Abstract
The fate of carbaryl in the Seto Inland Sea (west Japan) was predicted using a mass distribution model using carbaryl concentrations in river and sea water samples, degradation data, and published data. The predicted carbaryl concentrations in water in Kurose River and the Seto Inland Sea were 4.320 and 0.2134 μg/L, respectively, and the predicted concentrations in plankton, fish, and sediment were 0.4140, 2.436, and 1.851 μg/g dry weight, respectively. The carbaryl photodegradation and biodegradation rates were higher for river water (0.330 and 0.029 day−1, respectively) than sea water (0.23 and 0.001 day−1, respectively). The carbaryl photodegradation rates for river and sea water (0.33 and 0.23 day−1, respectively) were higher than the biodegradation rates (0.029 and 0.001 day−1, respectively). The hydrolysis degradation rate for carbaryl in sea water was 0.003 day−1, and the half-life was 231 days. Land (via rivers) was the main source of carbaryl to the Seto Inland Sea. The model confirmed carbaryl is distributed between sediment, plankton, and fish in the Seto Inland Sea. Degradation, loss to the Open Ocean, and sedimentation are the main carbaryl sinks in the Seto Inland Sea, accounting for 43.81, 27.90, and 17.68%, respectively, of total carbaryl inputs. Carbaryl source and sink data produced by the model could help in the management of the negative impacts of carbaryl on aquatic systems and human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides have greatly improved crop productivity. However, improper pesticide application has led to most environmental media, particularly aquatic media, around the world becoming contaminated. Pesticide contamination of water has become a serious environmental problem in the last few decades, and the long-term effects of contaminated water on human health and on other species are of great concern (Sudo et al. 2002; Derbalah et al. 2013, 2014, 2016).
Carbaryl is a carbamate insecticide that is used to control insect pests of crops and some other insect pests, such as mosquitoes and lice (EPA 2004). The United States Environmental Protection Agency has classed carbaryl as a possible carcinogen (Bazrafshan et al. 2017). Carbaryl can damage the human immune system, central nervous system, and endocrine system. Carbaryl is one of the most frequently detected insecticides in water according to the United States National Water-Quality Assessment Program (Chattoraj et al. 2014). This means carbaryl could have adverse effects on aquatic organisms and humans (Bazrafshan et al. 2017).
Pesticides can be removed from the aquatic environment through biodegradation (Tixier et al. 2001; Derbalah et al. 2013, 2014, 2016). Aquatic organisms can biodegrade some pesticides, and this a key survival strategy (Singh 2008). Some pesticides act as sources of carbon and nitrogen to aquatic organisms (Cullington and Walker 1999; Barragan-Huerta et al. 2007; Derbalah et al. 2013, 2014, 2016). It has been found that various bacterial isolates (e.g., Aeromonas sp., Bacillus spp., Corynebacterium spp., Morganella spp., and Pseudomonas sp.) can biodegrade carbaryl (Hamada et al. 2015).
Photodegradation (direct and indirect) is considered to be the most important abiotic pesticide degradation process in aquatic environments (Katagi 2018). Indirect photolysis produces hydroxyl radicals that can degrade pesticides. Hydroxyl radicals are produced naturally in water through several photolysis mechanisms, including NO2− and NO3− photolysis (Mopper and Zhou 1990). Hydroxyl radicals react with organic pollutants at high reaction rates, so hydroxyl radicals can be important contributors to organic pollutant (e.g., pesticide) degradation (Haag and Hoigné 1986).
Chemical hydrolysis decomposes a proportion of organic compounds such as pesticides but does not completely remove many organic compounds from most natural water at neutral pH (Lacorte and Barcelo 1994). Thuyet et al. (2013) found that some pesticides in alkaline water can be degraded through hydrolysis. Carbaryl is easily degraded in alkaline water but is resistant to degradation at acid and neutral pH values (Roberts and Hutson 1999).
Most pesticides that are resistant to degradation in aquatic environments accumulate in plankton, fish, and other aquatic organisms. Pesticides accumulate more in organisms higher up the food chain (e.g., fish) than in organisms lower down the food chain (Senthilkumar et al. 2001). Fish accumulate pesticide residues and may be consumed by humans and large marine animals. Decomposing dead fish will release pesticides and other pollutants into the water and sediment. Many small organisms live in sediment, so pesticide residues on the surfaces of sediment particles are effectively biologically available to humans through consuming animals that consume sediment-dwelling organisms (Chapman et al. 2002).
It is important to predict the fates of pesticides in aquatic environments and the distributions of pesticides in different aquatic environment components to allow the risks posed by pesticides to be assessed and to help decision-makers develop appropriate regulations. Predicting the fate of a pesticide requires appropriate information on the characteristics of the pesticide to be available. Some characteristics can be measured directly, but other characteristics cannot be measured (Mackay et al. 2001). It is therefore important to use models to predict the fates of pesticides in aquatic systems and the distributions of pesticides in sediment and biota. Very few studies of pesticides in the Seto Inland Sea, in western Japan, have been performed. The fate of carbaryl in the Seto Inland Sea was investigated in the study described here.
In this study, carbaryl residues in water from the Kurose River collected between March 2016 and February 2017 and in water, sediment, plankton, and fish from the Seto Inland Sea collected between 3 and 7 July and between 5 and 6 August 2017 were determined. Carbaryl hydrolysis, photodegradation, and biodegradation in river and sea water were assessed. The carbaryl residue, biodegradation, and photodegradation data and previously published data were used to predict the fate of carbaryl using a mass distribution model.
