinus compared with all other treated groups. The inhibition of AChE levels of contaminated fish compared with the control was 68%, 62.3%, 67%, 67%, 66%, 54%, 65.9%, and 53% for Pb (0.01), Pb (0.04), TiO2 NPs (0.09), TiO2 NPs (0.20), Pb (0.01) + TiO2 NPs (0.09), Pb (0.01) + TiO2 NPs (0.20), Pb (0.04) + TiO2 NPs (0.09), and Pb (0.04) + TiO2 NPs (0.20) μM, respectively. Furthermore, there was a significant p < 0.05 decrease of AChE. The interaction of binary mixtures showed a significant p < 0.05 inhibitory effect of both compounds on AChE activity (Table 2).
Glutathione reductase in C. gariepinus
Figure 1e demonstrated that GR (4.41 mmol g−1) was the highest in the control of C. gariepinus compared with all other treated groups. The inhibitory effect of Pb and TiO2 NPs in contaminated fish was 1.41-, 1.44-, 1.43-, 1.42-, 1.41-, 1.46-, 1.46-, and 1.37-fold for Pb (0.01), Pb (0.04), TiO2 NPs (0.09), TiO2 NPs (0.20), Pb (0.01) + TiO2 NPs (0.09), Pb (0.01) + TiO2 NPs (0.20), Pb (0.04) + TiO2 NPs (0.09), and Pb (0.04) + TiO2 NPs (0.20) μM, respectively. Furthermore, there was a significant p < 0.05 decrease of GR in fish; however, the interaction of both compounds showed significant p < 0.05 decrease of GR activity (Table 2).
Glutathione peroxidase in C. gariepinus
Figure 1f showed that the GPx (3.19 mmol g−1) was the highest in the control of C. gariepinus compared with all other treatment. Glutathione peroxidase decrease in contaminated fish was 1.42-, 1.38-, 1.52-, 1.53-, 1.44-, 1.19-, 1.49-, and 1.89-fold for Pb (0.01), Pb (0.04), TiO2 NPs (0.09), TiO2 NPs (0.20), Pb (0.01) + TiO2 NPs (0.09), Pb (0.01) + TiO2 NPs (0.20), Pb (0.04) + TiO2 NPs (0.09), and Pb (0.04) + TiO2 NPs (0.20) μM, respectively. Furthermore, there was a significant p < 0.05 decrease of GPx in contaminated fish. The inhibitory effect of the interaction of binary compounds on GPx activity was significantly p < 0.05 (Table 2).
Discussion
The stability of aqueous nanoparticles and toxicity depends on the size, physicochemical properties, precipitation, and dispersion of the particles (Gonçalves et al. 2018). Zeta potential measures the magnitudes of the electrostatic charges between particles in solution, and it is known to affect the colloidal stability of suspensions (Luo et al. 2010). In this study, particles had a negative zeta potential that account for the unstable colloidal particulates in the medium and elemental concentration (Ates et al. 2014). In order to prepare a stable suspension of nanoparticles in the present study, we sonicated samples to improve the dispersion of nanoparticles in the medium.
Nevertheless, aggregation of nanoparticles was observed under SEM, as reported for TiO2 NPs (Sadiq et al. 2011). According to the Sherrer equation, the size of nanoparticles ranged was 22.66 ± 0.18, which was within the value range reported by the manufacturer (21 nm). However, the DLS data of TiO2 NPs in nanopore water and after addition of Pb showed a relatively low dispersion of nanoparticles ranging from 2384 to 2206, respectively, and similar to report TiO2 NPs/Cd2+ (Miao et al. 2015).