Materials and methods
Reagents
Carbaryl was purchased from Sigma-Aldrich Laborchemikalien (Seelze, Germany). Acetonitrile (99.8%) and methanol (99.7%) were purchased from Nacalai Tesque (Kyoto, Japan).
Sampling sites
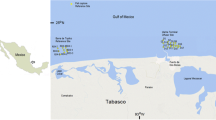
A map of the study area with the sampling sites marked is shown in Fig. 1. Water samples from the Kurose River, which runs through the city of Higashi–Hiroshima, were collected at sites K1, K2, K3, K4, K5, and K6 (Fig. 1a). Samples were collected each month over the year March 2016 to February 2017. The sampling sites were selected to represent the different uses of land near the river (agricultural, residential, industrial, and remote areas). Site K1 was in the upstream part of the river, and sites K2–K5 were close to areas with low population densities and intensive agricultural activities. Site K3 was near an industrial area with a high population density, and site K4 was next to the Higashi–Hiroshima Purification Centre wastewater treatment facility. Site K6 was downstream of the other samples near the Seto Inland Sea and was surrounded by farmland. Several sampling sites in the sea were selected, and their locations are shown in Fig. 1b. The characteristics of the sampling sites and areas are presented in Table 1S. For each site, the characteristics presented are the name of the coast near the sampling sites, the main activities in the catchment area, water depth, salinity, and water temperature. A total of 17 sampling sites were used during the 2017 cruises. Marine samples (sea water, sediment, plankton, and fish) were collected on cruises by the Hiroshima University research ship Toyoshio Maru on the Seto Inland Sea on July 3–7, 2017 and August 5–6, 2017. During the July 2017 cruise, sea water, plankton, and sediment samples were collected from Aki-nada (St. 1 to St. 3), Kii Channel (St. 5 to St. 7), Osaka Bay (St. 8 to St. 11 and St. 16), Harima-nada (St. 12 to St. 15), Bisan Seto (St. B2), and Hiuchi-Nada (St. B10). During the August 2017 cruise, sea water and plankton samples were collected from four sites (St. 1HB to St. 4HB) in Hiroshima Bay.
River water sample collection, preparation, and extraction
Each river water sample was passed through a glass-fiber filter paper (GC-50, pore size 0.45 μm, diameter 47 mm; Advantec MFS, Dublin, CA, USA) and transferred to a 1-L amber glass bottle. Carbaryl was extracted by passing the sample through a Sep-Pak Classic C18 cartridge mounted on an S.P.C. CONT automated solid-phase extraction (SPE) system (Waters, Tokyo, Japan), as described in detail by Chidya et al. (2018). The cartridge was pre-conditioned with 5 mL of dichloromethane, then 5 mL of methanol, and then 10 mL of Milli-Q (MQ) water. A 1-L aliquot of a water sample was passed through the cartridge at a flow rate of 12 mL/min. The cartridge was then washed with 10 mL of MQ water and dried under vacuum for 20 min using a SPE manifold system. The carbaryl that was adsorbed to the adsorbent in the Sep-Pak C18 cartridge was eluted with 5 mL of dichloromethane into a test tube over a period of 5 min, and then a vacuum was applied for 10 min. The extract was evaporated to 50 μL under a stream of nitrogen at a flow rate of ~ 1.0 L/min. Acetone (1 mL) was then added, and the solution was evaporated to a final volume of ~ 100 μL under a stream of nitrogen. The sample was kept at < 4 °C until analysis.
Seawater sample collection, preparation, and extraction
Sea water samples were collected using a 10-L Niskin sampler (General Oceanics, Miami, FL, USA) using a SBE-9 plus Rosset system with CTD sensors (Sea-Bird Scientific, Bellevue, WA, USA). Surface water samples were collected from 0 to 5 m deep, and bottom water samples were collected from 10 to 38 m deep. Each sample was passed through a filter and extracted following a procedure for river water described by Chidya (2018).
Plankton sample collection, preparation, and extraction
Each plankton sample was collected using an NXX13 vertical plankton net (100 μm mesh; North Pacific Standard-NORPAC, Saitama, Japan) fitted with a flow meter (Rigosha, Saitama, Japan). The collected plankton was transferred into a 250-mL plastic bottle and passed through a weighed glass fiber filter (GC-50, pore size 0.45 μm, diameter 47 mm). The filter paper with plankton attached was wrapped in aluminum foil and stored in a refrigerator at 4 °C. In the laboratory, the filter paper with plankton attached was dried using an FDU-506 freeze dryer (EYELA, Okayama, Japan). The filter paper was then weighed, cut into small pieces, and transferred to a 100-mL volumetric flask containing 30 mL of methanol. The mixture was shaken for 1 h at 254 rpm using an EYELA MMS 310 mechanical multi-shaker obtained from Rikakikai (Tokyo, Japan). The mixture was transferred to a separatory funnel. The flask was washed with 25 mL dichloromethane into the separatory funnel, and then 300 mL of 5% NaCl and 50 mL of dichloromethane were added to the separatory funnel. The separatory funnel was gently shaken, and then the contents were allowed to settle until two layers formed. Anhydrous Na2SO4 was added to remove water. The organic extract was passed through a filter into a round-bottomed flask and evaporated to ~ 5 mL using a rotary evaporator at 40 °C. The extract was then evaporated almost to dryness under a stream of nitrogen, and then the residue was dissolved in 1 mL of acetone. The acetone solution was evaporated to ~ 100 μL under a gentle stream of nitrogen and then diluted to 2 mL with a 1:1 v/v mixture of methanol and MQ water. The plankton mass per unit volume of sea water was calculated by dividing the mass of the freeze-dried plankton by the volume of water passed through the plankton net.
Sediment sample collection, preparation, and extraction
Surface sediment (0–5 cm deep) samples were collected using a Smith–McIntyre type sampler (sampling area 33 cm × 33 cm) (Rigosha & Co., Saitama, Japan). Each sample was immediately transferred to a polyethylene bag and stored at 4 °C in the dark until it was extracted. A wet sediment sample was transferred to a Petri dish and placed in an FDU-506 freeze dryer (EYELA). A 10-g aliquot of the dried sample was transferred to a 100-mL flask containing 35 mL of acetonitrile and some anhydrous Na2SO4. The mixture was shaken using an EYELA MMS 310 mechanical shaker (Rikakikai) for 1 h to extract carbaryl. The mixture was allowed to settle for 0.5–1.0 h, and then the organic layer was passed through a filter and transferred to a round-bottomed flask. The solution was evaporated to 0.5 mL using a rotary evaporator at 40 °C. The sample was cleaned up using a preconditioned Waters OASIS HLB 60 mg, 3 cm3 SPE cartridge. The cartridge had been conditioned with 3 mL of acetonitrile, then 3 mL of methanol, and then 5 mL of MQ water. Carbaryl was eluted by passing 5 mL of dichloromethane through the column. The extract was collected in a test tube and then evaporated under a stream of nitrogen to ~ 200 μL. The extract was diluted with 1 mL of a 1:1 mixture of methanol and MQ water and then analyzed by high-performance liquid chromatography (HPLC).
Fish sample collection, preparation, and extraction
Fish were caught during the 2017 cruise on the Seto Inland Sea. Red seabream (Pagrus major) (n = 3) and Japanese horse mackerel (Trachurus japonicus) (n = 3) were collected. The mean length and wet weight of the smaller fish were 22 cm and 224 g, respectively. The mean length and wet weight of the larger fish were 31 cm and 790 g, respectively. A 20-g wet weight aliquot of a fillet or whole fish sample was cut into small pieces and homogenized with 100 mL of methanol using a Blendtec 1-800 blender (Blendtec, Orem, UT, USA). Selected organs (liver, gills, and viscera) were treated in the same way without removing the organ contents. The homogenized sample was transferred to a 100-mL conical flask and shaken using an MMS 310 mechanical shaker (EYELA) for 1 h. The mixture was then centrifuged at 3000 rpm using a (KubotaKN-70 centrifuge (Kubota, Osaka, Japan) for 30 min. The supernatant was passed through a DISMIC disposable membrane filter (25HP020AN, 0.20 μm pores) (Advantec MFS) into 1-L amber glass bottle and then diluted to 1 L with MQ water. The liquid was passed through a C18 SPE cartridge to extract carbaryl and then concentrated following a procedure similar to that described by Chidya (2018).
HPLC analysis and quality control
The HPLC system had a Prominence LC-20AD pump (Shimadzu, Kyoto, Japan) that delivered the mobile phase (a 1:1 v/v mixture of acetonitrile and MQ water) at a flow rate of 1 mL/min. The injection volume was 20 μL. Separation was achieved using a Cosmosil MS-II C18 column (250 mm long, 4.6 mm i.d., 5 μm particle size; Nacalai Tesque). The HPLC system had a Prominence LC-20 AD UV-visible detector (Shimadzu), which was used with a detection wavelength of 220 nm. The limits of detection and quantification) were determined using the method described by Chidya (2018). The carbaryl limits of detection and quantification were 0.005 and 0.010 μg/L, respectively.
Water quality analysis
The NO3− and NO2− concentrations in the river water samples were measured using ion chromatography (Dionex ICS 1600, Thermo Scientific Dionex, Tokyo Japan). The NO3− and NO2− concentrations in the sea water samples were determined using a Shimadzu UV-1200 spectrophotometer (Shimadzu). The pH of each water sample was determined using a Horiba D-24 pH/conductivity meter (Horiba, Kyoto, Japan). The dissolved organic carbon (DOC) concentrations in the water samples were determined using a TOC-VCSH total organic carbon analyzer (Shimadzu).
Biodegradation experiment
All used glassware was sterilized at 180 °C for 2 h using the Drying Sterilizer SH401 (Yamato Scientific Co. Ltd., Tokyo, Japan). An unfiltered sea or river water sample (250 mL) was placed in a 300-mL conical flask, which was sealed with a silicone membrane. Each test was performed in triplicate. Each sample was spiked with carbaryl to give a final concentration of 500 μg/L (Derbalah et al. 2004; Kaonga et al. 2016). The samples were kept at 25 ± 2 °C in a Biotron incubator for 5 weeks, and an aliquot of each sample was removed each week for HPLC analysis.