Freshwater biodiversity, more especially fish, are of critical importance in human nutrition since they are a source of protein. Concerns about the harmful effects of ENM and xenobiotic compounds on the food chain have arisen because lower trophic levels can ingest and bioaccumulate ENM and heavy metal to higher organisms that feed on them. Uptake of ENM and heavy metal by fish via dietary means has been documented previously (Soto-Jimenez et al. 2011; Gambardella et al. 2014). Ates et al. (2014) found out that nanoparticles such as ZnO and CuO were transferred and bioaccumulated to higher trophic level fish Carassius auratus through the dietary intake of Artemia. Similarly, Soto-Jimenez et al. (2011) reported the transfer of non-essential metal lead (Pb) in the simple food chain from the primary producer Tetraselmis suecica, primary consumer Artemia franciscana, and secondary consumer fish Litopenaeus vannamei. The results in the present study indicated that despite the disparity of bioaccumulation factors in individual and binary treatments, lead (Pb2+) and titanium could be transferred through dietary ways from zooplankton to fish. These results are consistent with those observed for Escherichia coli and Caenorhabditis elegans (Luo et al. 2016). The specific physicochemical properties such as size and aggregation pattern of titanium nanoparticles accounted for their bioaccumulation in the present food chain as a result of poor solubility and sedimentation of the nanomaterial (Ates et al. 2014). The bulk BAFs of TiO2 NPs in the exposed fish was higher than one (1) in almost all treatments indicating that intake of contaminated primary consumers with TiO2 NPs led to the accumulation of TiO2 NPs in freshwater C. gariepinus. In this study, except for the binary mixture, the highest concentrations of TiO2 NPs + Pb, the BAF of Pb2+ tend to decrease in fish tissues. The values of TiO2 NPs BAF in this study corroborated with the findings of Chen et al. (2010), where the transfer from daphnia to zebrafish through dietary route revealed a BAF of 25.38 > 1.
Trophic transfer of Pb2+ depends on the structure and composition of the aquatic food chain (Soto-Jimenez et al. 2011). The generally weak increase of Ti4+ and Pb2+ uptake over time could account for the deposition and the accumulation of these compounds in the tissues and subsequently to the muscles of fish (Zhu et al. 2010). Presumably, in this study, the time for the uptake of Pb alone to appear up the higher trophic level after consumption of exposed copepods was lower compared with binary mixtures. This result agreed with the biological responses of simulated marine food chains exposed to lead (Pb). The report showed a poor transfer of Pb at a higher trophic level (Soto-Jimenez et al. 2011).
Antioxidant generally plays a defensive mechanism responsible for the reduction of oxygen free radicals. Superoxide dismutase (SOD) is capable of reducing free radicals; the anion superoxide (O2−) overwhelms the cells against ROS. In this attack, the dismutation of O2− by this enzyme produces O2 and H2O2, and the latter is degraded by catalase to produce water and oxygen (Kalayci et al. 2012). Variation in concentration significantly influenced SOD activity in the fish tissues (muscles) in this study. When Pb and TiO2 NPs were exposed and uptake separately by fish, there was a decrease of SOD compared with the control. Our result failed to agree with the increase of SOD in Hoplias intermedius exposed to Pb and TiO2 NPs, respectively (Vicari et al., 2018). The result could be due to the massive production of reactive oxygen species (ROS) that inhibited the effects of SOD. However, when analyzing the effects of the binary mixture (TiO2 NPs + Pb) on SOD activity in fish tissue, a smaller decrease of SOD compared with that exposed separately was observed. The result could suggest that the interaction of both compounds might have reduced the H2O2 production after preventing the large number of O2− from causing damage in the tissues. Our finding agreed with decreased SOD reported in fish exposed to the combination of TiO2 NPs + Pb by Guiloski et al. (2019).
Nanoparticles and metals in tissues can induce an array of cellular changes in fish tissues which may produce an increase of ROS and damage the membrane integrity, resulting to MDA production of a by-product of cellular lipid peroxidation (Orun et al. 2008). The effect of Pb2+ and TiO2 NPs in fish caused the activation of free radicals that could bind covalently to the macromolecules and induced peroxidative degeneration of the lipid membrane. The increase of MDA in treated fish with Pb or TiO2 NPs and the observed further increase in binary mixture in this study is due to stress-induced in the fish after the uptake of contaminated food embedded with either Pb or TiO2 NPs and TiO2 NPs + Pb, respectively. This suggests that the combination of both compounds could induct MDA increment in fish fed contaminated compounds. This study agreed with the findings on the effects of sub-acute exposure of zebrafish to TiO2 NPs (Hao et al. 2009).