Photodegradation experiment
Carbaryl photodegradation was investigated using a solar simulator. Each test sample contained carbaryl at a concentration of 0.5 mg/L. Tests were performed using each type of water. The tests were performed using a method described by Kaonga et al. (2016). The test samples were irradiated using an Oriel 81160-1000 solar simulator unit equipped with a 300-W model 81160 xenon lamp. A sample was placed in a 60-mL quartz cell and irradiated while being stirred using a magnetic stirrer. The temperature was kept at 20 °C. At regular intervals, an aliquot of the sample being irradiated was removed and analyzed by HPLC using the conditions described above.
Hydrolysis
Hydrolysis is one of the processes through which carbaryl can be degraded in water at > pH 7 (Carpenter 1990). Carbaryl hydrolysis rates in sea water were therefore determined without altering the pH. Sea water was passed through a syringe filter to remove microorganisms, and then the water was spiked with carbaryl to give a concentration of 500 μg/L. Each bottle containing spiked sea water was wrapped in aluminum foil to exclude sunlight and help maintain a constant temperature. At specified time intervals, an aliquot of each sample was removed and analyzed by HPLC using the method described above.
Determining of the degradation kinetics
The degradation rate constants k and half-lives t1/2 for hydrolysis, biodegradation, and photodegradation of carbaryl were determined using the method described by Kaonga et al. (2016). The amount of carbaryl that would be photodegraded in natural water in a year was determined using the rate constants produced using the solar simulator experiment data normalized and corrected by a factor of 0.10 (Finlayson-Pitts and Pitts-Jr 1986; Diamond et al. 2001; Takeda et al. 2014; Kaonga et al. 2016).
Carbaryl mass distribution calculations
Carbaryl residue concentrations in river water and marine samples as well as degradation data were used for the mass distribution model of carbaryl in the Seto Inland Sea. The published data used to calculate the total carbaryl distributions in the aquatic environment are presented in Table 1. The annual water exchange rate was calculated using a water exchange rate of 90% in 1.5 years (Yamazaki and Uejima 2005), meaning the water exchange rate for 1 year would be 60% ((1/1.5) × 90%). Carbaryl inputs in river water were estimated by multiplying the total water volume input into the Seto Inland Sea by the mean carbaryl concentration in river water. Carbaryl input via precipitation will depend on the volume of precipitation falling over the Seto Inland Sea, and the carbaryl concentration in rainwater derived from non-agricultural areas. No carbaryl concentrations in rainwater in Japan were available, so the carbaryl concentration found in rainwater in California was used (Lee 2005). The total carbaryl mass in sea water was estimated by multiplying the total water volume by the mean carbaryl concentration. The total sediment mass was calculated by multiplying the total sedimentation yield by the mean carbaryl concentration. The total mass of fish was estimated by multiplying the amount of fish caught (in t) by the mean carbaryl concentration. The total mass biodegraded was estimated by multiplying the yield indicated by the biodegradation rate constant by the mean carbaryl concentration in water and the total water volume. The amount of carbaryl that was photodegraded was estimated by multiplying the total product of the photodegradation rate constant by the mean carbaryl concentration in water and the volume of water in the Seto Inland Sea to 5 m deep. The depth 5 m was used because of the mean photon intensity values, as explained by Kaonga et al. (2016). It was assumed that the carbaryl remaining in the water without any decomposition, biological uptake, and sedimentation outflows to open ocean within a certain period of time. The amount of carbaryl lost to the open ocean was calculated from the annual water exchange rate and the amount of carbaryl remaining in the water (without any chemical and biological processes occurring). The carbaryl residence time was calculated using a standard method that has been published previously. The pesticide residence time could not be equated to the water residence time and so needed to be calculated in a different way. The mean carbaryl residence time in the Seto Inland Sea was calculated using the equation published by Takeoka (1984), Monsen et al. (2002), and Keller (2007) and shown below.
The carbaryl mass distribution calculations were modified versions of calculations used in a mass distribution model published by Kaonga et al. (2016).
Results and discussion
Carbaryl residues in water and marine samples
The carbaryl concentrations in river water and marine samples (sea, fish, plankton, and sediments) are summarized in Table 2. The mean carbaryl concentrations in the Kurose River water and Seto Inland Sea water were 4.320 and 0.2134 μg/L, respectively. The mean carbaryl concentration in plankton was 2.436 μg/g dw, which was higher than the mean concentrations in fish (1.851 μg/g dw) and sediment (0.4140 μg/g dw).
The carbaryl concentrations being higher in the plankton, fish, and sediment than in the water indicated that carbaryl had bioaccumulated. Carbaryl is used in all of the prefectures surrounding the Seto Inland Sea (Fig. 2). In total, 6.26 t of carbaryl is used each year in the prefectures around the Seto Inland Sea (NIES 2018). This is 13% of the total amount of carbaryl used in Japan. Fukuoka, Hiroshima, and Wakayama prefectures are major users of carbaryl, and the carbaryl use rates in these prefectures are 1.78, 1.28, and 0.80 t/year, respectively. Pesticide concentrations in river water can increase during a storm event, meaning there will be great uncertainty in carbaryl inputs in river water calculated using only carbaryl concentrations found during non-storm periods. However, carbaryl is used mostly on paddy fields in the study area. These fields hold water, and the water is only released at certain times of year. Paddy fields can flood during a storm, but this happens rarely because of the ways paddy fields are constructed.
Biodegradation and photodegradation
The biodegradation and photodegradation kinetics data for carbaryl in the Kurose River and Seto Inland Sea water are summarized in Table 3. The biodegradation rate constants for carbaryl in river and sea water were 2.9 × 10−2 and 1.0 × 10−3 day−1, respectively. The carbaryl half-lives for biodegradation in river and sea water were 23.90 and 693 days, respectively. The photodegradation rate constants for carbaryl in river and sea water were 0.33 and 0.23 day−1, respectively. The carbaryl half-lives for photodegradation in river and sea water were 2.10 and 3.01 days, respectively. The carbaryl hydrolysis rate for sea water was 0.003 day−1, and the half-life was 231 days.
The carbaryl biodegradation rate and half-life were markedly higher for sea water than river water, possibly because of the different natures, qualities, and numbers of microbes in sea and river water (Hobbie 1988; Kaonga et al. 2016). Also, the large amounts of salts in sea water may inhibit microbial activity and therefore biological decomposition of pesticides (Kodama and Kuwatsuka 1980; Kaonga et al. 2016). The results agreed with the results of studies by Bondarenko et al. (2004) and Kaonga et al. (2016), who found that pesticides are biologically degraded much faster in river water than sea water.
The carbaryl photodegradation rate was higher for river water than sea water. This could have been caused by the nitrite and nitrate concentrations being higher in Kurose River water that Seto Inland Sea water (Takeda et al. 2004). Nitrate and nitrite are important sources of hydroxyl radicals (•OH) in natural water (Takeda et al. 2004; Olasehinde et al. 2012). Olasehinde et al. (2012) found that direct photolysis of NO2− accounted for up to 73% of the •OH formation rate and suggested that photolysis of NO2− is an important source of •OH in upwelling areas. Takeda et al. (2004) found markedly higher •OH formation rates for water containing high nitrate and nitrite concentrations than for water containing lower nitrate and nitrite concentrations, and they found that nitrate and nitrite photolysis produces 10–80% of the •OH formed in river and sea water. The higher photodegradation rate for river water than sea water may also have been caused by the DOC concentration being higher for the river water than sea water, because DOC is one of the main sources of •OH (Mopper and Zhou 1990). We concluded that the high nitrite, nitrate, and DOC concentrations in river water caused more •OH to be formed in the river water than sea water (Mopper and Zhou 1990; Takeda et al. 2004). The higher •OH concentration in river water than sea water will have caused faster carbaryl photolysis in the river water than sea water.
The carbaryl degradation rate constants were high for both biodegradation and photodegradation and for both river and sea water (Table 3). This may have been because of the low bond energies of the C–S bonds in carbaryl, meaning the C–S bonds can easily be broken (Doong and Chang 1998). A considerable amount of carbaryl was hydrolyzed at alkaline pH values (Table 4). This agreed with the results of a study by Armbrust and Corsby (1991), who found that the fate of carbaryl in sea water is largely controlled by hydrolysis, biodegradation, and photolysis (which, under natural conditions, can completely degrade carbaryl within a day). The carbaryl residence time was longer than the photodegradation half-life, probably because the photodegradation half-life was calculated for only 0–5 m deep (Kaonga et al. 2016).
Carbaryl mass distribution
The carbaryl mass distribution in the Seto Inland Sea is shown in Fig. 3. The total carbaryl input in rivers flowing into the Seto Inland Sea was estimated to be 216 t/year, which accounted for 99.5% of the total carbaryl input into the Seto Inland Sea. Atmospheric deposition contributed 0.5% of the total carbaryl input. The carbaryl residence time in the Seto Inland Sea was estimated to be 0.83 year (the data used in the calculation are summarized in Table 5).
As shown in Table 6 and Fig. 3, the carbaryl input mass to the semi-closed Seto Inland Sea was distributed between the sea water, sediment, plankton, and fish. Total input to sea water was estimated to be 217.20 t. A large proportion (43.81% of the total carbaryl input) of carbaryl in sea water was found to be photodegraded, biodegraded, and hydrolyzed. The separate contributions of biodegradation, photodegradation, and hydrolysis were 25.42%, 11.39%, and 7%, respectively, of the total carbaryl input. The amount of carbaryl lost to the open ocean accounted for 27.90% of the total carbaryl input. About 17.68% of the total amount of carbaryl input into the sea was lost to sediment. A considerable amount (10.4% of the total carbaryl input) of carbaryl was found to be taken up by plankton and fish. We concluded that degradation and loss to the open ocean were the main causes of carbaryl losses from the Seto Inland Sea water.
The larger losses of carbaryl through degradation than through other processes may be caused by the high degradation rates for chemical hydrolysis, photochemical degradation, and biodegradation of carbaryl (Chapalamadugu and Chaudhry 1991; Miller and Chin 2002; Zhu et al. 2008). Armbrust and Corsby (1991) found that the fate of carbaryl in sea water appears to be controlled by hydrolysis, biodegradation, and photolysis. This could be because the contribution of photodegradation was calculated only for the surface layer (0–5 m deep). The biodegradation and hydrolysis contributions calculated for up to 38 m deep agreed with the results of a study by Kaonga et al. (2016).