The next antioxidant defense line after SOD is catalase, and despite the increase of SOD activity when Pb and TiO2 NPs were uptakes individually. CAT is a biomarker enzyme majorly found in peroxisome, which can promote the decomposition of H2O2 into O2 and H2O2. It is one of the most prominent enzymes in protective defense system. The enzyme function is to reduce the content of ROS and protect cell from the damage of H2O2 (Winston 1991). In this study, CAT activity decreased significantly in individual compound used. Furthermore, the mixture of Pb and TiO2 NPs further reduced CAT during oxidative stress, which indicates a high increase of ROS, overwhelming the cells and causing damage. Excessive production of ROS can damage the redox system and inhibit activity of antioxidant enzymes thereby reducing their content and cause oxidative damage (Venditti et al. 2013). The results of this study corroborate with the findings reporting the reduction of CAT in Oreochromis niloticus exposed to titanium dioxide nanoparticles (Firat and Bozat 2019).
We used acetylcholinesterase (AChE) as a biomarker of pollution, which are found mainly in the brain, erythrocytes, and muscle tissues of fish (Kienzler et al. 2013). This enzyme’s function is to hydrolyze neurotransmitter acetylcholine (ACh) in the synapse thereby enhancing the transmission of impulses from neurons and consequently prevent the occurrence of continuous stimuli of the neuron (Pretto et al. 2010). This study analyzed the AChE in muscle tissues of fish. The fish fed contaminated copepods decreased in all treated groups compared with the control. This decrease of the enzyme was due to stress caused by the compounds in fish tissues. The result suggests that the presence of these compounds in fish food is responsible for the inhibition of AChE due to neurotoxic effects. Regarding the impact of Pb or TiO2 NPs and Pb + TiO2 NPs on AChE, these compounds might interfere with calcium-mediated neurotransmitter released at the neuromuscular junction. This study was a similar exposure of Pb or TiO2 NPs and Pb + TiO2 NPs on Hoplias intermedius that revealed no variation and inhibition of AChE (Vicari et al., 2018).
Glutathione reductase is one of the main components of the cell scavenging system of ROS. However, GPx and GR play crucial roles in enzymatic defense against hydrogen peroxide (H2O2) and are linked to each other (Saint-Denis et al. 1998). Some studies showed that TiO2 NPs or Pb can induce an increase in ROS production and oxidative products such as GPx, CAT, and MDA are depleted (Matouke and Mustapha 2018). GPx for instance has a complementary role in hydrogen peroxide (H2O2) detoxification and its mission is to mitigate the tissue injury by removing H2O2 (Du et al. 2017). In this present study, the GPx and GR activities in fish tissues decreased significantly after the uptake with contaminated copepods exposed to Pb or TiO2 NPs and Pb + TiO2 NPs, respectively. The reduction of GPx and GR is conventionally proportional to GST production; however, this depletion may account for the inability of GPx and GR to remove the large quantity of ROS through detoxification of H2O2 in cellular tissues. Firat and Bozat (2019) reported similar results in Oreochromis niloticus exposed to titanium dioxide nanoparticles.
Conclusion
This study clearly indicated that the chronic (28 days) uptake of contaminated copepods by C. gariepinus induced relatively high BAF > 1 of Ti4+ in binary mixtures. However, C. gariepinus uptake of individual and binary mixture impaired their protection against reactive oxygen species (ROS). Pb or TiO2 NPs and Pb + TiO2 NPs applied separately caused neurotoxicity due to inhibition of AChE. Furthermore, the co-exposure of Pb and TiO2 NPs have, as consequences, the significant decrease of CAT, GR, GPx, and MDA in fish tissues indicating alteration of the defensive mechanism caused by the binary mixture. Our findings indicated that chronic uptake of TiO2 NPs and Pb through the trophic transfer of freshwater food chains should be further studied.