The total amount of carbaryl used in all of the prefectures surrounding the Seto Inland Sea is 6.26 t each year, and the total carbaryl input to the Seto Inland Sea in rivers was estimated to be 217.20 t/year. This indicated that a much larger amount of carbaryl enters the Seto Inland Sea in river water than is used on land around the sea. This could be because the total amount of carbaryl used in all the prefectures around the Seto Inland Sea in a particular year was estimated from the amount of carbaryl bought from agro-dealers in that year. However, farmers may use carbaryl bought in a previous year, meaning carbaryl use may exceed carbaryl sales. It is also possible, depending on environmental conditions, that some carbaryl could accumulate in sediment and other media and be re-released into the water column because of churning caused by waves. The results indicated that hydrolysis removes a large amount of carbaryl considering the low k value for hydrolysis. This was because the k values shown in Table 3 are in units of per day but the hydrolysis flux is in units of per year. The flux will therefore have been large even though the k value for hydrolysis appeared to be small.
The results indicated that considerable amounts of carbaryl are taken up by fish, but the total mass of fish not caught may affect the mass distribution of carbaryl in the Seto Inland Sea. However, carbaryl not included in the other media would have been part of the amount lost to the open ocean. Similar modeling studies have been performed for other pesticides in the Seto Inland Sea (Kaonga et al. 2016). In one such study, by Kaonga et al. (2016), the main routes through which pesticides were lost from the Seto Inland Sea were found to be transfer to sediment and to the open ocean. The high carbaryl concentrations found in sediment from Japan, including in sediment from the Seto Inland Sea, support this conclusion for carbaryl (Kuniaki et al. 2005). We found that degradation, transfer to sediment, and loss to the open ocean are the main routes through which carbaryl is lost from the Seto Inland Sea. Many other studies have provided evidence for transfer to sediment and losses to the open ocean being major routes though which pesticides are lost from seas (Bidleman et al. 1995; Looser et al. 2000; Kronvang et al. 2003; Stemmler and Lammel 2009; Ramirez-Llodra et al. 2011; Sabatier et al. 2014; Kaonga et al. 2016). The Berkeley–Trent model also supports this conclusion (Toose et al. 2004).
Conclusions
Carbaryl concentrations in river and sea water and in marine organisms (plankton, sediment, and fish) were determined. High carbaryl concentrations in plankton, fish, and sediment relative to the carbaryl concentrations in water indicated that carbaryl may bioaccumulate in aquatic organisms. The carbaryl degradation rate was lower for sea water than river water. River water is the main source of carbaryl to the Seto Inland Sea. A model of the fate of carbaryl in water in the Seto Inland Sea confirmed that carbaryl becomes distributed between the sediment, plankton, and fish. The model also indicated that the main route through which carbaryl is lost from the Seto Inland Sea is degradation but that losses to the open ocean and sediment are also important.
References
Armbrust KL, Corsby AG (1991) Fate of carbaryl, I-naphthol, and atrazine in seawater. Pac Sci 45(3):314–320
Barragan-Huerta BE, Costa-Perez C, Peralta-Cruz J, Barrera-Cortes J, Esparza-Garcıa F, Rodriguez-Vazquez R (2007) Biodegradation of organochlorine pesticides by bacteria grown in microniches of the porous structure of green bean coffee. Int Biodeterior Biodegrad 59:239–244. https://doi.org/10.1016/j.ibiod.2006.11.001
Bazrafshan AA, Ghaedi M, Rafiee Z, Hajati S, Ostovan A (2017) Nano-sized molecularly imprinted polymer for selective ultrasound-assisted microextraction of pesticide carbaryl from water samples: spectrophotometric determination. J Colloid Interface Sci 498:313–322. https://doi.org/10.1016/j.jcis.2017.03.076
Bidleman TF, Antunen LM, Falconer RL, Barrie LA, Fellin P (1995) Decline of hexachlorocyclohexane in the Arctic atmosphere and reversal of air-sea gas exchange. Geophys Res Lett 22:219–222
Bondarenko S, Gan J, Haver DL, Kabashima JN (2004) Persistence of selected organophosphate and carbamate insecticides in waters from a coastal watershed. Environ Toxicol Chem 23:2649–2654
Carpenter M (1990) Hydrolysis of 14C-carbaryl in aqueous solutions buffered at pH 5, 7 and 9, vol 169-218 # 92535. Department of Pesticide Regulation, Sacramento
Chapalamadugu S, Chaudhry GR (1991) Hydrolysis of carbaryl by a Pseudomonas sp. and construction of a microbial consortium that completely metabolizes carbaryl. Appl Environ Microbiol:744–750
Chapman PM, Ho KT, Munns WR, Solomon K, Weinstein MP (2002) Issues in sediment toxicity and ecological risk assessment. Mar Pollut Bull 44:271–278. https://doi.org/10.1016/S0025-326X(01)00329-0
Chattoraj S, Mondal NK, Das B, Roy P, Sadhukhan B (2014) Carbaryl removal from aqueous solution by Lemna major biomass using response surface methodology and artificial neural network. J Environ Chem Eng 2:1920–1928. https://doi.org/10.1016/j.jece.2014.08.011
Chidya RCD (2018) Occurrence, dynamics, spatio-temporal variations and risk assessment of pesticide residues in Kurose river and Seto Inland Sea. Doctoral thesis, Hiroshima University, Japan pp 98-101
Cullington JE, Walker A (1999) Rapid biodegradation of Diuron and other phenylurea herbicides by a soil bacterium. Soil Biol Biochem 31:677–686
Derbalah AS, Nakatani N, Sakugawa H (2004) Photocatalytic removal of Fenitrothion in pure and natural waters by photo-Fenton reaction. Chemosphere 57:635–644. https://doi.org/10.1016/j.chemosphere.2004.08.025
Derbalah AS, Ismail AA, Shaheen SM (2013) Monitoring of organophosphorus pesticides and remediation technologies of the frequently detected compound (chlorpyrifos) in drinking water. Pol J Chem Technol 15(3):25–34
Derbalah AS, Ismail AA, Hamza AM, Shaheen SM (2014) Monitoring and remediation of organochlorine residues in water. Water Environ Res 86(7):584–593
Derbalah AS, Ismail AA, Shaheen SM (2016) The presence of organophosphorus pesticides in wastewater and its remediation technologies. Environ Eng Manag J 15(8):1777–1787. https://doi.org/10.2478/pjct-2013-0040
Diamond ML, Priemer DA, Law NL (2001) Developing a multimedia model of chemical dynamics in an urban area. Chemosphere 44:1655–1667
Doong R, Chang W (1998) Photoassisted iron compound catalytic degradation of organophosphorous pesticides with hydrogen peroxide. Chemosphere 37:2563–2572
EMECS (Environmental Conservation of the Seto Inland Sea) (2008). Outline of the Seto Inland Sea. Available online at: http://www.emecs.or.jp/englishver2/publication/Seto_Inland_Sea/index.html
EPA (2004) Reregistration eligibility decision for carbaryl. US Environmental Protection Agency, Washington, DC
Finlayson-Pitts BJ, Pitts-Jr JN (1986) Atmospheric chemistry: fundamentals and experimental techniques. Wiley, New York
Hamada M, Matar A, Bashir A (2015) Carbaryl degradation by bacterial isolates from a soil ecosystem of the Gaza Strip. Brazilian J of Microbiol 46(4): 1087–1091. https://doi.org/10.1590/S1517-838246420150177S
Haag RW, Hoigné J (1986) Singlet oxygen in surface waters. 3. Photochemical formation and steady-state concentrations in various types of waters. Environ Sci Technol 20:341–348
Hobbie JE (1988) A comparison of the ecology of planktonic bacteria in fresh and salt water. Limnol Oceanogr 33:750–764
Hoshika A, Shiozawa T, Kawana K, Tanimoto T (1991) Heavy metal pollution in sediment from the Seto Inland Sea. Jpn Mar Pollut Bull 23:101–105
Imai I, Yamaguchi M, Hori Y (2006) Eutrophication and occurences of harmful algal blooms in the Seto Inland Sea, Japan. Plankton Benthos Res 1:71–84
Kaonga CC, Takeda K, Sakugawa H (2015) Antifouling agents and Fenitrothion contamination in seawater, sediment, plankton, fish and selected marine animals from the Seto Inland Sea, Japan. Geochem J 49:23–37. https://doi.org/10.2343/geochemj.2.0327
Kaonga CC, Takeda K, Sakugawa H (2016) Concentration and degradation of alternative biocides and an insecticide in surface waters and their major sinks in a semi-enclosed sea, Japan. Chemosphere 145:256–264. https://doi.org/10.1016/j.chemosphere.2015.11.100
Katagi T (2018) Direct photolysis mechanism of pesticides in water. J Pestic Sci 43:57–72
Keller AA (2007) Steady state and residence time. School of Environmental Science and Management, University of California, Santa Barbara. Available online at: http://www2.bren.ucsb.edu/∼keller/courses/esm202/SteadyState&ResidenceTime.pdf
Kodama T, Kuwatsuka S (1980) Factors for the persistence of parathion, methyl-parathion and fenitrothion in seawater. J Pestic Sci 5:351–355 (in Japanese with English abstract)
Kronvang B, Laubel A, Larsen SE, Friberg N (2003) Pesticides and heavy metals in Danish streambed sediment. The interactions between sediments and water. Dev Hydrobiol 169:93–101
Kuniaki K, Takashi A, Kikuo O, Akiko T (2005) Multiresidue determination of pesticides in sediment by ultrasonically assisted extraction and gas chromatography/mass spectrometry. J AOAC Inter 88:1440–1145
Lacorte S, Barcelo D (1994) Rapid degradation of fenitrothion in estuarine waters. Environ Sci Technol 28:1159–1163
Lee P (2005) Concentrations of pesticides in Sacramento Metropolitan area rainwater during the 2004 orchard dormant spray season. Technical report by staff of the California Regional Water Quality Control Board, Central Valley Region, USA
Looser R, Froescheis O, Cailliet GM, Jarman WM, Ballschmiter K (2000) The deep-sea as a final global sink of semivolatile persistent organic pollutants? Part II: organochlorine pesticides in surface and deep-sea dwelling fish of the North and South Atlantic and the Monterey Bay Canyon (California). Chemosphere 40:661–670. https://doi.org/10.1016/S0045-6535(99)00462-2
Mackay D, Webster E, Cousins I, Cahill T, Foster K, Gouin T (2001) An introduction to multimedia models: a final report prepared as a background paper for OECD Workshop Ottawa, October 2001 (2001) CEMC report no. 200102. Available online at: http://www.trentu.ca/academic/aminss/envmodel/CEMC200102.pdf