References
Arnot JA, Gobas FA (2006) A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ Rev 14(2):257–268
Ates M, Arslan Z, Demir V, Daniels J, Farah IO (2014) Accumulation and toxicity of CuO and ZnO nanoparticles through waterborne and dietary exposure of goldfish (Carassius auratus). Environ Toxicol 30(3):119–127
Beauchamp C, Fridovish I (1971) Superoxidase dismutase improved assay and an assay applicable to acrylamide gels. Anal Biochem 44(2):276–287
Bour A, Mouchet F, Cadarsi SP, Silvestre JR, Verneuil L, Baque D, Chauvet E, Bonzom JM, Pagnout C, Clivot H (2015) Toxicity of CeO2 nanoparticles on a freshwater experimental trophic chain: a study in environmentally relevant conditions through the use of mesocosms. Nanotoxicol 10(3):245–559
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254
Carvalho FP (2014) Polonium (210 Po) and lead (210 Pb) in marine organisms and their transfer in marine food chains. J Environ Radioact 102(3):462–473
Chen Y, Zhu X, Wang J, Zhang X, Chang Y (2010) Trophic transfer of TiO2 nanoparticles from Daphnia to zebrafish in a simplified freshwater food chain. Chemosphere 79(9):928–933
Davis MJ (2009) Nanomaterial case study: nanoscale titanium dioxide in water treatment and in topical sunscreen. United States Environmental Protection Agency, Washington
Du J, Ca J, Wang S, You H (2017) Oxidative stress and apotosis to zebrafish (Danio rerio) embryo. Int J Occup Med Environ Health 30(2):213–229
Federici G, Shaw BJ, Handy RD (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol 84:415–430
Firat O, Bozat RC (2019) Assessment of biochemical and toxic responses induced by titanium dioxide nanoparticles in Nile tilapia Oreochromis niloticus. Hum Ecol Risk Assess 25(6):1438–1447
Fitzsimmons K, 2017. Lead price forecast, May 2017: global demand to grow. Sourcing & Trading Intelligence for Global Metals Markets, https://agmetalminer.com/2017/05/19/85082/
Gambardella C, Gallus L, Gatti AM, Faimali M, Carbone S, Antisari LV, Falugi C, Ferrando S (2014) Toxicity and transfer of metal oxide nanoparticles from microalgae to sea urchin larvae. Chem Ecol 30(1):308–327
García-Lestón J, Méndez J, Pásaro E, Laffon B (2010) Genotoxic effects of lead: an updated review. Environ Int 36:623–636. https://doi.org/10.1016/j.envint.2010.04.011
Gazquez MJ, Bolivar JP, Garcia-Tenorio R, Vaca F (2014) A review of the production cycle of titanium dioxide pigment. Mater Sci Appl 5(2):441–461
Gonçalves S, Domingues MM, Carvalho PM, Felício MR, Santos NC (2018) Application of light scattering techniques to nanoparticle characterization and development. Front Chem 6:237–254
Guiloski IC, Vicari T, Delmond KA, Dagostim AC, Voigt CL, Silva-de-Assis HC, Ramsdorf WA, Cestari MM (2019) Antioxidant imbalance and genotoxicity detected in fish induced by titanium dioxide nanoparticles (NpTiO2) and inorganic lead (PbII). Environ Toxicol Pharmacol 67:42–52. https://doi.org/10.1016/j.etap.2019.01.009.Epub2019
Hafeman DG, Lang PZ (1997) Effect of dietary selenium and erythrocyte and liver glutathione peroxidase in the rat. J Nutr 104(1):580–592
Hall S, Bradley T, Moore JT, Kuykindall T, Minella L (2009) Acute and chronic toxicity of nano-scale TiO2 particles to freshwater fish, cladocerans, and green algae, and effects of organic and inorganic substrate on TiO2 toxicity. Nanotoxicol 3(2):91–104
Hansen SF, Michelson ES, Kamper A, Borling P, Stuer-Lauridsen F, Baun A (2008) Categorization framework to aid exposure assessment of nanomaterials in consumer products. Ecotoxicol 17:438–447
Hao L, Wang Z, Xing B (2009) Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in Juvenile Carp (Cyprinus carpio). J Environ Sci 21(10):1459–1466