MAFF (Ministry of Agriculture, Forestry and Fisheries) Japan (2011) Seto Inland Sea Environmental Protection Work. Available online at: http://www.env.go.jp/council/former2013/11seto/y111-02/mat02_5-1.pdf (In Japanese)
Miller PL, Chin Y (2002) Photoinduced degradation of carbaryl in a wetland surface water. J Agric Food Chem 50:6758–6765. https://doi.org/10.1021/jf025545m
Monsen NE, Cloem JE, Lucas LV, Monismith SG (2002) A comment on the use of flushing time, residence time and age transport time scales. Limnol Oceanogr 47:1545–1553. https://doi.org/10.4319/lo.2002.47.5.1545
Mopper K, Zhou X (1990) Hydroxyl radical photoproduction in the sea and its potential impact on marine processes. Sci 250:661–664
NIES (National Institute of Environmental Studies) (2018) Japan, 2012NIES (National Institute of Environmental Studies) Japan Agricultural Chemicals Database (2018) Available online at: http://db-out.nies.go.jp/kis-plus/index_3.html
Olasehinde EF, Ogunsuyi HO, Sakugawa H (2012) Determination of hydroxyl radical in Seto Inland Sea and its potential to degrade Irgarol IOSR. J Appl Chem 1(5):07–14. https://doi.org/10.9790/5736-0150714
Ramirez-Llodra E, Tyler PA, Baker MC, Bergstad OA, Clark MR, Escobar E, Levin LA, Menot L, Rowden AA, Smith CR, Van-Dover CL (2011) Man and the last great wilderness: human impact on the deep sea. PlOS-One 6:1–25. https://doi.org/10.1371/journal.pone.0022588
Roberts TR, Hutson DH (1999) The agrochemicals handbook; the Royal Society of Chemistry, Information Services. MPG Books, Bodmin
Sabatier P, Poulenard J, Fanget B, Reyss J, Develle A, Wilhelm B, Ployon E, Pignol C, Naffrechoux E, Dorioz J, Montuelle B, Arnaud F (2014) Long-term relationship among pesticide applications, mobility and soil erosion in a vineyard watershed. PNAS 111:15647–15652. https://doi.org/10.1073/pnas.1411512111
Senthilkumar K, Kannan K, Subramanian A, Tanabe S (2001) Accumulation of organochlorine pesticides and polychlorinated biphenyls. Environ Sci Pollut Res 8:35–47. https://doi.org/10.1007/BF02987293
Singh DK (2008) Biodegradation and bioremediation of pesticide in soil: concept, method and recent developments. Indian J Microbiol 48:35–40. https://doi.org/10.1007/s12088-008-0004-7
Stemmler I, Lammel G (2009) Cycling of DDT in the global environment 1950–2002: world ocean returns the pollutant. Geophys Res Lett 36:1–15
Sudo M, Kunimatsu T, Okubo T (2002) Concentration and loading of pesticide residues in Lake Biwa basin (Japan). Water Res 36:315–329
Takeda K, Takedoi H, Yamaji S, Ohta K, Sakugawa H (2004) Determination of hydroxyl radical photoproduction rates in natural waters. Anal Sci 20:153–158. https://doi.org/10.2116/analsci.20.153
Takeda K, Katoh S, Mitsui Y, Nakano S, Nakatani N, Sakugawa H (2014) Spatial distributions of and diurnal variations in low molecular weight carbonyl compounds in coastal seawater, and the controlling factors. Sci Total Environ 493:454–462. https://doi.org/10.1016/j.scitotenv.2014.05.126
Takeoka H (1984) Fundamental concepts of exchange and transport time scales in a coastal sea. Cont Shelf Res 3:311–326
Thuyet DO, Watanabe H, Ok J (2013) Effect of pH on the degradation of imidacloprid and fipronil in paddy water. J Pestic Sci 38(4):223–227. https://doi.org/10.1584/jpestics.D12-080
Tixier C, Sancelme M, Bonnemoy F, Cuer A, Veschambre H (2001) Degradation products of a phenylurea herbicide, diuron: synthesis, ecotoxicity, and biotransformation. Environ Toxicol Chem 20:1381–1389. https://doi.org/10.1002/etc.5620200701
Toose L, Woodfine DG, MacLeod M, Mackay D, Gouin J (2004) BETR-World: a geographically explicit model of chemical fate: application to transport of α-HCH to the Arctic. Environ Pollut 128:233–240. https://doi.org/10.1016/j.envpol.2003.08.037
Trapp S, Matthies M (1995) Generic one-compartment model for uptake of organic chemicals by foliar vegetation. Environ Sci Technol 29:2333–2338
Yamazaki M, Uejima H (2005) World largest scale analog simulator (Seto Inland Sea large sized hydrological model) history and results. Nihon Engan-iki Gakkaishi 18:33–35 (In Japanese)
Zhu SH, Wu HL, Xia AL, Han QJ, Zhang Y, Yu RQ (2008) Quantitative analysis of hydrolysis of carbaryl in tap water and river by excitation-emission matrix fluorescence coupled with second-order calibration. Talanta 74(5):1579–1585. https://doi.org/10.1016/j.talanta.2007.10.002
Acknowledgments
We would like to acknowledge the JSPS for funding an invitation research fellowship to Professor Aly Derbalah at Hiroshima University, Japan. We thank Natalie Kim, PhD, and Gareth Thomas, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. We are also grateful for the support of our laboratory members throughout the study period.
Funding
This research was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant number 16KT0149).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Christian Gagnon
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Derbalah, A., Chidya, R., Kaonga, C. et al. Carbaryl residue concentrations, degradation, and major sinks in the Seto Inland Sea, Japan. Environ Sci Pollut Res 27, 14668–14678 (2020). https://doi.org/10.1007/s11356-020-08010-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08010-0