Hodges DM, Delong JM, Forney C, Prange PK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Plant 207(1):604–611. https://doi.org/10.1007/s004250050524
Kalayci MD, Birben E, Sahiner MU, Sackesen CM, Erzurum S (2012) Oxidative stress and antioxidant defense. World Allergy Organ 5(1):9–19. https://doi.org/10.1097/WOX.0b013e3182439613
Kienzler A, Bony S, Devaux A (2013) DNA repair activity in fish and interest in ecotoxicology: a review. Aquat Toxicol 134–135:47–56. https://doi.org/10.1016/j.aquatox.2013.03.005
Kim JH, Kang J (2016) Changes in hematological parameters, plasma cortisol and accetylcholinesterase of Juvenile Rockfish, Sebastes schlegelii supplemented with the dietary ascorbic acid. Aqua Rep 4(1):80–85
Kulacki KJ, Cardinale BJ (2012) Effects of nano-titanium dioxide on freshwater algal population dynamics. PLoS One 7:e47130
Leigh K, Bouldin J, Buchanan R (2012) Effects of exposure to semiconductor nanoparticles on aquatic organisms. J Toxicol 2012(397657):9
Luo J, Cote LJ, Tung VC, Tan AT, Goins PE, Wu J (2010) Graphene oxide nanocolloids. J Am Chem Soc 132(50):17667–17676
Luo X, Xu S, Yang Y, Li S, Chen A, Wu L (2016) Insights into the ecotoxicity of silver nanoparticles transferred from Escherichia coli to Caenorhabditis elegans. Sci Rep 6(1):36465
Mahmoud UM, Mekkawy IA, Ibrahim AT (2012) Biochemical response of the African catfish, Clarias gariepinus (Burchell, 1822) to sublethal concentrations of mercury chloride with supplementation of selenium and vitamin. Toxicol Environ Heal Sci 4(1):218–234
Marimuthu K, Muthu N, Xavier R, Arockiaraj J, Rahman MA, Subramaniam S (2013) Toxicity of buprofezin on the survival of embryo and larvae of African catfish, Clarias gariepinus (Bloch). PLoS One 8:e75545
Matouke MM, Mustapha M (2018) Bioaccumulation and physiologica effects of copepods sp (Eucyclops sp.) fed Chlorella ellipsoides exposed to titanium dioxide (TiO2 NPs) and lead (Pb2+). Aquat Toxicol 198:30–39
Matouke MM, Elewa D, Abdulhahi K (2018) Binary effect of TiO2 NPs and phosphorus on microalgae Chlorella ellipsoidea Gerneck, 1907. Aquat Toxicol 198:1233–1332.
Matranga V, Corsi I (2012) Toxic effects of engineered nanoparticles in the marine environment: model organisms and molecular approaches. Mar Environ Res 76(2):32–46
Miao W, Zhu B, Xiao X, Li Y, Dirbaba NB, Zhou B, Wu H (2015) Effects of titanium dioxide nanoparticles on lead bioconcentration and toxicity on thyroid endocrine system and neuronal development in zebrafish larvae. Aquat Toxicol 161:117–129
Morato-Fernandes J, Tavares R, Rocha C, Pouey JL, Piedras SR (2013) Benzocaine and clove oil as anesthetics for pejerrey (Odontesthes bonariensis) fingerlings. Arq Bras Med Vet Zootech 65(5):1441–1446
Nguyen LT, Janssen CR (2002) Embryo-larval toxicity tests with the African catfish (Clarias gariepinus): comparative sensitivity of endpoints. Arch Environ Contam Toxicol 42:256–306
Oberdörster G, Oberdörster E, Oberdörster J (2005) Reviews: Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113(7):823–839
Orun I, Talas ZS, Ozdemir I, Alkan A, Erdogan K (2008) Antioxidative role of selenium on some tissues of (Cd2+, Cr3+)-induced rainbow trout. Ecotoxicol Environ Saf 71:71–89
Pretto A, Loro VL, Morsch VM, Moraes BS, Menezes C, Clasen B, Hoehne L, Dressler B (2010) Acetylcholinesterase activity, lipid peroxidation, and bioaccumulation in silver catfish (Rhamdia quelen) exposed to cadmium. Arch Environ Contam Toxicol 58(4):1008–1014. https://doi.org/10.1007/s00244-009-9419-3
Qian H, Li J, Sun L, Chen W, Sheng GD, Liu W, Fu Z (2009) Combined effect of copper and cadmium on Chlorella vulgaris growth and photosynthesis-related gene transcription. Aquat Toxicol 94:56–74
Robichaud CO, Uyar AE, Darby MR, Zucker LG, Wiesner MR (2009) Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ Sci Technol 43:4227–4233
Sadiq IM, Dalai S, Chandrasekaran N, Mukherjee A (2011) Ecotoxicity study of titania (TiO 2) NPs on two microalgae species: Scenedesmus sp. and Chlorella sp. Ecotoxicol Environ Saf 74(1):1180–1194
Saint-Denis M, Labrot F, Narbonne JF, Ribera D (1998) Glutathione, glutathione-related enzymes, and catalase activities in the earthworm Eisenia fetida andrei. Archiv Environ ContamToxicol 35(1):602–626
Skjolding LM, Soerensen SN, Hartmann NB, Hjorth R, Hansen SF, Baun A (2016) Aquatic ecotoxicity testing of nanoparticles: the quest to disclose nanoparticle effects. Angew Chem Int Ed 55(2):15224–15265
Smith IK, Vierheller TL, Thome CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5.5 dithiobis (2-nitrobenzoic acid). Anal Biochem 175(1):408–413
Soto-Jimenez MF, Arellano-Fiore C, Rocha-Velarde R, Jara-Marini ME, Ruelas-Inzunza J, Paj-Osuna F (2011) Trophic transfer of lead through a model marine four-level food chain: Tetraselmis suecica, Artemia franciscana, Litopenaeus vannamei, and Haemulon scudderi. Arch Environ Contam Toxicol 61(1):280–298
Tangaa SR, Selck H, Winther-Nielsen M, Khan FR (2016) Trophic transfer of metal-based nanoparticles in aquatic environments: a review and recommendations for future research focus. Environ Sci, Nanomat 3(1):966–984
Travis RJ, Solon O, Quimbo SA, Tan CMC, Butrick E, Peabody JW (2007) Elevated blood-Lead levels among children living in the rural Philippines. Bull World Health Organ 85(9):674–680
Venditti P, Di-Stefano L, Di-Meo S (2013) Mitochondrial metabolism of reactive oxygen species. Mitochondrion 13:71–82
Vicaria T, Dagostima AC, Klingelfusa T, Galvana GL, Monteirob PS, Pereirab LS, Assisb HC, Cestaria MM (2018) Co-exposure to titanium dioxide nanoparticles (NpTiO2) and lead at environmentally relevant concentrations in the neotropical fish species Hoplias intermedius. Toxicol Rep 5:1032–1043
Winston GW (1991) Oxidants and antioxidants in aquatic animals. Comp Biochem Physiol Pharmacol Toxicol 100(1–2):173–179
Wu H, Hu S, Jian H, Lihua Y, Sen L, Yongyong G, Zhou B (2019) Impact of co-exposure to titanium dioxide nanoparticles and Pb on zebrafish embryos. Chemosphere 233:579–589
Wu H, Miao W, Zhub B, Xiao X, Li Y, Dirbaba NB, Zhou B (2015) Effects of titanium dioxide nanoparticles on lead bioconcentration and toxicity on thyroid endocrine system and neuronal development in zebrafish larvae. Aquat Toxicol 161:117–126
Xu JB, Yuan XF, Lang PZ (1997) Determination of catalase activity and catalase inhibition by ultraviolet spectrophotometry. Chinese Environ Chem 16(1):73–76
Zhang X, Sun H, Zhang Z, Niu Q, Chen Y, Crittenden JC (2007) Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles. Chemosphere 67(1):160–172
Zhu X, Chang Y, Chen Y (2010) Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere 78(1):209–224
Acknowledgments
The National Institute for Fisheries and Freshwater Research (NIFFR), New Bussa, Nigeria, partly supported the work. We would like to thank the anonymous reviewers for their critical comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We conducted all animal protocols in this study under the supervision and approval of the ethical board committee (UERC/LSC/029) of the University of Ilorin, Ilorin, Nigeria.
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 267 kb)
Rights and permissions
About this article
Cite this article
Matouke, M., Mustapha, M. Impact of co-exposure to titanium dioxide nanoparticles (TiO2 NPs) and lead (Pb) on African catfish Clarias gariepinus (Burchell, 1922) fed contaminated copepods (Eucyclop sp.). Environ Sci Pollut Res 27, 16876–16885 (2020). https://doi.org/10.1007/s11356-020-08234-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08234-0